StSUT2 Regulates Cell Wall Architecture and Biotic Stress Responses in Potatoes (Solanum tuberosum)
Abstract
1. Introduction
2. Results
2.1. StSUT2 Silencing Affected Cell Wall Structure
2.2. StSUT2 Silencing Affected the Content of Cell Wall Composition
2.3. StSUT2 Silencing Affected the Activities of Cell Wall Degrading Enzymes
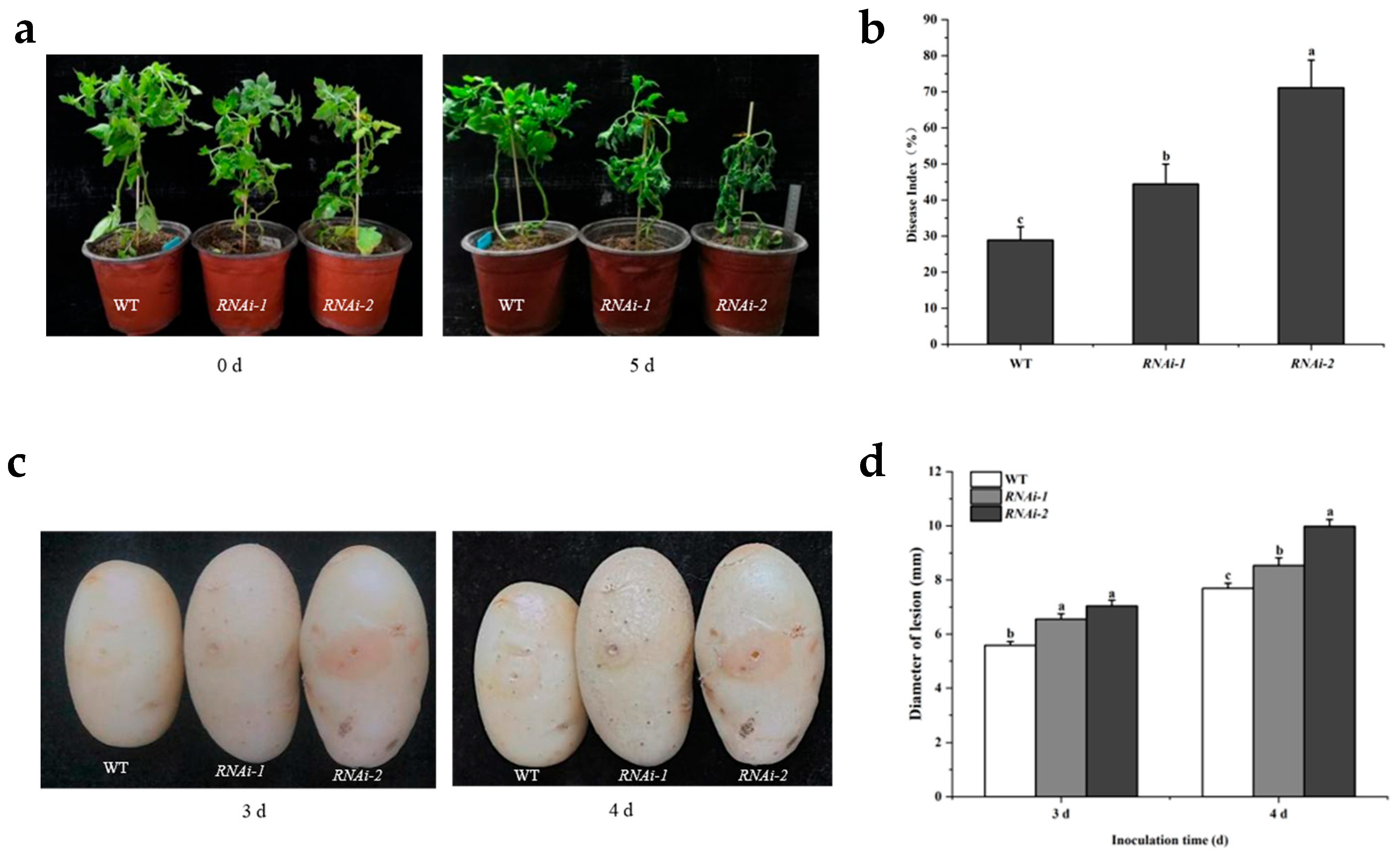
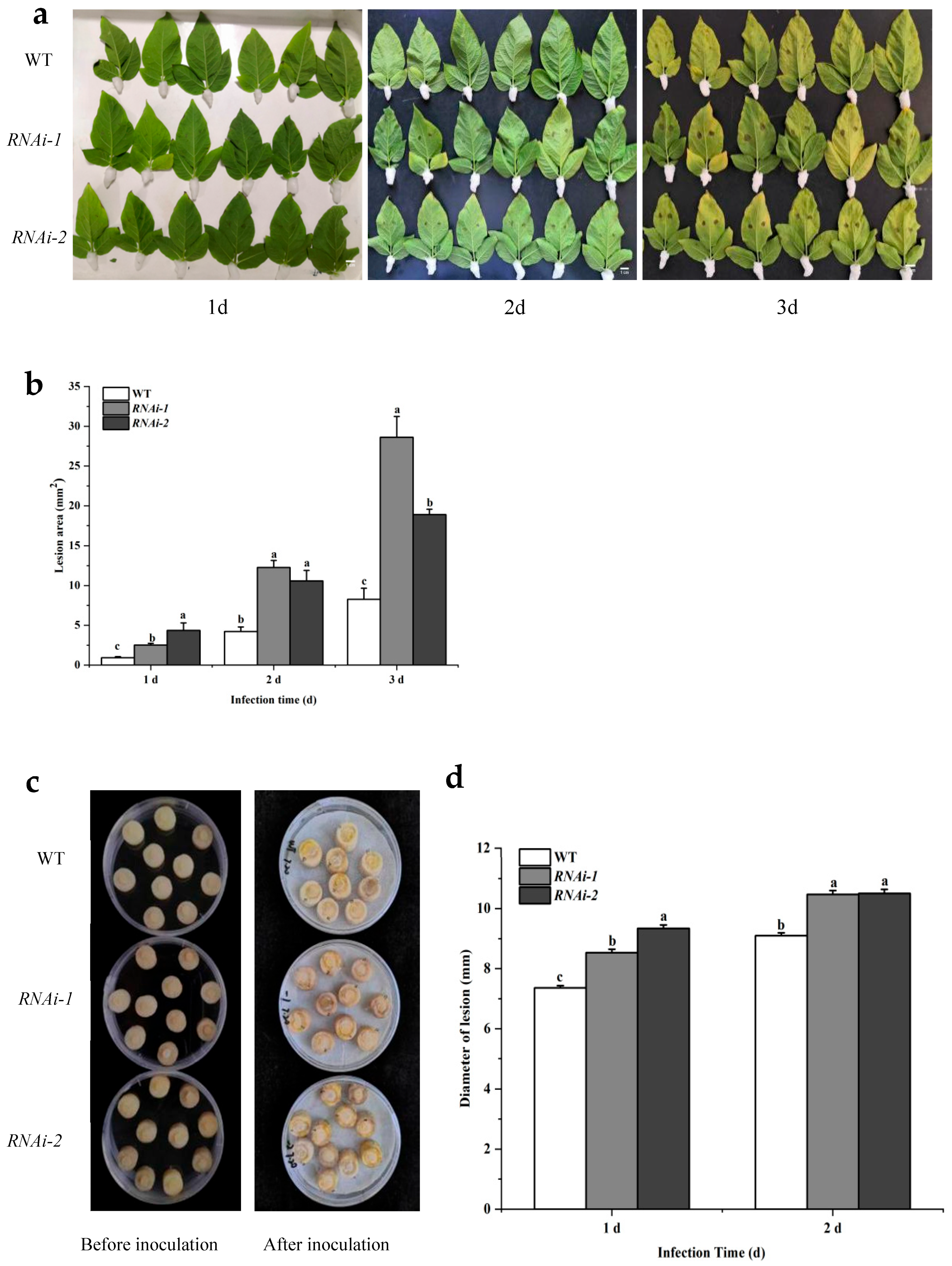
2.4. Silencing of StSUT2 Increases Susceptibility to Dry Rot and Bacterial Wilt Pathogens in Potato
3. Discussion
3.1. StSUT2 Silencing Induces Changes in Cell Wall Structure and Composition
3.2. StSUT2 Silencing Impairs Resistance to Bacterial and Fungal Pathogens
3.3. Comparative Roles of SUT2 in Potatoes and Other Crops: Implications for Crop Improvement
4. Materials and Methods
4.1. Plant Materials and Pathogenic Bacteria
4.1.1. Plant Materials
4.1.2. Pathogen Materials
4.2. Determination of Cell Wall Structure
4.3. Pectin Content Determination
4.4. Analysis of Cellulose Content
4.5. Hemicellulose Content Determination
4.6. Determination of Lignin Content
4.7. Determination of Pectinase (PME) Activity
4.8. Cellulase (CEL) Activity Assay
4.9. Determination of Xyloglucan Glycosyltransferase/Hydrolase (XTH) Activity in Plant Xyglucan
4.10. Determination of Polygalacturonidase (PG) Activity
4.11. Identification of Pathogen Resistance
4.12. Data Processing
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Riesmeier, J.W.; Hirner, B.; Frommer, W.B. Potato sucrose transporter expression in minor veins indicates a role in phloem loading. Plant Cell 1993, 5, 1591–1598. [Google Scholar]
- Kühn, C.; Grof, C.P.L. Sucrose transporters of higher plants. Curr. Opin. Plant Biol. 2010, 13, 288–298. [Google Scholar] [CrossRef]
