Polyphosphate Polymerase—A Key Enzyme for the Phosphorus Economy of the Microalgal Cell and the Sustainable Usage of This Nutrient
Abstract
1. Introduction
2. Localization in the Cell
3. Polyphosphate Polymerases in Microalgae
4. Structure and Function
5. Regulation of Polyphosphate Polymerase Function
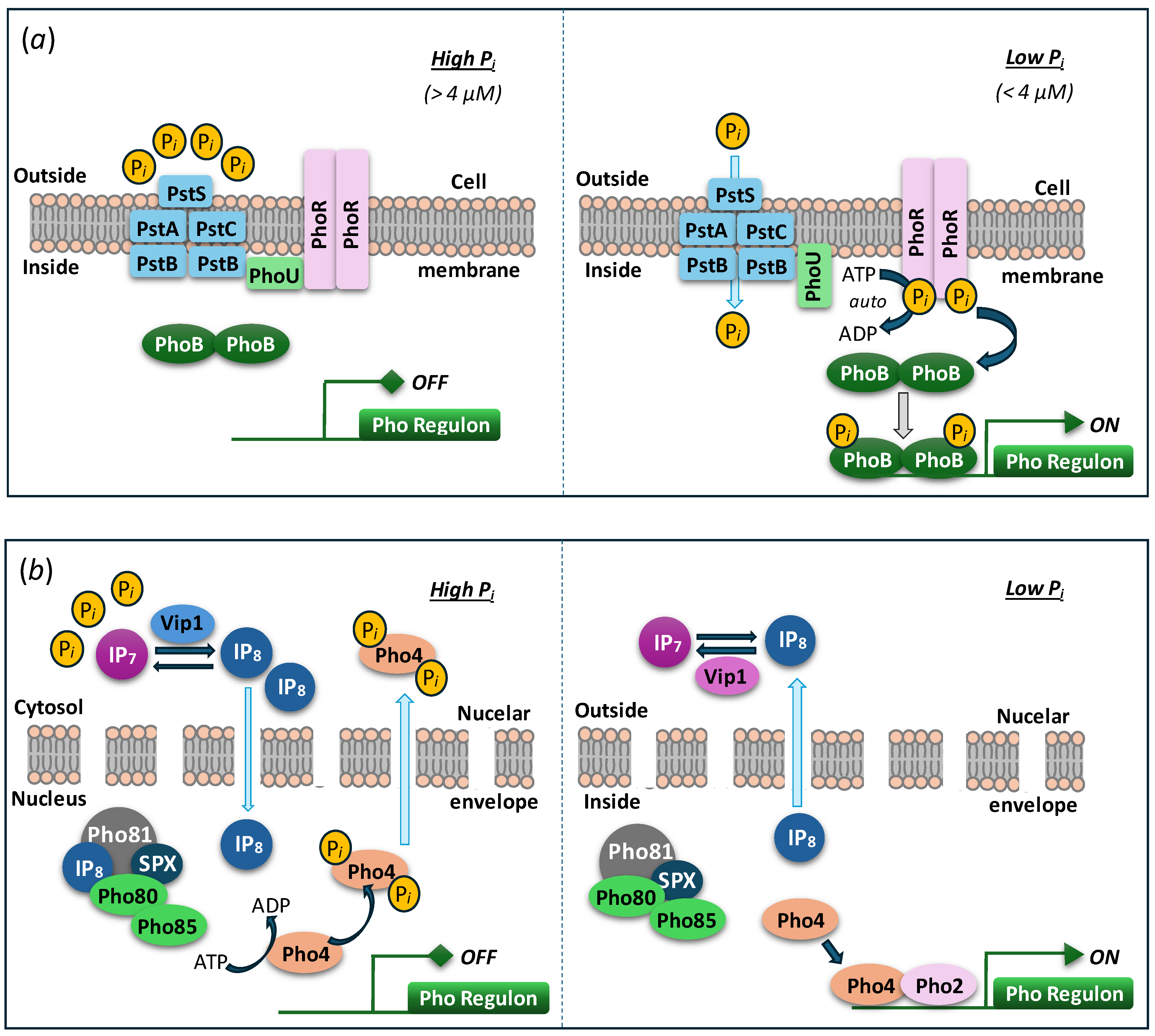
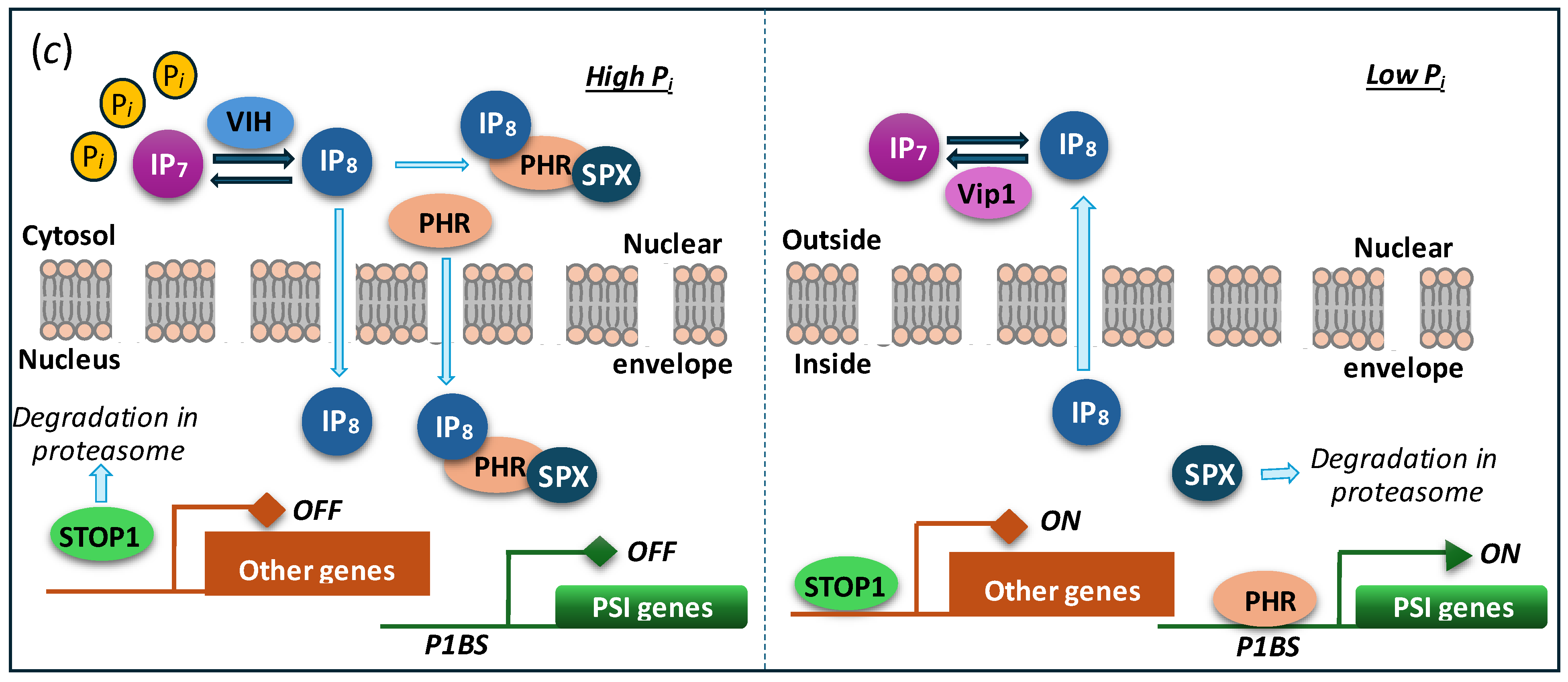
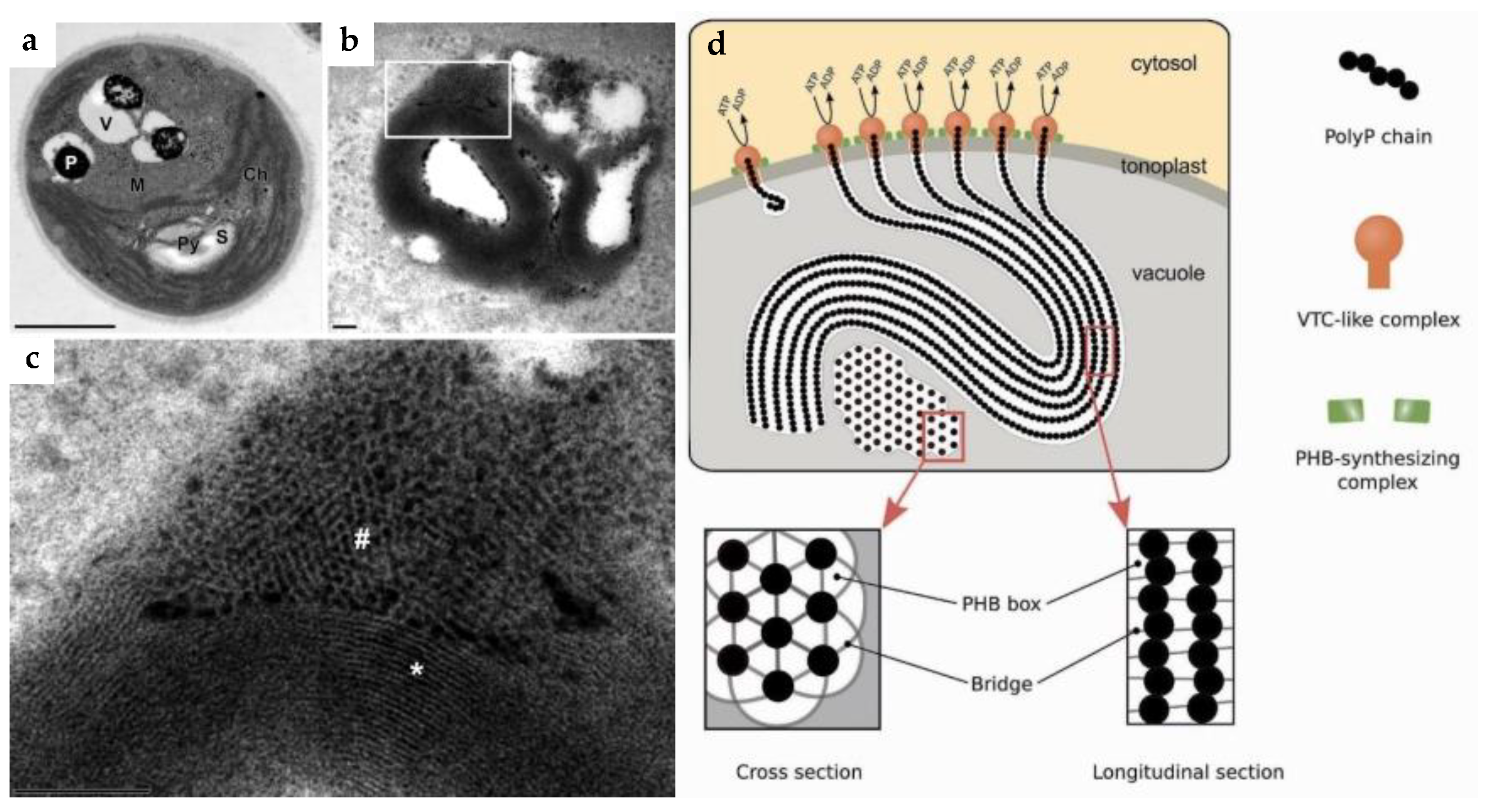
6. Eco-Physiological Significance of Polyphosphate Polymerases
7. Implications for Biotechnology and Sustainability of P Usage
8. Conclusions and Outlook
Funding
Conflicts of Interest
Abbreviations
| P | Phosphorus |
| PHB | Poly-(R)-3-hydroxybutyrate |
| polyP | Polyphosphate |
| PP-InsP | Inositol pyrophosphate |
| PPK | Polyphosphate kinase |
| VTC | Vacuolar Transport Chaperone |
References
- Brown, M.R.; Kornberg, A. Inorganic polyphosphate in the origin and survival of species. Proc. Natl. Acad. Sci. USA 2004, 101, 16085–16087. [Google Scholar] [CrossRef]
- Rao, N.N.; Gómez-García, M.R.; Kornberg, A. Inorganic polyphosphate: Essential for growth and survival. Annu. Rev. Biochem. 2009, 78, 605–647. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, J.L.E.; Kleinermanns, K.; Martin, W.F. Pyrophosphate and Irreversibility in Evolution, or why PPi Is Not an Energy Currency and why Nature Chose Triphosphates. Front. Microbiol. 2021, 12, 759359. [Google Scholar] [CrossRef] [PubMed]
- Dyhrman, S.T. Nutrients and Their Acquisition: Phosphorus Physiology in Microalgae. In The Physiology of Microalgae; Borowitzka, M.A., Beardall, J., Raven, J.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 155–183. [Google Scholar] [CrossRef]
- Cembella, A.D.; Antia, N.J.; Harrison, P.J. The utilization of inorganic and organic phosphorous compounds as nutrients by eukaryotic microalgae: A multidisciplinary perspective: Part I. Crit. Rev. Microbiol. 1982, 10, 317–391. [Google Scholar] [CrossRef] [PubMed]
- Blank, L.M. The cell and P: From cellular function to biotechnological application. Curr. Opin. Biotechnol. 2012, 23, 846–851. [Google Scholar] [CrossRef]
- Thomas, M.R.; O’Shea, E.K. An intracellular phosphate buffer filters transient fluctuations in extracellular phosphate levels. Proc. Natl. Acad. Sci. USA 2005, 102, 9565–9570. [Google Scholar] [CrossRef]
- Azevedo, C.; Saiardi, A. Eukaryotic phosphate homeostasis: The inositol pyrophosphate perspective. Trends Biochem. Sci. 2017, 42, 219–231. [Google Scholar] [CrossRef]
- Tiwari, B. Chapter 7-Phosphate metabolism in cyanobacteria: Fundamental prospective and applications. In Cyanobacteria; Mishra, A.K., Singh, S.S., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 159–175. [Google Scholar] [CrossRef]
- Mason-Jones, K.; Robinson, S.L.; Veen, G.F.C.; Manzoni, S.; van der Putten, W.H. Microbial storage and its implications for soil ecology. ISME J. 2021, 16, 617–629. [Google Scholar] [CrossRef]
- Blaby-Haas, C.E.; Merchant, S.S. Regulating cellular trace metal economy in algae. Curr. Opin. Plant. Biol. 2017, 39, 88–96. [Google Scholar] [CrossRef]
- Achbergerová, L.; Nahálka, J. Polyphosphate-an ancient energy source and active metabolic regulator. Microb. Cell Fact. 2011, 10, 14170–14175. [Google Scholar] [CrossRef]
- Sanz-Luque, E.; Bhaya, D.; Grossman, A.R. Polyphosphate: A Multifunctional Metabolite in Cyanobacteria and Algae. Front. Plant Sci. 2020, 11, 938. [Google Scholar] [CrossRef]
- Denoncourt, A.; Downey, M. Model systems for studying polyphosphate biology: A focus on microorganisms. Curr. Genet. 2021, 67, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Cliff, A.; Guieysse, B.; Brown, N.; Lockhart, P.; Dubreucq, E.; Plouviez, M. Polyphosphate synthesis is an evolutionarily ancient phosphorus storage strategy in microalgae. Algal Res. 2023, 7, 103161. [Google Scholar] [CrossRef]
- Abel, S.; Naumann, C. Evolution of phosphate scouting in the terrestrial biosphere. Philos. Trans. B 2024, 379, 20230355. [Google Scholar] [CrossRef]
- Wang, L.; Fraley, C.D.; Faridi, J.; Kornberg, A.; Roth, R.A. Inorganic polyphosphate stimulates mammalian TOR, a kinase involved in the proliferation of mammary cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 11249–11254. [Google Scholar] [CrossRef]
