Quasi-Targeted Metabonomics Reveals Metabolites Associated with Antioxidant Activity of Mesona chinensis Benth Cultivar Xiaoye
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Collection and Preparation
2.2. Determination of Antioxidant Activity
2.3. Determination of Polyphenol Content
2.4. Determination of Flavonoid Content
2.5. Quasi-Targeted Metabolomics
2.6. Treatment of Data
2.7. Antioxidant and Metabolite Correlation Network Diagram
3. Results
3.1. Antioxidant Activity Analysis of Different Varieties of M. chinensis Benth
3.2. Determination of TPC, TFC, and MPC
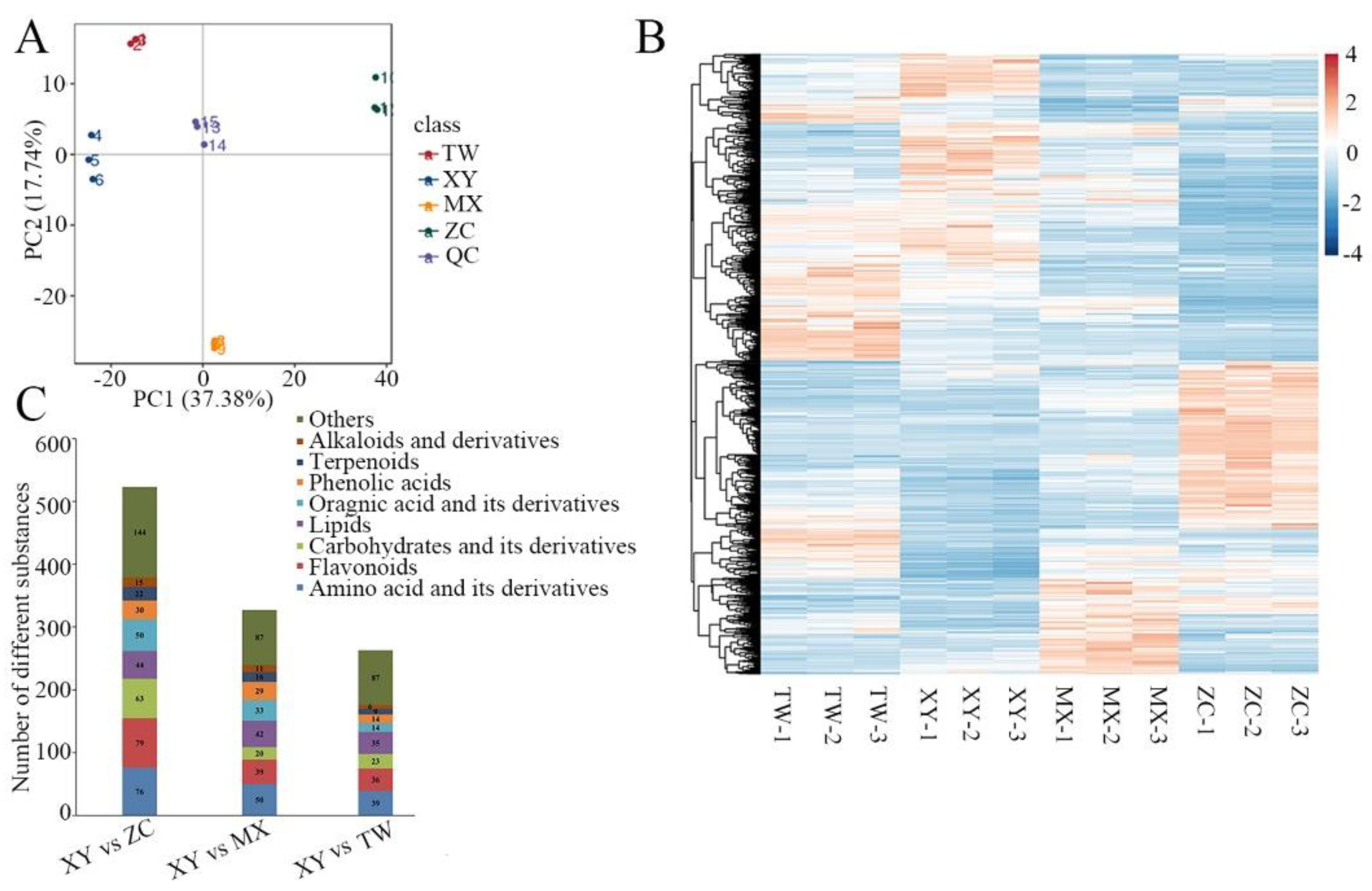
3.3. Detection Metabolites of M. chinensis Benth by Quasi-Targeted Metabonomics
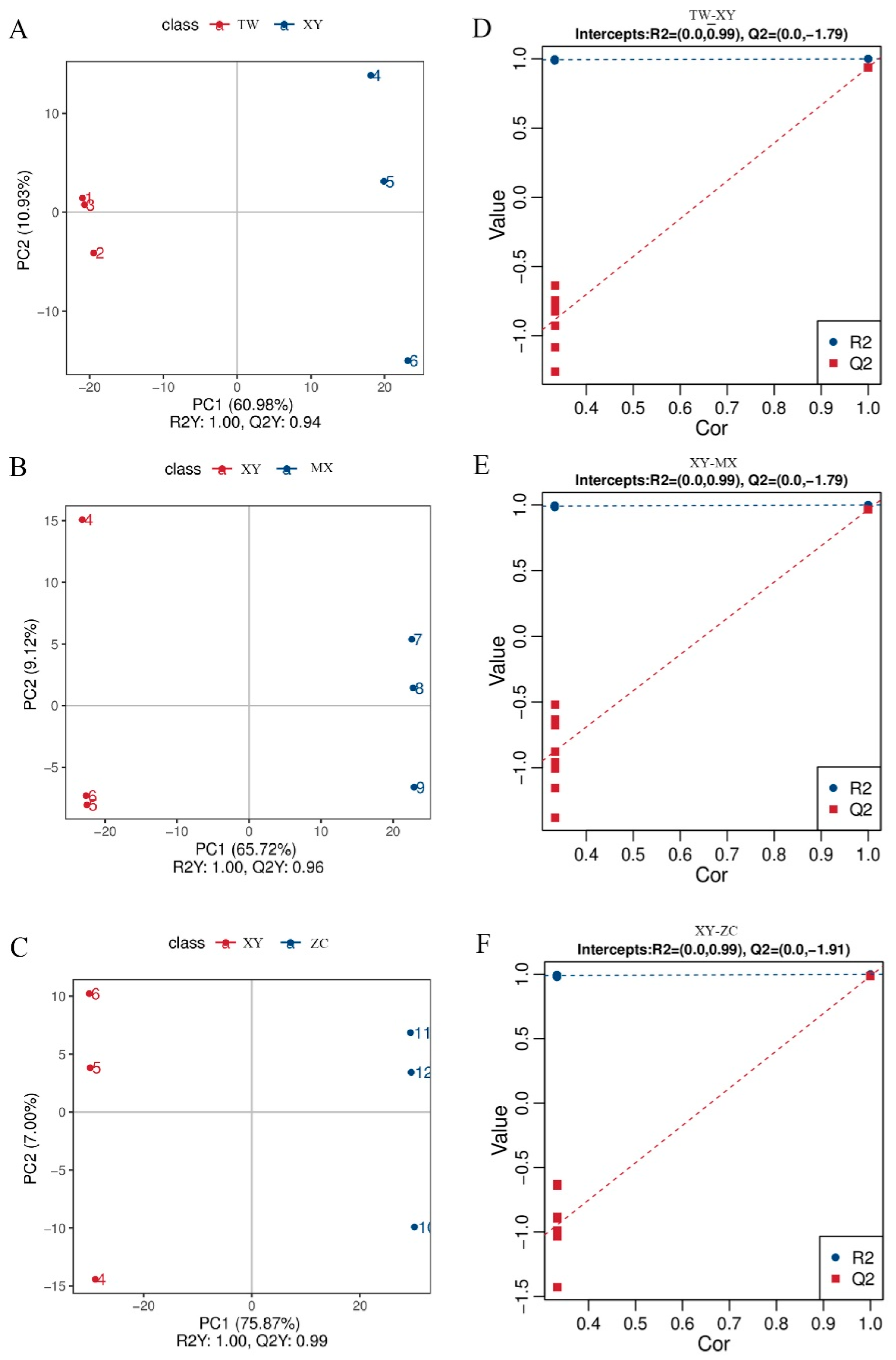
3.4. Principal Component Analysis and Orthogonal Projections to Latent Structures Discriminant Analysis of Four Varieties M. chinensis Benth
3.5. Sample Quality Control and PLS-DA Results
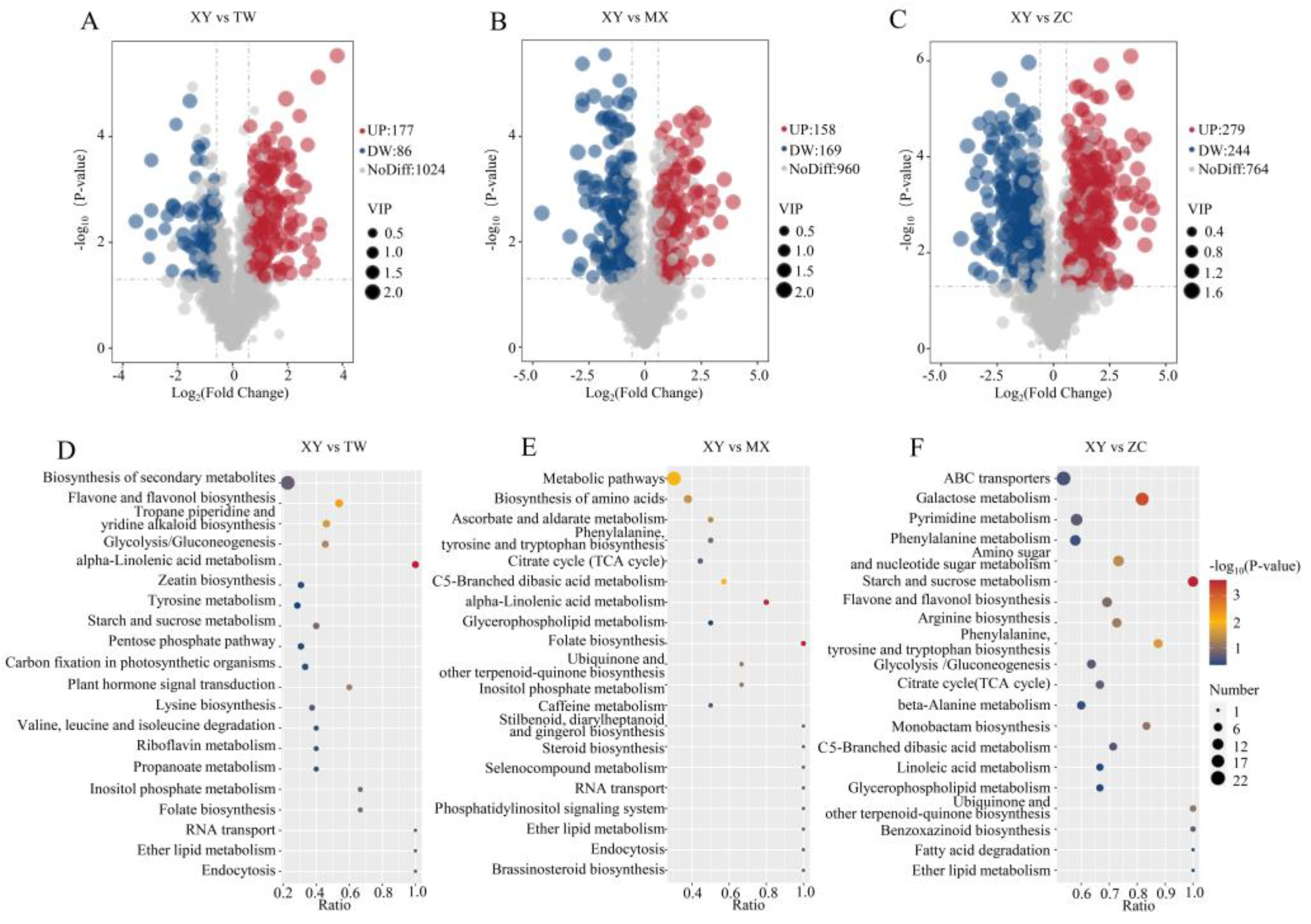
3.6. Differential Metabolite Screening, Functional Annotation, and Enrichment Analysis of XY Compared to the Other Three Varieties
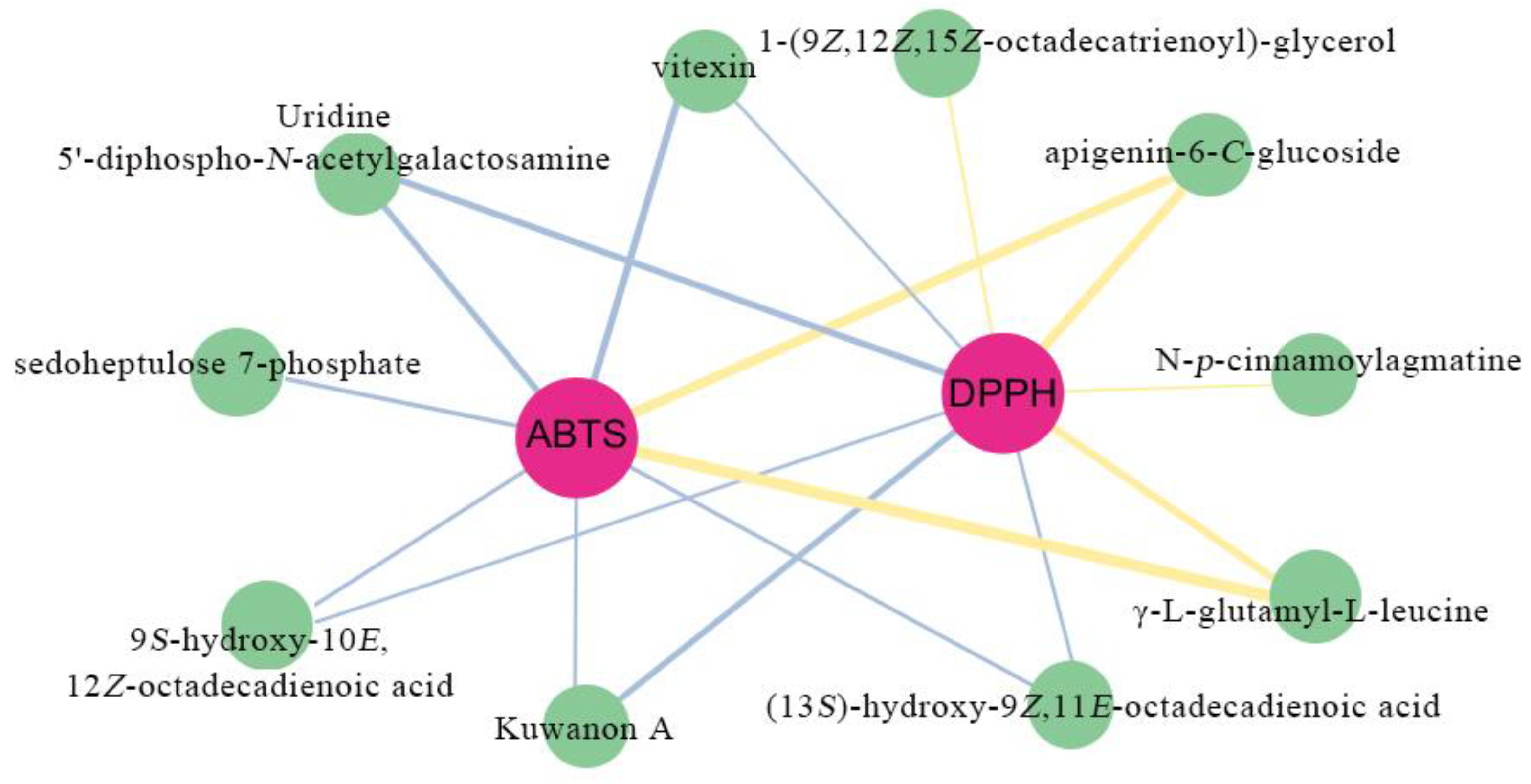
3.7. Correlation Analysis of Secondary Metabolites in M. chinensis Benth and Their Antioxidant Activity