- Bitterlich, M.; Krügel, U.; Boldt-Burisch, K.; Franken, P.; Kühn, C. Interaction of brassinosteroid functions and sucrose transporter SlSUT2 regulate the formation of arbuscular mycorrhiza. Plant Signal. Behav. 2014, 9, e970426. [Google Scholar] [CrossRef] [PubMed]
- Bitterlich, M.; Krügel, U.; Boldt-Burisch, K.; Franken, P.; Kühn, C. The sucrose transporter SlSUT2 from tomato interacts with brassinosteroid functioning and affects arbuscular mycorrhiza formation. Plant J. 2014, 78, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, F.; Batailler, B.; Thorpe, M.R.; Razan, F.; Le Hir, R.; Vilaine, F.; Bouchereau, A.; Martin-Magniette, M.-L.; Eveillard, S.; Dinant, S. Involvement of SUT1 and SUT2 sugar transporters in the impairment of sugar transport and changes in phloem exudate contents in phytoplasma-infected plants. Int. J. Mol. Sci. 2021, 22, 745. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Xiao, G.; Liu, J.B.; Yi, Z.F.; Bai, B.; Zhou, B.; Wu, J. Functional analysis of sucrose transporter OsSUT2 in rice disease resistance. Mol. Plant Breed. 2022, 22, 7432–7438. [Google Scholar]
- Leach, K.A.; Tran, T.M.; Slewinski, T.L.; Meeley, R.B.; Braun, D.M. Sucrose transporter2 contributes to maize growth, development, and crop yield. J. Integr. Plant Biol. 2017, 59, 390–408. [Google Scholar] [CrossRef]
- Hou, Q.L.; Gao, J.G.; Qin, Z.L.; Sun, H.; Wang, H.; Yuan, S.; Zhang, F.; Yang, W. Genome-Wide Identification and Expression Analysis of Sucrose Transporter Gene Family in Wheat Lines under Heat Stress. Agronomy 2024, 14, 1549. [Google Scholar] [CrossRef]
- Julius, B.T.; Leach, K.A.; Tran, T.M.; Mertz, R.A.; Braun, D.M. Sugar Transporters in Plants: New Insights and Discoveries. Plant Cell Physiol. 2017, 58, 1442–1460. [Google Scholar] [CrossRef]
