Priming with Porcine Blood Polypeptide Enhances Salt Tolerance in Wheat Seedlings
Abstract
1. Introduction
2. Results
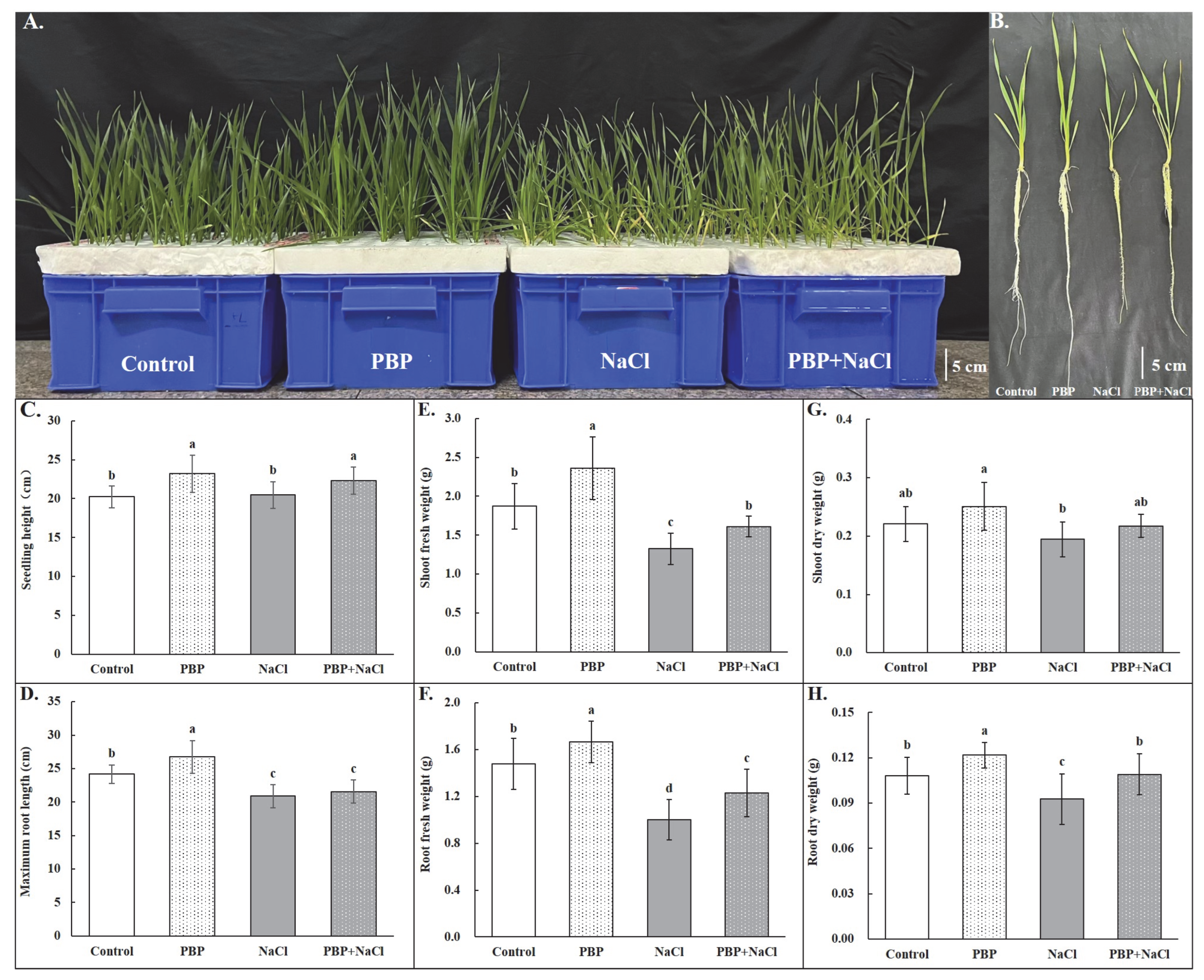
2.1. Exogenous PBP Promoted the Growth and Dry Matter Accumulation of Wheat Seedlings Under Salt Stress
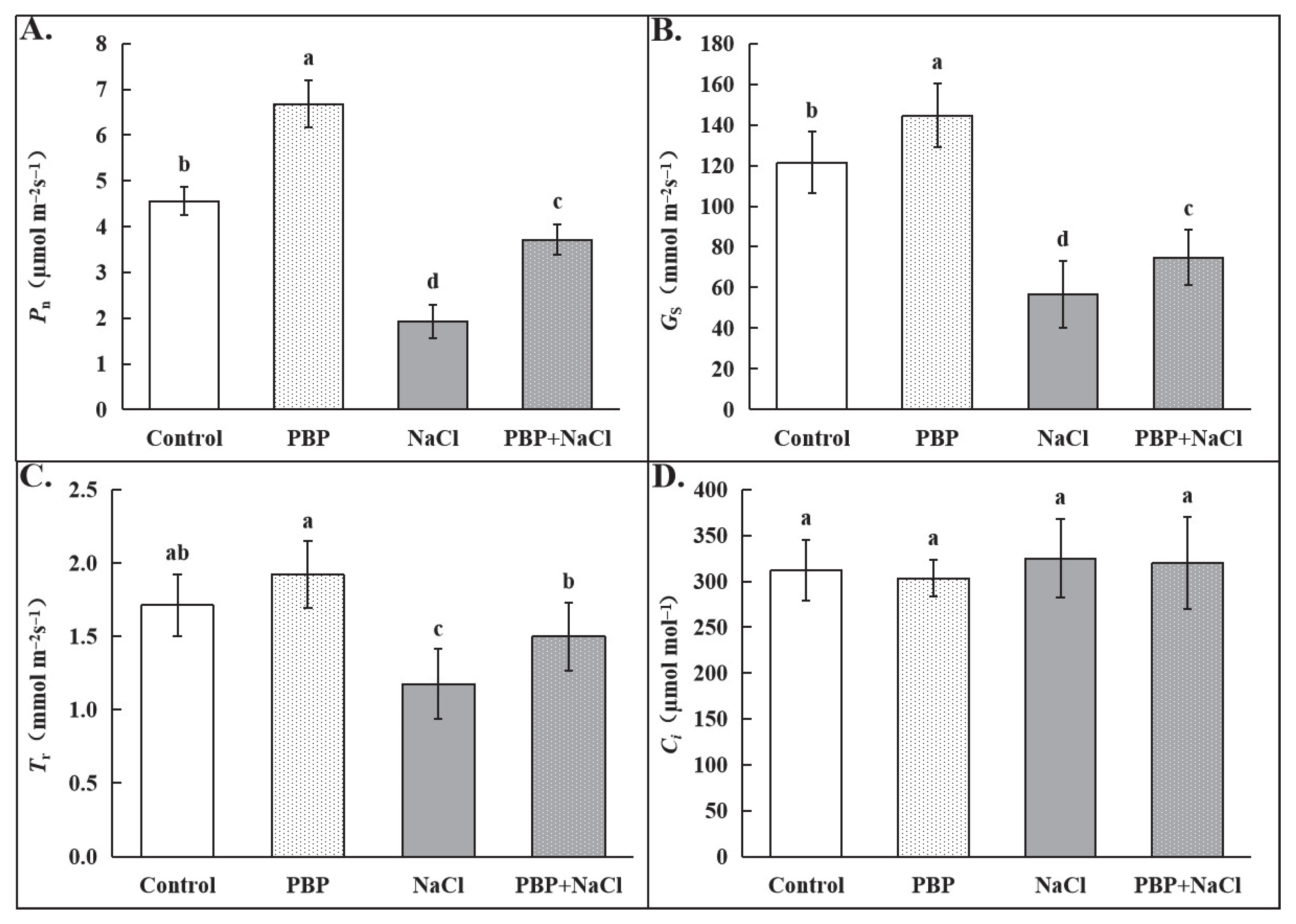
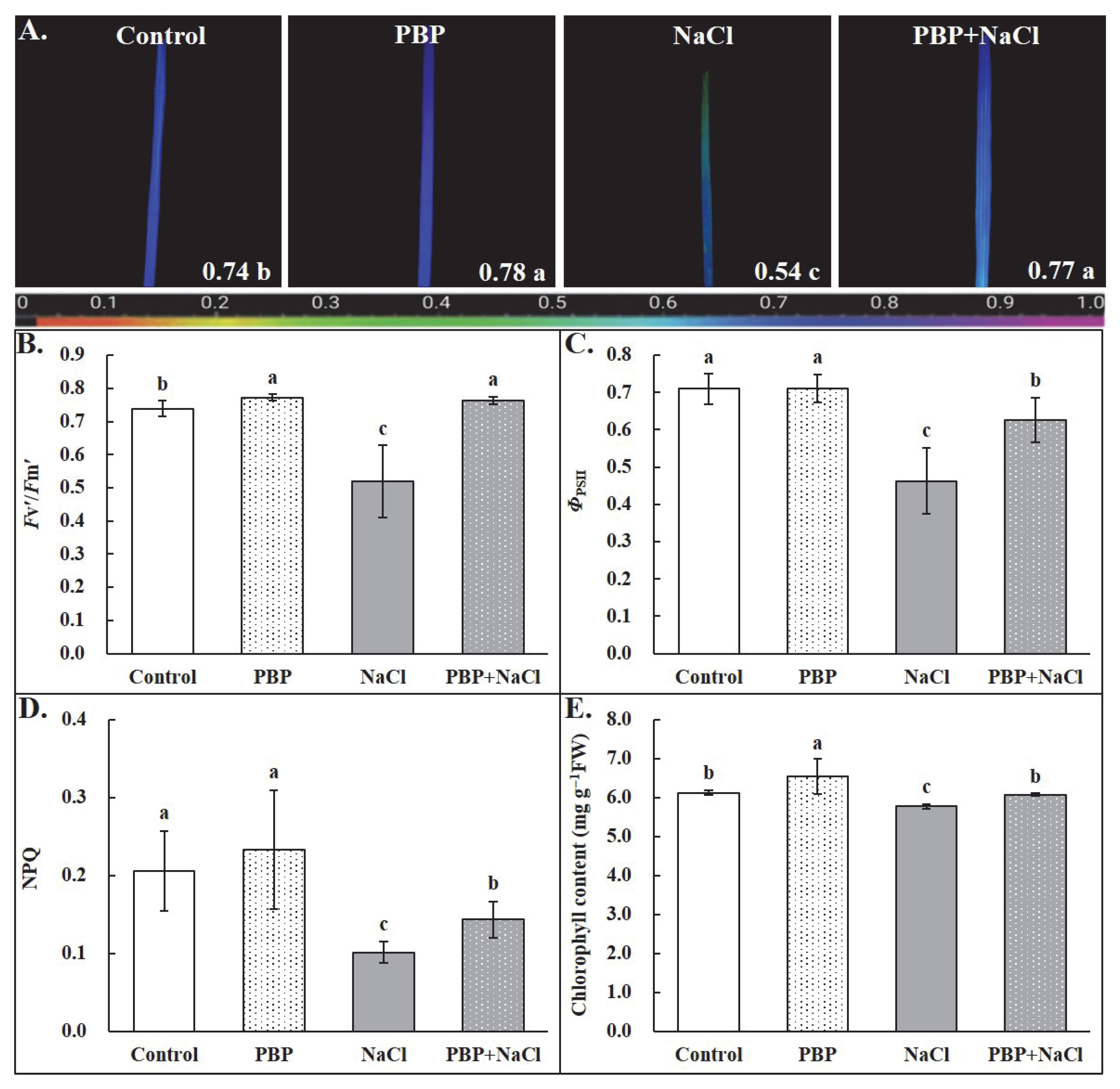
2.2. Exogenous PBP Alleviated the Inhibition of Salt Stress on Photosynthesis in Wheat Seedlings
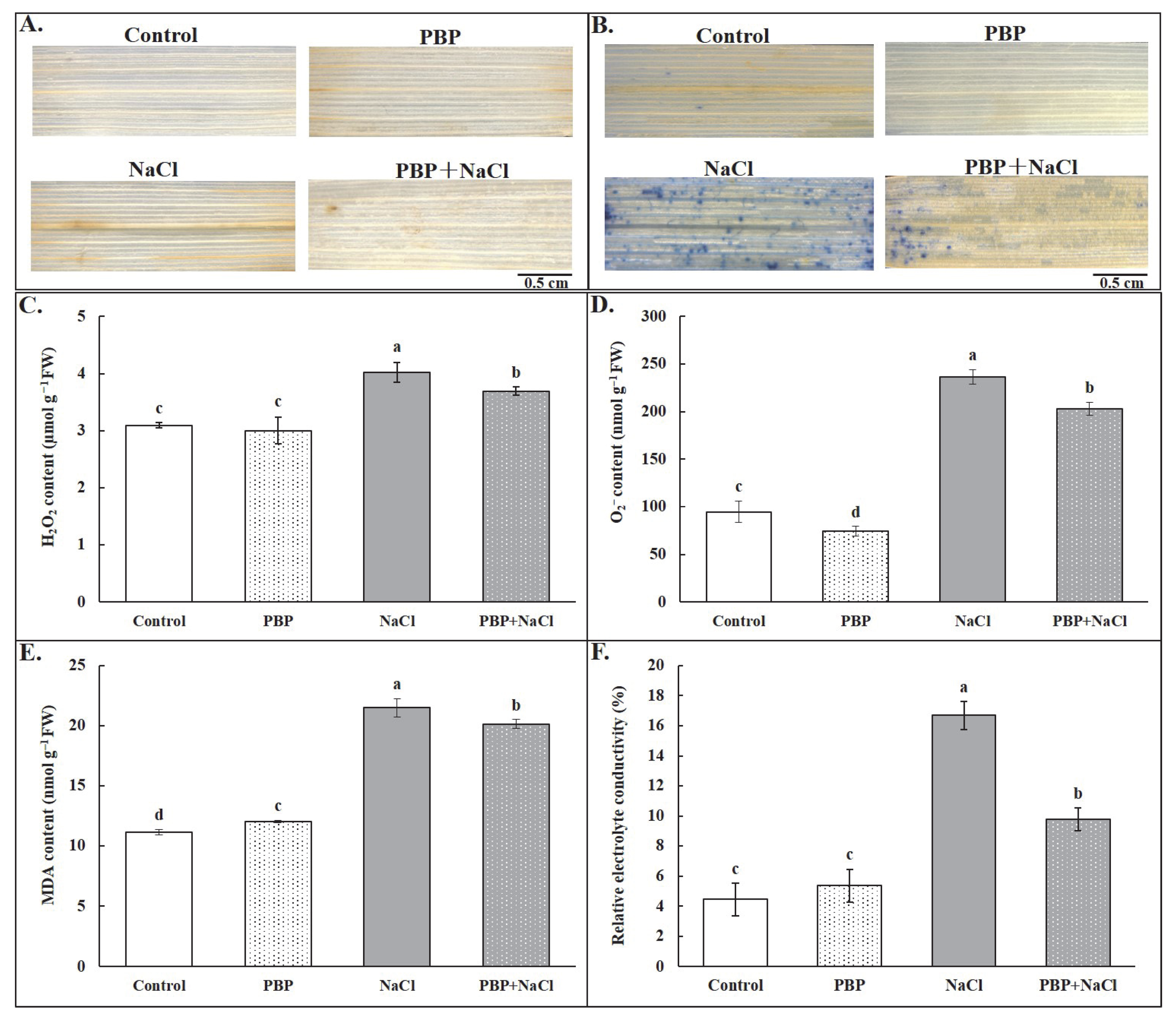
2.3. Exogenous PBP Reduced ROS Accumulation and Alleviated Membrane Damage to Wheat Seedlings Under Salt Stress
2.4. Exogenous PBP Enhanced the Activities of Antioxidant Enzymes and Increased the Contents of Osmotic Regulatory Substances in Wheat Seedlings Under Salt Stress
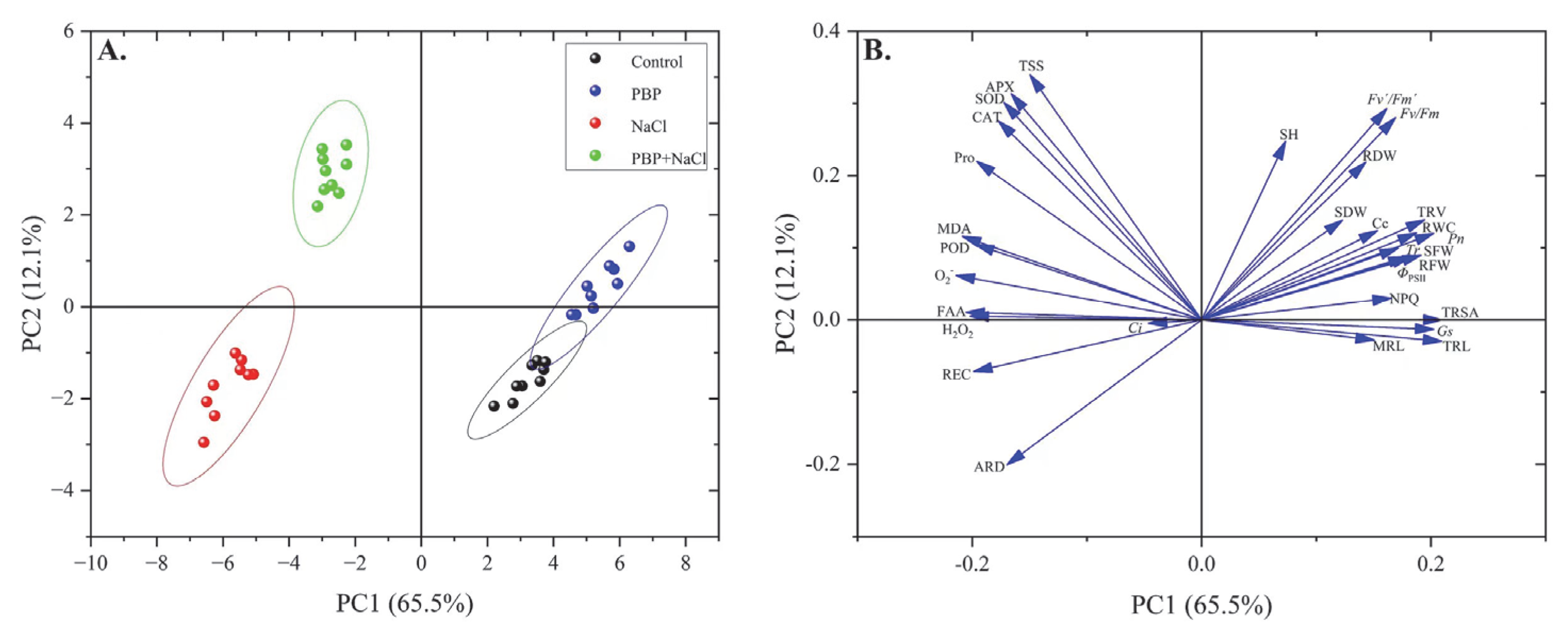
2.5. Principal Component Analysis
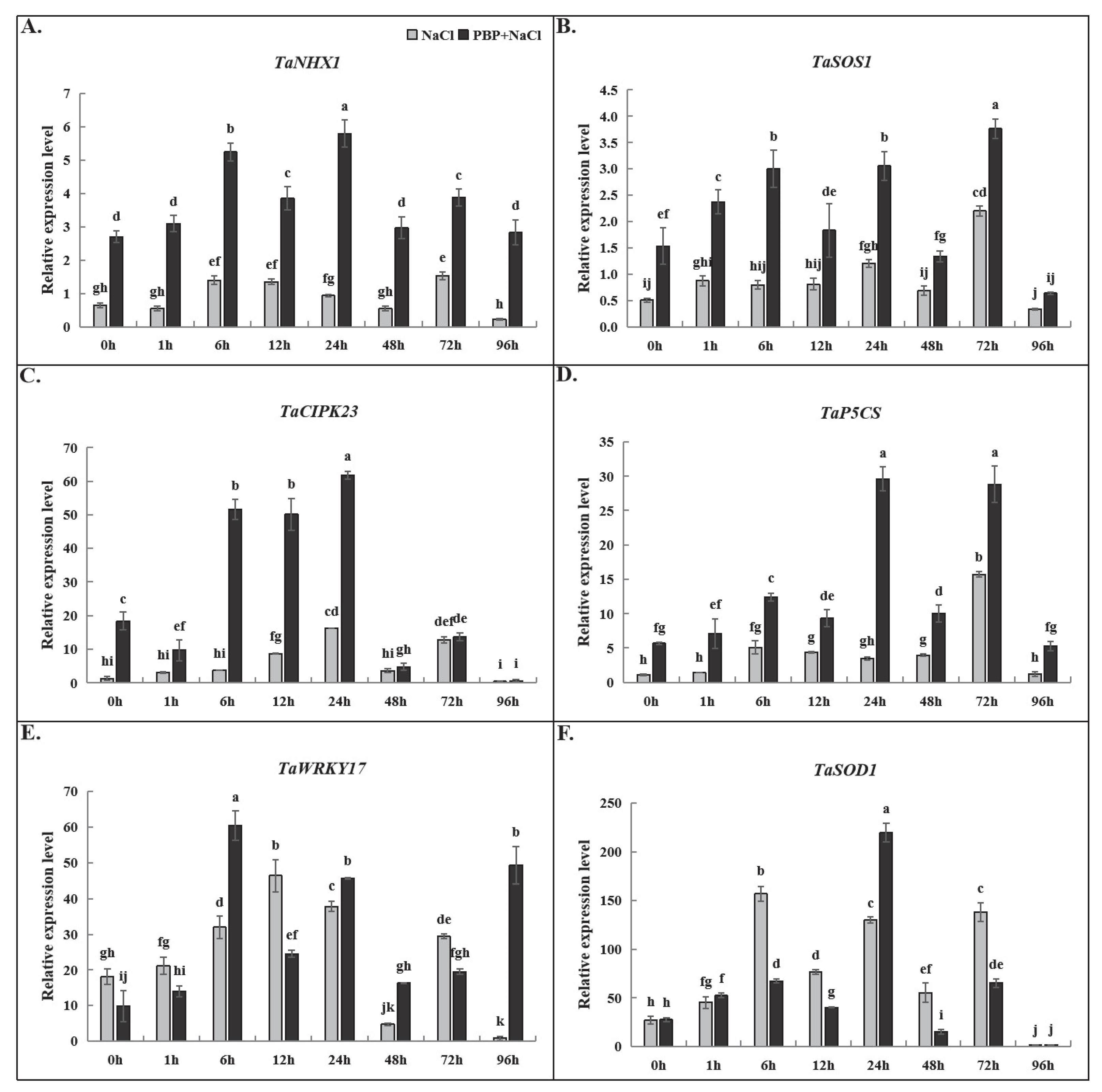
2.6. The Influence of Exogenous PBP on the Expression of Stress Response Genes in Wheat Seedlings Under Salt Stress
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experimental Design
4.3. Measurement of Morphological Indicators
4.4. Determination of Photosynthetic Indexes
4.5. Measurement of RWC and REC
4.6. Accumulation Analysis of H2O2, O2−, and MDA
4.7. Activity Analysis of Antioxidant Enzymes
4.8. Content Determination of Osmotic Regulatory Substances
4.9. RNA Isolation and Expression Analysis of Stress Response Genes
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PBP | Porcine blood polypeptide |
| SH | Seedling height |
| MRL | Maximum root length |
| SFW | Shoot fresh weight |
| SDW | Shoot dry weight |
| RFW | Root fresh weight |
| RDW | Root dry weight |
| TRL | Total root length |
| TRSA | Total root surface area |
| TRV | Total root volume |
| ARD | Average root diameter |
| Pn | Net photosynthetic rate |
| Gs | Stomatal conductance |
| Tr | Transpiration rate |
| Ci | Intercellular CO2 concentration |
| Fv/Fm | Maximal photochemical efficiency of photosystem II (PSII) |
| Fv′/Fm′ | Photochemical activity of PSII |
| ΦPSII | Quantum efficiency of PSII photochemistry |