4. Discussion
4.1. Variability in Secondary Metabolites Among M. chinensis Benth Varieties
4.2. Relationship Between Composition and Antioxidant Capacity of Secondary Metabolites in M. chinensis Benth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, L.; Huang, L.; Xie, J. Review on main chemical constituents and biological activities of Mesona chinensis. Sci. Technol. Food Ind. 2016, 37, 356–359. [Google Scholar]
- Ma, J.Y.; Li, K.L.; Shi, S.L.; Li, J.; Tang, S.; Liu, L.H. The Application of UHPLC-HRMS for Quality Control of Traditional Chinese Medicine. Front. Pharmacol. 2022, 13, 922488. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.F.; Liu, L.; Nie, Q.; Huang, M.J.; Luo, C.L.; Sun, Y.D.; Ma, Y.Y.; Yu, J.X.; Du, F.Q. HPLC-based metabolomics of Dendrobium officinale revealing its antioxidant ability. Front. Plant Sci. 2023, 14, 1060242. [Google Scholar] [CrossRef]
- Tang, Y.C.; Liu, Y.J.; He, G.R.; Cao, Y.W.; Bi, M.M.; Song, M.; Yang, P.P.; Xu, L.F.; Ming, J. Comprehensive analysis of secondary metabolites in the extracts from different Lily Bulbs and their antioxidant ability. Antioxidants 2021, 10, 1634. [Google Scholar] [CrossRef]