- Molina, A.; Miedes, E.; Bacete, L.; Rodríguez, T.; Mélida, H.; Denancé, N.; Sánchez-Vallet, A.; Rivière, M.P.; López, G.; Freydier, A.; et al. Arabidopsis cell wall composition determines disease resistance specificity and fitness. Proc. Natl. Acad. Sci. USA 2021, 118, e2010243118. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, X.; Li, S.; Wang, Q.; Liu, Y.; Zhang, Z.; Yang, C.; Xu, S.; Mao, K.; Ma, F.; et al. MdMYB54 reduces disease severity caused by Fusarium solani in apple by modulating cell wall cellulose and pectate lyase-dependent defense. Plant J. 2025, 121, e17206. [Google Scholar] [CrossRef]
- Ma, Q.; Zhu, H.; Qiao, M. Contribution of both lignin content and sinapyl monomer to disease resistance in tobacco. Plant Pathol. 2018, 67, 642–651. [Google Scholar] [CrossRef]
- Xie, W.; Ke, Y.; Cao, J.; Wang, S.; Yuan, M. Knock out of transcription factor WRKY53 thickens sclerenchyma cell walls, confers bacterial blight resistance. Plant Physiol. 2021, 187, 1746–1761. [Google Scholar] [CrossRef]
- Gong, H.L.; Tian, Z.; Xu, J.; Tang, X.X. Progress of sucrose transporters in Solanaceae plants. Plant Physiol. J. 2017, 53, 1819–1823. [Google Scholar]
- Gong, H.L.; Liu, J.B.; Igiraneza, C.; Dusengemungu, L. Sucrose transporter StSUT2 affects potato plants growth, flowering time, and tuber yield. Curr. Issues Mol. Biol. 2023, 45, 2629–2643. [Google Scholar] [CrossRef] [PubMed]
