Abstract
Evidence indicates that light can trigger an increase in triacylglycerol (TAG) accumulation in eukaryotic microalgae without reducing cell division. In connection with this, we have recently reported that the expression of the chloroplast enzyme diacylglycerol acyltransferase 3 (DGAT3) is induced by light in concert with TAG accumulation in Chlamydomonas reinhardtii. In this work, we report the identification of two phytyl ester synthases (PES) in C. reinhardtii, named PESα and PESβ. These are homologous to chloroplast PES1 and PES2 of Arabidopsis thaliana, which play a role in the synthesis of fatty acid phytyl esters (FAPEs) and TAGs. We demonstrate that PESα and PESβ transcript levels are transiently induced upon transferring cell cultures from a growth condition of low light to high light, and this occurs in parallel to an increase in TAG levels. In a pesα knockdown mutant, DGAT3 transcripts and TAG levels are significantly higher than in the parental strain at the end of the low-light period, and remain elevated after shifting pesα cells to the high-light condition. On the contrary, in a pesβ knockdown mutant, TAG levels, as well as DGAT3 expression, are similar to those of the control strain. These results suggest that PESα and PESβ are non-redundant in TAG metabolism and that PESα is functionally related to DGAT3.
1. Introduction
Eukaryotic microalgae are considered important oil producers [1]. One of the major lipid classes in these organisms are storage lipids in the form of triacylglycerols (TAGs) [1]. Nowadays, there is great interest in using native or engineered eukaryotic microalgae as a source of TAGs for biotechnological applications, such as biofuel and biopolymer manufacture [2]. In many species of microalgae, several stress conditions such as nutrient limitations induce TAG accumulation [1,3]. Nitrogen (N) starvation is the best characterized culture condition that stimulates TAG synthesis [1,3]. The enzymes triggered by this stress are mainly part of the conventional cytosolic/microsomal lipid synthesis pathway [4]. For industrial applications, the key challenge of such a scheme is that N depletion limits amino acid and protein synthesis, thereby reducing the rate of cell division and diminishing total lipid productivity [5]. For this reason, it is crucial to explore growth conditions that maximize TAG production without affecting cell growth.
Chlamydomonas reinhardtii is a unicellular green alga employed as an experimental model organism [6]. Goold et al. reported that high light induces TAG production in C. reinhardtii without affecting the rate of cell division [7]. Accordingly, it was observed that increasing light intensity across different spectral qualities boosts biomass concentration and neutral lipid accumulation in C. reinhardtii [8]. It was proposed that TAGs serve as key reservoirs for excess reducing power generated by the photosynthetic electron transport chain when energy input surpasses cellular utilization capacity, thereby mitigating photodamage [9]. The fact that high light can increase neutral lipid productivity without compromising cell growth, makes it a promising culture condition for the microalgal oil industry. However, the knowledge of non-conventional (i.e., non-microsomal) enzymatic pathways and their potential contribution to light-induced TAG synthesis remains limited.
Diacylglycerol acyltransferases (DGATs) are enzymes that catalyze the formation of TAGs by performing an acylation of an activated fatty acid onto the sn-3 position of a molecule of diacylglycerol [10]. The best characterized DGAT families are DGAT1 and DGAT2, which are membrane-bound enzymes of the cytosolic/microsomal pathway [10]. Interestingly, DGAT activity was also detected in chloroplast envelopes, though the specific isoform responsible for this activity remained unidentified [11]. Consistent with this finding, electron microscopy analysis revealed close associations between lipid droplets and both the endoplasmic reticulum and the outer membrane of the chloroplast envelope in C. reinhardtii and Dunaliella bardawil [12,13]. Given that DGAT1 harbors a predicted chloroplast transit peptide and is upregulated during senescence in Arabidopsis thaliana leaves—paralleling chloroplast TAG accumulation—this isoform is hypothesized to mediate lipid droplet formation at the chloroplast envelope [14,15].
In recent years, our group reported a DGAT exclusive to green algae, named DGAT3, which is moderately related to its plant homolog and structurally different from the canonical DGAT1 and DGAT2 families [15]. In silico analyses suggest that DGAT3 is a soluble protein that is targeted to the chloroplast [15,16]. Previous work showed that heterologous expression of C. reinhardtii DGAT3 in Escherichia coli cells increased the levels of TAGs in the presence of oleate, which indicates that DGAT3 has indeed DGAT activity [15]. We later reported that DGAT3 transcripts were augmented in a C. reinhardtii wild-type strain upon transferring cell cultures from low light to high light [16]. Similar results were obtained in starch-deficient C. reinhardtii cells after shifting them from darkness to moderate light [16]. In both strains, the increase in DGAT3 transcripts occurred in concert with a transient accumulation of TAGs [16]. In addition, we reported that DGAT1 expression was induced in C. reinhardtii cells, together with DGAT3, upon shifting cell cultures from low light to high light [16]. These results suggest that DGAT3 and DGAT1 play a role in chloroplast TAG production, and support the hypothesis about the existence of a non-conventional TAG synthesis pathway.
Phospholipid diacylglycerol acyltransferase (PDAT) transfers the acyl group from the sn-2 position of a phospholipid to the sn-3 position of DAG [10]. In C. reinhardtii, PDAT localizes to chloroplasts, with compelling evidence supporting its significant contribution to TAG biosynthesis under nitrogen-deprived conditions [15,17,18]. Furthermore, Chouhan et al. demonstrated that high light increases PDAT protein levels in C. reinhardtii cells in agreement with TAG accumulation [19]. Altogether, these results suggest that changes in light conditions might trigger the involvement of distinct enzymes in TAG production and point to the importance of further exploring these pathways.
Phytyl ester synthases (PESs) have been proposed as an alternative chloroplast TAG synthesis pathway in plants. Leaf senescence and stress conditions trigger the disintegration of the chloroplast membrane. This produces, among other metabolites, free phytol and free fatty acids (FFAs), which are derived from chlorophyll and lipid hydrolysis, respectively [20,21]. Both phytol and FFAs are cytotoxic metabolites and, consequently, must be rapidly recycled in order to avoid cell damage [22]. Thus, PESs provide a protective mechanism by catalyzing the acylation of a FFA onto a phytol molecule, resulting in the formation of fatty acid phytyl esters (FAPEs) [22]. Two PES isoforms were characterized in A. thaliana: PES1 (At1g54570) and PES2 (At3g26840), which belong to the estearase/lipase/thioestearase family [23]. Both proteins localize to the chloroplast and have an acyltransferase/DGAT domain [23,24,25]. In vivo and in vitro assays using heterologously expressed PES1 and PES2 demonstrated that both proteins generate FAPEs and TAGs [23]. Interestingly, the contents of both FAPEs and TAGs are strongly reduced in pes1pes2 double mutants grown under N starvation [23]. Accordingly, heterologous expression of AtPES2 together with a Fatty Acid Reductase, which synthesizes fatty acid alcohols, and the transcription factor WRINKLED1, known to be involved in the regulation of fatty acid biosynthesis, enhanced wax esters and TAG accumulation in Nicotiana benthamiana [26]. From these results, it could be concluded that PES enzymes play a role in both FAPE and TAG synthesis in higher plants.
Thus far, FAPEs were detected in seven species of Chlorophyta and PES proteins were identified by proteomic analyses in the halotolerant alga Dunaliella bardawil [13,27,28]. This suggests that homologs of plant PES might exist in algae, which opens the possibility of their participation in FAPE and TAG synthesis.
In this work, we describe the identification of two PES isoforms in C. reinhardtii, named PESα and PESβ, homologous to PES1 and PES2 of A. thaliana. Our data reveal light-dependent upregulation of PESα and PESβ transcripts together with increased TAG levels in C. reinhardtii following transition from low-light to high-light conditions. Comparative analysis of DGAT3, DGAT1, and PDAT expression patterns, along with TAG accumulation profiles, showed significant differences between the pesα knockdown mutant and the parental strain. The putative role of PESα in non-conventional TAG synthesis is discussed.
2. Results
2.1. PESα and PESβ In Silico Analyses
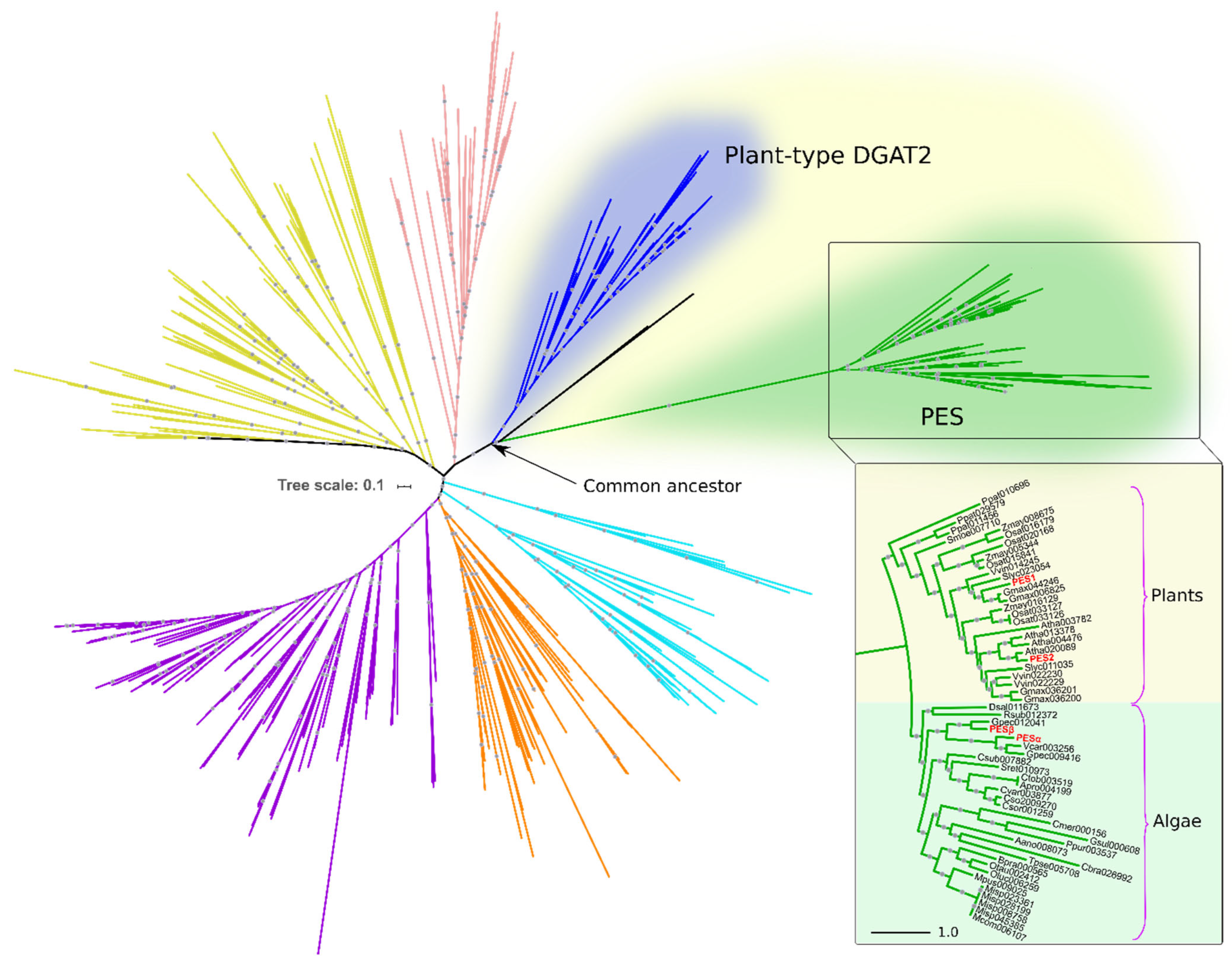
To identify PES homologs in algae, we conducted a HMMER iterative search using A. thaliana PES1 and PES2 as queries against predicted proteomes from algal species and from a set of representative eukaryotes (Table S1). The resulting candidates were then subjected to clustering and phylogenetic analyses, in order to establish their positioning within the superfamily of DAGAT domain-containing proteins (Pfam ID PF03982). Figure 1 shows the phylogenetic relationships of the DAGAT domain, present in DGAT2 and several related proteins, including PES.
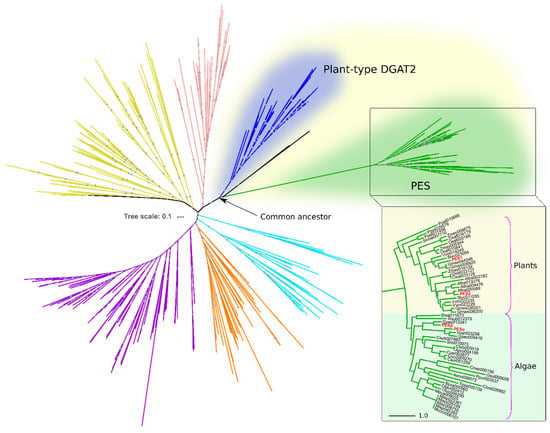
Figure 1.
Algal and plant PES belong to the DAGAT superfamily. Rooted circular phylogram representation of the tree generated by the maximum likelihood (ML) method (500 bootstraps) on the proteins with DAGAT domain of the species detailed in Table S1. Gray circles represent ML bootstrap values > 50. Different colors represent distinct DAGAT protein clades previously reported by our group [15]. The inset shows the plant and algal PES clade. Numbers after the abbreviated species names are internal IDs. The names in red correspond to PES1 (At1g54570) and PES2 (At3g26840) from A. thaliana, and PESα (Cre08.g365950) and PESβ (Cre12.g521650) from C. reinhardtii. The scale bars represent the number of amino acid substitutions per site.