| NPQ | Non-photochemical quenching |
| Cc | Chlorophyll content |
| ROS | Reactive oxygen species |
| H2O2 | Hydrogen peroxide |
| O2− | Superoxide anion |
| MDA | Malondialdehyde |
| REC | Relative electrolyte conductivity |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| POD | Peroxidase |
| APX | Ascorbate peroxidase |
| Pro | Proline |
| TSS | Total soluble sugar |
| FAA | Free amino acids |
| RWC | Relative water content |
References
- Parvin, K.; Nahar, K.; Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Mohsin, S.M.; Fujita, M. Exogenous vanillic acid enhances salt tolerance of tomato: Insight into plant antioxidant defense and glyoxalase systems. Plant Physiol. Biochem. 2020, 150, 109–120. [Google Scholar] [CrossRef]
- Wei, H.; Geng, X.; Zhu, W.; Zhang, X.; Zhang, X.; Chen, Y.; Huo, Z.; Xu, K.; Zhou, G.; Meng, T.; et al. Individual and combined influences of salinity and drought stress on the agro-physiological traits and grain yield of rice. Field Crops Res. 2023, 304, 109172. [Google Scholar] [CrossRef]
- Li, L.; Huang, Z.; Zhang, Y.; Mu, Y.; Li, Y.; Nie, L. Regulation of 2-acetyl-1-pyrroline (2-AP) biosynthesis and grain quality in fragrant rice under salt stress. Field Crops Res. 2025, 322, 109747. [Google Scholar] [CrossRef]
- Yu, D.H.; Xu, H.L.; Shao, Q.L. Introduction and selection of salt-tolerant wheat in Yellow River Delta. Agric. Sci. Tech. 2017, 18, 1232–1234. [Google Scholar]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Han, Y.; Li, X. Current progress in research focused on salt tolerance in Vitis vinifera L. Front. Plant Sci. 2024, 15, 1353436. [Google Scholar]
- Etesami, H.; Beattie, G.A. Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front. Microbiol. 2018, 9, 148. [Google Scholar] [CrossRef]
- Mauro, R.P.; Pérez-Alfocea, F.; Cookson, S.J.; Ollat, N.; Vitale, A. Editorial: Physiological and molecular aspects of plant rootstock-scion interactions. Front. Plant Sci. 2022, 13, 852518. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, F.; Salas, J.J.; Nouairi, I.; Smaoui, A.; Abdelly, C.; Martínez-Force, E.; Youssef, N.B. Changes in chloroplast lipid contents and chloroplast ultrastructure in Sulla carnosa and Sulla coronaria leaves under salt stress. J. Plant Physiol. 