- Kühn, C.; Hajirezaei, M.R.; Fernie, A.R.; Roessner-Tunali, U.; Czechowski, T.; Hirner, B.; Frommer, W.B. The sucrose transporter StSUT1 localizes to sieve elements in potato tuber phloem and influences tuber physiology and development. Plant Physiol. 2003, 131, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Barker, L.; Kühn, C.; Weise, A.; Schulz, A.; Gebhardt, C.; Hirner, B.; Hellmann, H.; Schulze, W.; Ward, J.M.; Frommer, W.B. SUT2, a putative sucrose sensor in sieve elements. Plant Cell 2000, 12, 1153–1164. [Google Scholar] [CrossRef][Green Version]
- Chincinska, I.A.; Liesche, J.; Krügel, U.; Michalska, J.; Geigenberger, P.; Grimm, B.; Kühn, C. Sucrose transporter StSUT4 from potato affects flowering, tuberization, and shade avoidance response. Plant Physiol. 2008, 146, 515–528. [Google Scholar] [CrossRef]
- Chincinska, I.A.; Gier, K.; Krügel, U.; Liesche, J.; He, H.; Grimm, B.; Harren, F.J.M.; Cristescu, S.M.; Kühn, C. Photoperiodic regulation of the sucrose transporter StSUT4 affects the expression of circadian-regulated genes and ethylene production. Front. Plant Sci. 2013, 4, 26. [Google Scholar] [CrossRef]
- Hackel, A.; Schauer, N.; Carrari, F.; Fernie, A.R.; Grimm, B.; Kühn, C. Sucrose transporter LeSUT1 and LeSUT2 inhibition affects tomato fruit development in different ways. Plant J. 2006, 45, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Li, L.; Cao, R.X.; Xu, S.; Cheng, L.L.; Yu, M.Y.; Lv, Z.F.; Lu, G.Q. Changes in cell wall components and polysaccharide-degrading enzymes in relation to differences in texture during sweetpotato storage root growth. J. Plant Physiol. 2020, 254, 153282. [Google Scholar] [CrossRef]
- Cheng, S.-S.; Ku, Y.-S.; Cheung, M.-Y.; Lam, H.-M. AtGAP1 promotes the resistance to Pseudomonas syringae pv. tomato DC3000 by regulating cell-wall thickness and stomatal aperture in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 7540. [Google Scholar]