The PES clade evolved from a most recent ancestor in common with plant DGAT2 (Figure 1). The sequences within this clade are restricted to photosynthetic, chlorophyll-containing, organisms. Among the species within our analysis, it includes ten plant species and nineteen species of green and red algae, showing a clear taxonomic division between them (Figure 1). PES1 and PES2 of A. thaliana reported by Lippold et al. are included within the plant PES group (Figure 1) [23]. In the algae group, we detected two PES sequences from C. reinhardtii, which we named PESα and PESβ (Figure 1). These are identified in Phytozome as genes Cre08.g365950 (https://phytozome-next.jgi.doe.gov/report/transcript/Creinhardtii_v5_6/Cre08.g365950.t1.2 (accessed on 9 September 2019)) and Cre12.g521650 (https://phytozome-next.jgi.doe.gov/report/gene/Creinhardtii_v5_6/Cre12.g521650 (accessed on 9 September 2019)), respectively.
PES1 and PES2 have very similar sequences, mainly differing in the 100 amino-terminal amino acids [23]. PESα and PESβ share 50% amino acid identity and 66% similarity with each other. Their most significant differences occur in the N-terminal regions, along with several distinct internal extensions present in both proteins. When compared to Arabidopsis PES proteins, PESα shows 33–34% identity and 48–49% similarity to PES1 and PES2 (Figure S1). PESβ exhibits slightly higher conservation, with 37% identity and 49–53% similarity to PES1 and PES2 (Figure S1).
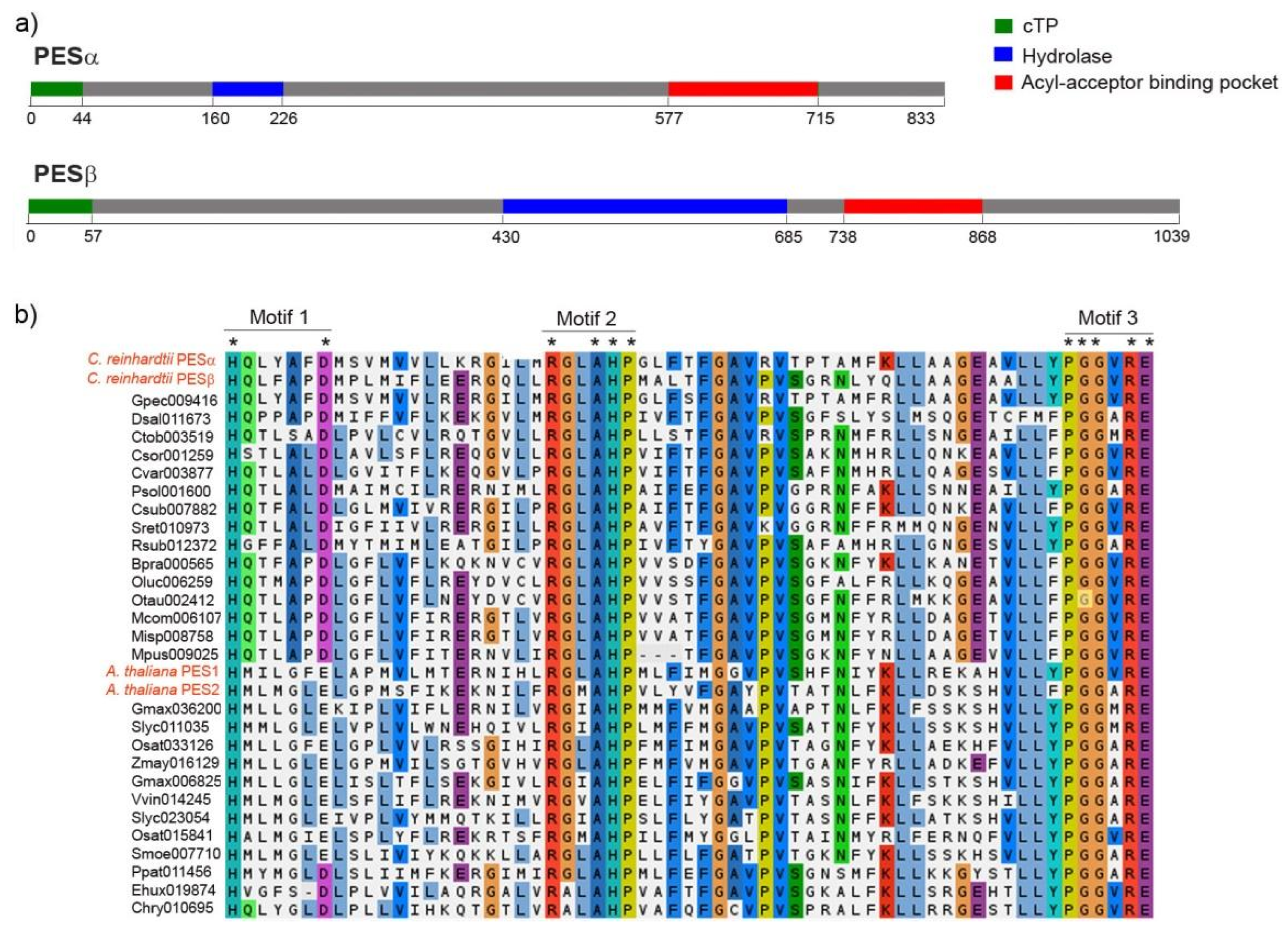
The estimated molecular weight of PESα and PESβ is 94 kDa and 107 kDa, respectively, and this difference is mainly due to the presence of a longer amino-terminal extension in PESβ. Both proteins have predicted chloroplast localization, and the start of the mature proteins is presumed to be at positions Asp-45 and Gly-58 for PESα and PESβ, respectively (Figure 2a, green segments).
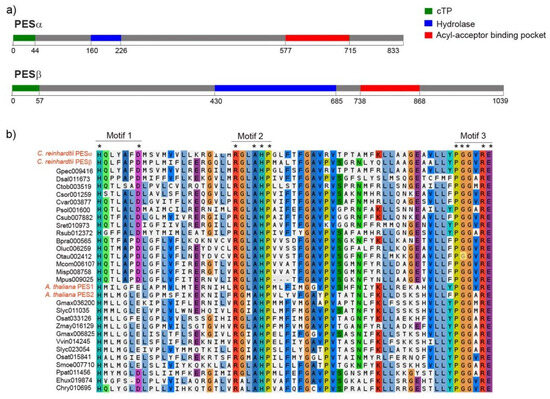
Figure 2.
Protein sequence features of PESα and PESβ. (a) Sequence scanning with PredAlgo predicted that both, PESα and PESβ, have a chloroplast transit peptide (cTP, in green) of 44 and 57 amino acids, respectively. Conserved Domain Database analysis of PESα and PESβ revealed the presence of a hydrolase-like sequence (in blue: PESα: amino acids 160–226, PESβ: amino acids 430–685) and an acyl-acceptor binding pocket (in red: PESα: amino acids 577–715, PESβ: amino acids 738–868). (b) Multiple sequence alignment of the acyl-acceptor binding pocket motifs from PESα and PESβ, fifteen PES sequences from algae and fourteen PES sequences from plants, including PES1 and PES2 from A. thaliana. The alignment was performed with MAFFT using BLOSUM62 and default settings. The coloring reflects charge and polarity properties of the majority-rule consensus residues. The three characteristic motifs are shown. Asterisks indicate conserved residues. The C. reinhardtii and A. thaliana name tags are written in red.
The in silico analysis of conserved domains revealed the presence of a predicted region of α/β hydrolase fold and a putative acyl acceptor-binding pocket (Figure 2a). In PESα, the α/β hydrolyase fold lies in the amino-terminal portion, while in PESβ it is situated centrally and is larger (Figure 2a, blue segments). In both sequences, the putative acyl acceptor-binding pocket is localized at the carboxy terminus (Figure 2a, red segments). According to this analysis, there are three motifs in the predicted acyl-binding pocket of C. reinhardtii PESs that are similar to acyltransferase motifs. These were detected by multiple sequence alignment of putative PES sequences from algal and plant species (Figure 2b). The residues histidine (H) and aspartic acid (D)/glutamic acid (E) of motif 1 are conserved in all the analyzed PES sequences, including PESα and PESβ (Figure 2b). The second motif is composed of strictly conserved arginine (R), alanine (A), H and proline (P) and two extra positions that correspond to conserved non-polar hydrophobic glycine (G)/A and leucine (L)/isoleucine (I)/valine (V)/methionine (M) residues (Figure 2b). The third motif is formed by strictly conserved P, two G, R and E and a conserved position with non-polar hydrophobic V/A/M residues (Figure 2b).
According to in silico predictions, PESα and PESβ do not have transmembrane regions, which suggests that both are soluble proteins. Hydrophobicity analysis indicated that PESα and PESβ have 46.6% and 54% hydrophobic amino acid residues, respectively, which is consistent with their likely association to membranes and interaction with lipid substrates.
2.2. Analysis of PESα and PESβ Transcripts in the Parental Strain, cc-5325, After Shifting Cells from Low Light to High Light
RNA-seq results reported by Wittkopp et al. showed that the expression of PESα and PESβ is rapidly and transiently induced in C. reinhardtii cells by changes in the light conditions [29]. Based on this evidence, we hypothesized that PESα and PESβ could play a role in light-induced lipid synthesis. With the purpose of evaluating this assumption, we first examined the response of PESα and PESβ to light. We cultured the cc-5325 strain of C. reinhardtii on Tris-acetate-phosphate (TAP) medium under continuous illumination (50 µmol photon m−2 s−1) until reaching exponential phase (2 × 106 cells mL−1). Then, the cells were transferred to low light (14 µmol photon m−2 s−1) for 16 h and subsequently shifted to high light (140 µmol photon m−2 s−1) for 1, 4 and 7 h. Expression was determined by reverse transcription quantitative polymerase chain reaction (RT-qPCR). The Chlamydomonas Guanine nucleotide-binding protein subunit β-Like Polypeptide (GBLP) gene was used as an endogenous control, since its transcript levels were previously demonstrated to remain stable during dark/low-light to high-light transitions [16]. Transcript levels were analyzed in relation to the End of the Low Light (ELL) period.
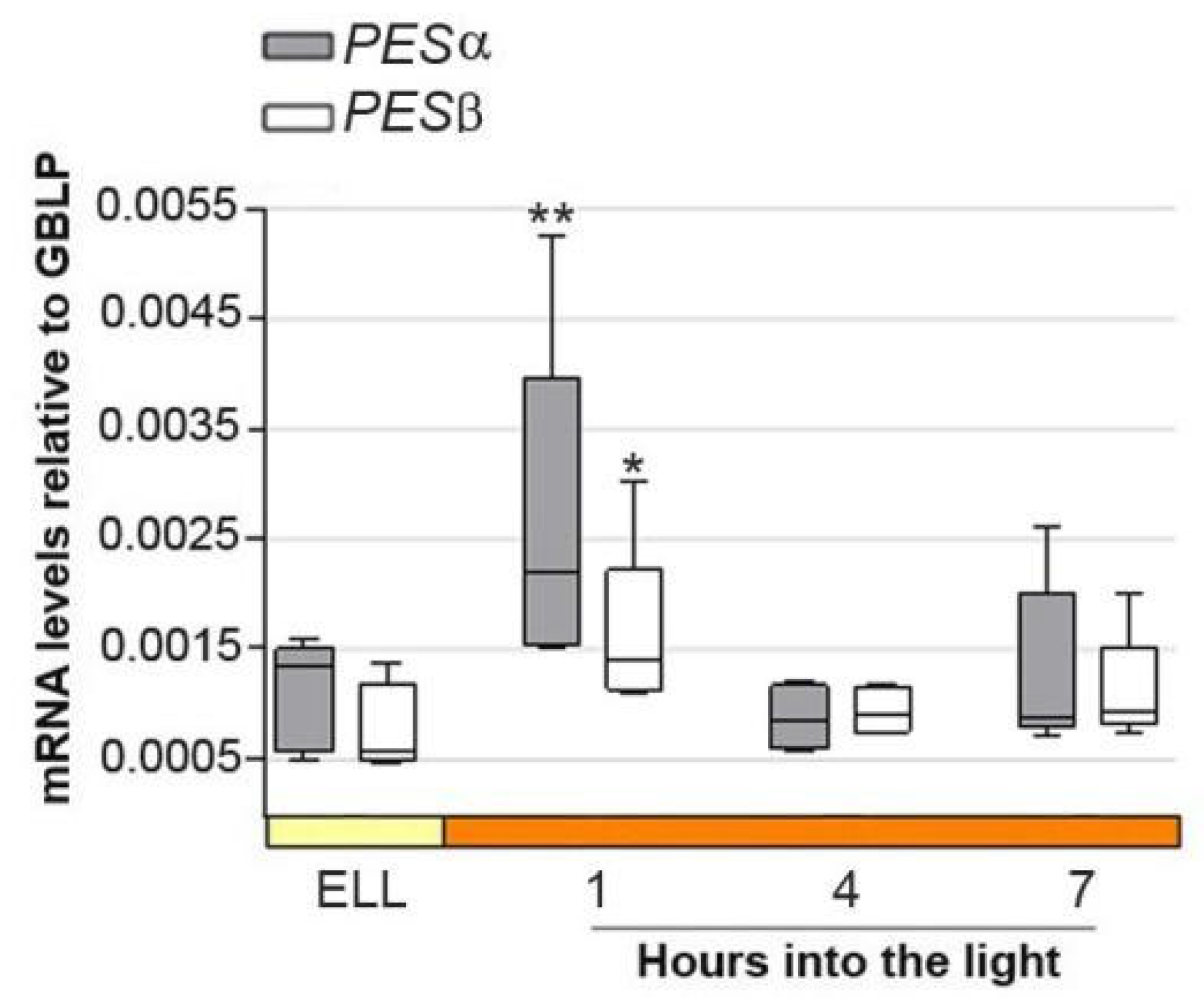
Figure 3 shows that PESα and PESβ transcript levels peaked 2.4 and 2.6-fold, respectively, as early as 1 h into the high-light period, and then decreased at 4 h and 7 h. This result suggests that PESα and PESβ are light-responsive genes.
