Non-Targeted Metabolomics and Network Pharmacology Reveal Bioactive Metabolites and the Medicinal Potential of Three Ornamental Camellia Flowers
Abstract
1. Introduction
2. Results
2.1. Overview of Metabolomics Analysis
2.1.1. Multivariate Statistical Analysis of Three Camellias
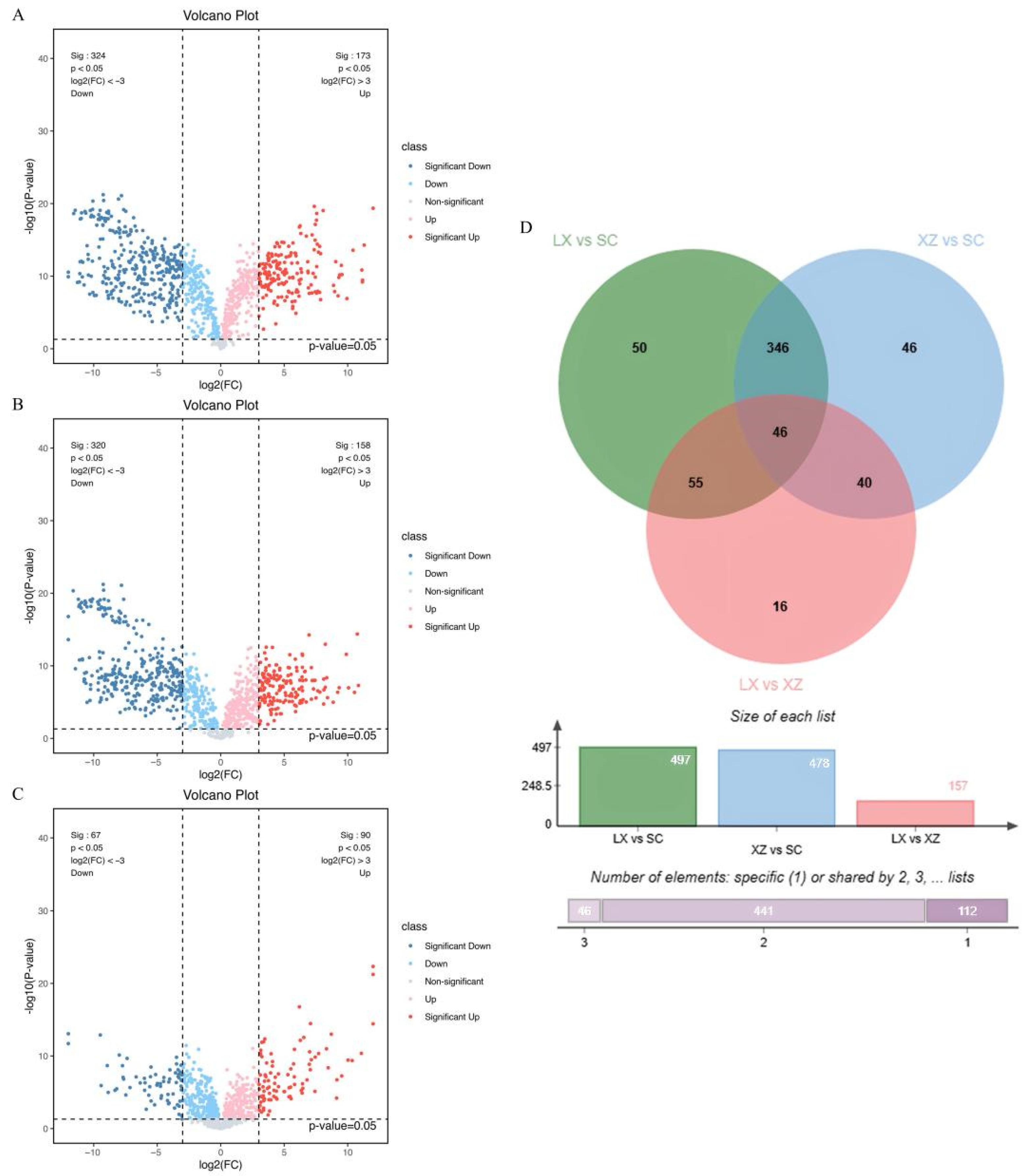
2.1.2. Screening of Different Metabolites Among Three Camellias
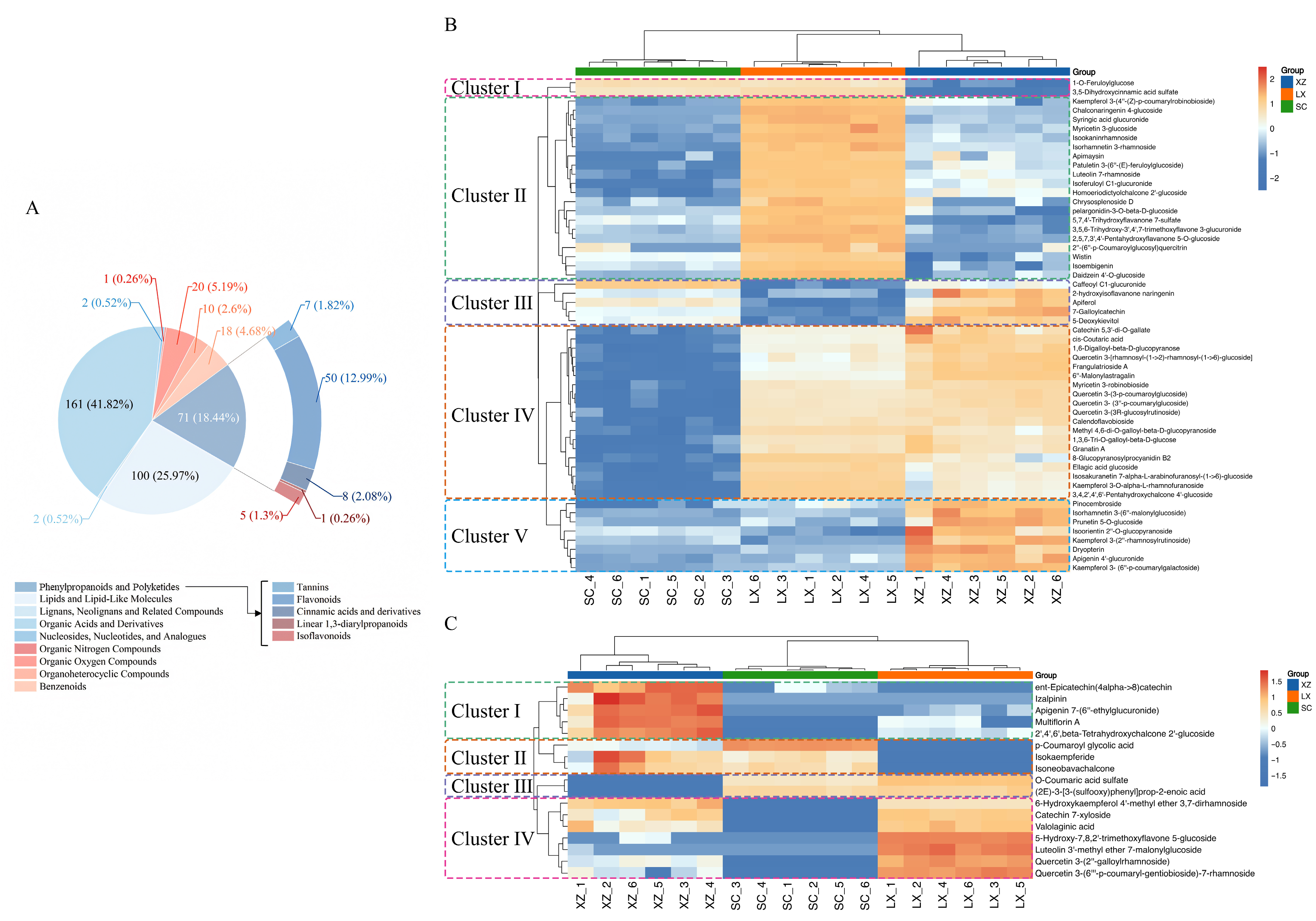
2.2. Deep Analysis of Significantly Different Phenylpropanoids and Polyketides in Three Camellias
2.2.1. In-Depth Examination of Differential Metabolites
2.2.2. Differential Phenylpropanoids and Polyketides Analysis Among the Three Camellias
2.3. Prediction of Potential Active Compounds and Targets via Network Pharmacology
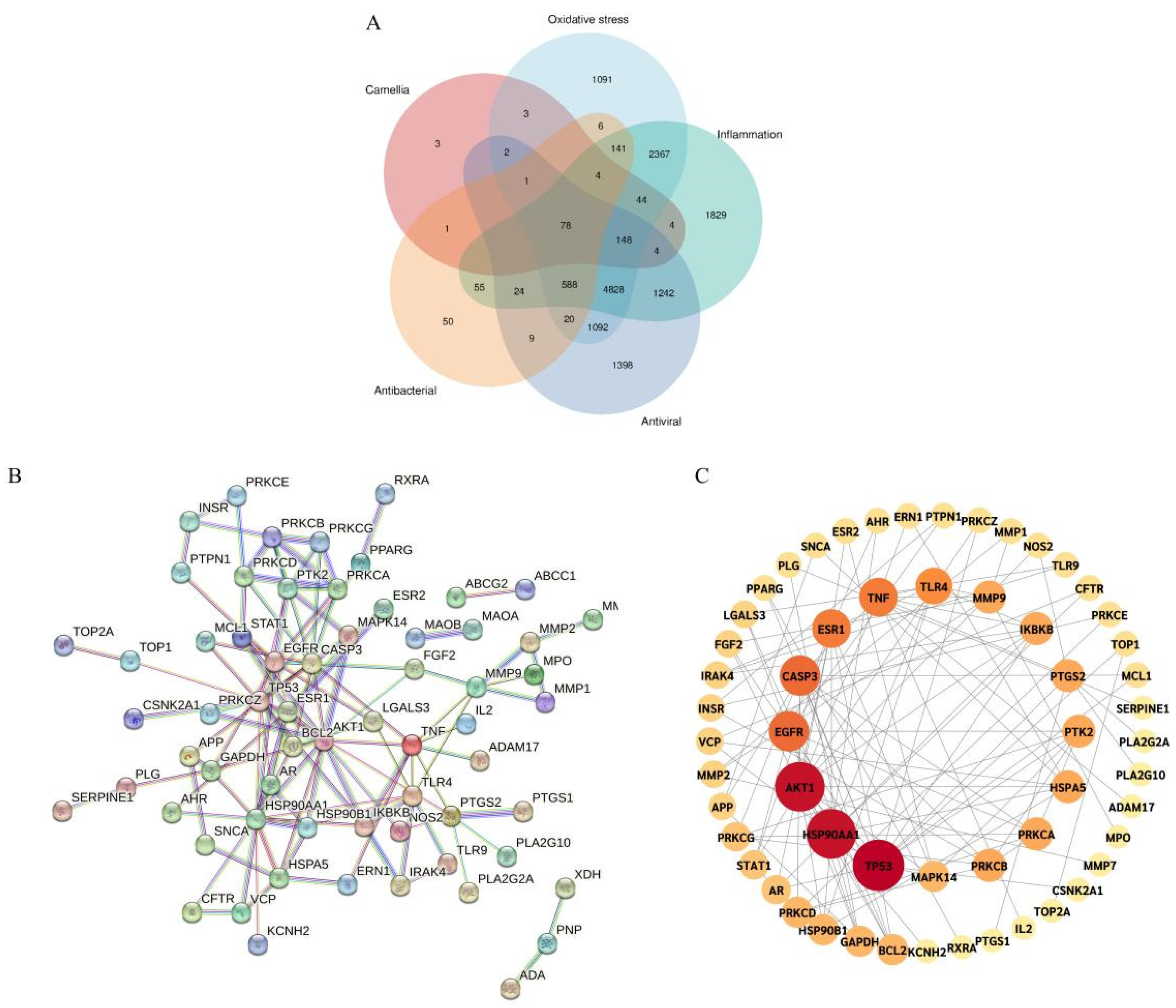
2.3.1. Active Compounds and Disease-Associated Targets of the Studied Camellias
2.3.2. Construction of PPI and Selection of Core Targets
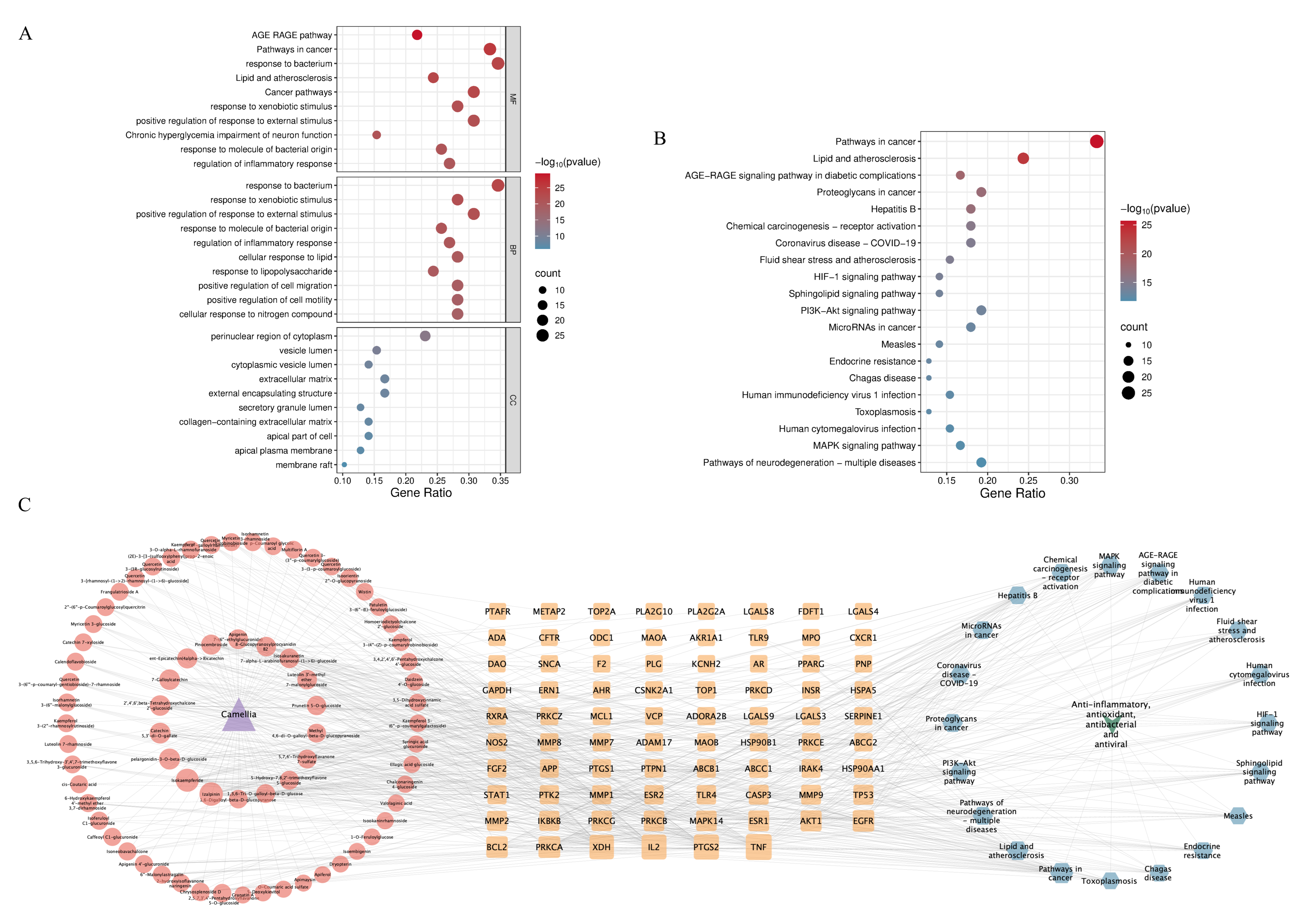
2.3.3. GO Function and KEGG Pathway Enrichment Analysis
2.3.4. Network Diagram Construction and Key Ingredients Identification
3. Discussion
3.1. Valuable Resources Improve the Application of Genus Camellia
3.2. More Flavonoid Components Are Identified as Vital Bioactivities
3.3. Unique Bioactive Floavonoids in Genus Camellia May Play Important Role
4. Materials and Methods
4.1. Materials
4.1.1. Preparation of Flowers
4.1.2. Preparation of Flower Extracts
4.2. Metabolomics Analysis
4.2.1. Condition for Metabolomics Analysis
4.2.2. Metabolite Identification
4.2.3. Data Analysis
4.3. Network Pharmacology Analysis
4.3.1. Screening of Cross-Targets Between Ingredient-Related Targets and Disease-Associated Targets
4.3.2. Protein–Protein Interaction (PPI) Construction and Core Target Selection
4.3.3. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis
4.3.4. Network Diagram Construction and Key Ingredient Identification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| XZ | Camellia japonica ‘Kōshi’ |
| LX | Camellia ‘High Fragrance’ |
| SC | Camellia japonica |
| LC | Liquid Chromatograph |
| MS | Mass Spectrometer |
| ESI | Electrospray ionization |
| PCA | Principle Component Analysis |
| OPLS-DA | Orthogonal Partial Least-Squares–Discriminant Analysis |
| PLS-DA | Partial Least-Squares–Discriminant Analysis |
