Field Performance of Novel Citrus Rootstocks Grafted with ‘Valencia’ Orange and Their Response to Systemic Delivery of Oxytetracycline
Abstract
1. Introduction
2. Results
2.1. Candidatus Liberibacter asiaticus (CLas) Detection
2.2. Tree Growth
2.3. Tree Health
2.4. Pre-Harvest Fruit Drop (%), Fruit Yield, and Yield Efficiency
2.5. Fruit and Juice Quality
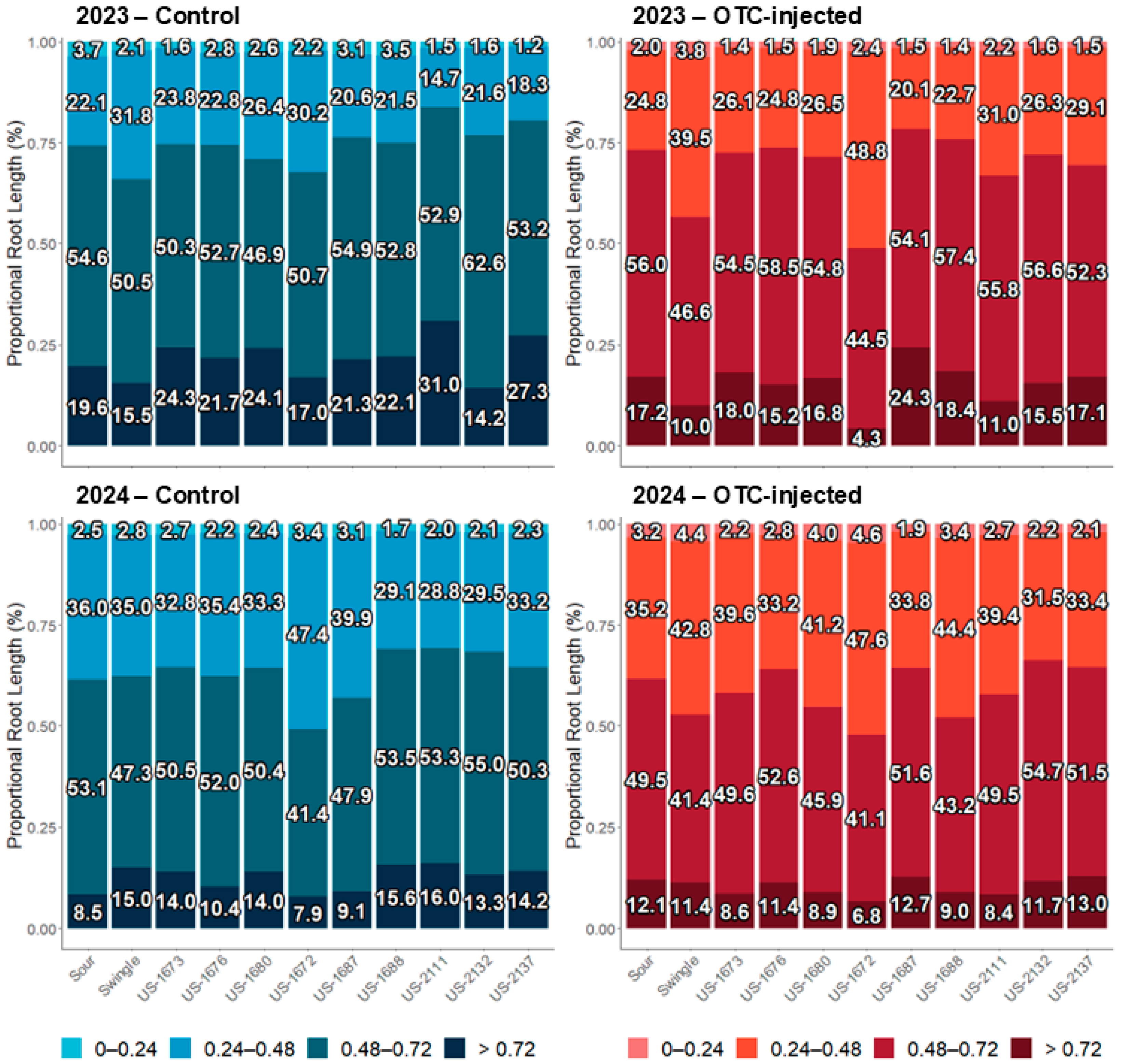
2.6. Fibrous Root Characterization
2.7. Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Oxytetracycline Trunk Injection
4.3. Candidatus Liberibacter asiaticus (CLas) Detection
4.4. Biometric Assessments
4.4.1. Tree Growth
4.4.2. Pre-Harvest Fruit Drop, Fruit Yield, and Yield Efficiency
4.4.3. Fruit and Juice Quality
4.5. Fibrous Root Characterization
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bové, J.M. Huanglongbing: A Destructive, Newly-Emerging, Century-Old Disease of Citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Gottwald, T.R. Citrus Canker and Citrus Huanglongbing, Two Exotic Bacterial Diseases Threatening the Citrus Industries of the Western Hemisphere. Outlooks Pest Manag. 2007, 18, 274–279. [Google Scholar] [CrossRef]
- Wang, N.; Trivedi, P. Citrus Huanglongbing: A Newly Relevant Disease Presents Unprecedented Challenges. Phytopathology 2013, 103, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Gottwald, T.R.; Graça, J.V.d.; Bassanezi, R.B. Citrus Huanglongbing: The Pathogen and Its Impact. Plant Health Prog. 2007, 8, 31. [Google Scholar] [CrossRef]
- Graham, J.; Gottwald, T.; Setamou, M. Status of Huanglongbing (HLB) Outbreaks in Florida, California and Texas. Trop. Plant Pathol. 2020, 45, 265–278. [Google Scholar] [CrossRef]
- Tiwari, S.; Mann, R.S.; Rogers, M.E.; Stelinski, L.L. Insecticide Resistance in Field Populations of Asian Citrus Psyllid in Florida. Pest Manag. Sci. 2011, 67, 1258–1268. [Google Scholar] [CrossRef]
- Chen, X.D.; George, J.; Diepenbrock, L.M.; Gossett, H.; Liu, G.; Qureshi, J.A.; Stelinski, L.L. Feeding Behavior and Hormoligosis Associated with Imidacloprid Resistance in Asian Citrus Psyllid, Diaphorina Citri. Insect Sci. 2024, 31, 1211–1221. [Google Scholar] [CrossRef]
- da Graça, J.V.; Kunta, M.; Sétamou, M.; Rascoe, J.; Li, W.; Nakhla, M.K.; Salas, B.; Bartels, D.W. Huanglongbing in Texas: Report on the First Detections in Commercial Citrus. J. Citrus Pathol. 2015, 2. [Google Scholar] [CrossRef]