- Müller, F.; Mutch, N.J.; Schenk, W.A.; Smith, S.A.; Esterl, L.; Spronk, H.M.; Schmidbauer, S.; Gahl, W.A.; Morrissey, J.H.; Renné, T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell 2009, 139, 1143–1156. [Google Scholar] [CrossRef]
- Omelon, S.J.; Grynpas, M.D. Relationships between polyphosphate chemistry, biochemistry and apatite biomineralization. Chem. Rev. 2008, 108, 4694–4715. [Google Scholar] [CrossRef]
- Hernandez-Ruiz, L.; González-García, I.; Castro, C.; Brieva, J.A.; Ruiz, F.A. Inorganic polyphosphate and specific induction of apoptosis in human plasma cells. Haematologica 2006, 91, 1180–1186. [Google Scholar]
- Abramov, A.Y.; Fraley, C.; Diao, C.T.; Winkfein, R.; Colicos, M.A.; Duchen, M.R.; French, R.J.; Pavlov, E. Targeted polyphosphatase expression alters mitochondrial metabolism and inhibits calcium-dependent cell death. Proc. Natl. Acad. Sci. USA 2007, 104, 18091–18096. [Google Scholar] [CrossRef]
- Gray, M.J.; Wholey, W.-Y.; Wagner, N.O.; Cremers, C.M.; Mueller-Schickert, A.; Hock, N.T.; Krieger, A.G.; Smith, E.M.; Bender, R.A.; Bardwell, J.C. Polyphosphate is a primordial chaperone. Mol. Cell 2014, 53, 689–699. [Google Scholar] [CrossRef]
- Kuroda, A.; Nomura, K.; Ohtomo, R.; Kato, J.; Ikeda, T.; Takiguchi, N.; Ohtake, H.; Kornberg, A. Role of inorganic polyphosphate in promoting ribosomal protein degradation by the Lon protease in E. coli. Science 2001, 293, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Cremers, C.M.; Knoefler, D.; Gates, S.; Martin, N.; Dahl, J.-U.; Lempart, J.; Xie, L.; Chapman, M.R.; Galvan, V.; Southworth, D.R. Polyphosphate: A conserved modifier of amyloidogenic processes. Mol. Cell 2016, 63, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Jakob, U. Inorganic polyphosphate, a multifunctional polyanionic protein scaffold. J. Biol. Chem. 2019, 294, 2180–2190. [Google Scholar] [CrossRef] [PubMed]
- Abdelkarim, O.H.; Verhagen, R.A.; Wijffels, R.H.; Barbosa, M.J. Physiological, biochemical, and morphological responses to nitrogen starvation and biomass-specific photon supply rates of Nannochloropsis oceanica and Microchloropsis gaditana. J. Appl. Phycol. 2025. [Google Scholar] [CrossRef]
- Cohen, A.; Perzov, N.; Nelson, H.; Nelson, N. A novel family of yeast chaperons involved in the distribution of V-ATPase and other membrane proteins. J. Biol. Chem. 1999, 274, 26885–26893. [Google Scholar] [CrossRef]
- Sato, N.; Endo, M.; Nishi, H.; Fujiwara, S.; Tsuzuki, M. Polyphosphate-kinase-1 dependent polyphosphate hyperaccumulation for acclimation to nutrient loss in the cyanobacterium, Synechocystis sp. PCC 6803. Front. Plant Sci. 2024, 15, 1441626. [Google Scholar] [CrossRef]
- Yagisawa, F.; Fujiwara, T.; Yamashita, S.; Hirooka, S.; Tamashiro, K.; Izumi, J.; Kanesaki, Y.; Onuma, R.; Misumi, O.; Nakamura, S. A fusion protein of polyphosphate kinase 1 (PPK1) and a Nudix hydrolase is involved in inorganic polyphosphate accumulation in the unicellular red alga Cyanidioschyzon merolae. Plant Mol. Biol. 2025, 115, 1–21. [Google Scholar] [CrossRef]
- Guan, Z.; Chen, J.; Liu, R.; Chen, Y.; Xing, Q.; Du, Z.; Cheng, M.; Hu, J.; Zhang, W.; Mei, W.; et al. The cytoplasmic synthesis and coupled membrane translocation of eukaryotic polyphosphate by signal-activated VTC complex. Nat. Commun. 2023, 14, 718. [Google Scholar] [CrossRef]
- Hothorn, M.; Neumann, H.; Lenherr, E.D.; Wehner, M.; Rybin, V.; Hassa, P.O.; Uttenweiler, A.; Reinhardt, M.; Schmidt, A.; Seiler, J.J.S. Catalytic core of a membrane-associated eukaryotic polyphosphate polymerase. Science 2009, 324, 513–516. [Google Scholar] [CrossRef]
- Plouviez, M.; Fernandez, E.; Grossman, A.R.; Sanz-Luque, E.; Sells, M.; Wheeler, D.; Guieysse, B. Responses of Chlamydomonas reinhardtii during the transition from P-deficient to P-sufficient growth (the P-overplus response): The roles of the vacuolar transport chaperones and polyphosphate synthesis. J. Phycol. 2021, 57, 988–1003. [Google Scholar] [CrossRef]
- Plouviez, M.; Brown, N. Polyphosphate accumulation in microalgae and cyanobacteria: Recent advances and opportunities for phosphorus upcycling. Curr. Opin. Biotechnol. 2024, 90, 103207. [Google Scholar] [CrossRef]