| VIP | Variable importance in projection |
| PPI | Protein–protein interaction |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| AGE-RAGE | Advanced glycation end product—(receptor for AGEs) |
References
- Pereira, A.G.; Fraga-Corral, M.; Silva, A.; Barroso, M.F.; Grosso, C.; Carpena, M.; Garcia-Perez, P.; Perez-Gregorio, R.; Cassani, L.; Simal-Gandara, J.; et al. Unraveling the bioactive potential of Camellia japonica edible flowers: Profiling antioxidant substances and in vitro bioactivity Assessment. Pharmaceuticals 2024, 17, 946. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Zhou, C.; Wang, D.; Qian, M.; Huang, L. Network pharmacology integrated with experimental validation revealed potential molecular mechanisms of Camellia nitidissima C. W. Chi in the treatment of lung cancer. J. Ethnopharmacol. 2023, 314, 116576. [Google Scholar]
- Song, Q.; Gong, W.; Yu, X.; Ji, K.; Chang, Y.; Wang, L.; Yuan, D. Integrative analysis of the metabolome and transcriptome provides novel insights into the mechanisms of flavonoid biosynthesis in Camellia lanceoleosa. Sci. Hortic. 2022, 304, 111357. [Google Scholar] [CrossRef]
- Piao, M.J.; Yoo, E.S.; Koh, Y.S.; Kang, H.K.; Kim, J.; Kim, Y.J.; Kang, H.H.; Hyun, J.W. Antioxidant effects of the ethanol extract from flower of Camellia japonica via scavenging of reactive oxygen species and induction of antioxidant enzymes. Int. J. Mol. Sci. 2011, 12, 2618–2630. [Google Scholar] [CrossRef]
- Jung, E.; Lee, J.; Baek, J.; Jung, K.; Lee, J.; Huh, S.; Kim, S.; Koh, J.; Park, D. Effect of Camellia japonica oil on human type I procollagen production and skin barrier function. J. Ethnopharmacol. 2007, 112, 127–131. [Google Scholar] [CrossRef]
- Lee, S.Y.; Bae, C.S.; Seo, N.S.; Na, C.S.; Yoo, H.Y.; Oh, D.S.; Bae, M.S.; Kwon, M.S.; Cho, S.S.; Park, D.H. Camellia japonica oil suppressed asthma occurrence via GATA-3 & IL-4 pathway and its effective and major component is oleic acid. Phytomedicine 2019, 57, 84–94. [Google Scholar] [CrossRef]
- Ramachandran, G.; Rajivgandhi, G.N.; Murugan, S.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Almanaa, T.N.; Manoharan, N.; Li, W.J. Anti-carbapenamase activity of Camellia japonica essential oil against isolated carbapenem resistant klebsiella pneumoniae (MN396685). Saudi J. Biol. Sci. 2020, 27, 2269–2279. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Pereira, E.L.; Ramalhosa, E.; Saraiva, J.A. Effect of high hydrostatic pressure on the quality of four edible flowers: Viola × wittrockiana, Centaurea cyanus, Borago officinalis and Camellia japonica. Int. J. Food Sci. Technol. 2017, 52, 2455–2462. [Google Scholar] [CrossRef]