- Pan, J.J.; Shi, Y.J.; Zou, J.B.; Zhang, X.F.; Xin, B.; Zhai, B.T.; Guo, D.Y.; Sun, J.; Luan, F. Preparation technologies, structural features, and biological activities of polysaccharides from Mesona chinensis Benth.: A review. J. Ethnopharmacol. 2024, 326, 117979. [Google Scholar] [CrossRef]
- Tang, D.F.; Wei, F.; Cai, Z.Q.; Wei, Y.Y.; Khan, A.; Miao, J.H.; Wei, K.H. Analysis of codon usage bias and evolution in the chloroplast genome of Mesona chinensis Benth. Dev. Genes. Evol. 2020, 231, 1–9. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.Q. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug. Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life. Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Huang, L.X.; Huang, M.; Shen, M.Y.; Wen, P.W.; Wu, T.; Hong, Y.Z.; Yu, Q.; Chen, Y.; Xie, J.H. Sulfated modification enhanced the antioxidant activity of Mesona chinensis Benth polysaccharide and its protective effect on cellular oxidative stress. Int. J. Biol. Macromol. 2019, 136, 1000–1006. [Google Scholar] [CrossRef]
- Huang, L.X.; Shen, M.Y.; Wu, T.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J.H. Mesona chinensis Benth polysaccharides protect against oxidative stress and immunosuppression in cyclophosphamide-treated mice via MAPKs signal transduction pathways. Int. J. Biol. Macromol. 2020, 152, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ding, L.; Tian, W.; Zhi, H.; Chen, J.; Wu, L.; Wang, L.; Xie, J.; Bai, J.; Fan, H.; et al. Polyphaenolic profiling, antioxidant properties, and inhibition of α-glucosidase of Mesona chinensis benth from Southern China. Microchem. J. 2021, 168, 106399. [Google Scholar] [CrossRef]
- Zhang, W.; Tan, B.P.; Ye, G.L.; Wang, J.X.; Dong, X.H.; Yang, Q.H.; Chi, S.Y.; Liu, H.Y.; Zhang, S.; Zhang, H.T. Identiffcation of potential biomarkers for soybean meal-induced enteritis in juvenile pearl gentian grouper, Epinephelus lanceolatus male × Epinephelus fuscoguttatus female. Aquaculture 2019, 512, 14. [Google Scholar] [CrossRef]
- Wang, J.B.; Pu, S.B.; Sun, Y.; Li, Z.F.; Niu, M.; Yan, X.Z.; Zhao, Y.L.; Wang, L.F.; Qin, X.M.; Ma, Z.J.; et al. Metabolomic Proffling of Autoimmune Hepatitis: The Diagnostic Utility of Nuclear Magnetic Resonance Spectroscopy. J. Proteome Res. 2014, 13, 3792–3801. [Google Scholar] [CrossRef]
- Xiao, L.; Lu, X.; Yang, H.; Lin, C.; Li, L.; Ni, C.; Fang, Y.; Mo, S.; Zhan, R.; Yan, P. The Antioxidant and Hypolipidemic Effects of Mesona Chinensis Benth Extracts. Molecules 2022, 27, 3423. [Google Scholar] [CrossRef]