- Yu, D.; Yan, L.; Shi, J.; Liu, Y.; Zhang, A.; Wang, Y.; Zhang, Y.; Xie, T. Phosphorus Removal and Recovery During Microalgae-Based Wastewater Treatment: A Mini-review. Int. J. Environ. Res. 2024, 18, 34. [Google Scholar] [CrossRef]
- Demling, P.; Baier, M.; Deitert, A.; Fees, J.; Blank, L.M. Biotechnological polyphosphate as an opportunity to contribute to the circularization of the phosphate economy. Curr. Opin. Biotechnol. 2024, 87, 103107. [Google Scholar] [CrossRef] [PubMed]
- Blank, L.M. (Poly)phosphate biotechnology: Envisaged contributions to a sustainable P future. Microb. Biotechnol. 2023, 16, 1616–1622. [Google Scholar] [CrossRef]
- Solovchenko, A.; Plouviez, M.; Khozin-Goldberg, I. Getting Grip on Phosphorus: Potential of Microalgae as a Vehicle for Sustainable Usage of This Macronutrient. Plants 2024, 13, 1834. [Google Scholar] [CrossRef]
- Solovchenko, A.E.; Ismagulova, T.T.; Lukyanov, A.A.; Vasilieva, S.G.; Konyukhov, I.V.; Pogosyan, S.I.; Lobakova, E.S.; Gorelova, O.A. Luxury phosphorus uptake in microalgae. J. Appl. Phycol. 2019, 31, 2755–2770. [Google Scholar] [CrossRef]
- Solovchenko, A.; Khozin-Goldberg, I.; Selyakh, I.; Semenova, L.; Ismagulova, T.; Lukyanov, A.; Mamedov, I.; Vinogradova, E.; Karpova, O.; Konyukhov, I.; et al. Phosphorus starvation and luxury uptake in green microalgae revisited. Algal Res. 2019, 43, 101651. [Google Scholar] [CrossRef]
- Solovchenko, A.; Gorelova, O.; Karpova, O.; Selyakh, I.; Semenova, L.; Chivkunova, O.; Baulina, O.; Vinogradova, E.; Pugacheva, T.; Scherbakov, P. Phosphorus feast and famine in cyanobacteria: Is luxury uptake of the nutrient just a consequence of acclimation to its shortage? Cells 2020, 9, 1933. [Google Scholar] [CrossRef]
- Voronkov, A.; Sinetova, M. Polyphosphate accumulation dynamics in a population of Synechocystis sp. PCC 6803 cells under phosphate overplus. Protoplasma 2019, 256, 1153–1164. [Google Scholar] [CrossRef]
- Plouviez, M.; Demling, P.; Deitert, A.; Fees, J.; Baier, M.; Karmainski, T.; Blank, L.M.; Guieysse, B. Coming to Terms: The Mechanisms of Overplus and Luxury Phosphorus Uptake for Polyphosphate Storage in Microalgae and Yeast. Biotechnol. Bioeng. 2025. [Google Scholar] [CrossRef]
- McCarthy, L.; Abramchuk, I.; Wafy, G.; Denoncourt, A.; Lavallée-Adam, M.; Downey, M. Ddp1 Cooperates with Ppx1 to Counter a Stress Response Initiated by Nonvacuolar Polyphosphate. mBio 2022, 13. [Google Scholar] [CrossRef]
- Gerasimaite, R.; Sharma, S.; Desfougeres, Y.; Schmidt, A.; Mayer, A. Coupled synthesis and translocation restrains polyphosphate to acidocalcisome-like vacuoles and prevents its toxicity. J. Cell Sci. 2014, 127, 5093–5104. [Google Scholar] [CrossRef]
- Goodenough, U.; Heiss, A.A.; Roth, R.; Rusch, J.; Lee, J.-H. Acidocalcisomes: Ultrastructure, Biogenesis, and Distribution in Microbial Eukaryotes. Protist 2019, 170, 287–313. [Google Scholar] [CrossRef] [PubMed]
- Docampo, R.; de Souza, W.; Miranda, K.; Rohloff, P.; Moreno, S.N. Acidocalcisomes? Conserved from bacteria to man. Nat. Rev. Microbiol. 2005, 3, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Docampo, R.; Huang, G. Acidocalcisomes of eukaryotes. Curr. Opin. Cell Biol. 2016, 41, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, F.A.; Marchesini, N.; Seufferheld, M.; Govindjee; Docampo, R. The Polyphosphate Bodies of Chlamydomonas reinhardtii Possess a Proton-pumping Pyrophosphatase and Are Similar to Acidocalcisomes. J. Biol. Chem. 2001, 276, 46196–46203. [Google Scholar] [CrossRef]
- Nagel, L. Volutin. Bot. Rev. 1948, 14, 174–184. [Google Scholar] [CrossRef]
- Lobakova, E.S.; Selyakh, I.O.; Semenova, L.R.; Scherbakov, P.N.; Fedorenko, T.A.; Chekanov, K.A.; Chivkunova, O.B.; Baulina, O.I.; Vasilieva, S.G.; Solovchenko, A.E. Hints for understanding microalgal phosphate-resilience from Micractinium simplicissimum IPPAS C-2056 (Trebouxiophyceae) isolated from a phosphorus-polluted site. J. Appl. Phycol. 2022, 34, 2409–2422. [Google Scholar] [CrossRef]