- Ramadugu, C.; Keremane, M.L.; Halbert, S.E.; Duan, Y.P.; Roose, M.L.; Stover, E.; Lee, R.F. Long-Term Field Evaluation Reveals Huanglongbing Resistance in Citrus Relatives. Plant Dis. 2016, 100, 1858–1869. [Google Scholar] [CrossRef]
- Souza, U.; da Silva Gesteira, A.; Júnior, L.L.R.; Sinico, T.E.; Moreira, A.S.; Ferreira, C.F.; Harakava, R.; Stuchi, E.S.; Freitas-Astúa, J.; Girardi, E.A. How Scion/Rootstock Biometric, Anatomical, and DNA Methylation Dynamics Affect Citrus-Candidatus Liberibacter Asiaticus Interaction. Eur. J. Plant Pathol. 2024, 171, 459–476. [Google Scholar] [CrossRef]
- Wang, N. The Citrus Huanglongbing Crisis and Potential Solutions. Mol. Plant 2019, 12, 607–609. [Google Scholar] [CrossRef]
- Boava, L.P.; Sagawa, C.H.D.; Cristofani-Yaly, M.; Machado, M. Incidence of ‘Candidatus Liberibacter asiaticus’-Infected Plants Among Citrandarins as Rootstock and Scion Under Field Conditions. Phytopathology 2015, 105, 518–524. [Google Scholar] [CrossRef]
- Rodrigues, J.D.B.; Moreira, A.S.; Stuchi, E.S.; Bassanezi, R.B.; Laranjeira, F.F.; Girardi, E.A. Huanglongbing Incidence, Canopy Volume, and Sprouting Dynamics of ‘Valencia’ Sweet Orange Grafted onto 16 Rootstocks. Trop. Plant Pathol. 2020, 45, 611–619. [Google Scholar] [CrossRef]
- Carvalho, L.M.d.; Carvalho, H.W.L.d.; Barros, I.d.; Martins, C.R.; Soares Filho, W.d.S.; Girardi, E.A.; Passos, O.S. New Scion-Rootstock Combinations for Diversification of Sweet Orange Orchards in Tropical Hardsetting Soils. Sci. Hortic. 2019, 243, 169–176. [Google Scholar] [CrossRef]
- Eissenstat, D.M. On the Relationship between Specific Root Length and the Rate of Root Proliferation: A Field Study Using Citrus Rootstocks. New Phytol. 1991, 118, 63–68. [Google Scholar] [CrossRef]
- Bowman, K.D.; Joubert, J. Citrus Rootstocks. In The Genus Citrus; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Johnson, E.G.; Wu, J.; Bright, D.B.; Graham, J.H. Association of ‘Candidatus Liberibacter Asiaticus’ Root Infection, but Not Phloem Plugging with Root Loss on Huanglongbing-Affected Trees Prior to Appearance of Foliar Symptoms. Plant Pathol. 2014, 63, 290–298. [Google Scholar] [CrossRef]
- Tardivo, C.; Archer, L.; Nunes, L.; Alferez, F.; Albrecht, U. Root System Reductions of Grafted ‘Valencia’ Orange Trees Are More Extensive Than Aboveground Reductions after Natural Infection with Candidatus Liberibacter Asiaticus. HortScience 2024, 59, 595–604. [Google Scholar] [CrossRef]