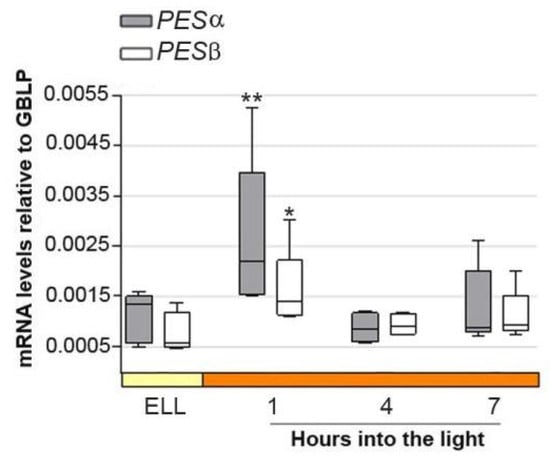
Figure 3.
PESα and PESβ mRNAs increase in cc-5325 after shifting cells from low light to high light. Cells from the parental cc-5325 strain were grown in TAP medium under continuous light (50 µmol photon m−2 s−1) to approximately 2 × 106 cells mL−1, then transferred to 14 µmol photon m−2 s−1 (low light period) for 16 h and subsequently switched to 140 µmol photon m−2 s−1 (high light). Samples were harvested at the end of the low-light period (ELL) and at 1, 4 and 7 h after transferring the cells to high light. Total RNAs were extracted from each sample and mRNAs were analyzed by RT-qPCR. GBLP was used as endogenous control. Box plots show the expression of PESα and PESβ. The pale yellow box and the orange box indicate the ELL and high-light periods, respectively. Results are expressed as mRNA levels relative to GBLP. Vertical bars indicate minimum and maximum values and horizontal black strips indicate median values (n = 5). Asterisks indicate significant difference from the corresponding ELL sample according to one-way ANOVA, post hoc Dunnett’s: ** p < 0.05. * p < 0.1.
2.3. Molecular Characterization of Pesα and Pesβ Mutants
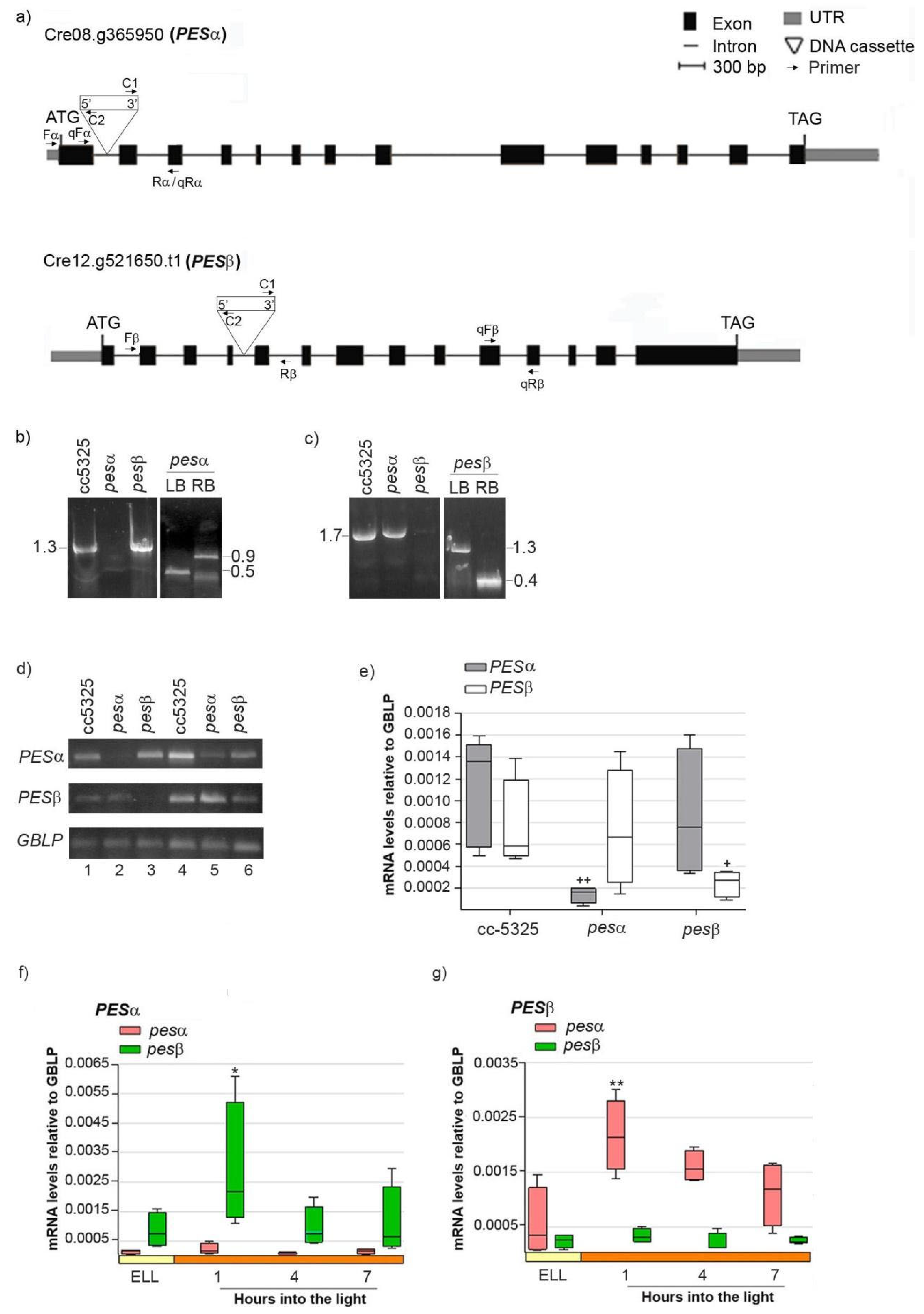
In order to study if PESα and PESβ participate in a light-induced lipid synthesis pathway, we acquired mutants from the Chlamydomonas Library Project (CLiP, https://www.chlamylibrary.org/ (accessed on 4 April 2020)). This is a collection of C. reinhardtii strains generated by random insertion of a 2.2 kb paromomycin-resistance cassette into the cc-5325 strain [30]. We selected the lines LMJ.RY0402.052315 (pesα) and LMJ.RY0402.046050 (pesβ), which contain the DNA cassette inserted in intron 1 and intron 4 of the PESα and PESβ genes, respectively, based on a 95% mapping confidence (Figure 4a) [30].
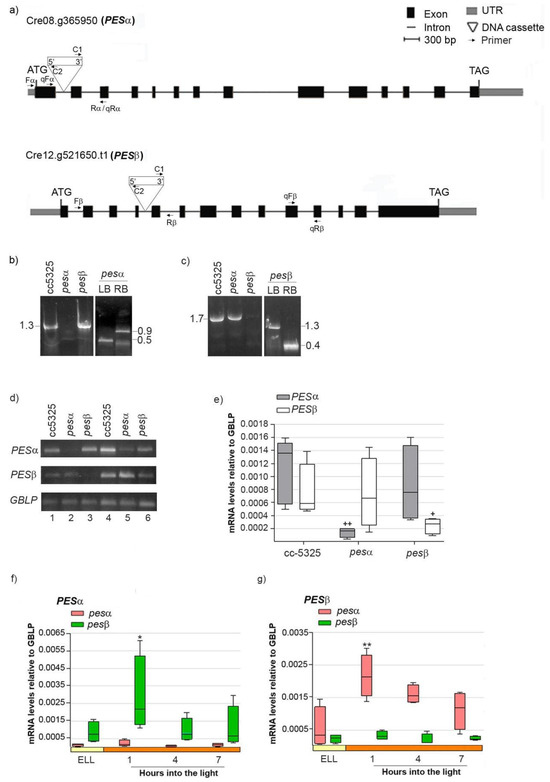
Figure 4.
Molecular characterization of C. reinhardtii pesα and pesβ mutants. (a) Exon/intron structure and location of the DNA cassette insertion in genes Cre08.g365950 (PESα) and Cre12.g521650 (PESβ). UTRs, exons and introns are designated by gray boxes, black boxes and lines, respectively. Binding sites for primers employed in the PCR with genomic DNA analysis Fα (Forward PESα), Rα (reverse PESα), Fβ (Forward PES β), Rβ (Reverse PESβ), C1 (Forward DNA cassette) and C2 (Reverse DNA cassette), are indicated, as well as the binding sites for primers employed in the semi-quantitative and quantitative RT-PCR analysis qFα (qPCR Forward PESα), qRα (qPCR reverse PESα), qFβ (qPCR Forward PES β), qRβ (qPCR Reverse PESβ). (b) PCR using genomic DNA of cc-5325, pesα and pesβ and the primer pair “Fα + Rα” to amplify PESα (1.3 kb, left panel). Confirmation of PESα disruption by PCR with genomic DNA of pesα and the primer pairs “Fα + C2” and “C1 + Rα”, to amplify the left border (LB, 0.5 kb) and right border (RB, 0.9 kb) of the DNA cassette insertion, respectively (right panel). (c) PCR with genomic DNA of cc-5325, pesα and pesβ and the primer pairs “Fβ + Rβ” to amplify PESβ (1.7 kb, left panel). Confirmation of PESβ disruption by PCR with genomic DNA of pesβ and the primer pairs “Fβ + C2” and “C1 + Rβ”, to amplify the left border (LB, 1.3 kb) and right border (RB, 0.4 kb) of the DNA cassette insertion, respectively (right panel). (d–g) Expression of PESα and PESβ in the parental and mutant backgrounds: Cells from cc-5325, pesα and pesβ strains were grown in TAP medium under continuous light (50 µmol photon m−2 s−1) to approximately 2 × 106 cells mL−1, then transferred to 14 µmol photon m−2 s−1 (low-light period) for 16 h and subsequently switched to 140 µmol photon m−2 s−1 (high light). Samples were harvested at the end of the low light period (ELL) and at 1, 4 and 7 h after transferring the cells to high light. (d) Semi-quantitative RT-PCR of PESα and PESβ using RNAs from the ELL samples of cc-5325, pesα and pesβ mutants. GBLP was used as endogenous control. The PCR conditions were: 35 or 40 cycles for PESα (upper panel, lanes1–3 and 4–6, respectively); 30 or 33 cycles for PESβ (middle panel, lanes 1–3 and 4–6, respectively) and 20 or 23 cycles for the endogenous control, GBLP (lower panel, lanes 1–3 and 4–6, respectively). (e) RT-qPCR using ELL samples of cc-5325, pesα and pesβ. Box plots show mRNA levels of PESα and PESβ normalized to GBLP. (f,g) Expression of PESα and PESβ in pesα and pesβ cells following a transition from low light to high light analyzed by RT-qPCR. Results are expressed as mRNA levels relative to GBLP. The pale yellow box and the orange box indicate the ELL and high-light periods, respectively. Semi-quantitative RT-PCR and RT-qPCR were performed with the primer pairs “qFα + qRα” and “qFβ + qRβ” to amplify PESα and PESβ, respectively. Vertical bars indicate minimum and maximum values and horizontal black strips indicate median values (n = 5). Significant differences between cc-5325 and pesα/pesβ mutants are indicated by plus symbols (+), while significant differences between ELL and high-light conditions within each strain are indicated by asterisks (*) according to one-way ANOVA, post hoc Dunnett’s: ++ ** p < 0.05, + * p < 0.1.
To confirm that the insertions were indeed present in the PESα and PESβ genes, we mapped the location of each insertion by PCR using oligonucleotides specific to both the genes and the insertion sites. Figure 4b (left panel) shows that, in the cc-5325 and pesβ strains, we obtained a PCR product of 1.3 kb that spans the PESα insertion site, whereas this product was not observed when using the genomic DNA of pesα as template. The insertion site within the first intron of PESα was verified by amplifying the left border (LB, 0.5 kb) and right border (RB, 0.9 kb) junctions of the DNA cassette (Figure 4b, right panel).
A 1.7 kb amplicon spanning the PESβ insertion site was obtained from the cc-5325 and pesα strains, but not from pesβ (Figure 4c, left panel). We confirmed the disruption of the fourth intron of PESβ by amplifying the LB (1.3 kb) and RB (0.4 kb) junctions of the DNA cassette (Figure 4c, right panel). Semi-quantitative RT-PCR detected PESα transcript expression in cc-5325 and pesβ at 35 PCR cycles, while they were only detectable at 40 PCR cycles in pesα (Figure 4d, upper panel). Similarly, PESβ mRNAs were detected in cc-5325 and pesα at 30 PCR cycles, while these were only detectable at 33 PCR cycles in pesβ (Figure 4d, middle panel). GBLP was equally amplified in the three strains at 20 and 23 PCR cycles, indicating that equivalent amounts of RNA were used in the semi-quantitative RT-PCR analysis. Therefore, these results suggest that pesα and pesβ are knockdown mutants rather than complete knockouts. Quantification of PESα and PESβ transcripts by RT-qPCR revealed detectable mRNA levels in the respective pesα and pesβ mutants, though significantly reduced compared to the parental strain (Figure 4e). In contrast, PESα mRNAs in pesβ and PESβ mRNAs in pesα were similar to those in cc-5325 (Figure 4e). This confirms the results obtained by semi-quantitative RT-PCR.
Next, we evaluated the light-dependent expression of PESα and PESβ in the pesα and pesβ mutant backgrounds, as previously performed with the parental strain. Analysis by RT-qPCR revealed that PESα was not induced in pesα, nor was PESβ in pesβ cells (Figure 4f,g). Conversely, PESα and PESβ transcripts were transiently increased in the pesβ and pesα strains, respectively, at 1 h into the high-light period (Figure 4f,g). The induction levels were similar to those observed in cc-5325 (Figure 3). These results suggest that the high-light induction of PESα and PESβ mRNAs is not affected by the mutation of the alternative isoform.
To further investigate the phenotype of pesα and pesβ mutants, we analyzed seven-day growth curves and the cell areas of cc-5325, pesα and pesβ cells cultured under standard conditions. Figure S2a shows that the growth curves of cc-5325, pesα and pesβ were similar. A transient increase in cell density was detected in the pesβ mutant at day five, but comparable cell densities were reached at the end of the exponential phase. Consistent with this result, analysis of growth rates revealed no significant differences between any of the strains (Figure S2b). This suggests that knockdown of PESα and PESβ does not impair normal growth.