- Yagisawa, F.; Kuroiwa, H.; Fujiwara, T.; Kuroiwa, T. Intracellular structure of the unicellular red alga Cyanidioschyzon merolae in response to phosphate depletion and resupplementation. Cytologia 2016, 81, 341–347. [Google Scholar] [CrossRef]
- Shebanova, A.; Ismagulova, T.; Solovchenko, A.; Baulina, O.; Lobakova, E.; Ivanova, A.; Moiseenko, A.; Shaitan, K.; Polshakov, V.; Nedbal, L.; et al. Versatility of the green microalga cell vacuole function as revealed by analytical transmission electron microscopy. Protoplasma 2017, 254, 1323–1340. [Google Scholar] [CrossRef]
- Reusch, R. Transmembrane ion transport by polyphosphate/poly-(R)-3-hydroxybutyrate complexes. Biochem. Moscow 2020, 65, 280–295. [Google Scholar]
- Ota, S.; Yoshihara, M.; Yamazaki, T.; Takeshita, T.; Hirata, A.; Konomi, M.; Oshima, K.; Hattori, M.; Bisova, K.; Zachleder, V.; et al. Deciphering the relationship among phosphate dynamics, electron-dense body and lipid accumulation in the green alga Parachlorella kessleri. Sci. Rep. 2016, 6, 25731. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jia, X.; Zhang, Y.; Xu, L.; Menand, B.; Zhao, H.; Zeng, H.; Dolan, L.; Zhu, Y.; Yi, K. Loss of two families of SPX domain-containing proteins required for vacuolar polyphosphate accumulation coincides with the transition to phosphate storage in green plants. Mol. Plant 2021, 14, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Loubéry, S.; Broger, L.; Zhang, Y.; Lorenzo-Orts, L.; Utz-Pugin, A.; Fernie, A.R.; Young-Tae, C.; Hothorn, M. A genetically validated approach for detecting inorganic polyphosphates in plants. Plant J. 2020, 102, 507–516. [Google Scholar] [CrossRef]
- Silva, V.M.; Putti, F.F.; White, P.J.; Dos Reis, A.R. Phytic acid accumulation in plants: Biosynthesis pathway regulation and role in human diet. Plant Physiol. Biochem. 2021, 164, 132–146. [Google Scholar] [CrossRef]
- Lorenzo-Orts, L.; Couto, D.; Hothorn, M. Identity and functions of inorganic and inositol polyphosphates in plants. New Phytol. 2020, 225, 637–652. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Zuckerkandl, E.; Pauling, L. Evolutionary divergence and convergence in proteins. In Evolving Genes and Proteins; Elsevier: Amsterdam, The Netherlands, 1965; pp. 97–166. [Google Scholar]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetic Analysis version 12 for adaptive and green computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef]
- Okazaki, K.; Hori, K.; Iwai, M.; Kurita, T.; Shimizu, S.; Nomura, S.; Saito, F.; Maeda, S.; Takami, A.; Yamamoto, T.; et al. Knockout of an SPX-related gene for polyphosphate synthetase accelerates phosphate starvation responses in the oleaginous microalga Nannochloropsis oceanica. J. Exp. Bot. 2025, 76, 3593–3610. [Google Scholar] [CrossRef]
- Whitehead, M.P.; Hooley, P.; Brown, M.R. Horizontal transfer of bacterial polyphosphate kinases to eukaryotes: Implications for the ice age and land colonisation. BMC Res. Notes 2013, 6, 221. [Google Scholar] [CrossRef]
- Sun, Q.-W.; Gao, Y.; Wang, J.; Fu, F.-x.; Yong, C.-W.; Li, S.-Q.; Huang, H.-L.; Chen, W.-Z.; Wang, X.-W.; Jiang, H.-B. Molecular mechanism of a coastal cyanobacterium Synechococcus sp. PCC 7002 adapting to changing phosphate concentrations. Mar. Life Sci. Technol. 2024, 6, 562–575. [Google Scholar] [CrossRef]
- Srouji, J.R.; Xu, A.; Park, A.; Kirsch, J.F.; Brenner, S.E. The evolution of function within the Nudix homology clan. Proteins: Struct. Funct. Bioinform. 2017, 85, 775–811. [Google Scholar] [CrossRef] [PubMed]
- Blaby, I.K.; Blaby-Haas, C.E.; Tourasse, N.; Hom, E.F.Y.; Lopez, D.; Aksoy, M.; Grossman, A.; Umen, J.; Dutcher, S.; Porter, M.; et al. The Chlamydomonas genome project: A decade on. Trends Plant Sci. 2014, 19, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Grossman, A.R.; Aksoy, M. Algae in a phosphorus-limited landscape. In Annual Plant Reviews, Phosphorus Metabolism in Plants; Plaxton, W., Lambers, H., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; Volume 48, pp. 337–374. [Google Scholar]
- Plouviez, M.; Abyadeh, M.; Hasan, M.; Mirzaei, M.; Paulo, J.A.; Guieysse, B. The proteome of Chlamydomonas reinhardtii during phosphorus depletion and repletion. Algal Res. 2023, 71, 103037. [Google Scholar] [CrossRef]