- Brochado, C.D.O.; Almeida, A.P.D.; Barreto, B.P.; Costa, L.P.; Ribeiro, L.S.; Pereira, R.L.D.C.; Koatz, V.L.G.; Costa, S.S. Flavonol robinobiosides and rutinosides from Alternanthera brasiliana (Amaranthaceae) and their effects on lymphocyte proliferation in vitro. J. Braz. Chem. Soc. 2003, 14, 449–451. [Google Scholar] [CrossRef]
- Babaei, F.; Moafizad, A.; Darvishvand, Z.; Mirzababaei, M.; Hosseinzadeh, H.; Nassiri-Asl, M. Review of the effects of vitexin in oxidative stress-related diseases. Food Sci. Nutr. 2020, 8, 2569–2580. [Google Scholar] [CrossRef]
- Liu, C.X.; Zhao, M.D.; Chen, J.Y.; Xu, L.W.; Wang, K.Y.; Li, G.Y. Nodakenin alleviates ovariectomy-induced osteoporosis by modulating osteoblastogenesis and osteoclastogenesis. Eur. J. Pharmacol. 2023, 960, 176121. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Song, Q.Y.; Su, J.C.; Tang, W.; Song, J.G.; Huang, X.J.; An, J.M.; Li, Y.L.; Ye, W.C.; Wang, Y. Caffeic acid oligomers from Mesona chinensis and their In Vitro antiviral activities. Fitoterapia 2020, 144, 104603. [Google Scholar] [CrossRef]