- Albrecht, U.; Bowman, K.D. Tolerance of Trifoliate Citrus Rootstock Hybrids to Candidatus Liberibacter Asiaticus. Sci. Hortic. 2012, 147, 71–80. [Google Scholar] [CrossRef]
- Kunwar, S.; Meyering, B.; Grosser, J.; Gmitter, F.G.; Castle, W.S.; Albrecht, U. Field Performance of ‘Valencia’ Orange Trees on Diploid and Tetraploid Rootstocks in Different Huanglongbing-Endemic Growing Environments. Sci. Hortic. 2023, 309, 111635. [Google Scholar] [CrossRef]
- Alves, M.N.; Lopes, S.A.; Raiol-Junior, L.L.; Wulff, N.A.; Girardi, E.A.; Ollitrault, P.; Peña, L. Resistance to ‘Candidatus Liberibacter Asiaticus,’ the Huanglongbing Associated Bacterium, in Sexually and/or Graft-Compatible Citrus Relatives. Front. Plant Sci. 2021, 11, 617664. [Google Scholar] [CrossRef]
- Bowman, K.D.; McCollum, G.; Albrecht, U. SuperSour: A New Strategy for Breeding Superior Citrus Rootstocks. Front. Plant Sci. 2021, 12, 741009. [Google Scholar] [CrossRef]
- Bowman, K.D.; McCollum, G.; Seymour, D.K. Genetic Modulation of Valencia Sweet Orange Field Performance by 50 Rootstocks under Huanglongbing-Endemic Conditions. Front. Plant Sci. 2023, 14, 1061663. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Jiang, J.; Wang, N. Control of Citrus Huanglongbing via Trunk Injection of Plant Defense Activators and Antibiotics. Phytopathology 2018, 108, 186–195. [Google Scholar] [CrossRef]
- Archer, L.; Qureshi, J.; Albrecht, U. Efficacy of Trunk Injected Imidacloprid and Oxytetracycline in Managing Huanglongbing and Asian Citrus Psyllid in Infected Sweet Orange (Citrus sinensis) Trees. Agriculture 2022, 12, 1592. [Google Scholar] [CrossRef]
- Archer, L.; Kunwar, S.; Alferez, F.; Batuman, O.; Albrecht, U. Trunk Injection of Oxytetracycline for Huanglongbing Management in Mature Grapefruit and Sweet Orange Trees. Phytopathology 2023, 113, 1010–1021. [Google Scholar] [CrossRef]
- Albrecht, U.; Tardivo, C.; Moreno, G.; de Freitas, J.; Singerman, A.; Plotto, A.; Bai, J. Managing Endemic Huanglongbing in Commercial Citrus Production through Vascular Delivery of Oxytetracycline. Crop Prot. 2025, 195, 107250. [Google Scholar] [CrossRef]
- Roach, W.A. Plant Injection as a Physiological Method. Ann. Bot. 1939, 3, 155–226. [Google Scholar] [CrossRef]