- Kumar Sharma, A.; Mühlroth, A.; Jouhet, J.; Maréchal, E.; Alipanah, L.; Kissen, R.; Brembu, T.; Bones, A.M.; Winge, P. The Myb-like transcription factor phosphorus starvation response (PtPSR) controls conditional P acquisition and remodelling in marine microalgae. New Phytol. 2020, 225, 2380–2395. [Google Scholar] [CrossRef]
- Aksoy, M.; Pootakham, W.; Grossman, A.R. Critical function of a Chlamydomonas reinhardtii putative polyphosphate polymerase subunit during nutrient deprivation. Plant Cell 2014, 26, 4214–4229. [Google Scholar] [CrossRef]
- Sanz-Luque, E.; Grossman, A.R. Chapter 4-Phosphorus and sulfur uptake, assimilation, and deprivation responses. In The Chlamydomonas Sourcebook, 3rd ed.; Grossman, A.R., Wollman, F.-A., Eds.; Academic Press: London, UK, 2023; pp. 129–165. [Google Scholar] [CrossRef]
- Desfougeres, Y.; Gerasimaite, R.U.; Jessen, H.J.; Mayer, A. Vtc5, a Novel Subunit of the Vacuolar Transporter Chaperone Complex, Regulates Polyphosphate Synthesis and Phosphate Homeostasis in Yeast. J. Biol. Chem. 2016, 291, 22262–22275. [Google Scholar] [CrossRef]
- Ogawa, N.; DeRisi, J.; Brown, P.O. New components of a system for phosphate accumulation and polyphosphate metabolism in Saccharomyces cerevisiae revealed by genomic expression analysis. Mol. Biol. Cell 2000, 11, 4309–4321. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, W.; Lee, S.S.K.; Xu, W. Crystal structure of a polyphosphate kinase and its implications for polyphosphate synthesis. EMBO Rep. 2005, 6, 681–687. [Google Scholar] [CrossRef]
- Tzeng, C.-M.; Kornberg, A. The multiple activities of polyphosphate kinase of Escherichia coli and their subunit structure determined by radiation target analysis. J. Biol. Chem. 2000, 275, 3977–3983. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Shi, L.; Zhu, L.; Chen, Y.; Zhu, H.; Cheng, W.; Chen, A.F.; Fu, C. Functions, mechanisms, and therapeutic applications of the inositol pyrophosphates PP-InsP5 and InsP8 in mammalian cells. J. Cardiovasc. Transl. Res. 2024, 17, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Nguyen, H.-N.; Hofer, A.; Jessen, H.J.; Dai, X.; Wang, H.; Shears, S.B. The significance of the bifunctional kinase/phosphatase activities of diphosphoinositol pentakisphosphate kinases (PPIP5Ks) for coupling inositol pyrophosphate cell signaling to cellular phosphate homeostasis. J. Biol. Chem. 2017, 292, 4544–4555. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lau, K.; Puschmann, R.; Harmel, R.K.; Zhang, Y.; Pries, V.; Gaugler, P.; Broger, L.; Dutta, A.K.; Jessen, H.J.; et al. Two bifunctional inositol pyrophosphate kinases/phosphatases control plant phosphate homeostasis. eLife 2019, 8, e43582. [Google Scholar] [CrossRef]
- Austin, S.; Mayer, A. Phosphate Homeostasis − A Vital Metabolic Equilibrium Maintained Through the INPHORS Signaling Pathway. Front. Microbiol. 2020, 11, 1367. [Google Scholar] [CrossRef]
- Szijgyarto, Z.; Garedew, A.; Azevedo, C.; Saiardi, A. Influence of Inositol Pyrophosphates on Cellular Energy Dynamics. Science 2011, 334, 802–805. [Google Scholar] [CrossRef]
- Wild, R.; Gerasimaite, R.; Jung, J.-Y.; Truffault, V.; Pavlovic, I.; Schmidt, A.; Saiardi, A.; Jessen, H.J.; Poirier, Y.; Hothorn, M.; et al. Control of eukaryotic phosphate homeostasis by inositol polyphosphate sensor domains. Science 2016, 352, 986–990. [Google Scholar] [CrossRef]
- Zhou, J.; Hu, Q.; Xiao, X.; Yao, D.; Ge, S.; Ye, J.; Li, H.; Cai, R.; Liu, R.; Meng, F.; et al. Mechanism of phosphate sensing and signaling revealed by rice SPX1-PHR2 complex structure. Nat. Commun. 2021, 12, 7040. [Google Scholar] [CrossRef]
- Li, X.; Gu, C.; Hostachy, S.; Sahu, S.; Wittwer, C.; Jessen, H.J.; Fiedler, D.; Wang, H.; Shears, S.B. Control of XPR1-dependent cellular phosphate efflux by InsP8 is an exemplar for functionally-exclusive inositol pyrophosphate signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 3568–3574. [Google Scholar] [CrossRef]
- Rubio, V.; Linhares, F.; Solano, R.; Martín, A.C.; Iglesias, J.; Leyva, A.; Paz-Ares, J. A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev. 2001, 15, 2122–2133. [Google Scholar] [CrossRef]