In contrast, analysis of cell morphology revealed an altered phenotype for the pesα mutant. These cells exhibited a significantly larger cell area during the exponential growth phase and a strong tendency to form palmelloids (i.e., non-dividing cell aggregates), a typical algal stress response (Figure S2c,d) [31]. Conversely, morphological analysis of the pesβ mutant revealed only a significantly smaller cell area on the fourth day of growth, a phenotype that was not sustained by the fifth day.
2.4. Analysis of DGAT3, DGAT1, PDAT Expression and TAG Levels in cc-5325, Pesα and Pesβ Strains After Shifting Cells from Low Light to High Light
Substantial evidence suggests that DGAT3, DGAT1 and PDAT are chloroplast enzymes involved in TAG synthesis [11,14,15,16,18]. In contrast, DGAT2, which is encoded by a family of six isoforms, functions exclusively in microsomal TAG synthesis [15]. Because PESα and PESβ are predicted to localize to the chloroplast, we investigated whether they are related to DGAT3, DGAT1 and PDAT.
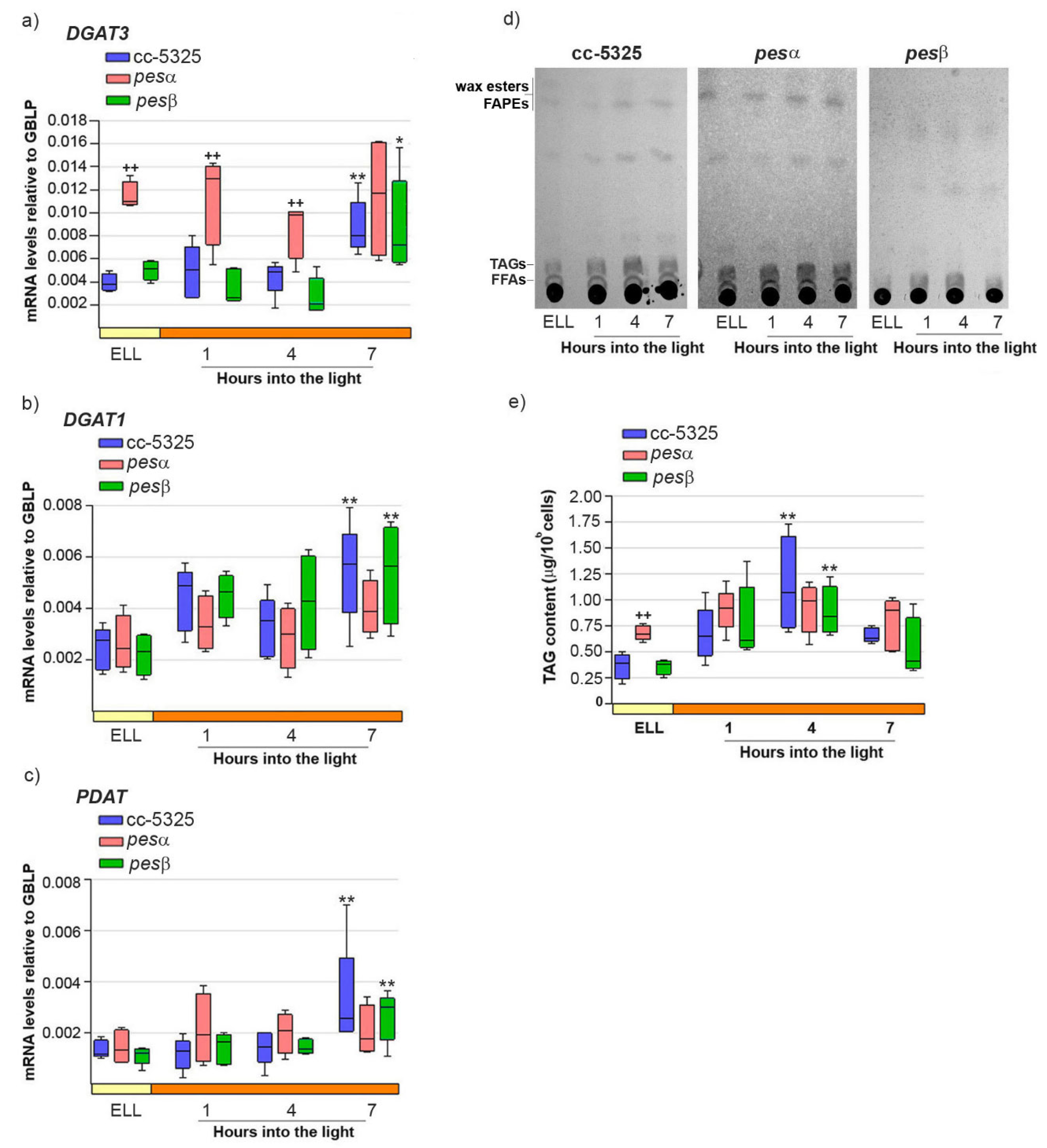
Our previous work demonstrated that high-light conditions upregulate both DGAT3 and DGAT1 mRNA levels while concurrently increasing TAG accumulation in C. reinhardtii [16]. We also proposed a functional role for PDAT in this stress response [16]. Therefore, we analyzed whether the expression pattern of DGAT3, DGAT1 and PDAT, as well as TAG content, were modified in pesα and pesβ strains. Figure 5a–c shows that DGAT3, DGAT1 and PDAT mRNAs were significantly increased in cc-5325, 7 h after shifting cells from low light to high light.
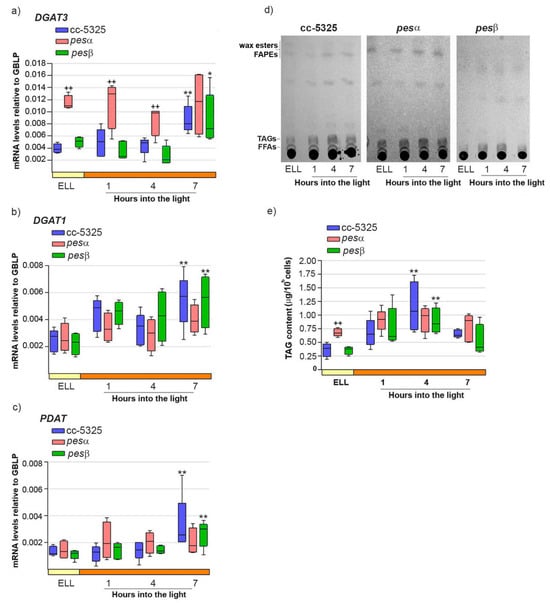
Figure 5.
Response of DGAT3, DGAT1, PDAT mRNAs and TAGs upon shifting cc-5325, pesα and pesβ cells from low light to high light. Cells from the parental cc-5325 (control), pesα and pesβ strains were grown in TAP medium under continuous light (50 µmol photon m−2 s−1) to approximately 2 × 106 cells mL−1, then transferred to 14 µmol photon m−2 s−1 (low-light period) for 16 h and subsequently switched to 140 µmol photon m−2 s−1 (high light). Samples were harvested at the end of the low light period (ELL) and at 1, 4 and 7 h after transferring the cells to high light. (a–c) Total RNAs were extracted from each sample and specific mRNAs were analyzed by RT-qPCR. GBLP was used as endogenous control. Box plots show the expression of DGAT3 (a), DGAT1 (b) and PDAT (c) in cc-5325, pesα and pesβ strains. Results are expressed as mRNA levels relative to GBLP. (d) Total lipids were extracted from each sample and analyzed by thin-layer chromatography (TLC). Sample volumes equivalent to 35 million cells were loaded on each lane. Dashes show the spots of free fatty acid (FFA), triacylglycerol (TAG) and wax ester/FAPE standards. A representative image of five independent biological replicates is shown. (e) TAGs eluted from the spots on (d) were quantified using a colorimetric assay. Box plots show the content of TAGs in cc-5325, pesα and pesβ strains. The pale yellow box and the orange box indicate the ELL and high-light periods, respectively. Vertical bars indicate minimum and maximum values and horizontal black strips indicate median values (n = 5). Significant differences between cc-5325 and pesα/pesβ mutants are indicated by plus symbols (+), while significant differences between ELL and high-light conditions within each strain are indicated by asterisks (*) according to one-way ANOVA, post hoc Dunnett’s: ++ ** p < 0.05, * p < 0.1.
In the same conditions, TAGs reached a 3.1-fold increase in cc-5325 cells at 4 h into the high-light period, and decreased at 7 h (Figure 5d,e).
In the pesα mutant, DGAT3 transcript levels were 2.9-fold higher than in cc-5325 at the ELL condition, and remained elevated throughout the subsequent high-light period (Figure 5a). In line with DGAT3 expression, the pesα strain had 1.9-fold more TAGs than cc-5325 at ELL (Figure 5d,e). The higher TAG content in pesα at ELL remained at similar levels until the end of the experiment, consistent with our observations of DGAT3 transcripts. These results demonstrate that the pesα knockdown strain exhibits elevated DGAT3 expression and increased TAG accumulation prior to high-light exposure compared to the parental strain. Consistent with this result, we observed that pesα cells had an enlarged cell morphology that was similar to the phenotype shown in Supplemental Figure S2c,d.
In contrast to DGAT3, both DGAT1 and PDAT transcript levels were comparable in pesα and cc-5325 strains in the ELL condition. Notably, neither gene showed significant upregulation in pesα following the transition to high-light conditions (Figure 5b,c). Thus, it could be concluded that PESα knockdown affects the expression of DGAT3, DGAT1, and PDAT differently.
In the pesβ mutant, DGAT3, DGAT1, and PDAT exhibited expression patterns similar to cc-5325, showing 2–3 fold induction within 7 h following the transfer from low-light to high-light conditions (Figure 5a–c). TAG levels were transiently augmented at 4 h after switching pesβ cells from low light to high light, as observed in cc-5325 (Figure 5d,e). No morphological differences with the control were observed under high-light conditions, in concert with a lack of increased TAG accumulation. These results suggest that PESβ knockdown does not affect the high-light induction of DGAT3, DGAT1, and PDAT mRNAs, nor the content of TAGs.
2.5. Analysis of FAPEs, Phytol and Chlorophyll Levels in cc-5325, Pesα and Pesβ Strains upon Shifting Cells from Low Light to High Light
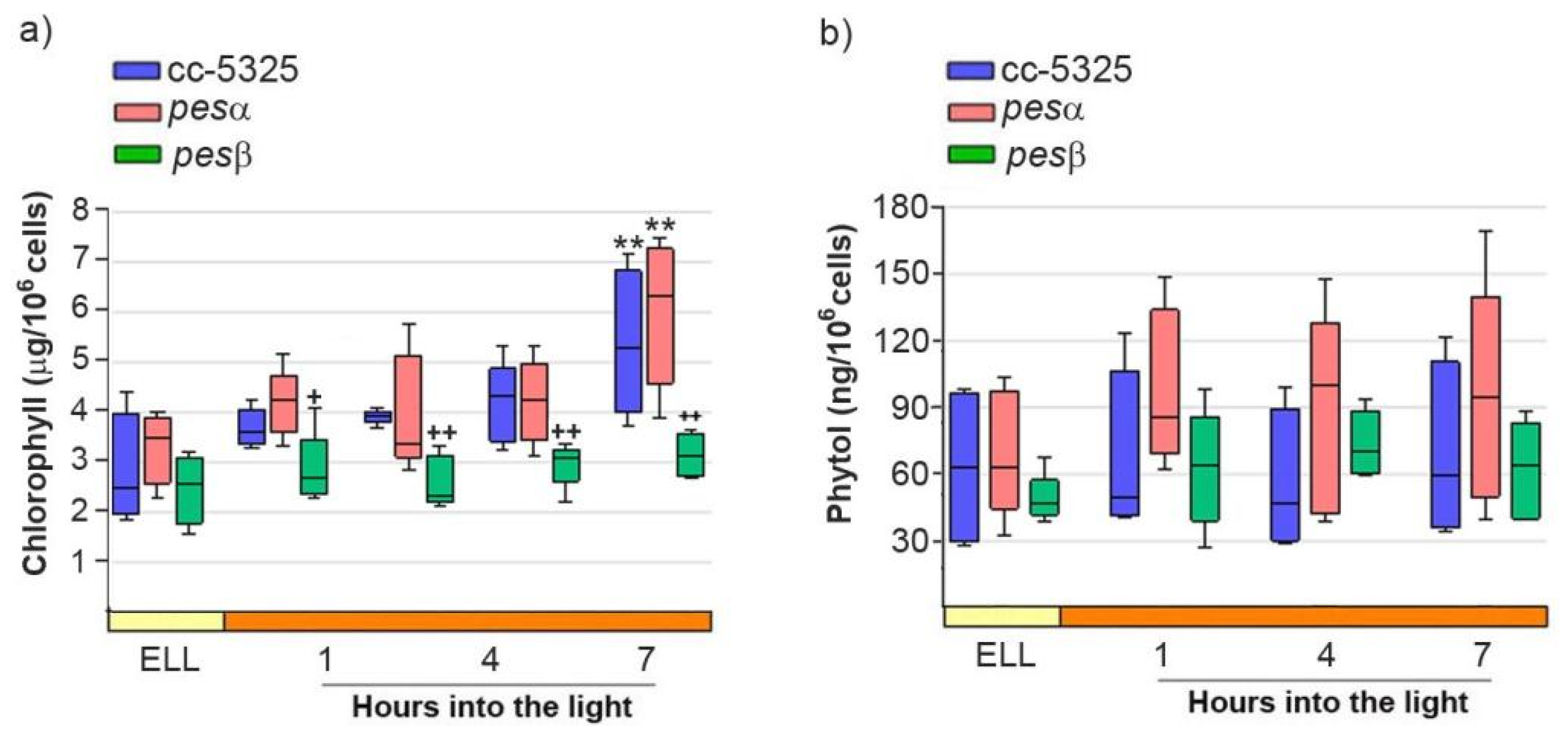
We further analyzed FAPE levels in the cc-5325, pesα and pesβ strains upon shifting the cells from low light to high light. Given that FAPEs and wax esters co-migrate in TLC, we isolated the wax ester/FAPE fractions from the TLCs shown in Figure 5d [32,33]. We then attempted to identify and quantify FAPEs in these fractions using gas chromatography-mass spectrometry (GC-MS). No detectable FAPEs were found in any of the strains grown under both low light and high light. Since FAPE synthesis depends on free phytol produced from chlorophyll degradation, we further analyzed chlorophyll and phytol content in cc-5325, pesα and pesβ cells grown under both low-light and high-light conditions. As shown in Figure 6a, chlorophyll content did not differ significantly between the parental cc-5325 strain and the pesα mutant throughout the experimental period. Following 7 h of exposure to high light, chlorophyll levels increased significantly in both cc-5325 and pesα. In contrast, pesβ showed significantly lower chlorophyll levels compared to cc-5325 and pesα during the high-light period.