- Li, J.; Pang, Z.; Duan, S.; Lee, D.; Kolbasov, V.G.; Wang, N. The in Planta Effective Concentration of Oxytetracycline Against ‘Candidatus Liberibacter Asiaticus’ for Suppression of Citrus Huanglongbing. Phytopathology 2019, 109, 2046–2054. [Google Scholar] [CrossRef]
- Aubert, B.; Bové, J.M. Effect of Penicillin or Tetracycline Injections of Citrus Trees Affected by Greening Disease Under Field Conditions in Reunion Island. Proc. Int. Org. Citrus Virol. (IOCV) 1980, 8. [Google Scholar] [CrossRef]
- Schwarz, R.E.; Moll, J.N.; van Vuuren, S.P. Control of Citrus Greening and Its Psylla Vector by Trunk Injections of Tetracyclines and Insecticides. Proc. Int. Org. Citrus Virol. (IOCV) 1974, 6. [Google Scholar] [CrossRef]
- Van Vuuren, S.P. The Determination of Optimal Concentration and pH of Tetracycline Hydrochloride for Trunk Injection of Greening-Infected Citrus Trees. Phytophylactica 1977, 9, 77–81. [Google Scholar]
- Hu, J.; Wang, N. Evaluation of the Spatiotemporal Dynamics of Oxytetracycline and Its Control Effect Against Citrus Huanglongbing via Trunk Injection. Phytopathology 2016, 106, 1495–1503. [Google Scholar] [CrossRef]
- Shin, K.; Ascunce, M.S.; Narouei-Khandan, H.A.; Sun, X.; Jones, D.; Kolawole, O.O.; Goss, E.M.; van Bruggen, A.H.C. Effects and Side Effects of Penicillin Injection in Huanglongbing Affected Grapefruit Trees. Crop Prot. 2016, 90, 106–116. [Google Scholar] [CrossRef]
- Tatineni, S.; Sagaram, U.S.; Gowda, S.; Robertson, C.J.; Dawson, W.O.; Iwanami, T.; Wang, N. In Planta Distribution of ‘Candidatus Liberibacter Asiaticus’ as Revealed by Polymerase Chain Reaction (PCR) and Real-Time PCR. Phytopathology 2008, 98, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Tardivo, C.; Qureshi, J.; Bowman, K.D.; Albrecht, U. Relative Influence of Rootstock and Scion on Asian Citrus Psyllid Infestation and Candidatus Liberibacter Asiaticus Colonization. HortScience 2023, 58, 395–403. [Google Scholar] [CrossRef]
- Bodaghi, S.; Pugina, G.; Meyering, B.; Bowman, K.D.; Albrecht, U. Different Sweet Orange–Rootstock Combinations Infected by Candidatus Liberibacter Asiaticus under Greenhouse Conditions: Effects on the Roots. HortScience 2022, 57, 56–64. [Google Scholar] [CrossRef]
- Girardi, E.A.; Sola, J.G.P.; Scapin, M. da S.; Moreira, A.S.; Bassanezi, R.B.; Ayres, A.J.; Peña, L. The Perfect Match: Adjusting High Tree Density to Rootstock Vigor for Improving Cropping and Land Use Efficiency of Sweet Orange. Agronomy 2021, 11, 2569. [Google Scholar] [CrossRef]