- Huang, H.T.; Liaw, C.C.; Chiou, C.T.; Kuo, Y.H.; Lee, K.T. Triterpene acids from Mesona procumbens exert anti-inflammatory effects on LPS-stimulated murine macrophages by regulating the MAPK signaling pathway. J. Agric. Food Chem. 2021, 69, 6271–6280. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Wang, G.M.; Bo, X.U. Study on antioxidant activity and constituents of Radix Paeonia veitchii. Food Sci. 2007, 28, 76–82. [Google Scholar]
- Lin, Z.J.; Bao, Y.Y.; Hong, B.; Wang, Y.Y.; Zhang, X.M.; Wu, Y.P. Salvianolic acid B attenuated cisplatin-induced cardiac injury and oxidative stress via modulating Nrf2 signal pathway. J. Toxicol. Sci. 2021, 46, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Hung, C.Y. Effects of alkaline and heat treatment on antioxidative activity and total phenolics of extracts from Hsian-tsao (Mesona procumbens Hemsl.). Food Res. Int. 2000, 33, 487–492. [Google Scholar] [CrossRef]
- Wongverawattanakul, C.; Suklaew, P.O.; Chusak, C.; Adisakwattana, S.; Thilavech, T. Encapsulation of Mesona chinensis Benth extract in alginate beads enhances the stability and antioxidant activity of polyphenols under simulated gastrointestinal digestion. Foods 2022, 11, 2378. [Google Scholar] [CrossRef]