2016, 198, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, N.; Hu, Z.; Liu, H.; Hu, Y.; Tan, Y.; Sun, Q.; Liu, X.; Xiao, L.; Wang, W.; et al. Mutation of leaf senescence 1 encoding a C2H2 zinc finger protein induces ROS accumulation and accelerates leaf senescence in rice. Int. J. Mol. Sci. 2022, 23, 14464. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Zhang, J.L.; Shi, H. Physiological and molecular mechanisms of plant salt tolerance. Photosynth. Res. 2013, 115, 1–22. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, H.; Ren, Y.; Wang, X.; Wang, B.; Yuan, F. The transmembrane protein LbRSG from the recretohalophyte Limonium bicolor enhances salt gland development and salt tolerance. Plant J. 2024, 2, 498–515. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Liang, X.; Lu, M.; Lai, J.; Song, W.; Jiang, C. A teosinte-derived allele of an HKT1 family sodium transporter improves salt tolerance in maize. Plant Biotechnol. J. 2023, 21, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Nahakpam, S. Heat exposure alters the expression of SOD, POD, APX and CAT isozymes and mitigates low cadmium toxicity in seedlings of sensitive and tolerant rice cultivars. Plant Physiol. Biochem. 2012, 57, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.M.; Osmond, C.B.; Turner, N.C. Accumulation of solutes in leaves of sorghum and sunflower in response to water deficits. Funct. Plant Biol. 1980, 7, 193–205. [Google Scholar] [CrossRef]
- Xiang, J.; Huang, Q.; Ju, C.Y.; Huang, L.; Zhao, Z. Effect of exogenous melatonin on seed germination and seedling growth of rice under salt stress. Plant Physiol. J. 2021, 57, 393–401, (In Chinese with English Abstract). [Google Scholar]
- Munns, R. Physiological processes limiting plant growth in saline soil: Some dogmas and hypotheses. Plant Cell Environ. 1993, 16, 15–24. [Google Scholar] [CrossRef]