- Schumann, A.W.; Singerman, A.; Ritenour, M.A.; Qureshi, J.; Alferez, F. 2024–2025 Florida Citrus Production Guide: Citrus under Protective Screen (CUPS) Production Systems: CPG Ch. 21, HS1304/CMG19, Rev. 5/2024. EDIS 2024. [Google Scholar] [CrossRef]
- Kallsen, C.E.; Parfitt, D.E. Comparisons of Scion/Rootstock Growth Rates among U.S. Pistachio Cultivars. HortScience 2011, 46, 197–200. [Google Scholar] [CrossRef]
- Martínez-Cuenca, M.-R.; Primo-Capella, A.; Forner-Giner, M.A. Influence of Rootstock on Citrus Tree Growth: Effects on Photosynthesis and Carbohydrate Distribution, Plant Size, Yield, Fruit Quality, and Dwarfing Genotypes. In Plant Growth; IntechOpen: London, UK, 2016; pp. 107–129. [Google Scholar] [CrossRef]
- Tardivo, C.; Patel, S.; Bowman, K.D.; Albrecht, U. Nursery Characteristics and Field Performance of Nine Novel Citrus Rootstocks under HLB-Endemic Conditions. HortScience 2025, 60, 931–939. [Google Scholar] [CrossRef]
- Sundin, G.W.; Wang, N. Antibiotic Resistance in Plant-Pathogenic Bacteria. Annu. Rev. Phytopathol. 2018, 56, 161–180. [Google Scholar] [CrossRef]
- Batuman, O.; Britt-Ugartemendia, K.; Kunwar, S.; Yilmaz, S.; Fessler, L.; Redondo, A.; Chumachenko, K.; Chakravarty, S.; Wade, T. The Use and Impact of Antibiotics in Plant Agriculture: A Review. Phytopathology 2024, 114, 885–909. [Google Scholar] [CrossRef]
- Glusberger, P.; Russell, J.T.; Cohn, A.R.; Petrone, J.R.; Lai, K.-K.; Triplett, E.W. Whole Genome Analysis of Spontaneous Antimicrobial Resistance in Liberibacter Crescens Suggests Long-Term Efficacy for Antimicrobial Treatment of Citrus Greening Disease. J. Citrus Pathol. 2024, 11. [Google Scholar] [CrossRef]
- Irigoyen, S.; Ramasamy, M.; Pant, S.; Niraula, P.; Bedre, R.; Gurung, M.; Rossi, D.; Laughlin, C.; Gorman, Z.; Achor, D.; et al. Plant Hairy Roots Enable High Throughput Identification of Antimicrobials against Candidatus Liberibacter spp. Nat. Commun. 2020, 11, 5802. [Google Scholar] [CrossRef]
- Aksenov, A.A.; Blacutt, A.; Ginnan, N.; Rolshausen, P.E.; Melnik, A.V.; Lotfi, A.; Gentry, E.C.; Ramasamy, M.; Zuniga, C.; Zengler, K.; et al. Spatial Chemistry of Citrus Reveals Molecules Bactericidal to Candidatus Liberibacter Asiaticus. Sci. Rep. 2024, 14, 20306. [Google Scholar] [CrossRef]