- Xiao, Y.H.; Liu, S.C.; Shen, M.Y.; Jiang, L.; Ren, Y.M.; Luo, Y.; Wen, H.L.; Xie, J.H. Physicochemical, rheological and thermal properties of Mesona chinensis polysaccharides obtained by sodium carbonate assisted and cellulase assisted extraction. Int. J. Biol. Macromol. 2019, 126, 30–36. [Google Scholar] [CrossRef]
- Huang, L.X.; Shen, M.Y.; Zhang, X.W.; Jiang, L.; Song, Q.Q.; Xie, J.H. Effect of high-pressure microfluidization treatment on the physicochemical properties and antioxidant activities of polysaccharide from Mesona chinensis Benth. Carbohydr. Polym. 2018, 200, 191–199. [Google Scholar] [CrossRef]
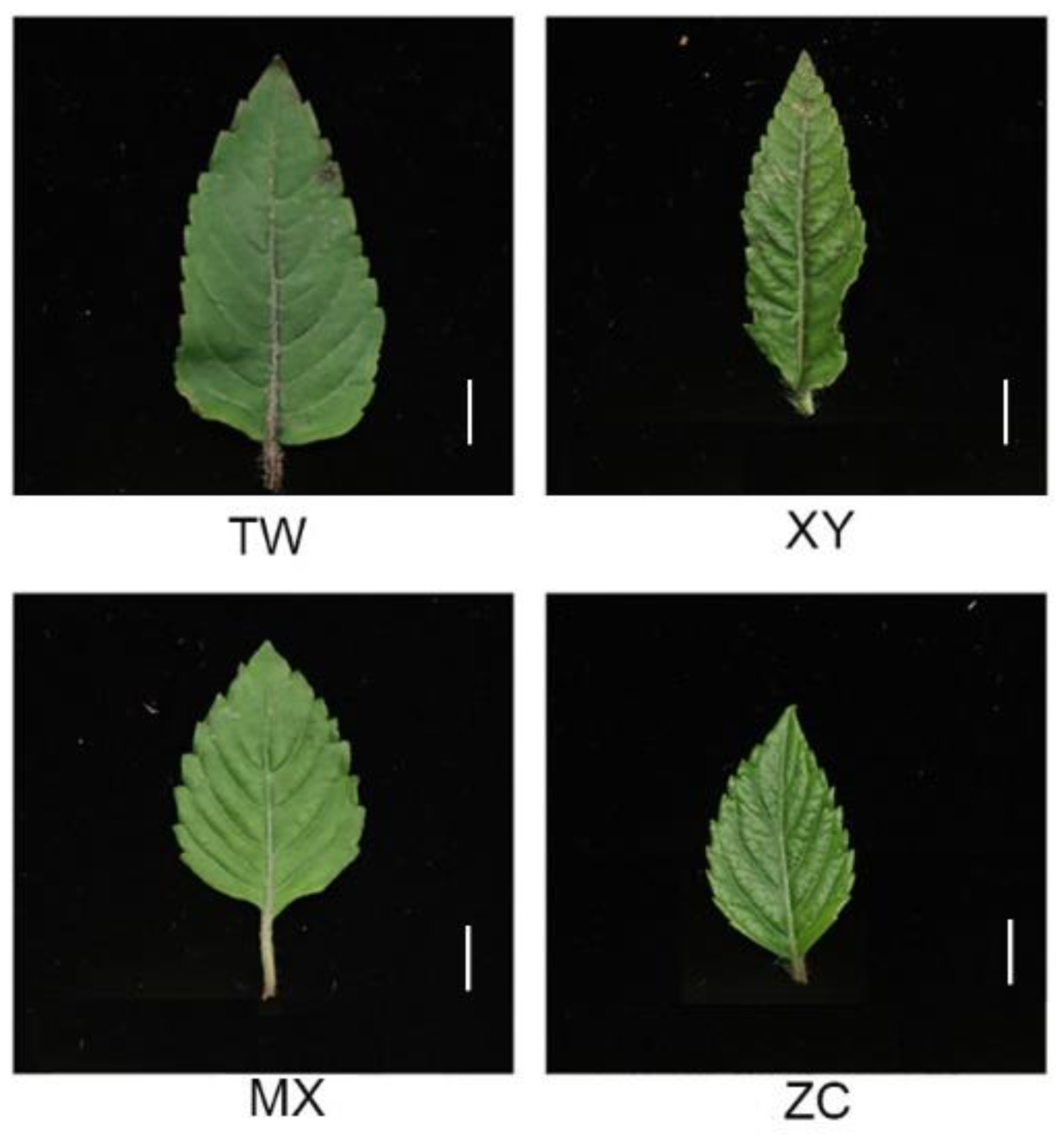
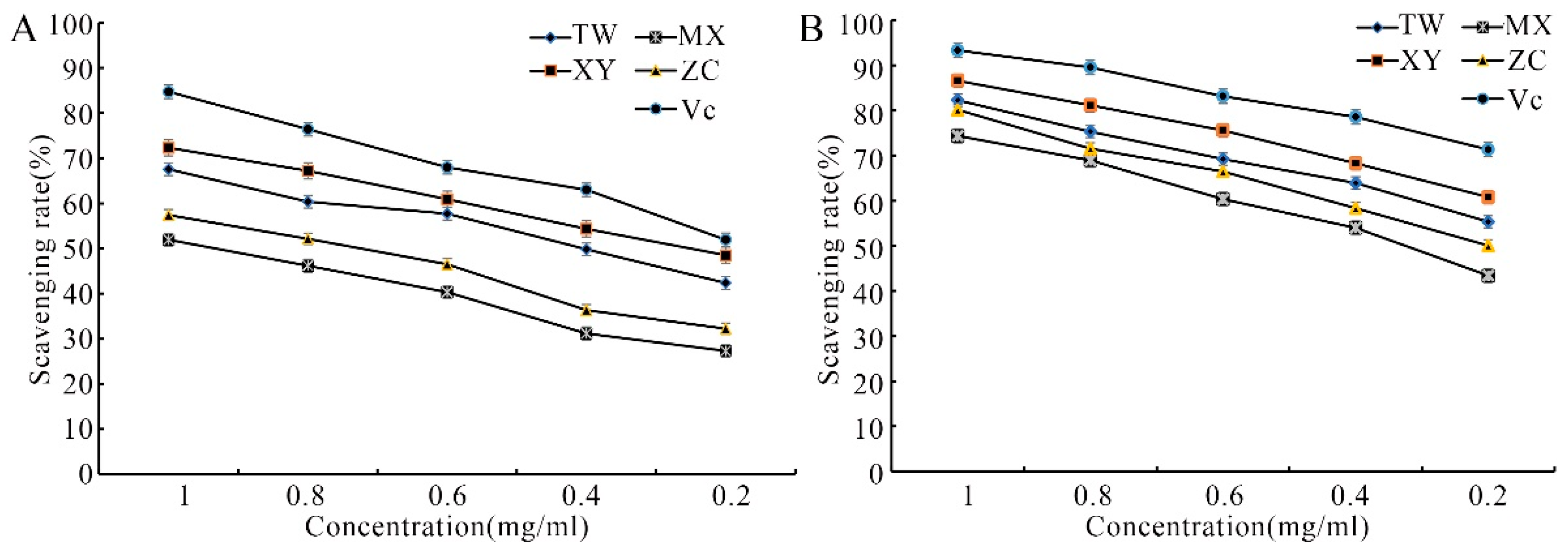
| Materials | IC50 (mg/mL) | |
|---|---|---|
| DPPH Radical Scavenging Effects | ABTS Radical Scavenging Effects | |
| TW | 0.36 ± 0.01 | 0.17 ± 0.02 |
| XY | 0.25 ± 0.01 | 0.13 ± 0.01 |
| MX | 1.02 ± 0.02 | 0.31 ± 0.03 |
| ZC | 0.72 ± 0.02 | 0.23 ± 0.01 |
| Vc | 0.20 ± 0.01 | 0.08 ± 0.00 |
| Materials | TPC (mg/g) | TFC (mg/g) | MPC (mg/g) |
|---|---|---|---|
| TW | 54.56 ± 0.61 a | 126.18 ± 13.91 a | 44.78 ± 1.20 b |
| XY | 55.81 ± 0.84 a | 125.88 ± 7.66 a | 114.19 ± 2.41 a |
| MX | 52.50 ± 0.39 b | 95.58 ± 4.20 b | 46.49 ± 0.81 b |