- Qiu, Z.; Guo, J.; Zhu, A.; Zhang, L.; Zhang, M. Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotoxicol. Environ. Saf. 2014, 104, 202–208. [Google Scholar] [CrossRef]
- Ke, Q.; Ye, J.; Wang, B.; Ren, J.; Yin, L.; Deng, X.; Wang, S. Melatonin mitigates salt stress in wheat seedlings by modulating polyamine metabolism. Front. Plant Sci. 2018, 9, 914. [Google Scholar] [CrossRef] [PubMed]
- Fu, N.X.; He, M.R.; Zhuge, Y.P.; Dai, X.L.; Hu, G.Q.; Dong, Y.J. Effects and mechanisms of exogenous SA alleviating the growth of winter wheat seedlings under salt stress. J. China Agric. Univ. 2019, 24, 10–17, (In Chinese with English Abstract). [Google Scholar]
- Li, X.; Li, S.; Wang, J.; Lin, J. Exogenous abscisic acid alleviates harmful effect of salt and alkali stresses on wheat seedlings. Int. J. Environ. Res. Public Health 2020, 17, 3770. [Google Scholar] [CrossRef]
- Sheng, L.; Han, J.; Sheng, Y.; Lu, J.; Ji, Y.; Zhang, Y.; Li, X. The role of exogenous (Z)-3-hexenyl acetate in alleviating salt stress in wheat. Plant Physiol. J. 2022, 58, 1263–1274, (In Chinese with English Abstract). [Google Scholar]
- Shen, S.H.; Zhu, Z.Q. Advances in research of novel plant growth regulating substance ‘hormone-like peptides’. Chin. Bull. Bot. 1999, 16, 648–652, (In Chinese with English Abstract). [Google Scholar]
- Kozaki, A.; Takeba, G.; Tanaka, O. A polypeptide that induces flowering in Lemna paucicostata at a very low concentration. Plant Physiol. 1991, 95, 1288–1290. [Google Scholar] [CrossRef]
- Yu, L.; Wang, Y.; Liu, Y.; Li, N.; Yan, J.; Luo, L. Wound-induced polypeptides improve resistance against Pseudomonas syringae pv. tomato DC3000 in Arabidopsis. Biochem. Biophys. Res. Commun. 2018, 504, 149–156. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Jin, S.; Shang, M.; Wang, M. Effect of foliar spraying of two functional water soluble fertilizers containing amino acids on yield and quality of wheat. Soil. Fert. Sci. Chin. 2022, 11, 127–132, (In Chinese with English Abstract). [Google Scholar]