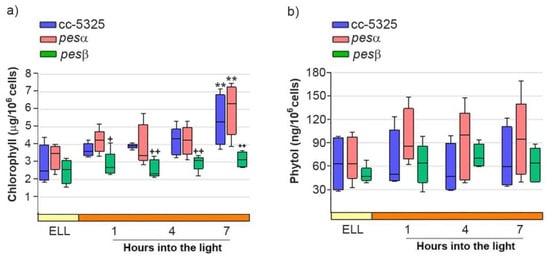
Figure 6.
Analysis of chlorophyll and phytol content after shifting cc-5325, pesα and pesβ cells from low light to high light. Cells from the parental cc-5325 (control), pesα and pesβ strains were grown in TAP medium under continuous light (50 µmol photon m−2 s−1) to approximately 2 × 106 cells mL−1, then transferred to 14 µmol photon m−2 s−1 (low-light period) for 16 h and subsequently switched to 140 µmol photon m−2 s−1 (high light). Samples were harvested at the end of the low-light period (ELL) and at 1, 4 and 7 h after transferring the cells to high light. (a) Chlorophyll was extracted from each sample and measured photometrically. Box plots show the content of chlorophyll in cc-5325, pesα and pesβ strains. (b) Total lipids were extracted from each sample and separated by TLC. Sample volumes equivalent to 35 million cells were loaded on each lane. Phytol spots were eluted from the silica of the TLC plates and quantified by HPLC. Box plots show the content of phytol in cc-5325, pesα and pesβ strains. The pale yellow box and the orange box indicate the ELL and high-light periods, respectively. Vertical bars indicate minimum and maximum values and horizontal black strips indicate median values (n = 5). Significant differences between cc-5325 and pesα/pesβ mutants are indicated by plus symbols (+), while significant differences between ELL and high-light conditions within each strain are indicated by asterisks (*) according to one-way ANOVA, post hoc Dunnett’s: ++ ** p < 0.05, + p < 0.1.
Figure 6b shows that phytol levels remained stable in cc-5325 following the transition from low light to high-light conditions and throughout the whole experimental period. Neither pesα nor pesβ showed significant differences in phytol content compared to the parental strain under either light regime (Figure 6b). This indicates that, under the experimental conditions tested, there is no significant chlorophyll degradation and, hence, phytol does not accumulate following high-light exposure.
3. Discussion
In this report, we identify two PES isoforms in C. reinhardtii, named PESα and PESβ. We demonstrate that PESα and PESβ are transiently induced by high light, consistent with an increase in TAG levels. Interestingly, pesα cells accumulate TAGs and upregulate DGAT3 transcripts—a gene proposed to be involved in light-dependent TAG synthesis—even under low-light conditions, prior to exposure to high light. Moreover, in pesα cells, DGAT1 and PDAT are not induced by high light, unlike in parental cells. This phenotype is absent in pesβ cells, where TAG levels and DGAT3, DGAT1 and PDAT transcript levels remain similar to those of control cells. These findings suggest that PESα and PESβ are functionally non-redundant in TAG metabolism and implicate PESα as a novel component of a non-conventional, light-induced, TAG synthesis pathway in C. reinhardtii, likely alongside DGAT3, DGAT1 and PDAT.
High light induces lipid accumulation in various algal species, directing excess energy toward chemical products and preventing photooxidative damage [34]. Under this growth condition, it was observed that TAGs are mainly of chloroplastic origin and accumulate in lipoproteic particles called lipid droplets, mostly associated with the chloroplast envelope [7]. Chloroplastic TAGs can also accumulate in plastoglobules, which are lipid droplets that are associated with thylakoids and proliferate in plants during senescence and oxidative stress conditions [35]. PES1 and PES2 have been described as proteins associated with plastoglobules that play a role in recycling chlorophyll and lipids derived from the chloroplastic membrane, by producing FAPEs and TAGs [23,24,25]. Our data mining analysis identified PESα and PESβ in C. reinhardtii as homologs of Arabidopsis PES1 and PES2, consistent with previous phylogenetic analyses [13,23,33,36].
Both, PESα and PESβ, have a putative hydrolase domain and an acyl-binding pocket, which are characteristic of acyltransferase proteins such as PES1 and PES2 [23]. The α/β hydrolase fold is common to several hydrolytic enzymes of widely differing phylogenetic origin and catalytic function [37]. The presence of this domain suggests that PESα and PESβ may have lipase activity, as previously postulated for PES1 and PES2 [23]. Regarding the putative acyl-binding pocket of PESα and PESβ, the first motif—containing the conserved H and D/E residues—represents a critical component of the acyltransferase catalytic domain and has been previously identified in DGAT3 [15,38]. PESα and PESβ are predicted to be hydrophobic proteins without transmembrane regions, as previously described for PES1 and PES2 in the proteomic analysis of Arabidopsis plastoglobules [25]. Their hydrophobicity suggests that PESα and PESβ could interact with membranes or in general with lipid compounds, as it has been proposed for DGAT3 [15].
The sequences of PESα and PESβ proteins differ in their N-terminal portions, in which both isoforms have specific extensions, being the one of PESβ longer than that of PESα. It has been reported that plant and algal proteins involved in light-dependent reactions are regulated by distinct post-translational modifications and by the binding of other proteins, which affect activity, stability, localization and other characteristics of the protein fate [39]. In this scenario, we could argue that the differences in the N-terminal portions of PESα and PESβ could provide different opportunities for post-translational regulation.
PESα and PESβ are predicted to have chloroplastic localization, as recently reported by Wang et al. [40]. Accordingly, Davidi et al. detected three PES proteins in plastoglobule fractions of D. bardawil that are homologs of PESα and PESβ and have similar predicted molecular weights [13].
We demonstrate that PESα and PESβ mRNAs are transiently increased shortly after shifting cc-5325 cells from low light to high light conditions. This pulse pattern is consistent with light activation, in which the transient response is thought to meet the immediate requirements of photosynthetic activity following such a shift. As the light period progresses, these requirements diminish as cells adapt through a light-dependent genetic program. In C. reinhardtii, this response is regulated mainly at the transcriptional and/or RNA stability level, particularly in chloroplast-targeted genes such as DGAT3, which are functionally linked to photosynthetic activity [16,41,42,43,44,45,46]. Additionally, this is consistent with RNA-seq results showing that PESα and PESβ expression is rapidly and transiently induced in C. reinhardtii within 0.5–1 h after transferring cells from dark to high light [29,42]. Similarly, it has been reported that Arabidopsis PES1 protein levels and tomato (Solanum lycopersicum) PES2 mRNAs increase in plants grown under high light [25,47]. Despite this evidence, little is known about the mechanisms that regulate PES expression. RNA-seq analysis revealed that biliverdin—a product of the heme catabolism involved in plastid-to-nucleus retrograde signaling in plants—attenuates the light-induced expression of PESα and PESβ in C. reinhardtii [29]. Additionally, Kamranfar et al. reported that PES1 is regulated by the transcription factor RD26, which is involved in metabolic reprogramming during senescence [48]. Future work will be focused on studying the regulatory mechanisms of PESα and PESβ.
We previously proposed that DGAT3 and DGAT1 are induced in cc-125 wild-type cells when light intensity is increased, which occurs in concert with an increase in TAG content [16]. We obtained similar results in cc-5325 cells, from which we conclude that these light-dependent responses are reproducible in both C. reinhardtii strains. In order to analyze the roles of PESα and PESβ in the light-dependent lipid synthesis pathway, we acquired two insertional mutants, pesα and pesβ, which we characterized as knockdown mutants. In this type of transformants, a knockdown mutation may result from a small population of mRNAs in which the entire insertion cassette is spliced out together with the flanking intron sequence, leading to considerably lower, but not null, mRNA expression, as previously reported [49,50]. Despite this, we observed that the mutated genes are not induced by high light, as are the wild-type genes, and that the mutation of one PES isoform does not affect the expression of the other. We show, as well, that DGAT3 mRNAs were significantly higher in pesα cells than in the parental strain in the ELL sample, and this was maintained along the high-light period. Interestingly, we observed that pesα cells had higher TAG levels than the parental strain under the same growth condition. These results support our hypothesis regarding a role of DGAT3 in TAG synthesis in C. reinhardtii, as DGAT3 overexpression coincided with an increase in TAG content in pesα cells. The upregulation of DGAT3 in pesα cells may be attributed to genetic compensation, a mechanism observed in the cellular networks of various eukaryotic species. In this process, the disruption of a specific gene can lead to changes in the expression of other genes within the same network, in order to preserve cellular viability [51]. Specifically, Lee et al. reported that DGAT2-2 knockout triggered the overexpression of DGAT2-1, DGAT2-3 and PDAT in P-deprived C. reinhardtii cultures [52]. In contrast, DGAT2-1 knockout only induced PDAT overexpression [52]. In both cases, this response occurred in concert with the overaccumulation of TAGs [52]. The authors concluded that compensating for mutations in genes involved in key steps of lipid biosynthesis with related genes mitigates their impact, thereby enhancing the genetic robustness of microalgal TAG production [52]. We hypothesize that a similar mechanism may apply to PESα and DGAT3, where DGAT3 overexpression compensates for PESα knockdown, resulting in TAG over-accumulation under low-light conditions. This suggests that PESα and DGAT3 are functionally related genes involved in non-conventional TAG synthesis. In contrast to the results observed in pesα cells, the A. thaliana pes1pes2 double mutant, along with the PES-related mutants slr2103 from Synechocystis sp. PCC 6803 and A0918 from Synechococcus sp. PCC 7002, exhibited reduced TAG levels compared to their respective wild-type strains [23,33,36]. Since these were null mutants, it could be hypothesized that: (i) the combined mutation of both PES1 and PES2 creates a different and/or more severe phenotype or (ii) distinct phenotypes arise from knockout versus knockdown PES strains. Further studies will be performed in order to analyze TAG production in a pesα knockout background, as well as in pesαpesβ double mutants.
Unlike DGAT3, the levels of DGAT1 and PDAT mRNAs in pesα cells under low-light conditions were comparable to those in the parental strain, but did not increase when exposed to high light. This suggests that PESα is necessary, either directly or indirectly, for the high-light dependent expression of DGAT1 and PDAT. Alternatively, the elevated levels of TAGs in the pesα background might inhibit the expression of DGAT1 and PDAT induced by light, as we previously proposed [16].
In contrast to the pesα phenotype, pesβ knockdown had no effect on the expression of DGAT3, DGAT1, or PDAT, nor on TAG content under both low-light and high-light conditions. Overall, these data suggest that PESα and PESβ are likely non-redundant isoforms in TAG metabolism, and that PESβ does not have a noticeable role in light-dependent TAG accumulation.
It has been postulated that FAPEs serve as a temporary storage for free phytol and free fatty acids, which are released from chlorophyll and galactolipids during chlorotic stress and later recycled when the stress subsides [22]. No FAPEs were detected in cc-5325 in our culture conditions, consistent with the lack of observable chlorophyll degradation or phytol accumulation in any of the strains across both light regimes. Plants grown under standard conditions contain very few FAPEs, but their levels significantly increase under chlorotic stresses, such as senescence and nitrogen deprivation, in concert with chlorophyll decrease and phytol increase [23,32,53,54]. Conversely, Spicher et al. showed that only two out of nine FAPE species, 14:0-phytol and 16:0-phytol, are increased by high light (850 µmol m−2 s−1) in tomato plants, while phytol levels remain constant [47]. These reports suggest that FAPEs are primarily produced under conditions that induce massive chlorophyll degradation and increased phytol levels. In addition, it has been demonstrated that chlorophyll levels do not change significantly in C. reinhardtii cells grown for up to 48 h at 500 µmol m−2 s−1 [55,56]. These findings support our results, which show that the light intensities used in our assays did not induce substantial chlorophyll degradation. This could explain the lack of phytol accumulation and, consequently, the absence of FAPEs in C. reinhardtii cells.
These results further suggest that, during low- to high-light transitions, PESα functions primarily in the TAG synthesis pathway, with no detectable role on FAPE accumulation.
4. Materials and Methods
4.1. Chlamydomonas reinhardtii Strains and Culture Conditions
The C. reinhardtii mutant strains LMJ.RY0402.052315 (pesα) and LMJ.RY0402.046050 (pesβ), as well as the parental strain cc-5325 (cw15 mt-), were obtained from the Chlamydomonas Resource Center (https://www.chlamycollection.org). The mutant strains were generated by the Chlamydomonas Library Project (CLiP, https://www.chlamylibrary.org/) by random insertion of a 2.2 kb paromomycin-resistance cassette named CIB1 into cc-5325 cells [30]. The strains were maintained on TAP agar plates [57]. For experiments, the cells were inoculated at 104 cells mL−1 in 100 mL Erlenmeyer flasks containing liquid TAP. The cultures were grown on a rotary shaker at 21 °C under continuous white light (6500 K) at 50 µmol photon m−2 s−1 until they reached a cell density of 2 × 106 cells mL−1. Then, they were transferred to low light (14 µmol photon m−2 s−1) for 16 h and subsequently shifted to high light (140 µmol photon m−2 s−1). Cell cultures were grown in batch mode until the end of the low light period and in semi-continuous mode after transferring them to the light [16]. Samples were taken at the ELL and then 1, 4 and 7 h after shifting the cultures to high light. Cell harvesting was performed as previously described [16]. Five independent experiments were conducted for each culture condition and strain.