- Majumder, S.; Ghosh, A.; Chakraborty, S.; Bhattacharya, M. Brewing and biochemical characterization of Camellia japonica petal wine with comprehensive discussion on metabolomics. Food Prod. Process. Nutr. 2022, 4, 29. [Google Scholar] [CrossRef]
- He, D.; Li, X.; Sai, X.; Wang, L.; Li, S.; Xu, Y. Camellia nitidissima C.W. Chi: A review of botany, chemistry, and pharmacology. Phytochem. Rev. 2018, 17, 327–349. [Google Scholar] [CrossRef]
- Nguyen, P.N.; Do, T.Y.; Do, T.N.; Gontier, E.; Nguyen, H.T.L.; Thi, V.A.L.; Mai, N.T.P.; Sato, M.; Hirai, M.Y.; Thi, K.O.N. Widely targeted metabolomics reveals the species-specific, matureness-specific and post-harvest-specific discriminations in the chemical profiles of Vietnamese endemic golden camellias. Int. J. Food Sci. Technol. 2024, 59, 7873–7886. [Google Scholar] [CrossRef]
- Nguyen, P.N.; Le, H.L.; Gontier, E.; Thi, H.C.H.; Thi, V.A.L.; Mai, N.T.P.; Thi, K.O.N. A comprehensive strategy for metabolites profiling of flowers and leaves from Camellia tienii, an endemic golden tea of Vietnam. Chem. Biodivers. 2024, 21, e202400997. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.G.; Cassani, L.; Oludemi, T.; Chamorro, F.; Calhelha, R.C.; Prieto, M.A.; Barros, L.; Simal-Gandara, J.; Lucini, L.; Garcia-Perez, P. Untargeted metabolomics and in vitro functional analysis unravel the intraspeciffc bioactive potential of flowers from underexplored Camellia japonica cultivars facing their industrial application. Ind. Crop. Prod. 2023, 204, 117389. [Google Scholar] [CrossRef]
- Zhai, Y.; Liu, L.; Zhang, F.; Chen, X.; Wang, H.; Zhou, J.; Chai, K.; Liu, J.; Lei, H.; Lu, P.; et al. Network pharmacology: A crucial approach in traditional Chinese medicine research. Chin. Med. 2025, 20, 8. [Google Scholar] [CrossRef]
- Joshi, C.P.; Baldi, A.; Kumar, N.; Pradhan, J. Harnessing network pharmacology in drug discovery: An integrated approach. Naunyn-Schmiedebergs Arch. Pharmacol. 2025, 398, 4689–4703. [Google Scholar] [CrossRef]