| ZC | 52.40 ± 0.15 b | 100.81 ± 3.06 b | 35.04 ± 2.68 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, Y.; Zheng, M.; Tian, D.; Zhu, Y.; Chen, H.; Zhu, Y.; Su, H. Quasi-Targeted Metabonomics Reveals Metabolites Associated with Antioxidant Activity of Mesona chinensis Benth Cultivar Xiaoye. Plants 2025, 14, 1585. https://doi.org/10.3390/plants14111585
Niu Y, Zheng M, Tian D, Zhu Y, Chen H, Zhu Y, Su H. Quasi-Targeted Metabonomics Reveals Metabolites Associated with Antioxidant Activity of Mesona chinensis Benth Cultivar Xiaoye. Plants. 2025; 14(11):1585. https://doi.org/10.3390/plants14111585
Chicago/Turabian StyleNiu, Yuqing, Meixia Zheng, Dagang Tian, Yanming Zhu, Hong Chen, Yujing Zhu, and Hailan Su. 2025. "Quasi-Targeted Metabonomics Reveals Metabolites Associated with Antioxidant Activity of Mesona chinensis Benth Cultivar Xiaoye" Plants 14, no. 11: 1585. https://doi.org/10.3390/plants14111585
APA StyleNiu, Y., Zheng, M., Tian, D., Zhu, Y., Chen, H., Zhu, Y., & Su, H. (2025). Quasi-Targeted Metabonomics Reveals Metabolites Associated with Antioxidant Activity of Mesona chinensis Benth Cultivar Xiaoye. Plants, 14(11), 1585. https://doi.org/10.3390/plants14111585