4.2. Determination of Growth Curve, Growth Rate and Cell Area
Aliquots from cultures of cc-5325, pesα, and pesβ grown at 50 µmol photon m−2 s−1 were harvested at 3, 4, 5, 6, and 7 days of growth, thereby covering the lag, exponential, and stationary phases. Cell density was determined by cell counting using an Olympus BH2 microscope (Olympus Corporation of the Americas, Breinigsville, PA, USA) and a hemocytometer. Cell density values were plotted against time, and the growth rate (µ) was calculated according to the following formula [58]:
where “x2” and “x1” correspond to the cell densities recorded on day 6 (t2, end of the exponential phase) and day 3 (t1, beginning of the exponential phase), respectively.
µ = (ln(x2) − ln(x1))/(t2 − t1),
To determine cell area, cells from the three strains harvested at 4 and 5 days of growth were visualized using an Olympus CKX53 microscope (Olympus Corporation of the Americas, Breinigsville, PA, USA) and digitized with an AmScope MU1000 camera (United Scope LLC, Irvine, CA, USA) attached to the microscope. Cell area was measured using the ImageJ software (version 1.54g, Wayne Rasband, NIH, Bethesda, MD, USA).
4.3. In Silico Analyses
PES homologs were identified with the phmmer tool within HMMER version 3 using A. thaliana PES1 and PES2 as seeds [59]. These sequences were used to search a compiled FASTA file containing the complete proteomes of selected eukaryotic species. Sequences above a specified inclusion threshold (E-value = 0.001) were retrieved and redundant sequences showing 100% identity were eliminated using CD-HIT [60]. The positive hits were aligned using MAFFT online with default settings, available at http://mafft.cbrc.jp/alignment/server/index.html (accessed on 9 September 2019) [61]. The resulting multiple sequence alignment (MSA) was used to generate a position-specific scoring table (hidden Markov model, hmm) using the hmmbuild tool from the HMMER suite. This profile was used again against the complied FASTA file, using hmmsearch. Positive hits were aligned again and used for the generation of a new hmm file, re-starting the whole cycle. This process was repeated until convergence, at which point no new information was obtained when a new data-mining cycle was done. The final MSA was trimmed using BMGE v1.2 (Block Mapping and Gathering with Entropy), with the following optional arguments: -m BLOSUM45 -h 0.6 -g 0.5:0.3 -b 3, and used for phylogeny reconstruction [62]. This was performed by the Maximum Likelihood method using PhyML v3.0, with the LG model, default settings and 500 bootstraps [63]. Final tree editing was performed using iTOL (accessed on 24 October 2019) [64].
The analysis of conserved domains was performed with NCBI Conserved Domain Database (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi (accessed on 10 June 2024)). Subcellular localization was analyzed using PredAlgo (https://lobosphaera.ibpc.fr/cgi-bin/predalgodb2.perl?page=main (accessed on 6 June 2024)), an algorithm specifically trained with green algal proteins. The presence of transmembrane regions was studied with the programmes DeepTMHMM (https://dtu.biolib.com/DeepTMHMM (accessed on 6 June 2024)), SOUSI (https://harrier.nagahama-i-bio.ac.jp/sosui/mobile/ (accessed on 6 June 2024)) and CCTOP (https://cctop.ttk.hu/). Hydrophobicity analysis was performed with Peptide 2.0 (https://www.peptide2.com/N_peptide_hydrophobicity_hydrophilicity.php (accessed on 4 June 2024)).
4.4. DNA Isolation and PCR Mapping
DNA isolation was performed as described by Sambrook et al. [65]. The control strain cc-5325 and the mutant strains pesα and pesβ were genotyped by PCR using 0.25 µM of oligonucleotide primers targeting the genomic sequence and primers flanking the left and right borders of the paromomycin-resistance cassette, CIB1 (Table S2). PCR reactions for PESα amplification contained 0.05 U·µL−1 GoTaq G2 Hot Start Polymerase from Promega (cat. no. M7401; Madison, WI, USA) and were performed under the following conditions: holding stage, 95 °C for 5 min; cycling stage, 40 cycles at 95 °C for 30 s (melting), 50 °C for 20 s (annealing) and 72 °C for 2 min (extension), with a final extension cycle at 72 °C for 5 min. PCR reactions for PESβ amplification contained 0.015 U·µL−1 KAPA HiFi DNA Polymerase from KAPA Biosystems, Inc. (cat. no. 7958897001; Wilmington, MA, USA) with 1× KAPA HiFi GC buffer, and were run using the following parameters: holding stage, 95 °C for 5 min; cycling stage, 40 cycles at 95 °C for 30 s (melting), 62 °C for 20 s (annealing) and 72 °C for 2 min (extension), with a final extension cycle at 72 °C for 5 min. PCR products obtained from pesα and pesβ mutants were cloned into pCR4 Blunt TOPO vector from Thermo Fisher Scientific (cat. no. 450245; Waltham, MA, USA), following the manufacturer’s instructions. DNA sequencing was performed by Macrogen Facility (https://dna.macrogen.com/ (accessed on 28 September 2021)).
4.5. RNA Isolation, cDNA Synthesis and Quantitative PCR Analyses
The harvested cells were lysed by incubating them with 70 mM Tris-HCl, pH 8, 200 mM NaCl, 20 mM EDTA, pH 8, and 2.7% SDS, at room temperature for 30 min [16]. Total RNAs were extracted with phenol:chloroform:isoamyl alcohol (25:24:1), as previously described [16]. Copy DNA (cDNA) was synthesized using MMLV reverse transcriptase (RT) from Thermo Fisher Scientific (cat. no. 28025013; Waltham, MA, USA) and an oligo-dT primer on 1 µg of total RNA as a template, following the manufacturer’s instructions. The cDNA was diluted to a final volume of 50 µL and 2 µL were used for quantitative PCR (qPCR). The Fast Universal SYBR Green Master mix from Millipore Sigma (cat. no. 04913914001; Burlington, MA, USA) was employed, using an Applied Biosystems StepOneTM Real-Time PCR System from Thermo Fisher Scientific (Waltham, MA, USA). The standard amplification program was used. The nucleotide sequences of the specific primers for qPCR analysis of DGAT3, DGAT1, PDAT and GBLP were previously reported [16]. The oligo sequences for PESα and PESβ are detailed in Table S2. Data was analyzed using LinRegPCR v11.0, a program for determining PCR efficiency and calculating starting concentrations of target sequences [66]. To ensure the comparability of Ct values despite differences in expression levels, we used template concentrations that showed acceptable linearity (R2 ≥ 0.98). Furthermore, only genes with amplification efficiencies between 1.8 and 2.2, and with less than 10% efficiency differences between each other were compared [67].
4.6. Lipid Extraction and Thin-Layer Chromatography
Lipid extraction was performed according to the method described by Bligh and Dyer [68]. Briefly, cells were resuspended in 200 µL of phosphate-buffered saline and vortexed for 20 s. Total lipids were extracted with 700 µL of chloroform:methanol (1:2, v/v) followed by overnight incubation at −20 °C. For phase partitioning, 233 µL of chloroform and 200 µL of H2O were added, samples were vortexed for 20 s, and the tubes were centrifuged at 1000× g for 20 min at room temperature. Successful lipid extraction was confirmed by the white color of the protein solid phase, indicating complete partitioning of chlorophyll into the lower chloroform phase, which was transferred to a new tube. Lipid extracts were dried under a nitrogen stream and resuspended in 30 µL of chloroform. For the resolution of TAGs and wax esters/FAPEs, lipid extracts were spotted onto 500-µm silica gel G-60 TLC plates (20 × 15 cm) under a nitrogen stream. A mixture of n-hexane:diethyl ether (96:4, v/v) was used as the mobile phase. Plates were pre-equilibrated for 10 min in a tank containing the solvent mixture and then developed until the solvent front reached 4 cm from the top. After drying the TLC plates for 10 min, lipid bands were visualized under blue light following spraying with 0.03% 2,7-dichlorofluorescein in methanol and exposure to ammonia vapor. Lipid classes were identified by comparison with co-spotted standards. Olive oil and jojoba oil were used as TAG and wax ester/FAPE standards, respectively. Oleic acid (product code MATSOL 101 OD; MATERIA Hnos SACIF, Mar del Plata, Argentina) was kindly provided as a free fatty acid standard. Commercial phytol from Sigma, which is a mix of cis-/trans-isomers (W502200; St Louis, MI, USA), was used as a standard. TAGs and wax esters/FAPEs were eluted from the silica with chloroform:methanol:water (5:5:1, v/v) and partitioned by adding 0.8 volumes of 1 N ammonia (for TAGs) or 0.8 volumes of water (for wax esters/FAPEs and phytol) to recover them in the organic phase. Each organic fraction was dried under a nitrogen stream. Purified TAGs were resuspended in 25 µL of isopropyl alcohol, and their total quantity was determined using an enzymatic assay (GPO-PAP method, TG Color, Wiener Lab, (Catalog number 1780111; Ciudad Autónoma de Buenos Aires, Argentina) following the manufacturer’s instructions. Wax ester/FAPE fractions were further analyzed by gas chromatography–mass spectrometry (GC–MS) to detect FAPE molecular species.
4.7. Detection of FAPEs by GC-MS
For identification and quantification purposes, the FAPE standard (phytylpalmitate) was prepared and purified as previously described [69]. GC-MS analyses were performed in full-scan mode (m/z 50–800) for identification, as well as in selected ion-monitoring (SIM) mode for quantification. The wax ester/FAPE fractions were analyzed on a GC-MS Agilent GC8890-MSD5977C system from Agilent Technologies (Santa Clara, CA, USA), which was equipped with a split/splitless injector operated in splitless mode and an HP-5MS GC (Hewlett-Packard/Agilent, Waldbronn, Germany).
4.8. Quantification of Phytol by HPLC
For phytol separation, lipid extracts were co-spotted with a commercial phytol standard onto silica gel H 60 TLC plates under a nitrogen stream. Plates were developed using a mobile phase of benzene:ethyl acetate (19:1, v/v) [70]. Lipid bands were visualized by spraying with 0.03% 2,7-dichlorofluorescein in methanol and exposing the plate to ammonia vapor. The phytol band was identified by co-migration with the standard, scraped from the silica, and eluted with chloroform:methanol:water (5:5:1, v/v). Dried phytol samples were reconstituted in methanol and analyzed using an Agilent 1260 Infinity HPLC system equipped with an Eclipse Plus C18 reverse-phase column (100 × 4.6 mm, 3.5 µm spherical particles, octadecylsilane-coated; Agilent Technologies; Waldbronn, Germany), as reported by Narai-Kanayama, with the following modifications [71]. Methanol was used as the mobile phase, delivered by a quaternary pump at 40 °C. The flow rate was 0.5 mL min−1. Phytol elution was monitored at 210 nm using a UV detector. An external calibration curve was constructed using standard solutions of phytol in the range of 0.5–16 μg mL−1 in methanol.
4.9. Chlorophyll Quantification
The harvested cells were resuspended in 1 mL of 80% (v/v) acetone and vortexed for 1 min. The extracts were then heated at 100 °C for 1 min and centrifuged at 6000× g for 5 min at room temperature. Successful chlorophyll extraction was confirmed by the white color of the pelleted cellular debris. The supernatant was transferred to a new tube for chlorophyll quantification. Absorbance measurements were taken at 663 nm (chlorophyll a) and 646 nm (chlorophyll b), and their concentrations were calculated using the equations described by Lichtenthaler [72]. The entire procedure was performed under dim light to avoid chlorophyll bleaching.
5. Conclusions
We demonstrate that PESα and PESβ are transiently induced by high light in parallel with TAGs. In addition, PESα knockdown results in overexpression of DGAT3, which may be functionally linked to PESα, along with TAG accumulation, under low light. We propose that PESα plays a role in light-dependent TAG production, but not in FAPE synthesis, in C. reinhardtii cells grown under light conditions in which phytol levels are not increased above basal levels. A follow-up to our work will involve analyzing the enzymatic activity of PESα and its regulatory mechanisms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14193044/s1, Figure S1: Multiple sequence alignment performed with MAFFT of PESα and PESβ from C. reinhardtii and PES1 and PES2 from A. thaliana; Figure S2. Knockdown of PESα and PESβ does not affect growth rate but increases cell area in the pesα mutant under standard light conditions. Table S1: Sources of the proteome files used for DAGAT superfamily biocomputational HHMER iterative profiling; Table S2: qPCR and PCR oligonucleotides [73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96].
Author Contributions
Conceptualization, M.V.B. and G.G.; methodology, G.M.O., M.V.B. and G.G.; investigation, F.E.Z.B., G.M.O., N.P., M.V.B. and G.G.; supervision, G.M.O., M.V.B. and G.G.; writing—original draft preparation, G.G.; writing—review and editing, M.V.B. and G.G.; funding acquisition, M.V.B. and G.G.; project administration, M.V.B. and G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants to G.G., M.V.B. and G.O.M. from the Argentinean institutions Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, PIP-2021-0942 to M.V.B and G.G.), Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación (Agencia I+D+i, PICT-2021-00098 to G.G. and PICT-2021-00190 to M.V.B), and Secretaría General de Ciencia y Técnica-Universidad Nacional del Sur, Argentina (SGCyT-UNS, PGI-24/B341 to G.O.M.). G.G., M.V.B. and G.M.O. are CONICET researchers; F.E.Z.B. is an Agencia I+D+i doctoral fellow; N.P. is a CONICET doctoral fellow.