- Duan, X.; Wang, N.; Peng, D. Application of network pharmacology in synergistic action of Chinese herbal compounds. Theory Biosci. 2024, 143, 195–203. [Google Scholar] [CrossRef]
- Lu, S.; Wang, R.; Gao, X.; Lin, H.; Chen, Z.; Zhang, Y.; Chen, X.; Huang, Q.; Li, B.; Lin, X. Exploring analytics of mechanisms behind the anti-inflammatory activity of black tea from Camellia ptilophylla chang based on high-resolution mass spectrum and network pharmacology. Sci. Technol. Food Ind. 2024, 45, 30–39. [Google Scholar]
- Li, N.; Wang, Z.; Sun, Z. Exploring mechanism of Camellia plants in treating osteoporosis based on network pharmacology and molecular docking technology. New J. Tradit. Chin. Med. 2024, 56, 198–204. [Google Scholar]
- Wahyuni, S.; Jamil, A.S.; Muchlisin, M.A. A network pharmacology of Camellia sinensis (Green Tea). In Proceedings of International Pharmacy Ulul Albab Conference and Seminar (PLANAR), 3rd ed.; Program Studi Farmasi, Fakultas Kedokteran dan Ilmu Kesehatan: Malang, Indonesia, 2023; Volume 3, pp. 216–224. [Google Scholar]
- Patel, K.R.; Chahwala, F.D.; Yadav, U.C.S. Flavonoids as functional food. In Functional Food and Human Health; Rani, V., Yadav, U., Eds.; Springer: Singapore, 2018; pp. 83–106. [Google Scholar]
- Mahmud, A.R.; Ema, T.I.; Siddiquee, M.F.R.; Shahriar, A.; Ahmed, H.; Mosfeq-Ul-Hasan, M.; Rahman, N.; Islam, R.; Uddin, M.R.; Mizan, M.F.R. Natural favonols: Actions, mechanisms, and potential therapeutic utility for various diseases. Beni-Suef Univ. J. Basic Appl. Sci. 2023, 12, 47. [Google Scholar] [CrossRef]
- Awasthi, K.; Jain, N.; Srivastava, M.P.; Verma, S.; Khanna, A. Therapeutic potential of natural pharmacological agents (Flavonoids and other henolic compounds) found in plants. In Sustainability and Health Informatics. Advances in Geographical and Environmental Sciences; Tripathi, G., Shakya, A., Kanga, S., Guite, L.T.S., Singh, S.K., Eds.; Springer: Singapore, 2024; pp. 283–297. [Google Scholar]
- Lotf, M.S.; Rassouli, F.B. Natural flavonoid apigenin, an efective agent against nervous system cancers. Mol. Neurobiol. 2024, 61, 5572–5583. [Google Scholar] [CrossRef]