- Pustika, A.B.; Subandiyah, S.; Holford, P.; Beattie, G.A.C.; Iwanami, T.; Masaoka, Y. Interactions between Plant Nutrition and Symptom Expression in Mandarin Trees Infected with the Disease Huanglongbing. Australas. Plant Dis. Notes. 2008, 3, 112–115. [Google Scholar] [CrossRef]
- Bassanezi, R.B.; Primiano, I.V.; Vescove, H.V. Effect of Enhanced Nutritional Programs and Exogenous Auxin Spraying on Huanglongbing Severity, Fruit Drop, Yield and Economic Profitability of Orange Orchards. Crop Prot. 2021, 145, 105609. [Google Scholar] [CrossRef]
- Bassanezi, R.B.; Primiano, I.V.; Mattos, D.; Quaggio, J.A.; Boaretto, R.M.; Ayres, A.J.; Bové, J.M. Calcium and Magnesium Input Did Not Decrease Huanglongbing Progress and Yield Loss of Sweet Orange Trees. Crop Prot. 2023, 172, 106338. [Google Scholar] [CrossRef]
- Kadyampakeni, D.M.; Morgan, K.T.; Nkedi-Kizza, P.; Kasozi, G.N. Nutrient Management Options for Florida Citrus: A Review of NPK Application and Analytical Methods. J. Plant Nut. 2015, 38, 568–583. [Google Scholar] [CrossRef]
- Bowman, K.D.; Albrecht, U. Efficient Propagation of Citrus Rootstocks by Stem Cuttings. Sci. Hortic. 2017, 225, 681–688. [Google Scholar] [CrossRef]
- Li, W.; Hartung, J.S.; Levy, L. Quantitative Real-Time PCR for Detection and Identification of Candidatus Liberibacter Species Associated with Citrus Huanglongbing. J. Microb. Methods 2006, 66, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Seethepalli, A.; Dhakal, K.; Griffiths, M.; Guo, H.; Freschet, G.T.; York, L.M. RhizoVision Explorer: Open-Source Software for Root Image Analysis and Measurement Standardization. AoB Plants 2021, 13, plab056. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistica Lcomputing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 25 September 2025).
- Sneath, P.H.A.; Sokal, R.R. Numerical Taxonomy. In The Principles and Practice of Numerical Classification; W.H. Freeman: San Francisco, CA, USA, 1973. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Suzuki, R.; Shimodaira, H. Pvclust: An R package for assessing the uncertainty in hierarchical clustering. Bioinformatics 2006, 22, 1540–1542. [Google Scholar] [CrossRef] [PubMed]





| Factor | 2023 | 2024 | ||
|---|---|---|---|---|
| Leaf Ct-Values | Fibrous Roots Ct-Values | Leaf Ct-Values | Fibrous Roots Ct-Values | |
| Injection treatment | ||||
| OTC-injected | 28.1 ± 0.77 a | 33.3 ± 0.66 | 28.5 ± 0.69 a | 31.9 ± 0.54 a |