- Zhou, W.; Zheng, W.; Wang, W.; Lv, H.; Liang, B.; Li, J. Exogenous pig blood-derived protein hydrolysates as a promising method for alleviation of salt stress in tomato (Solanum lycopersicum L.). Sci. Hortic. Amst. 2022, 294, 110779, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Zheng, W.; Zhou, W.; Sun, F.; Liu, H.; Li, J. Effects of porcine blood polypeptide on seed germination and seedling growth of Pakchoi under salt stress. Shandong Agric. Sci. 2021, 53, 66–71, (In Chinese with English Abstract). [Google Scholar]
- Zhao, X.; Zhang, C.; Fang, Z.; Dong, L.; Liu, Q.; Li, J. Effects of spraying functional substances on growth and nutrient uptake of sweet potato seedlings under low temperature stress. Shandong Agric. Sci. 2025, 57, 54–60, (In Chinese with English Abstract). [Google Scholar]
- Li, X.; Topbjerg, H.B.; Jiang, D.; Liu, F. Drought priming at vegetative stage improves the antioxidant capacity and photosynthesis performance of wheat exposed to a short-term low temperature stress at jointing stage. Plant Soil. 2015, 393, 307–318. [Google Scholar] [CrossRef]
- Wang, W.B.; Kim, Y.H.; Lee, H.S.; Kim, K.Y.; Deng, X.P.; Kwak, S.S. Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol. Biochem. 2009, 47, 570–577. [Google Scholar] [CrossRef]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
- Petrov, P.; Petrova, A.; Dimitrov, I.; Tashev, T.; Olsovska, K.; Brestic, M.; Misheva, S. Relationships between leaf morpho-anatomy, water status and cell membrane stability in leaves of wheat seedlings subjected to severe soil drought. J. Agron. Crop Sci. 2018, 204, 219–227. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Shigeoka, S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef]
- Foyer, C.H.; Harbinson, J.C. Oxygen metabolism and the regulation of photosynthetic electron transport. In Causes of Photooxidative Stress and Amelioration of Defense Systems in Plant; Foyer, C.H., Mullineaux, P.M., Eds.; CRC: Boca Raton, FL, USA, 1994; pp. 1–42. [Google Scholar]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The roles of reactive oxygen metabolism in drought: Not so cut and dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef]