- Fang, W.; Du, J.; Nie, M.; Wang, X. Recent advances in flavonoid compounds for the treatment of prostate cancer. Mol. Biol. Rep. 2024, 51, 653. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Thi, P.T.; Vo, T.K.; Pham, T.H.T.; Nguyen, T.T.; Vo, G.V. Natural flavonoids as potential therapeutics in the management of Alzheimer’s disease: A review. 3 Biotech 2024, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, Y.; Zhan, L.; Rayat, G.R.; Xiao, J.; Jiang, H.; Li, X.; Chen, K. Anti-diabetic effects of natural antioxidants from fruits. Trends Food Sci. Technol. 2021, 117, 3–14. [Google Scholar] [CrossRef]
- Das, S.K.; Sen, K.; Sanyal, T.; Saha, A.; Madhu, N.R. Flavonoids: A promising neuroprotectant and its salutary effects on age-related neurodegenerative disorders. In Neuroprotective Effects of Phytochemicals in Brain Ageing; Pathak, S., Banerjee, A., Eds.; Springer: Singapore, 2024; pp. 221–255. [Google Scholar]
- Wang, Y.; Wu, X.; Li, H.; Pang, Y.; Tang, L.; Feng, B. Research of Camellia Linn. on the used to drugs. J. Dalian Univ. 2006, 27, 47–55; 58. [Google Scholar]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Lozano, R.; Gómez-Serranillos, M.P. The pharmacological activity of Camellia sinensis (L.) kuntze on metabolic and endocrine disorders: A systematic review. Biomolecules 2020, 10, 603. [Google Scholar] [CrossRef]
- Luo, S.; Dong, L.; Fan, L.; Fang, M.; Chen, Z. Protective effect of total flavone of Camellia against cerebral ischemic injury. Tradit. Chin. Drug Res. Clin. Pharmacol. 2004, 15, 376–379. [Google Scholar]
- Yuan, L.; Fan, L.; Dong, L.; Fang, M.; Zhao, W.; Chen, Z. Protective effects of total flavone C on myocardial ischemia in rats. Tradit. Chin. Drug Res. Clin. Pharmacol. 2004, 15, 25–28. [Google Scholar]
- Huang, C.; Ding, Z.; Yang, S.; Cai, J.; Fu, G. Study on scavenging free radical and antibacterial activities of flavonoids from Camellia oleifera leaves. Cereals Oils 2011, 12, 12–15. [Google Scholar]
- Zhong, X.; Liu, S.; Li, W.; Tan, Z.; Yang, Y. Study on antioxidant function of tea flavonoid through the elimination of hydroxyl radical. J. Tea Commun. 2009, 36, 16–18. [Google Scholar]