| Control | 22.2 ± 0.79 b | 31.8 ± 0.66 | 21.6 ± 0.73 b | 29.1 ± 0.57 b |
| p-value | <0.0001 | 0.0722 | <0.0001 | 0.0002 |
| Rootstock cultivar | ||||
| Sour orange | 25.4 ± 1.59 | 29.3 ± 1.21 b | 23.9 ± 1.60 | 27.2 ± 1.24 b |
| Swingle | 23.7 ± 1.43 | 34.2 ± 1.16 ab | 26.6 ± 1.65 | 30.9 ± 1.28 a |
| US-1673 | 26.1 ± 1.52 | 31.1 ± 1.26 ab | 25.1 ± 1.59 | 29.6 ± 1.23 ab |
| US-1676 | 24.7 ± 1.67 | 33.4 ± 1.41 ab | 21.6 ± 1.94 | 29.2 ± 1.49 ab |
| US-1680 | 25.0 ± 1.63 | 33.8 ± 1.31 ab | 26.0 ± 1.76 | 32.6 ± 1.38 ab |
| US-1672 | 24.0 ± 1.52 | 30.2 ± 1.16 ab | 23.9 ± 1.86 | 30.7 ± 1.31 ab |
| US-1687 | 22.5 ± 1.58 | 32.3 ± 1.27 ab | 26.3 ± 1.78 | 28.7 ± 1.38 ab |
| US-1688 | 26.4 ± 1.36 | 31.1 ± 1.10 ab | 23.3 ± 1.51 | 30.3 ± 1.17 ab |
| US-2111 | 25.1 ± 1.36 | 33.8 ± 1.10 ab | 24.2 ± 1.60 | 31.5 ± 1.25 ab |
| US-2132 | 25.5 ± 1.43 | 34.6 ± 1.11 a | 25.3 ± 1.52 | 33.3 ± 1.17 a |
| US-2137 | 28.0 ± 1.30 | 34.2 ± 1.05 ab | 29.5 ± 1.53 | 31.6 ± 1.13 ab |
| p-value | 0.3426 | 0.0064 | 0.0873 | 0.0308 |
| Rootstock cultivar × Injection treatment | ||||
| p-value | 0.2284 | 0.2778 | 0.1915 | 0.4876 |
| Block | ||||
| p-value | 0.8996 | 0.5279 | 1.0000 | 0.5915 |
| Factor | 2024 | 2025 | ||||
|---|---|---|---|---|---|---|
| Yield (kg/Tree) | Fruit Weight (g) | TSS | Yield (kg/Tree) | Fruit Weight (g) | TSS | |
| Injection treatment | ||||||
| OTC-injected | 26.3 ± 1.39 a | 160 ± 3.6 a | 9.9 ± 0.08 a | 26.9 ± 1.45 a | 153 ± 3.8 a | 9.6 ± 0.12 a |
| Control | 19.4 ± 1.42 b | 145 ± 3.7 b | 8.9 ± 0.08 b | 15.8 ± 1.42 b | 126 ± 3.8 b | 8.8 ± 0.12 b |
| p-value | 0.0018 | 0.0024 | <0.0001 | 0.0005 | <0.0001 | 0.0002 |
| Rootstock cultivar | ||||||
| Sour orange | 26.8 ± 2.12 ab | 154 ± 4.6 ab | 9.0 ± 0.13 c | 24.9 ± 1.98 ab | 145 ± 5.9 | 9.1 ± 0.18 |
| Swingle | 20.6 ± 2.17 bcd | 131 ± 5.0 d | 9.8 ± 0.14 ab | 19.2 ± 2.12 b | 123 ± 6.7 | 9.2 ± 0.21 |
| US-1673 | 21.4 ± 2.27 abcd | 159 ± 5.2 ab | 9.2 ± 0.14 bc | 24.2 ± 2.21 ab | 143 ± 7.1 | 8.9 ± 0.22 |
| US-1676 | 23.0 ± 2.75 abcd | 168 ± 6.2 ab | 9.1 ± 0.18 bc | 19.8 ± 2.67 ab | 148 ± 8.7 | 9.3 ± 0.27 |
| US-1680 | 16.0 ± 2.56 cd | 158 ± 5.8 ab | 9.1 ± 0.16 c | 20.7 ± 2.48 ab | 145 ± 7.5 | 9.0 ± 0.23 |
| US-1672 | 31.9 ± 2.39 a | 162 ± 5.4 ab | 9.0 ± 0.15 c | 26.0 ± 2.33 ab | 145 ± 7.46 | 9.3 ± 0.23 |
| US-1687 | 26.5 ± 2.50 abc | 147 ± 5.7 bcd | 9.0 ± 0.16 c | 25.4 ± 2.43 ab | 142 ± 7.8 | 9.2 ± 0.24 |