- Neill, S.; Desikan, R.; Hancock, J. Hydrogen peroxide signalling. Curr. Opin. Plant Biol. 2002, 5, 388–395. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Tarczynski, M.C.; Jensen, R.G.; Bohnert, H.J. Stress protection of transgenic tobacco by production of the osmolyte mannitol. Science 1993, 259, 508–510. [Google Scholar] [CrossRef]
- Westgate, M.E.; Boyer, J.S. Osmotic adjustment and the inhibition of leaf, root, stem and silk growth at low water potentials in maize. Planta 1985, 164, 540–549. [Google Scholar] [CrossRef]
- Robinson, S.P.; Jones, G.P. Accumulation of glycinebetaine in chloroplasts provides osmotic adjustment during salt stress. Aust. J. Plant Physiol. 1986, 13, 659–668. [Google Scholar] [CrossRef]
- Jensen, R.G.; Bassham, J.A. Photosynthesis by isolated chloroplasts. Proc. Natl. Acad. Sci. USA 1966, 56, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Escalona, J.M.; Flexas, J.; Medrano, H. Stomatal and non-stomatal limitations of photosynthesis under water stress in field-grown grapevines. Aust. J. Plant Physiol. 2000, 27, 87. [Google Scholar] [CrossRef]
- Kriedemann, P.E.; Loveys, B.R.; Fuller, G.L.; Leopold, A.C. Abscisic acid and stomatal regulation. Plant Physiol. 1972, 49, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, E.A.H.; Ebskamp, M.J.M.; Paul, M.J.; Jeuken, M.J.W.; Weisbeek, P.J.; Smeekens, S.C.M. Improved performance of transgenic fructan-accumulating tobacco under drought stress. Plant Physiol. 1995, 107, 125–130. [Google Scholar] [CrossRef]
- Yang, L.; Wu, Q.; Liang, H.; Yin, L.; Shen, P. Integrated analyses of transcriptome and metabolome provides new insights into the primary and secondary metabolism in response to nitrogen deficiency and soil compaction stress in peanut roots. Front. Plant Sci. 2022, 13, 948742. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, X.; Zhang, Y.; Wang, J.; Yang, J.; Ishida, T.; Jiang, W.; Han, X.; Kang, J.; Wang, X.; et al. CLE9 peptide-induced stomatal closure is mediated by abscisic acid, hydrogen peroxide, and nitric oxide in Arabidopsis thaliana. Plant Cell Environ. 2019, 42, 1033–1044. [Google Scholar] [CrossRef]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [PubMed]