- Zhao, Q.; Dixon, R.A. Altering the cell wall and its impact on plant disease: From forage to bioenergy. Annu. Rev. Phytopathol. 2014, 52, 69–91. [Google Scholar] [CrossRef]
- Chaudhary, A.; Hsiao, Y.-C.; Yeh, F.-L.J.; Zupunski, M.; Zhang, H.; Aizezi, Y.; Malkovskiy, A.; Grossmann, G.; Wu, H.-M.; Cheung, A.Y.; et al. FERONIA signaling maintains cell wall integrity during brassinosteroid-induced cell expansion in Arabidopsis. Mol. Plant 2025, 18, 603–618. [Google Scholar] [CrossRef]
- Percio, F.; Rubio, L.; Amorim-Silva, V.; Botella, M.A. Crucial roles of brassinosteroids in cell wall composition and structure across species: New insights and biotechnological applications. Plant Cell Environ. 2025, 48, 1751–1767. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, H.; Zeng, F. The sugar transporter proteins in plants: An elaborate and widespread regulation network—A review. Int. J. Biol. Macromol. 2025, 294, 139252. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Blanco, C.; Feng, D.X.; Hu, J.; Sánchez-Vallet, A.; Deslandes, L.; Llorente, F.; Berrocal-Lobo, M.; Keller, H.; Barlet, X.; Sánchez-Rodríguez, C.; et al. Impairment of cellulose synthases required for Arabidopsis secondary cell wall formation enhances disease resistance. Plant Cell 2007, 19, 890–903. [Google Scholar] [CrossRef] [PubMed]
- Eom, J.S.; Cho, J.I.; Reinders, A.; Lee, S.W.; Yoo, Y.; Tuan, P.Q.; Choi, S.B.; Bang, G.; Park, Y.I.; Cho, M.H.; et al. Impaired Function of the Tonoplast-Localized Sucrose Transporter in Rice, OsSUT2, Limits the Transport of Vacuolar Reserve Sucrose and Affects Plant Growth. Plant Physiol. 2011, 157, 109–119. [Google Scholar] [CrossRef]
- Siao, W.; Chen, J.Y.; Hsiao, H.H.; Chung, P.; Wang, S.J. Characterization of OsSUT2 Expression and Regulation in Germinating Embryos of Rice Seeds. Rice 2011, 4, 39–49. [Google Scholar] [CrossRef]