- Fiore, C.L.; Alexander, H.; Soule, M.C.K.; Kujawinski, E.B. A phosphate starvation response gene (psr1-like) is present and expressed in Micromonas pusilla and other marine algae. Aquat. Microb. Ecol. 2021, 86, 29–46. [Google Scholar] [CrossRef]
- Paz-Ares, J.; Puga, M.I.; Rojas-Triana, M.; Martinez-Hevia, I.; Diaz, S.; Poza-Carrión, C.; Miñambres, M.; Leyva, A. Plant adaptation to low phosphorus availability: Core signaling, crosstalks, and applied implications. Mol. Plant 2022, 15, 104–124. [Google Scholar] [CrossRef]
- Dyhrman, S.; Chappell, P.; Haley, S.; Moffett, J.; Orchard, E.; Waterbury, J.; Webb, E. Phosphonate utilization by the globally important marine diazotroph Trichodesmium. Nature 2006, 439, 68. [Google Scholar] [CrossRef]
- Dyhrman, S.T.; Haley, S.T.; Birkeland, S.R.; Wurch, L.L.; Cipriano, M.J.; McArthur, A.G.J.A.; Microbiology, E. Long serial analysis of gene expression for gene discovery and transcriptome profiling in the widespread marine coccolithophore Emiliania huxleyi. Appl. Environ. Microbiol. 2006, 72, 252–260. [Google Scholar] [CrossRef]
- Alexander, H.; Jenkins, B.D.; Rynearson, T.A.; Saito, M.A.; Mercier, M.L.; Dyhrman, S.T. Identifying reference genes with stable expression from high throughput sequence data. Front Microbiol. 2012, 3, 385. [Google Scholar] [CrossRef]
- Dyhrman, S.T.; Jenkins, B.D.; Rynearson, T.A.; Saito, M.A.; Mercier, M.L.; Alexander, H.; Whitney, L.P.; Drzewianowski, A.; Bulygin, V.V.; Bertrand, E.M.; et al. The Transcriptome and Proteome of the Diatom Thalassiosira pseudonana Reveal a Diverse Phosphorus Stress Response. PLoS ONE 2012, 7, e33768. [Google Scholar] [CrossRef]
- Xiao, M.; Burford, M.A.; Wood, S.A.; Aubriot, L.; Ibelings, B.W.; Prentice, M.J.; Galvanese, E.F.; Harris, T.D.; Hamilton, D.P. Schindler’s legacy: From eutrophic lakes to the phosphorus utilization strategies of cyanobacteria. FEMS Microbiol. Rev. 2022, 46. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, G.E. The paradox of the plankton. Am. Nat. 1961, 95, 137–145. [Google Scholar] [CrossRef]
- Sanz-Luque, E.; Saroussi, S.; Huang, W.; Akkawi, N.; Grossman, A. Metabolic control of acclimation to nutrient deprivation dependent on polyphosphate synthesis. Sci. Adv. 2020, 6, eabb5351. [Google Scholar] [CrossRef]
- De Mazancourt, C.; Schwartz, M.W. Starve a competitor: Evolution of luxury consumption as a competitive strategy. Theor. Ecol. 2010, 5, 37–49. [Google Scholar] [CrossRef][Green Version]
- Müller, W.E.; Schröder, H.C.; Wang, X. Inorganic polyphosphates as storage for and generator of metabolic energy in the extracellular matrix. Chem. Rev. 2019, 119, 12337–12374. [Google Scholar] [CrossRef]
- Pavlov, E.; Aschar-Sobbi, R.; Campanella, M.; Turner, R.J.; Gómez-García, M.R.; Abramov, A.Y. Inorganic polyphosphate and energy metabolism in mammalian cells. J. Biol. Chem. 2010, 285, 9420–9428. [Google Scholar] [CrossRef]
- Sebesta, J.; Cantrell, M.; Schaedig, E.; Hou, H.J.M.; Pastore, C.; Chou, K.J.; Xiong, W.; Guarnieri, M.T.; Yu, J. Polyphosphate kinase deletion increases laboratory productivity in cyanobacteria. Front. Plant Sci. 2024, 15, 1342496. [Google Scholar] [CrossRef]
- Kampinga, H.H. Chaperoned by prebiotic inorganic polyphosphate molecules: An ancient transcription-independent mechanism to restore protein homeostasis. Mol. Cell 2014, 53, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Fu, L.; Wang, Y.; Zhou, D.; Rittmann, B.E. Excessive phosphorus caused inhibition and cell damage during heterotrophic growth of Chlorella regularis. Bioresour. Technol. 2018, 268, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Lobakova, E.; Gorelova, O.; Selyakh, I.; Semenova, L.; Scherbakov, P.; Vasilieva, S.; Zaytsev, P.; Shibzukhova, K.; Chivkunova, O.; Baulina, O. Failure of Micractinium simplicissimum Phosphate Resilience upon Abrupt Re-Feeding of Its Phosphorus-Starved Cultures. Int. J. Mol. Sci. 2023, 24, 8484. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Li, Q.; Yan, G.; Zhou, D.; Crittenden, J.C. Hormesis effects of phosphorus on the viability of Chlorella regularis cells under nitrogen limitation. Biotechnol Biofuels 2019, 12, 121. [Google Scholar] [CrossRef]