Data Availability Statement
Dataset available upon request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mulgund, A. Increasing Lipid Accumulation in Microalgae through Environmental Manipulation, Metabolic and Genetic Engineering: A Review in the Energy NEXUS Framework. Energy Nexus 2022, 5, 100054. [Google Scholar] [CrossRef]
- Kong, F.; Blot, C.; Liu, K.; Kim, M.; Li-Beisson, Y. Advances in Algal Lipid Metabolism and Their Use to Improve Oil Content. Curr. Opin. Biotechnol. 2024, 87, 103130. [Google Scholar] [CrossRef]
- Du, Z.-Y.; Benning, C. Triacylglycerol Accumulation in Photosynthetic Cells in Plants and Algae. In Lipids in Plant and Algae Development; Nakamura, Y., Li-Beisson, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 179–205. ISBN 978-3-319-25979-6. [Google Scholar]
- Merchant, S.S.; Kropat, J.; Liu, B.; Shaw, J.; Warakanont, J. TAG, You’re It! Chlamydomonas as a Reference Organism for Understanding Algal Triacylglycerol Accumulation. Curr. Opin. Biotechnol. 2012, 23, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Rodolfi, L.; Chini Zittelli, G.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for Oil: Strain Selection, Induction of Lipid Synthesis and Outdoor Mass Cultivation in a Low-Cost Photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Benning, C. Lipid Metabolism in Microalgae Distinguishes Itself. Curr. Opin. Biotechnol. 2013, 24, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Goold, H.D.; Cuiné, S.; Légeret, B.; Liang, Y.; Brugière, S.; Auroy, P.; Javot, H.; Tardif, M.; Jones, B.; Beisson, F.; et al. Saturating Light Induces Sustained Accumulation of Oil in Plastidal Lipid Droplets in Chlamydomonas reinhardtii. Plant Physiol. 2016, 171, 2406–2417. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huff, J.; Crunkleton, D.W.; Johannes, T.W. Light Intensity and Spectral Quality Modulation for Improved Growth Kinetics and Biochemical Composition of Chlamydomonas reinhardtii. J. Biotechnol. 2023, 375, 28–39. [Google Scholar] [CrossRef]
- Roessler, P.G. Environmental Control of Glycerolipid Metabolism in Microalgae: Commercial Implications and Future Research Directions. J. Phycol. 1990, 26, 393–399. [Google Scholar] [CrossRef]
- Chen, G.; Harwood, J.L.; Lemieux, M.J.; Stone, S.J.; Weselake, R.J. Acyl-CoA:Diacylglycerol Acyltransferase: Properties, Physiological Roles, Metabolic Engineering and Intentional Control. Prog. Lipid Res. 2022, 88, 101181. [Google Scholar] [CrossRef]
- Martin, B.A.; Wilson, R.F. Subcellular Localization of Triacylglycerol Synthesis in Spinach Leaves. Lipids 1984, 19, 117–121. [Google Scholar] [CrossRef]
- Goodson, C.; Roth, R.; Wang, Z.T.; Goodenough, U. Structural Correlates of Cytoplasmic and Chloroplast Lipid Body Synthesis in Chlamydomonas reinhardtii and Stimulation of Lipid Body Production with Acetate Boost. Eukaryot. Cell 2011, 10, 1592–1606. [Google Scholar] [CrossRef]
- Davidi, L.; Shimoni, E.; Khozin-Goldberg, I.; Zamir, A.; Pick, U. Origin of β-Carotene-Rich Plastoglobuli in Dunaliella bardawil. Plant Physiol. 2014, 164, 2139–2156. [Google Scholar] [CrossRef]
- Kaup, M.T.; Froese, C.D.; Thompson, J.E. A Role for Diacylglycerol Acyltransferase during Leaf Senescence. Plant Physiol. 2002, 129, 1616–1626. [Google Scholar] [CrossRef]
- Bagnato, C.; Prados, M.B.; Franchini, G.R.; Scaglia, N.; Miranda, S.E.; Beligni, M.V. Analysis of Triglyceride Synthesis Unveils a Green Algal Soluble Diacylglycerol Acyltransferase and Provides Clues to Potential Enzymatic Components of the Chloroplast Pathway. BMC Genom. 2017, 18, 223. [Google Scholar] [CrossRef]
- Carro, M.; Gonorazky, G.; Soto, D.; Mamone, L.; Bagnato, C.; Pagnussat, L.; Beligni, M. Expression of Chlamydomonas reinhardtii Chloroplast Diacylglycerol Acyltransferase-3 Is Induced by Light in Concert with Triacylglycerol Accumulation. Plant J. 2022, 110, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Boyle, N.R.; Page, M.D.; Liu, B.; Blaby, I.K.; Casero, D.; Kropat, J.; Cokus, S.J.; Hong-Hermesdorf, A.; Shaw, J.; Karpowicz, S.J.; et al. Three Acyltransferases and Nitrogen-Responsive Regulator Are Implicated in Nitrogen Starvation-Induced Triacylglycerol Accumulation in Chlamydomonas. J. Biol. Chem. 2012, 287, 15811–15825. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.; Han, D.; Li, Y.; Sommerfeld, M.; Hu, Q. Phospholipid:Diacylglycerol Acyltransferase Is a Multifunctional Enzyme Involved in Membrane Lipid Turnover and Degradation While Synthesizing Triacylglycerol in the Unicellular Green Microalga Chlamydomonas reinhardtii. Plant Cell 2012, 24, 3708. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, N.; Devadasu, E.; Yadav, R.M.; Subramanyam, R. Autophagy Induced Accumulation of Lipids in Pgrl1 and Pgr5 of Chlamydomonas reinhardtii Under High Light. Front. Plant Sci. 2022, 12, 3308. [Google Scholar] [CrossRef]
- Christ, B.; Hörtensteiner, S. Mechanism and Significance of Chlorophyll Breakdown. J. Plant Growth Regul. 2014, 33, 4–20. [Google Scholar] [CrossRef]
- Cook, R.; Lupette, J.; Benning, C. The Role of Chloroplast Membrane Lipid Metabolism in Plant Environmental Responses. Cells 2021, 10, 706. [Google Scholar] [CrossRef]
- Gutbrod, K.; Romer, J.; Dörmann, P. Phytol Metabolism in Plants. Prog. Lipid Res. 2019, 74, 1–17. [Google Scholar] [CrossRef]
- Lippold, F.; vom Dorp, K.; Abraham, M.; Hölzl, G.; Wewer, V.; Yilmaz, J.L.; Lager, I.; Montandon, C.; Besagni, C.; Kessler, F.; et al. Fatty Acid Phytyl Ester Synthesis in Chloroplasts of Arabidopsis. Plant Cell 2012, 24, 2001–2014. [Google Scholar] [CrossRef]
- Vidi, P.A.; Kanwischer, M.; Baginsky, S.; Austin, J.R.; Csucs, G.; Dörmann, P.; Kessler, F.; Bréhélin, C. Tocopherol Cyclase (VTE1) Localization and Vitamin E Accumulation in Chloroplast Plastoglobule Lipoprotein Particles. J. Biol. Chem. 2006, 281, 11225–11233. [Google Scholar] [CrossRef] [PubMed]
- Ytterberg, A.J.; Peltier, J.B.; Van Wijk, K.J. Protein Profiling of Plastoglobules in Chloroplasts and Chromoplasts. A Surprising Site for Differential Accumulation of Metabolic Enzymes. Plant Physiol. 2006, 140, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Aslan, S.; Sun, C.; Leonova, S.; Dutta, P.; Dörmann, P.; Domergue, F.; Stymne, S.; Hofvander, P. Wax Esters of Different Compositions Produced via Engineering of Leaf Chloroplast Metabolism in Nicotiana benthamiana. Metab. Eng. 2014, 25, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Cranwell, A.; Jaworski, G.H.M.; Bickley, H.M. Hydrocarbons, Sterols, Esters and Fatty Acids in Six Freshwater Chlorophytes. Phytochemistry 1990, 29, 145–151. [Google Scholar] [CrossRef]
- Rager, M.N.; Metzger, P. Six Novel Tetraterpenoid Ethers, Lycopanerols B-G, and Some Other Constituents from the Green Microalga Botryococcus braunii. Phytochemistry 2000, 54, 427–437. [Google Scholar] [CrossRef]
- Wittkopp, T.M.; Schmollinger, S.; Saroussi, S.; Hu, W.; Zhang, W.; Fan, Q.; Gallaher, S.D.; Leonard, M.T.; Soubeyrand, E.; Basset, G.J.; et al. Bilin-Dependent Photoacclimation in Chlamydomonas reinhardtii. Plant Cell 2017, 29, 2711–2726. [Google Scholar] [CrossRef]
- Li, X.; Patena, W.; Fauser, F.; Jinkerson, R.E.; Saroussi, S.; Meyer, M.T.; Ivanova, N.; Robertson, J.M.; Yue, R.; Zhang, R.; et al. A Genome-Wide Algal Mutant Library and Functional Screen Identifies Genes Required for Eukaryotic Photosynthesis. Nat. Genet. 2019, 51, 627–635. [Google Scholar] [CrossRef]
- de Carpentier, F.; Lemaire, S.D.; Danon, A. When Unity Is Strength: The Strategies Used by Chlamydomonas to Survive Environmental Stresses. Cells 2019, 8, 1307. [Google Scholar] [CrossRef]
- Ischebeck, T.; Zbierzak, A.M.; Kanwischer, M.; Dörmann, P. A Salvage Pathway for Phytol Metabolism in Arabidopsis. J. Biol. Chem. 2006, 281, 2470–2477. [Google Scholar] [CrossRef]
- Shajil Das, A.; Shajil Das, A.; Chen, Z.; Peisker, H.; Gutbrod, K.; Hölzl, G.; Dörmann, P. Multifunctional Acyltransferases Involved in the Synthesis of Triacylglycerol, Fatty Acid Phytyl Esters and Plastoquinol Esters in Cyanobacteria. Planta 2025, 261, 123. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of Light Conditions on Microalgae Growth and Content of Lipids, Carotenoids, and Fatty Acid Composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef]
- Rottet, S.; Besagni, C.; Kessler, F. The Role of Plastoglobules in Thylakoid Lipid Remodeling during Plant Development. Biochim. Biophys. Acta (BBA)—Bioenerg. 2015, 1847, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Aizouq, M.; Peisker, H.; Gutbrod, K.; Melzer, M.; Hölzl, G.; Dörmann, P.; Niyogi, K.K. Triacylglycerol and Phytyl Ester Synthesis in Synechocystis sp. PCC6803. Proc. Natl. Acad. Sci. USA 2020, 117, 6216–6222. [Google Scholar] [CrossRef] [PubMed]
- Ollis, D.L.; Cheah, E.; Cygler, M.; Dijkstra, B.; Frolow, F.; Sybille, M.; Harel, M.; James Remington, S.; Silman, I.; Schrag, J.; et al. The α/β Hydrolase Fold. Protein Eng. Des. Sel. 1992, 5, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, A.P.; Rafferty, J.B.; Sedelnikova, S.E.; Slabas, A.R.; Schierer, T.P.; Kroon, J.T.M.; Simon, J.W.; Fawcett, T.; Nishida, I.; Murata, N.; et al. Analysis of the Structure, Substrate Specificity, and Mechanism of Squash Glycerol-3-Phosphate (1)-Acyltransferase. Structure 2001, 9, 347–353. [Google Scholar] [CrossRef]
- Grabsztunowicz, M.; Koskela, M.M.; Mulo, P. Post-Translational Modifications in Regulation of Chloroplast Function: Recent Advances. Front. Plant Sci. 2017, 8, 246787. [Google Scholar] [CrossRef]
- Wang, L.; Patena, W.; Van Baalen, K.A.; Xie, Y.; Singer, E.R.; Gavrilenko, S.; Warren-Williams, M.; Han, L.; Harrigan, H.R.; Hartz, L.D.; et al. A Chloroplast Protein Atlas Reveals Punctate Structures and Spatial Organization of Biosynthetic Pathways. Cell 2023, 186, 3499–3518.e14. [Google Scholar] [CrossRef]
- Beligni, M.V.; Yamaguchi, K.; Mayfield, S.P. The Translational Apparatus of Chlamydomonas reinhardtii Chloroplast. Photosynth. Res. 2004, 82, 315–325. [Google Scholar] [CrossRef]
- Strenkert, D.; Schmollinger, S.; Gallaher, S.D.; Salomé, P.A.; Purvine, S.O.; Nicora, C.D.; Mettler-Altmann, T.; Soubeyrand, E.; Weber, A.P.M.; Lipton, M.S.; et al. Multiomics Resolution of Molecular Events during a Day in the Life of Chlamydomonas. Proc. Natl. Acad. Sci. USA 2019, 116, 2374–2383. [Google Scholar] [CrossRef]
- Idoine, A.D.; Boulouis, A.; Rupprecht, J.; Bock, R. The Diurnal Logic of the Expression of the Chloroplast Genome in Chlamydomonas reinhardtii. PLoS ONE 2014, 9, e108760. [Google Scholar] [CrossRef] [PubMed]
- Coragliotti, A.T.; Beligni, M.V.; Franklin, S.E.; Mayfield, S.P. Molecular Factors Affecting the Accumulation of Recombinant Proteins in the Chlamydomonas reinhardtii Chloroplast. Mol. Biotechnol. 2011, 48, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.C.; Sullivan, J.A.; Wang, J.-H.; Jerome, C.A.; MacLean, D. Coordination of Plastid and Nuclear Gene Expression. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2003, 358, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Salvador, M.L.; Klein, U.; Bogorad, L. Light-Regulated and Endogenous Fluctuations of Chloroplast Transcript Levels in Chlamydomonas. Regulation by Transcription and RNA Degradation. Plant J. 1993, 3, 213–219. [Google Scholar] [CrossRef]
- Spicher, L.; Almeida, J.; Gutbrod, K.; Pipitone, R.; Dörmann, P.; Glauser, G.; Rossi, M.; Kessler, F. Essential Role for Phytol Kinase and Tocopherol in Tolerance to Combined Light and Temperature Stress in Tomato. J. Exp. Bot. 2017, 68, 5845–5856. [Google Scholar] [CrossRef]