- Lahlali, R.; Kumar, S.; Wang, L.; Forseille, L.; Sylvain, N.; Korbas, M.; Muir, D.; Swerhone, G.; Lawrence, J.R.; Fobert, P.R.; et al. Cell Wall Biomolecular Composition Plays a Potential Role in the Host Type II Resistance to Fusarium Head Blight in Wheat. Front. Microbiol. 2016, 7, 910. [Google Scholar] [CrossRef] [PubMed]
- Tao, A.L.; Zhang, G.S. Study on the determination of pectin content in tofu firewood leaves in the mountainous area of Southern Anhui by carbazole sulfuric acid colorimetric method. J. Chifeng Univ. (Nat. Sci. Ed.) 2012, 28, 108–110. [Google Scholar]
- Zhao, Y.J. Effects of Yeast and Chitosan Treatment on the Peel Structure of Citrus Inoculated with Anthrax Bacteria. Master’s Thesis, Southwest University, Chongqing, China, 2017. [Google Scholar]
- Sun, Q. Research on the Mechanism of microRNA528 Regulating Maize Lodging under High Nitrogen Conditions. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2018. [Google Scholar]
- Liu, X.J. Study on the Changes of Leaf Structure and Cell Wall of Creeping Brachium Against Brown Spot Disease Induced by 2,3-BD. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2018. [Google Scholar]
- Ma, J. Poplar Pectin Methylase (PME) and Pectin Methylase Inhibitor (PMEI) Gene Families and Their Responses to Salt Stress. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2016. [Google Scholar]
- Yuan, L. Effects of Postharvest Heat Treatment on Induction of Disease Resistance and Storage Quality of Thick-Skinned Muskmelon. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2011. [Google Scholar]