- Vasilieva, S.; Lobakova, E.; Gorelova, O.; Baulina, O.; Scherbakov, P.; Chivkunova, O.; Semenova, L.; Selyakh, I.; Lukyanov, A.; Solovchenko, A. Photosynthetic and ultrastructural responses of the chlorophyte Lobosphaera to the stress caused by a high exogenic phosphate concentration. Photochem. Photobiol. Sci. 2022, 21, 2035–2051. [Google Scholar] [CrossRef]
- Haneklaus, S.; Bloem, H.; Schnug, E. Hungry Plants—A Short Treatise on How to Feed Crops under Stress. Agriculture 2018, 8, 43. [Google Scholar] [CrossRef]
- Cordell, D.; White, S. Life’s Bottleneck: Implications of Global Phosphorus Scarcity and Pathways for a Sustainable Food System. Annu. Rev. Environ. Resour. 2014, 39, 161–188. [Google Scholar] [CrossRef]
- Daneshgar, S.; Callegari, A.; Capodaglio, A.; Vaccari, D. The Potential Phosphorus Crisis: Resource Conservation and Possible Escape Technologies: A Review. Resources 2018, 7, 37. [Google Scholar] [CrossRef]
- Bennett, E.; Elser, J. A broken biogeochemical cycle. Nature 2011, 478, 29–31. [Google Scholar] [CrossRef]
- Elser, J.J.; Call, D.F.; Deaver, J.A.; Duckworth, O.W.; Mayer, B.K.; McLamore, E.; Rittmann, B.; Mahmood, M.; Westerhoff, P. The phosphorus challenge: Biotechnology approaches for a sustainable phosphorus system. Curr. Opin. Biotechnol. 2024, 90, 103197. [Google Scholar] [CrossRef]
- Chen, M.; Graedel, T. A half-century of global phosphorus flows, stocks, production, consumption, recycling, and environmental impacts. Glob. Environ. Change 2016, 36, 139–152. [Google Scholar] [CrossRef]
- Cordell, D.; Rosemarin, A.; Schroder, J.J.; Smit, A.L. Towards global phosphorus security: A systems framework for phosphorus recovery and reuse options. Chemosphere 2011, 84, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Solovchenko, A.; Verschoor, A.M.; Jablonowski, N.D.; Nedbal, L. Phosphorus from wastewater to crops: An alternative path involving microalgae. Biotechnol. Adv. 2016, 34, 550–564. [Google Scholar] [CrossRef]
- Cakmak, E.K.; Hartl, M.; Kisser, J.; Cetecioglu, Z. Phosphorus Mining from Eutrophic Marine Environment Towards a Blue Economy: The Role of Bio-Based Applications. Water Res. 2022, 219, 118505. [Google Scholar] [CrossRef]
- De-Bashan, L.E.; Bashan, Y. Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997–2003). Water Res. 2004, 38, 4222–4246. [Google Scholar] [CrossRef]
- Akuku, V.; Satognon, F. Sustainable phosphorus recovery from wastewater using microalgae: Economic, environmental, and qgronomic implications for future phosphorus fertilizer solutions. Clean. Waste Syst. 2025, 12, 100377. [Google Scholar] [CrossRef]
- Brown, N.; Shilton, A. Luxury uptake of phosphorus by microalgae in waste stabilisation ponds: Current understanding and future direction. Rev. Environ. Sci. Bio/Technol. 2014, 13, 321–328. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, Y.; Beardall, J.; Raven, J.A.; Peng, B.; Xu, L.; Zhang, H.; Li, J.; Xia, J.; Jin, P. The dynamics of adaptive evolution in microalgae in a high-CO(2) ocean. New Phytol. 2024, 245, 1608–1624. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Tahir, F.; Akbar, I.; Alessa, A.H.; Alsaigh, A.A.; Liu, C.-G.; Xin, F.; Chi, Z.; Syafiuddin, A.; Mehmood, M.A.; et al. Towards Environmental Sustainability: Employing Adaptive Laboratory Evolution to Develop Elite Algae Strains for Industrial and Environmental Applications. Curr. Pollut. Rep. 2025, 11. [Google Scholar] [CrossRef]
- Marchetto, F.; Conde, T.; Sliwinska, M.A.; Rewerski, B.; Lebiedzinska-Arciszewska, M.; Szymanski, J.; Wieckowski, M.R.; Matlakowska, R.; Domingues, M.R.; Kargul, J. Adaptive laboratory evolution of extremophilic red microalga Cyanidioschyzon merolae under high nickel stress enhances lipid production and alleviates oxidative damage. Bioresour. Technol. 2025, 434, 132826. [Google Scholar] [CrossRef] [PubMed]
- Kulakovskaya, T.V.; Vagabov, V.M.; Kulaev, I.S. Inorganic polyphosphate in industry, agriculture and medicine: Modern state and outlook. Process Biochem. 2012, 47, 1–10. [Google Scholar] [CrossRef]
- Lorenz, B.; Leuck, J.; Köhl, D.; Müller, W.E.; Schröder, H.C. Anti-HIV-1 activity of inorganic polyphosphates. JAIDS J. Acquir. Immune Defic. Syndr. 1997, 14, 110–118. [Google Scholar] [CrossRef]