- Zhen, R.R.; Qu, Y.J.; Zhang, L.M.; Gu, C.; Ding, M.; Chen, L.; Peng, X.; Hu, B.; An, H. Exploring the potential anti-Alzheimer disease mechanisms of Alpiniae oxyphyliae Fructus by network pharmacology study and molecular docking. Metab. Brain. Dis. 2023, 38, 933–944. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.W.; Ko, N.Y.; Mun, S.H.; Kim, D.K.; Kim, J.D.; Kim, H.S.; Lee, K.R.; Kim, Y.K.; Radinger, M.; et al. Camellia japonica suppresses immunoglobulin E-mediated allergic response by the inhibition of Syk kinase activation in mast cells. Clin. Exp. Allergy. 2008, 38, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, Q.; Wei, X.; Qin, X. Pancreatic lipase and cholesterol esterase inhibitory effect of Camellia nitidissima Chi flower extracts in vitro and in vivo. Food Biosci. 2020, 37, 100682. [Google Scholar] [CrossRef]
- Chen, Y.; Gong, S.; Xu, X.; Xu, J.; Zhang, T.; Hou, X.; Deng, J. Research progress on chemical constituents and pharmacological studies of Camellia nitidissima. Drug Eval. Res. 2022, 45, 575–582. [Google Scholar]
- Wu, J.; Bai, Q.; Chen, J.; Yang, Z.; Zhu, X. Systemic analyses of anti-cell-senescence active compounds in Camellia Sect. Chrysantha Chang and their mechanisms. Plants 2024, 13, 2139. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.R.; White, H.M.; McCormack, J.D.; Niemeyer, E.D. Catechin composition, phenolic content, and antioxidant properties of commercially-available bagged, gunpowder, and matcha green teas. Plant Foods Hum. Nutr. 2023, 78, 662–669. [Google Scholar] [CrossRef]
- Li, M.; Xu, J.; Shi, T.; Yu, H.; Bi, J.; Chen, G. Epigallocatechin-3-gallate augments therapeutic effects of mesenchymal stem cells in skin wound healing. Clin. Exp. Pharmacol. Physiol. 2016, 43, 1115–1124. [Google Scholar] [CrossRef]
- Ran, Z.; Li, Z.; Xiao, X.; Yan, C.; An, M.; Chen, J.; Tang, M. Extensive targeted metabolomics analysis reveals the identification of major metabolites, antioxidants, and disease-resistant active pharmaceutical components in Camellia tuberculata (Camellia L.) seeds. Sci. Rep. 2024, 14, 8709. [Google Scholar] [CrossRef]
- Qiu, Y.; He, D.; Yang, J.; Ma, L.; Zhu, K.; Cao, Y. Kaempferol separated from Camellia oleifera meal by high-speed countercurrent chromatography for antibacterial application. Eur. Food Res. Technol. 2020, 246, 2383–2397. [Google Scholar] [CrossRef]
- Lee, H.H.; Cho, J.Y.; Moon, J.H.; Park, K.H. Isolation and identification of antioxidative phenolic acids and flavonoid glycosides from Camellia japonica flowers. Hortic. Environ. Biotechnol. 2011, 52, 270–277. [Google Scholar] [CrossRef]