- Carvajal, F.; Palma, F.; Jamilena, M.J.; Garrido, D. Cell wall metabolism and chilling injury during postharvest cold storage in zucchini fruit. Postharvest Biol. Technol. 2015, 108, 68–77. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, S.; Joyce, D.C.; Jiang, Y.; Qu, H.; Duan, X. Physiology and quality response of harvested banana fruit to cold shock. Postharvest Biol. Technol. 2010, 55, 154–159. [Google Scholar] [CrossRef]
- Yuan, L. Induction of Disease Resistance and Effects of Postharvest Heat Treatment on Fruit Storage Quality of Thick-Skinned Melons. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2011. [Google Scholar]
- Cellier, G.; Prior, P. Deciphering phenotypic diversity of Ralstonia solanacearum strains pathogenic to potato. Phytopathology 2010, 100, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.W.; Wang, Y.L.; Bi, Y.; Yun, J. Screening, identification and biological control effect of antagonistic bacteria for dry rot of potato plants. Chin. J. Microbiol. 2018, 45, 1726–1736. [Google Scholar]
- Zhang, W.N. Screening and Functional Verification of Receptor-Like Kinase Genes Responding to Stress Signals in Potatoes. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2020. [Google Scholar]
- Yang, Z.M.; Bi, Y.; Li, Y.C.; Kou, Z.H.; Bao, G.H.; Liu, C.K.; Wang, Y.; Wang, D. Changes of cell wall degrading enzymes in section tissues during the infection process of potato dry rot pathogen. Sci. Agric. Sin. 2012, 45, 127–134. [Google Scholar]

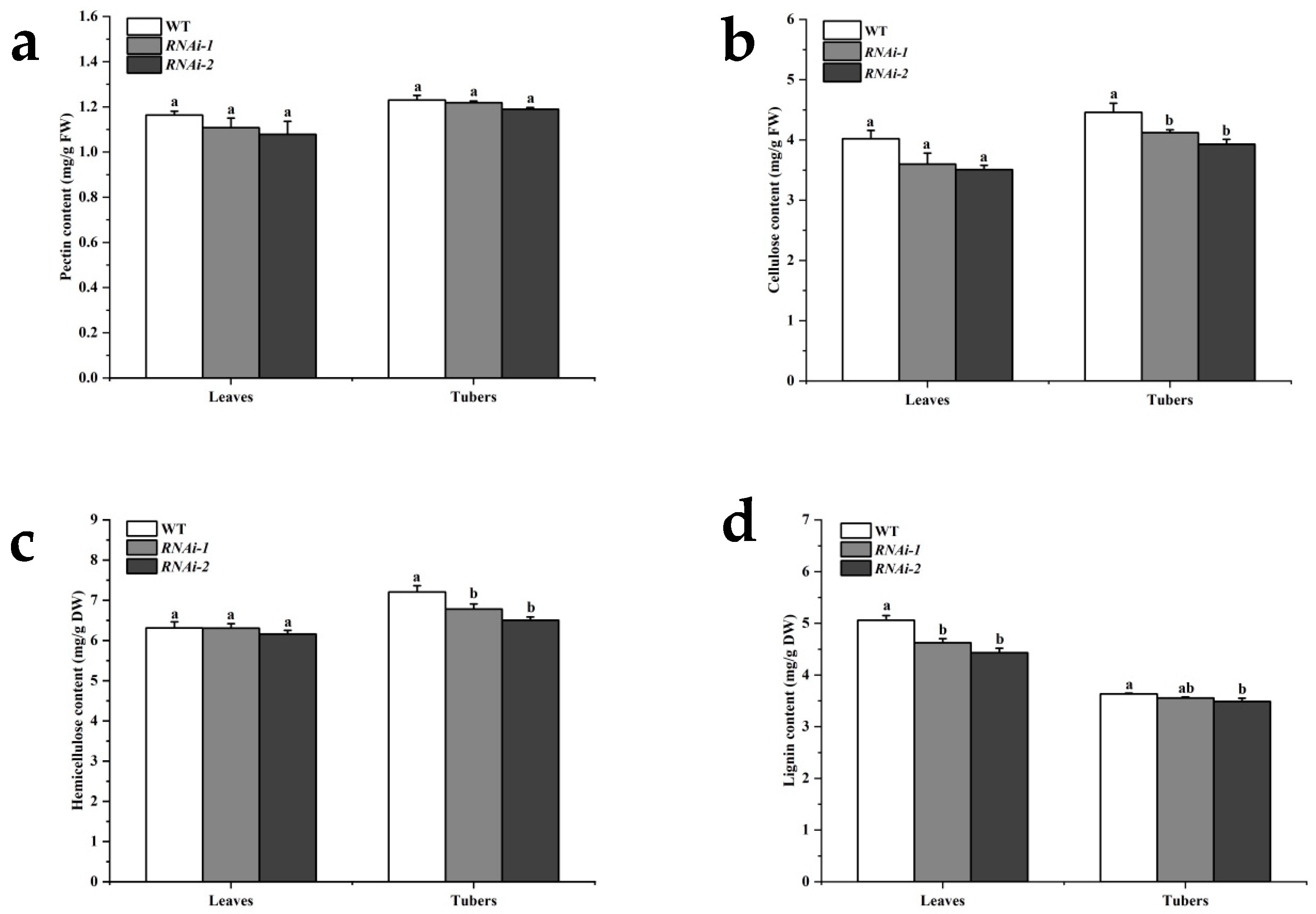
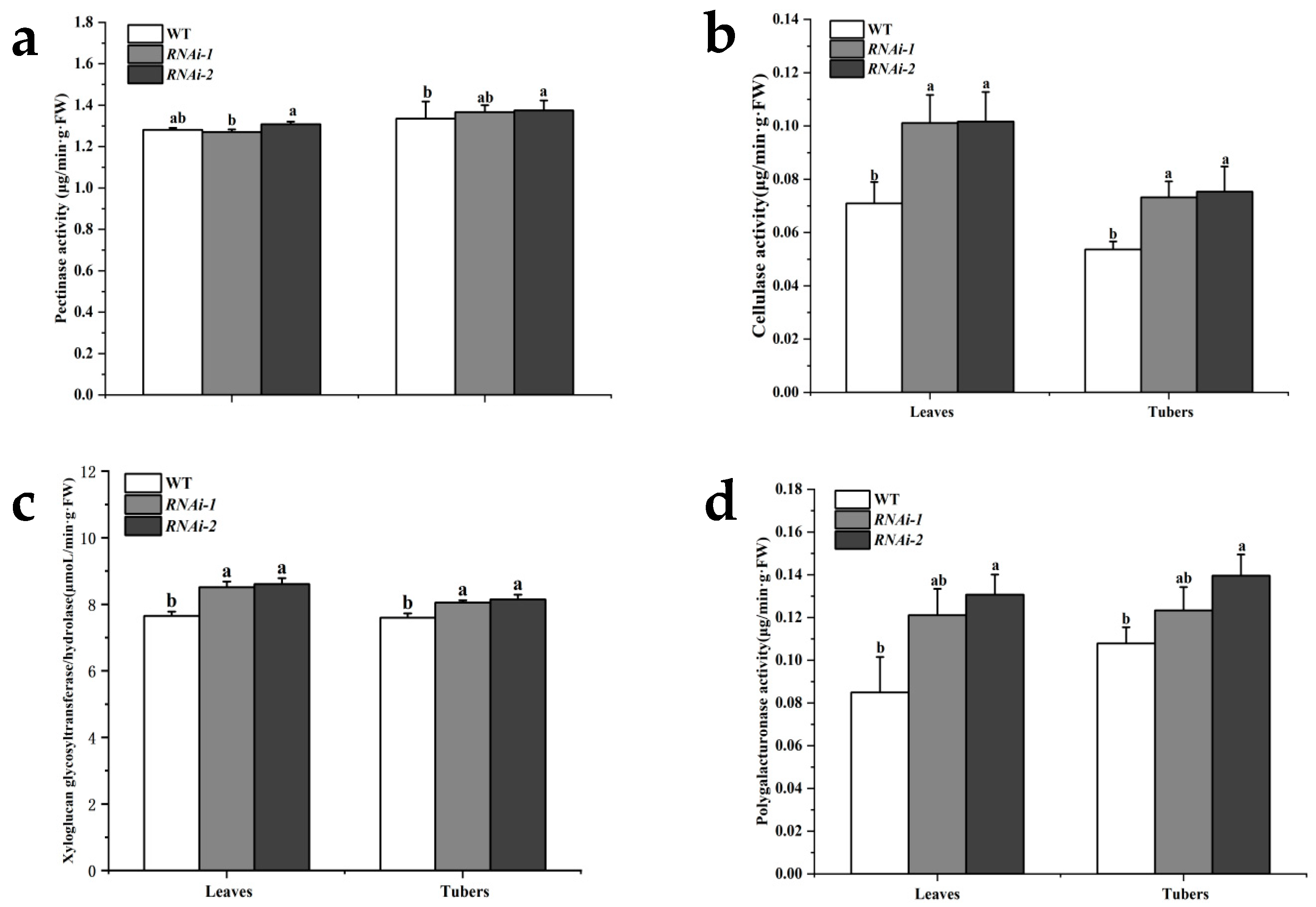
| Line | Organ | Pectin Content (mg/g FW) | Cellulose Content (mg/g FW) | Hemicellulose Content (mg/g DW) | Lignin Content (mg/g DW) |
|---|---|---|---|---|---|
| WT | Leaves | 1.16 | 4.02 | 6.31 | 5.06 |
| Tubers | 1.23 | 4.46 | 7.21 | 3.63 | |