- Kamranfar, I.; Xue, G.P.; Tohge, T.; Sedaghatmehr, M.; Fernie, A.R.; Balazadeh, S.; Mueller-Roeber, B. Transcription Factor RD26 Is a Key Regulator of Metabolic Reprogramming during Dark-Induced Senescence. New Phytol. 2018, 218, 1543–1557. [Google Scholar] [CrossRef]
- Gonzalez-Ballester, D.; Pootakham, W.; Mus, F.; Yang, W.; Catalanotti, C.; Magneschi, L.; De Montaigu, A.; Higuera, J.J.; Prior, M.; Galván, A.; et al. Reverse Genetics in Chlamydomonas: A Platform for Isolating Insertional Mutants. Plant Methods 2011, 7, 24. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, G.; Ke, W.; Zhao, L.; Lv, B.; Ma, X.; Xu, N.; Xia, X.; Deng, X.; Zheng, C.; et al. Building a Multipurpose Insertional Mutant Library for Forward and Reverse Genetics in Chlamydomonas. Plant Methods 2017, 13, 36. [Google Scholar] [CrossRef]
- El-Brolosy, M.A.; Stainier, D.Y.R. Genetic Compensation: A Phenomenon in Search of Mechanisms. PLoS Genet. 2017, 13, e1006780. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Park, R.; Miller, S.M.; Li, Y. Genetic Compensation of Triacylglycerol Biosynthesis in the Green Microalga Chlamydomonas reinhardtii. Plant J. 2022, 111, 1069–1080. [Google Scholar] [CrossRef]
- Peisker, C.; Düggelin, T.; Rentsch, D.; Matile, P. Phytol and the Breakdown of Chlorophyll in Senescent Leaves. J. Plant Physiol. 1989, 135, 428–432. [Google Scholar] [CrossRef]
- Gaude, N.; Bréhélin, C.; Tischendorf, G.; Kessler, F.; Dörmann, P. Nitrogen Deficiency in Arabidopsis Affects Galactolipid Composition and Gene Expression and Results in Accumulation of Fatty Acid Phytyl Esters. Plant J. 2007, 49, 729–739. [Google Scholar] [CrossRef]
- Baroli, I.; Gutman, B.L.; Ledford, H.K.; Shin, J.W.; Chin, B.L.; Havaux, M.; Niyogi, K.K. Photo-Oxidative Stress in a Xanthophyll-Deficient Mutant of Chlamydomonas. J. Biol. Chem. 2004, 279, 6337–6344. [Google Scholar] [CrossRef]
- Li, Z.; Keasling, J.D.; Niyogi, K.K. Overlapping Photoprotective Function of Vitamin E and Carotenoids in Chlamydomonas. Plant Physiol. 2012, 158, 313–323. [Google Scholar] [CrossRef]
- Gorman, D.S.; Levine, R.P. Cytochrome f and Plastocyanin: Their Sequence in the Photosynthetic Electron Transport Chain of Chlamydomonas reinhardi. Proc. Natl. Acad. Sci. USA 1965, 54, 1665–1669. [Google Scholar] [CrossRef] [PubMed]
- Fogg, G.E.; Thake, B. Algal Cultures and Phytoplankton Ecology; University of Wisconsin Press: Madison, WI, USA, 1987; ISBN 0-299-10560-1. [Google Scholar]
- Eddy, S.R. Profile Hidden Markov Models. Bioinformatics 1998, 14, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Godzik, A. Cd-Hit: A Fast Program for Clustering and Comparing Large Sets of Protein or Nucleotide Sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Criscuolo, A.; Gribaldo, S. BMGE (Block Mapping and Gathering with Entropy): A New Software for Selection of Phylogenetic Informative Regions from Multiple Sequence Alignments. BMC Evol. Biol. 2010, 10, 210. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v3: An Online Tool for the Display and Annotation of Phylogenetic and Other Trees. Nucleic Acids Res. 2016, 44, W242–W245. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Woodbury, NY, USA, 1989; ISBN 978-0-87969-309-1. [Google Scholar]
- Ruijter, J.M.; Lorenz, P.; Tuomi, J.M.; Hecker, M.; van den Hoff, M.J.B. Fluorescent-Increase Kinetics of Different Fluorescent Reporters Used for qPCR Depend on Monitoring Chemistry, Targeted Sequence, Type of DNA Input and PCR Efficiency. Microchim. Acta 2014, 181, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative C(T) Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Krauß, S.; Hammann, S.; Vetter, W. Phytyl Fatty Acid Esters in the Pulp of Bell Pepper (Capsicum annuum). J. Agric. Food Chem. 2016, 64, 6306–6311. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Fukushima, H.; Tamaki, E. Metabolism of Chlorophyll in Higher Plants—I.: A Thin-Layer Chromatography for the Quantitative Determination of Phytol. Phytochemistry 1963, 3, 641–645. [Google Scholar] [CrossRef]
- Narai-Kanayama, A.; Yokosaka, S.; Seo, Y.; Mikami, K.; Yoshino, T.; Matsuda, H. Evidence of Increases of Phytol and Chlorophyllide by Enzymatic Dephytylation of Chlorophylls in Smoothie Made from Spinach Leaves. J. Food Sci. 2023, 88, 2385–2396. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Y.; Shen, Y.; Yan, D.; He, X.; Dai, J.; Wu, Q. Oil Accumulation Mechanisms of the Oleaginous Microalga Chlorella Protothecoides Revealed through Its Genome, Transcriptomes, and Proteomes. BMC Genom. 2014, 15, 582. [Google Scholar] [CrossRef]
- Moreau, H.; Verhelst, B.; Couloux, A.; Derelle, E.; Rombauts, S.; Grimsley, N.; Van Bel, M.; Poulain, J.; Katinka, M.; Hohmann-Marriott, M.F.; et al. Gene Functionalities and Genome Structure in Bathycoccus Prasinos Reflect Cellular Specializations at the Base of the Green Lineage. Genome Biol. 2012, 13, R74. [Google Scholar] [CrossRef]
- Merchant, S.S.; Prochnik, S.E.; Vallon, O.; Harris, E.H.; Karpowicz, S.J.; Witman, G.B.; Terry, A.; Salamov, A.; Fritz-Laylin, L.K.; Maréchal-Drouard, L.; et al. The Chlamydomonas Genome Reveals the Evolution of Key Animal and Plant Functions. Science 2007, 318, 245–251. [Google Scholar] [CrossRef]
- Hovde, B.T.; Deodato, C.R.; Hunsperger, H.M.; Ryken, S.A.; Yost, W.; Jha, R.K.; Patterson, J.; Monnat, R.J., Jr.; Barlow, S.B.; Starkenburg, S.R.; et al. Genome Sequence and Transcriptome Analyses of Chrysochromulina Tobin: Metabolic Tools for Enhanced Algal Fitness in the Prominent Order Prymnesiales (Haptophyceae). PLoS Genet. 2015, 11, e1005469. [Google Scholar] [CrossRef]
- Blanc, G.; Agarkova, I.; Grimwood, J.; Kuo, A.; Brueggeman, A.; Dunigan, D.D.; Gurnon, J.; Ladunga, I.; Lindquist, E.; Lucas, S.; et al. The Genome of the Polar Eukaryotic Microalga Coccomyxa subellipsoidea Reveals Traits of Cold Adaptation. Genome Biol. 2012, 13, R39. [Google Scholar] [CrossRef] [PubMed]
- Blanc, G.; Duncan, G.; Agarkova, I.; Borodovsky, M.; Gurnon, J.; Kuo, A.; Lindquist, E.; Lucas, S.; Pangilinan, J.; Polle, J.; et al. The Chlorella variabilis NC64A Genome Reveals Adaptation to Photosymbiosis, Coevolution with Viruses, and Cryptic Sex. Plant Cell 2010, 22, 2943. [Google Scholar] [CrossRef] [PubMed]
- Polle Juergen, E.W.; Barry, K.; Cushman, J.; Schmutz, J.; Tran, D.; Hathwaik Leyla, T.; Yim Won, C.; Jenkins, J.; McKie-Krisberg, Z.; Prochnik, S.; et al. Draft Nuclear Genome Sequence of the Halophilic and Beta-Carotene-Accumulating Green Alga Dunaliella salina Strain CCAP19/18. Genome Announc. 2017, 5, 10–1128. [Google Scholar] [CrossRef]
- Hanschen, E.R.; Marriage, T.N.; Ferris, P.J.; Hamaji, T.; Toyoda, A.; Fujiyama, A.; Neme, R.; Noguchi, H.; Minakuchi, Y.; Suzuki, M.; et al. The Gonium Pectorale Genome Demonstrates Co-Option of Cell Cycle Regulation during the Evolution of Multicellularity. Nat. Commun. 2016, 7, 11370. [Google Scholar] [CrossRef]
- Worden, A.Z.; Lee, J.-H.; Mock, T.; Rouzé, P.; Simmons, M.P.; Aerts, A.L.; Allen, A.E.; Cuvelier, M.L.; Derelle, E.; Everett, M.V.; et al. Green Evolution and Dynamic Adaptations Revealed by Genomes of the Marine Picoeukaryotes Micromonas. Science 2009, 324, 268–272. [Google Scholar] [CrossRef]
- Bogen, C.; Al-Dilaimi, A.; Albersmeier, A.; Wichmann, J.; Grundmann, M.; Rupp, O.; Lauersen, K.J.; Blifernez-Klassen, O.; Kalinowski, J.; Goesmann, A.; et al. Reconstruction of the Lipid Metabolism for the Microalga Monoraphidium neglectum from Its Genome Sequence Reveals Characteristics Suitable for Biofuel Production. BMC Genom. 2013, 14, 926. [Google Scholar] [CrossRef]
- Palenik, B.; Grimwood, J.; Aerts, A.; Rouzé, P.; Salamov, A.; Putnam, N.; Dupont, C.; Jorgensen, R.; Derelle, E.; Rombauts, S.; et al. The Tiny Eukaryote Ostreococcus Provides Genomic Insights into the Paradox of Plankton Speciation. Proc. Natl. Acad. Sci. USA 2007, 104, 7705–7710. [Google Scholar] [CrossRef]
- Gonzalez-Esquer, C.R.; Twary Scott, N.; Hovde Blake, T.; Starkenburg Shawn, R. Nuclear, Chloroplast, and Mitochondrial Genome Sequences of the Prospective Microalgal Biofuel Strain Picochlorum soloecismus. Genome Announc. 2018, 6, 10–1128. [Google Scholar] [CrossRef]
- Suzuki, S.; Yamaguchi, H.; Nakajima, N.; Kawachi, M. Raphidocelis Subcapitata (=Pseudokirchneriella subcapitata) Provides an Insight into Genome Evolution and Environmental Adaptations in the Sphaeropleales. Sci. Rep. 2018, 8, 8058. [Google Scholar] [CrossRef]
- Prochnik, S.E.; Umen, J.; Nedelcu, A.M.; Hallmann, A.; Miller, S.M.; Nishii, I.; Ferris, P.; Kuo, A.; Mitros, T.; Fritz-Laylin, L.K.; et al. Genomic Analysis of Organismal Complexity in the Multicellular Green Alga Volvox carteri. Science 2010, 329, 223–226. [Google Scholar] [CrossRef] [PubMed]
- The Arabidopsis Genome Initiative. Analysis of the Genome Sequence of the Flowering Plant Arabidopsis thaliana. Nature 2000, 408, 796–815. [Google Scholar] [CrossRef] [PubMed]
- Dorrell, R.G.; Kuo, A.; Füssy, Z.; Richardson, E.H.; Salamov, A.; Zarevski, N.; Freyria, N.J.; Ibarbalz, F.M.; Jenkins, J.; Pierella Karlusich, J.J.; et al. Convergent Evolution and Horizontal Gene Transfer in Arctic Ocean Microalgae. Life Sci. Alliance 2023, 6, e202201833. [Google Scholar] [CrossRef] [PubMed]
- Read, B.A.; Kegel, J.; Klute, M.J.; Kuo, A.; Lefebvre, S.C.; Maumus, F.; Mayer, C.; Miller, J.; Monier, A.; Salamov, A.; et al. Pan Genome of the Phytoplankton Emiliania Underpins Its Global Distribution. Nature 2013, 499, 209–213. [Google Scholar] [CrossRef]
- Schmutz, J.; Cannon, S.B.; Schlueter, J.; Ma, J.; Mitros, T.; Nelson, W.; Hyten, D.L.; Song, Q.; Thelen, J.J.; Cheng, J.; et al. Genome Sequence of the Palaeopolyploid Soybean. Nature 2010, 463, 178–183. [Google Scholar] [CrossRef]
- International Rice Genome Sequencing Project. The Map-Based Sequence of the Rice Genome. Nature 2005, 436, 793. [Google Scholar] [CrossRef]
- Lang, D.; Ullrich, K.K.; Murat, F.; Fuchs, J.; Jenkins, J.; Haas, F.B.; Piednoel, M.; Gundlach, H.; Van Bel, M.; Meyberg, R.; et al. The Physcomitrella Patens Chromosome-Scale Assembly Reveals Moss Genome Structure and Evolution. Plant J. 2018, 93, 515–533. [Google Scholar] [CrossRef]
- Hosmani, P.S.; Flores-Gonzalez, M.; van de Geest, H.; Maumus, F.; Bakker, L.V.; Schijlen, E.; van Haarst, J.; Cordewener, J.; Sanchez-Perez, G.; Peters, S.; et al. An Improved de Novo Assembly and Annotation of the Tomato Reference Genome Using Single-Molecule Sequencing, Hi-C Proximity Ligation and Optical Maps. bioRxiv 2019. [Google Scholar] [CrossRef]
- Banks, J.A.; Nishiyama, T.; Hasebe, M.; Bowman, J.L.; Gribskov, M.; de Pamphilis, C.; Albert, V.A.; Aono, N.; Aoyama, T.; Ambrose, B.A.; et al. The Selaginella Genome Identifies Genetic Changes Associated with the Evolution of Vascular Plants. Science 2011, 332, 960–963. [Google Scholar] [CrossRef]
- Jaillon, O.; Aury, J.-M.; Noel, B.; Policriti, A.; Clepet, C.; Casagrande, A.; Choisne, N.; Aubourg, S.; Vitulo, N.; Jubin, C.; et al. The Grapevine Genome Sequence Suggests Ancestral Hexaploidization in Major Angiosperm Phyla. Nature 2007, 449, 463–467. [Google Scholar] [CrossRef]
- Gore, M.A.; Chia, J.-M.; Elshire, R.J.; Sun, Q.; Ersoz, E.S.; Hurwitz, B.L.; Peiffer, J.A.; McMullen, M.D.; Grills, G.S.; Ross-Ibarra, J.; et al. A First-Generation Haplotype Map of Maize. Science 2009, 326, 1115–1117. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).