- Itokawa, H.; Sawada, N.; Murakami, T. Structures of camelliagenins A, B, and C obtained from Camellia japonica L. Tetrahedron Lett. 1969, 17, 474–480. [Google Scholar]
- Yoshikawa, M.; Harada, E.; Murakami, T.; Matsuda, H.; Yamahara, J.; Murakami, N. Camelliasaponins B1, B2, C1 and C2, new type inhibitors of ethanol absorption in rats from the seeds of Camellia japonica L. Chem. Pharm. Bull. 1994, 42, 742–744. [Google Scholar] [CrossRef]
- Onodera, K.; Hanashiro, K.; Yasumoto, T. Camellianoside, a novel antioxidant glycoside from the leaves of Camellia japonica. Biosci. Biotechnol. Biochem. 2006, 70, 1995–1998. [Google Scholar] [CrossRef]
- Tsujita, T.; Matsuo, Y.; Saito, Y.; Tanaka, T. Enzymatic oxidation of ellagitannin and a new ellagitannin metabolite from Camellia japonica leaves. Tetrahedron 2017, 73, 500–507. [Google Scholar] [CrossRef]
- Lan, H.T.T.; Hoan, L.T.; Anh, B.T.M.; Mai, N.T.; Cuong, N.T.; Thoa, H.T.; Thao, V.M.; Dang, N.H.; Park, S.J.; Nhiem, N.X. Flavonol glycosides from theleaves of Camellia hirsuta and their α-Glucosidase, α-Amylase, and advanced glycation end-products inhibitory effects. Rev. Bras. Farmacogn. 2025, 35, 170–176. [Google Scholar] [CrossRef]
- Yu, X.; Xiao, J.; Chen, S.; Yu, Y.; Ma, J.; Lin, Y.; Li, R.; Lin, J.; Fu, Z.; Zhou, Q.; et al. Metabolite signatures of diverse Camellia sinensis tea populations. Nat. Commun. 2020, 11, 5586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Su, M.; Du, J. Zhou, H.; Li, X.; Ye, Z. Comparative UPLC-Q-TOF/MS-based metabolomics approach for distinguishing peach (Prunus persica (L.) Batsch) fruit cultivars with varying antioxidant activity. Food Res. Int. 2020, 137, 109531. [Google Scholar] [CrossRef]
- Jiang, L.; Yue, M.; Liu, Y.; Zhang, N.; Lin, Y.; Zhang, Y.; Wang, Y.; Li, M.; Luo, Y.; Zhang, Y.; et al. A novel R2R3-MYB transcription factor FaMYB5 positively regulates anthocyanin and proanthocyanidin biosynthesis in cultivated strawberries (Fragaria × ananassa). Plant Biotechnol. J. 2023, 21, 1140–1158. [Google Scholar] [CrossRef] [PubMed]

| Metabolites | Formula | PubChem ID | Sub Class | Degree | Accumulation Level Across the Three Cultivars 1 | ||
|---|---|---|---|---|---|---|---|
| Average (XZ) | Average (LX) | Average (SC) | |||||
| Izalpinin | C16H12O5 | 5318691 | Flavones | 28 | 16.09 | 7.23 | 7.23 |