| US-1688 | 31.4 ± 2.16 a | 146 ± 4.9 bcd | 9.0 ± 0.14 c | 30.1 ± 2.11 a | 140 ± 6.7 | 8.9 ± 0.21 |
| US-2111 | 18.3 ± 2.16 bcd | 151 ± 5.0 bc | 9.8 ± 0.14 ab | 16.7 ± 2.20 bc | 134 ± 7.0 | 9.6 ± 0.22 |
| US-2132 | 12.5 ± 2.17 d | 132 ± 5.0 cd | 10.2 ± 0.14 a | 7.6 ± 2.26 c | 130 ± 7.2 | 9.6 ± 0.22 |
| US-2137 | 22.9 ± 2.07 abc | 172 ± 4.8 a | 9.9 ± 0.13 a | 20.1 ± 2.02 b | 138 ± 6.4 | 9.3 ± 0.20 |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.2924 | 0.2282 |
| Rootstock cultivar × Injection treatment | ||||||
| p-value | 0.4606 | 0.6463 | 0.0623 | 0.2547 | 0.3802 | 0.6577 |
| Block | ||||||
| p-value | 0.5450 | 0.6883 | 1.0000 | 0.4727 | 0.4996 | 1.0000 |
| Rootstock | Parentage |
|---|---|
| Sour orange | C. aurantium |
| Swingle | C. paradisi ‘Duncan’ × P. trifoliata |
| US-1673 | C. maxima ‘Hirado’ × C. tachibana |
| US-1676 | C. maxima ‘Hirado’ × C. tachibana |
| US-1680 | C. maxima ‘Hirado’ × C. tachibana |
| US-1672 | C. maxima ‘Hirado’ × C. reticulata ‘Cleopatra’ |
| US-1687 | C. maxima ‘Hirado’ × C. reticulata ‘Cleopatra’ |
| US-1688 | C. maxima ‘Hirado’ × C. reticulata ‘Cleopatra’ |
| US-2111 | C. maxima ‘Hirado’ × US-942 (C. reticulata ‘Sunki’ × Poncirus trifoliata ‘Flying Dragon’) |
| US-2132 | C. maxima ‘Hirado’ × US-942 |
| US-2137 | C. maxima ‘Hirado’ × US-942 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tardivo, C.; Pugina, G.; Bowman, K.D.; Albrecht, U. Field Performance of Novel Citrus Rootstocks Grafted with ‘Valencia’ Orange and Their Response to Systemic Delivery of Oxytetracycline. Plants 2025, 14, 3020. https://doi.org/10.3390/plants14193020
Tardivo C, Pugina G, Bowman KD, Albrecht U. Field Performance of Novel Citrus Rootstocks Grafted with ‘Valencia’ Orange and Their Response to Systemic Delivery of Oxytetracycline. Plants. 2025; 14(19):3020. https://doi.org/10.3390/plants14193020
Chicago/Turabian StyleTardivo, Caroline, Gabriel Pugina, Kim D. Bowman, and Ute Albrecht. 2025. "Field Performance of Novel Citrus Rootstocks Grafted with ‘Valencia’ Orange and Their Response to Systemic Delivery of Oxytetracycline" Plants 14, no. 19: 3020. https://doi.org/10.3390/plants14193020
APA StyleTardivo, C., Pugina, G., Bowman, K. D., & Albrecht, U. (2025). Field Performance of Novel Citrus Rootstocks Grafted with ‘Valencia’ Orange and Their Response to Systemic Delivery of Oxytetracycline. Plants, 14(19), 3020. https://doi.org/10.3390/plants14193020