| RNAi-1 | Leaves | 1.11 | 3.60 | 6.31 | 4.63 |
| Tubers | 1.22 | 4.12 | 6.78 | 3.56 | |
| RNAi-2 | Leaves | 1.08 | 3.51 | 6.16 | 4.43 |
| Tubers | 1.19 | 3.93 | 6.51 | 3.49 |
| Line | Organ | Pectinase Activity (μg/min·g·FW) | Cellulase Activity (μg/min·g·FW) | Xyloglucan Glycosyltransfer-ase/Hydrolase Activity (µmoL/min·g·FW) | Polygalacturonase Activity (μg/min·g·FW) |
|---|---|---|---|---|---|
| WT | Leaves | 1.28 | 0.07 | 7.65 | 0.08 |
| Tubers | 1.34 | 0.05 | 7.60 | 0.11 | |
| RNAi-1 | Leaves | 1.27 | 0.10 | 8.52 | 0.12 |
| Tubers | 1.37 | 0.07 | 8.05 | 0.12 | |
| RNAi-2 | Leaves | 1.31 | 0.10 | 8.61 | 0.13 |
| Tubers | 1.37 | 0.08 | 8.14 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, H.; Li, H.; Wang, C.; Kui, Q.; Dusengemungu, L.; Cai, X.; Feng, Z. StSUT2 Regulates Cell Wall Architecture and Biotic Stress Responses in Potatoes (Solanum tuberosum). Plants 2025, 14, 2941. https://doi.org/10.3390/plants14182941
Gong H, Li H, Wang C, Kui Q, Dusengemungu L, Cai X, Feng Z. StSUT2 Regulates Cell Wall Architecture and Biotic Stress Responses in Potatoes (Solanum tuberosum). Plants. 2025; 14(18):2941. https://doi.org/10.3390/plants14182941
Chicago/Turabian StyleGong, Huiling, Hongmei Li, Chenxia Wang, Qian Kui, Leonce Dusengemungu, Xia Cai, and Zaiping Feng. 2025. "StSUT2 Regulates Cell Wall Architecture and Biotic Stress Responses in Potatoes (Solanum tuberosum)" Plants 14, no. 18: 2941. https://doi.org/10.3390/plants14182941
APA StyleGong, H., Li, H., Wang, C., Kui, Q., Dusengemungu, L., Cai, X., & Feng, Z. (2025). StSUT2 Regulates Cell Wall Architecture and Biotic Stress Responses in Potatoes (Solanum tuberosum). Plants, 14(18), 2941. https://doi.org/10.3390/plants14182941