| Isokaempferide | C21H22O10 | 42607834 | Flavonoid glycosides | 26 | 14.87 | 21.85 | 12.35 |
| pelargonidin-3-O-beta-D-glucoside | C21H20O10 | 443649 | Flavonoid glycosides | 18 | 9.51 | 15.92 | 10.44 |
| Catechin 5,3′-di-O-gallate | C29H22O14 | 15689619 | Flavans | 17 | 18.04 | 16.12 | 11.66 |
| 2′,4′,6′,beta-Tetrahydroxychalcone 2′-glucoside | C21H22O10 | 42607637 | Flavonoid glycosides | 16 | 15.57 | 10.55 | 7.23 |
| 7-Galloylcatechin | C22H18O10 | 74490567 | Flavans | 16 | 18.47 | 12.94 | 15.40 |
| Pinocembroside | C21H22O9 | 46881227 | Flavonoid glycosides | 14 | 17.49 | 11.54 | 8.17 |
| ent-Epicatechin(4alpha->8)catechin | C30H26O12 | 130556 | Biflavonoids and polyflavonoids | 14 | 16.16 | 7.23 | 9.02 |
| Apigenin 7-(6″-ethylglucuronide) | C23H22O11 | 14309759 | Flavonoid glycosides | 13 | 18.07 | 8.66 | 7.23 |
| Luteolin 3′-methyl ether 7-malonylglucoside | C25H24O14 | 131752190 | Flavonoid glycosides | 12 | 7.48 | 14.92 | 7.23 |
| 8-Glucopyranosylprocyanidin B2 | C36H36O17 | 21637579 | Biflavonoids and polyflavonoids | 12 | 17.65 | 20.26 | 12.70 |
| Isosakuranetin 7-alpha-L-arabinofuranosyl-(1->6)-glucoside | C27H32O14 | 42607954 | Flavonoid glycosides | 12 | 15.81 | 19.16 | 8.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, J.; Wu, Y.; Wu, Y.; Guo, W.; You, C. Non-Targeted Metabolomics and Network Pharmacology Reveal Bioactive Metabolites and the Medicinal Potential of Three Ornamental Camellia Flowers. Plants 2025, 14, 2967. https://doi.org/10.3390/plants14192967
Zhang Y, Zhang J, Wu Y, Wu Y, Guo W, You C. Non-Targeted Metabolomics and Network Pharmacology Reveal Bioactive Metabolites and the Medicinal Potential of Three Ornamental Camellia Flowers. Plants. 2025; 14(19):2967. https://doi.org/10.3390/plants14192967
Chicago/Turabian StyleZhang, Yali, Jianhua Zhang, Yani Wu, Yin Wu, Wenjiao Guo, and Chunshan You. 2025. "Non-Targeted Metabolomics and Network Pharmacology Reveal Bioactive Metabolites and the Medicinal Potential of Three Ornamental Camellia Flowers" Plants 14, no. 19: 2967. https://doi.org/10.3390/plants14192967
APA StyleZhang, Y., Zhang, J., Wu, Y., Wu, Y., Guo, W., & You, C. (2025). Non-Targeted Metabolomics and Network Pharmacology Reveal Bioactive Metabolites and the Medicinal Potential of Three Ornamental Camellia Flowers. Plants, 14(19), 2967. https://doi.org/10.3390/plants14192967