- Feng, G.; Dong, S.; Huang, M.; Zeng, M.; Liu, Z.; Zhao, Y.; Wu, H. Biogenic Polyphosphate Nanoparticles from a Marine Cyanobacterium Synechococcus sp. PCC 7002: Production, Characterization, and Anti-Inflammatory Properties In Vitro. Mar. Drugs 2018, 16, 322. [Google Scholar] [CrossRef]
- Glass, D.J. Government Regulation of the Uses of Genetically Modified Algae and Other Microorganisms in Biofuel and Bio-based Chemical Production. In Algal Biorefineries: Volume 2: Products and Refinery Design; Prokop, A., Bajpai, R.K., Zappi, M.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 23–60. [Google Scholar] [CrossRef]
- Vasilieva, S.; Petrova, E.; Lobakova, E.; Solovchenko, A.; Antal, T.; Gorelova, O. Effect of Hydrogenase Deficiency on Accumulation of Phosphorus-Rich Inclusions in Chlamydomonas reinhardtii. Russ. J. Plant Physiol. 2024, 71, 48. [Google Scholar] [CrossRef]
- Trebuch, L.M.; Sohier, J.; Altenburg, S.; Oyserman, B.O.; Pronk, M.; Janssen, M.; Vet, L.E.M.; Wijffels, R.H.; Fernandes, T.V. Enhancing phosphorus removal of photogranules by incorporating polyphosphate accumulating organisms. Water Res. 2023, 235, 119748. [Google Scholar] [CrossRef]
- Kulaev, I.; Vagabov, I.; Kulakovskaya, T. The Biochemistry of Inorganic Polyphosphates, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, England, 2004. [Google Scholar]
- Miyachi, S.; Kanai, R.; Mihara, S.; Miyachi, S.; Aoki, S. Metabolic roles of inorganic polyphosphates in Chlorella cells. Biochim. Et Biophys. Acta 1964, 93, 625–634. [Google Scholar] [CrossRef]
- Miyachi, S.; Miyachi, S. Modes of formation of phosphate compounds and their turnover in Chlorella cells during the process of life cycle as studied by the technique of synchronous culture. Plant Cell Physiol. 1961, 2, 415–424. [Google Scholar] [CrossRef]
- Miyachi, S.; Tamiya, H. Some observations on the phosphorus metabolism in growing Chlorella cells. Biochim. Et Biophys. Acta 1961, 46, 200–202. [Google Scholar] [CrossRef]
- Miyachi, S.; Tamiya, H. Distribution and turnover of phosphate compounds in growing Chlorella cells. Plant Cell Physiol. 1961, 2, 405–414. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]


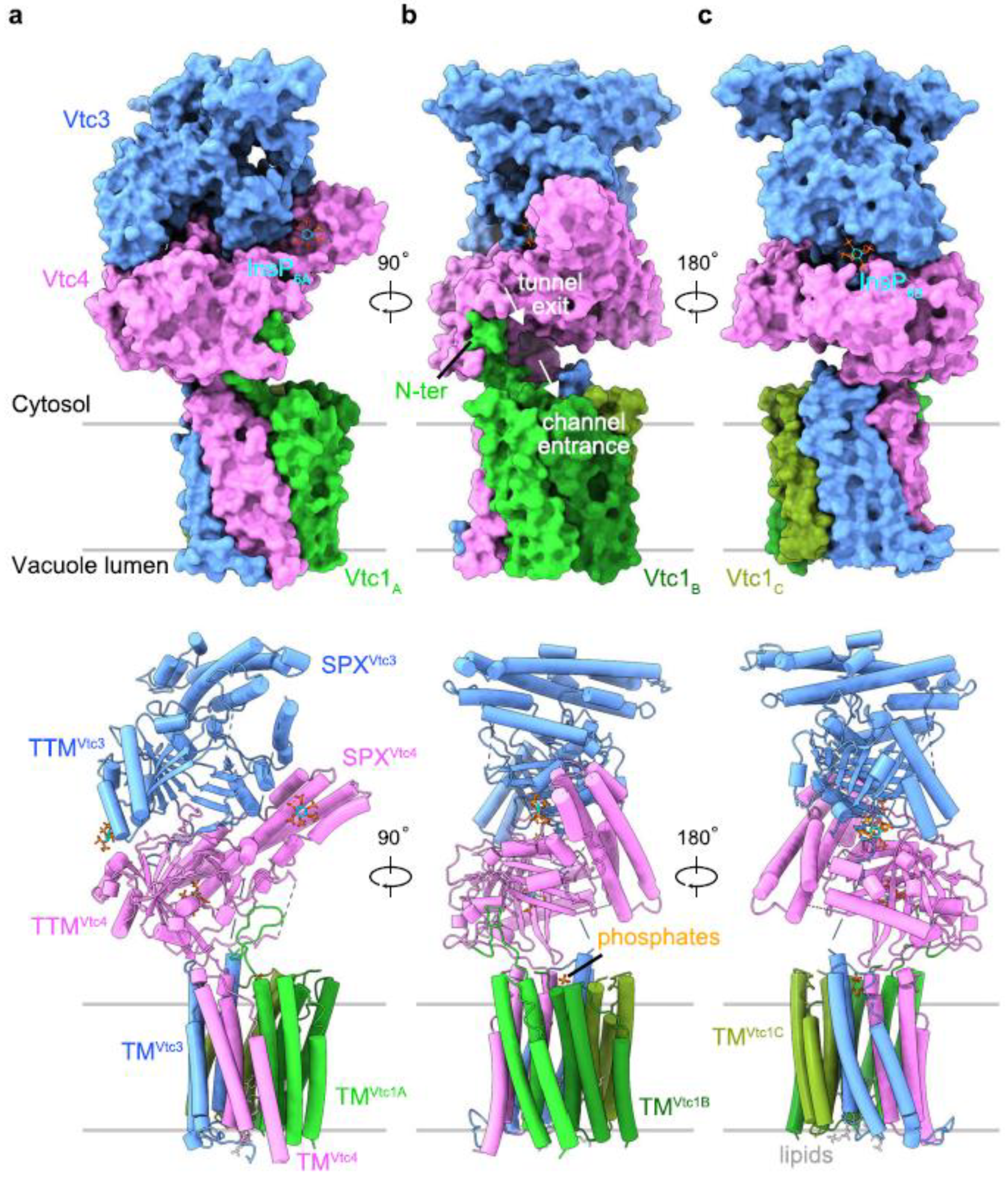
| Subunit | Domains Harbored [PFAM Accession] | Function |
|---|---|---|
| Vtc1 | Transmembrane domain, composed of three protomers (VTC1A, VTC1B, VTC1C) | Formation of the transmembrane channel guiding the nascent PolyP chain into the vacuole lumen |
| Vtc2 | Auxiliary subunit; catalytically inactive 1 | |
| Vtc3 | N-terminal SPX domain [PF03105] | Auxiliary subunit; not essential for VTC regulation 1 |
| TM1 domain | Discharging of polyP chain | |
| Vtc4 | Catalytic tunnel domain (triphosphate tunnel metalloenzyme, TTM domain) [PF09359] | Polymerization of polyP |
| N-terminal SPX domain [PF03105] | Regulation (Pi level sensing via PP-InsP) | |
| Transmembrane domain [PF02656] | Anchoring the whole complex to the vacuolar membrane and enabling translocation of the formed polyP chain into the vacuole | |
| Vtc5 | SPX domain [PF03105] | Accessory subunit for activation of the VTC complex. The only protein acting directly on the VTC complex to stimulate polyP production 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solovchenko, A. Polyphosphate Polymerase—A Key Enzyme for the Phosphorus Economy of the Microalgal Cell and the Sustainable Usage of This Nutrient. Plants 2025, 14, 3061. https://doi.org/10.3390/plants14193061
Solovchenko A. Polyphosphate Polymerase—A Key Enzyme for the Phosphorus Economy of the Microalgal Cell and the Sustainable Usage of This Nutrient. Plants. 2025; 14(19):3061. https://doi.org/10.3390/plants14193061
Chicago/Turabian StyleSolovchenko, Alexei. 2025. "Polyphosphate Polymerase—A Key Enzyme for the Phosphorus Economy of the Microalgal Cell and the Sustainable Usage of This Nutrient" Plants 14, no. 19: 3061. https://doi.org/10.3390/plants14193061
APA StyleSolovchenko, A. (2025). Polyphosphate Polymerase—A Key Enzyme for the Phosphorus Economy of the Microalgal Cell and the Sustainable Usage of This Nutrient. Plants, 14(19), 3061. https://doi.org/10.3390/plants14193061