- Teige, M.; Scheikl, E.; Eulgem, T.; Dóczi, R.; Ichimura, K.; Shinozaki, K.; Dangl, J.L.; Hirt, H. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol. Cell 2004, 15, 141–152. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, S. MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Li, X.; Hicks, L.M.; Xiong, L. A Raf-like MAPKKK gene DSM1 mediates drought resistance through reactive oxygen species scavenging in rice. Plant Physiol. 2010, 152, 876–890. [Google Scholar] [PubMed]
- Park, H.J.; Kim, W.Y.; Yun, A.D. New insight of salt stress signaling in plant. Mol. Cell 2016, 39, 447–459. [Google Scholar] [CrossRef]
- Li, L.; Kim, B.-G.; Cheong, Y.H.; Pandey, G.K.; Luan, S. A Ca2+ signaling pathway regulates a K+ channel for low-K response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 12625–12630. [Google Scholar] [CrossRef]
- Fang, H.; Gao, X.; Wu, Y.; Zhang, K.; Wu, Y.; Li, J.; Qian, D.; Li, R.; Gu, H.; Mehari, T.G.; et al. Unveiling the role of GhP5CS1 in cotton salt stress tolerance: A comprehensive genomic and functional analysis of P5CS genes. Plants 2025, 14, 231. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Khan, A.A.; Lin, Q.; Han, Y.; Mu, P.; Liu, Y.; Zhang, H.; Li, L.; Meng, X.; et al. Expression partitioning of homeologs and tandem duplications contribute to salt tolerance in wheat (Triticum aestivum L.). Sci. Rep. 2016, 6, 21476. [Google Scholar] [CrossRef]
- Li, X.; Ji, Y.; Sheng, Y.; Sheng, L.; Guo, W.; Wang, H.; Zhang, Y. Priming with the green leaf volatile (Z)-3-hexeny-1-yl acetate enhances drought resistance in wheat seedlings. Plant Growth Regul. 2022, 98, 477–490. [Google Scholar] [CrossRef]
- Tian, S.; Guo, R.; Zou, X.; Zhang, X.; Yu, X.; Zhan, Y.; Ci, D.; Wang, M.; Wang, Y.; Si, T. Priming with the green leaf volatile (Z)-3-hexeny-1-yl acetate enhances salinity stress tolerance in peanut (Arachis hypogaea L.) seedlings. Front. Plant Sci. 2019, 10, 785. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Ruan, Y.P.; Zhou, J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Brassinosteroid alleviates polychlorinated biphenyls-induced oxidative stress by enhancing antioxidant enzymes activity in tomato. Chemosphere 2013, 90, 2645–2653. [Google Scholar] [CrossRef] [PubMed]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fuorescence parameters for the determination of QA redox state and excitation energy fuxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Jensen, C.R.; Jacobsen, S.E.; Andersen, M.; Nunez, N.; Andersen, S.D.; Rasmussen, L.; Mogensen, V.O. Leaf gas exchange and water relation characteristics of field quinoa (Chenopodium quinoa Willd) during soil drying. Eur. J. Agron. 2000, 13, 11–25. [Google Scholar] [CrossRef]
- Griffith, M.; Mclntyre, H.C. The interrelationship of growth and frost tolerance in winter rye. Physiol. Plant 1993, 87, 335–344. [Google Scholar] [CrossRef]
- Li, X.; Ji, Y.; Guo, W.; Han, L.; Zhang, Y. Non-uniform salt distribution in the root zone alleviates salt damage in wheat. Can. J. Plant Sci. 2021, 101, 107–118. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Ma, Y.; Yuan, Y.; Dong, M.; Wang, Y.; Zhou, J.; Yang, J.; Guo, Y.; Guo, W.; Wang, H.; et al. Priming with Porcine Blood Polypeptide Enhances Salt Tolerance in Wheat Seedlings. Plants 2025, 14, 2968. https://doi.org/10.3390/plants14192968
Shen Y, Ma Y, Yuan Y, Dong M, Wang Y, Zhou J, Yang J, Guo Y, Guo W, Wang H, et al. Priming with Porcine Blood Polypeptide Enhances Salt Tolerance in Wheat Seedlings. Plants. 2025; 14(19):2968. https://doi.org/10.3390/plants14192968
Chicago/Turabian StyleShen, Yong, Yanling Ma, Yiming Yuan, Meitian Dong, Yanan Wang, Jilong Zhou, Jinpeng Yang, Yang Guo, Weiwei Guo, Huifang Wang, and et al. 2025. "Priming with Porcine Blood Polypeptide Enhances Salt Tolerance in Wheat Seedlings" Plants 14, no. 19: 2968. https://doi.org/10.3390/plants14192968
APA StyleShen, Y., Ma, Y., Yuan, Y., Dong, M., Wang, Y., Zhou, J., Yang, J., Guo, Y., Guo, W., Wang, H., Zhang, Y., & Li, X. (2025). Priming with Porcine Blood Polypeptide Enhances Salt Tolerance in Wheat Seedlings. Plants, 14(19), 2968. https://doi.org/10.3390/plants14192968




