Fruit Bag Removal Timing Influences Fruit Coloration, Quality, and Physiological Disorders in ‘Arisoo’ Apples
Abstract
1. Introduction
2. Results
2.1. Incidence of Fruit Cracking, Sunburn, and Pathogen Infection
2.2. Fruit Surface Temperature, Fruit Weight, and Size
2.3. Fruit Quality and Color
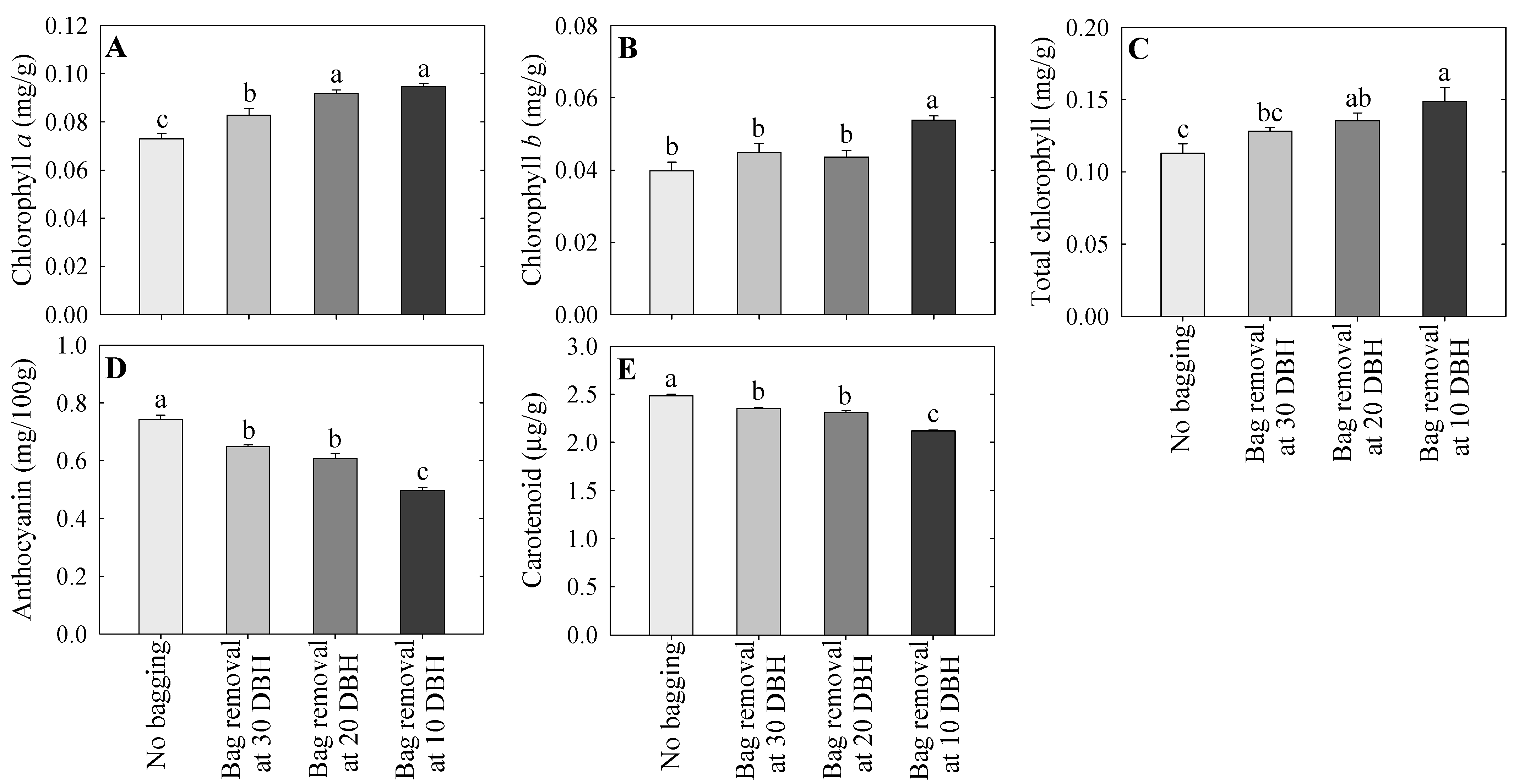
2.4. Chlorophyll, Anthocyanin, and Carotenoid Contents
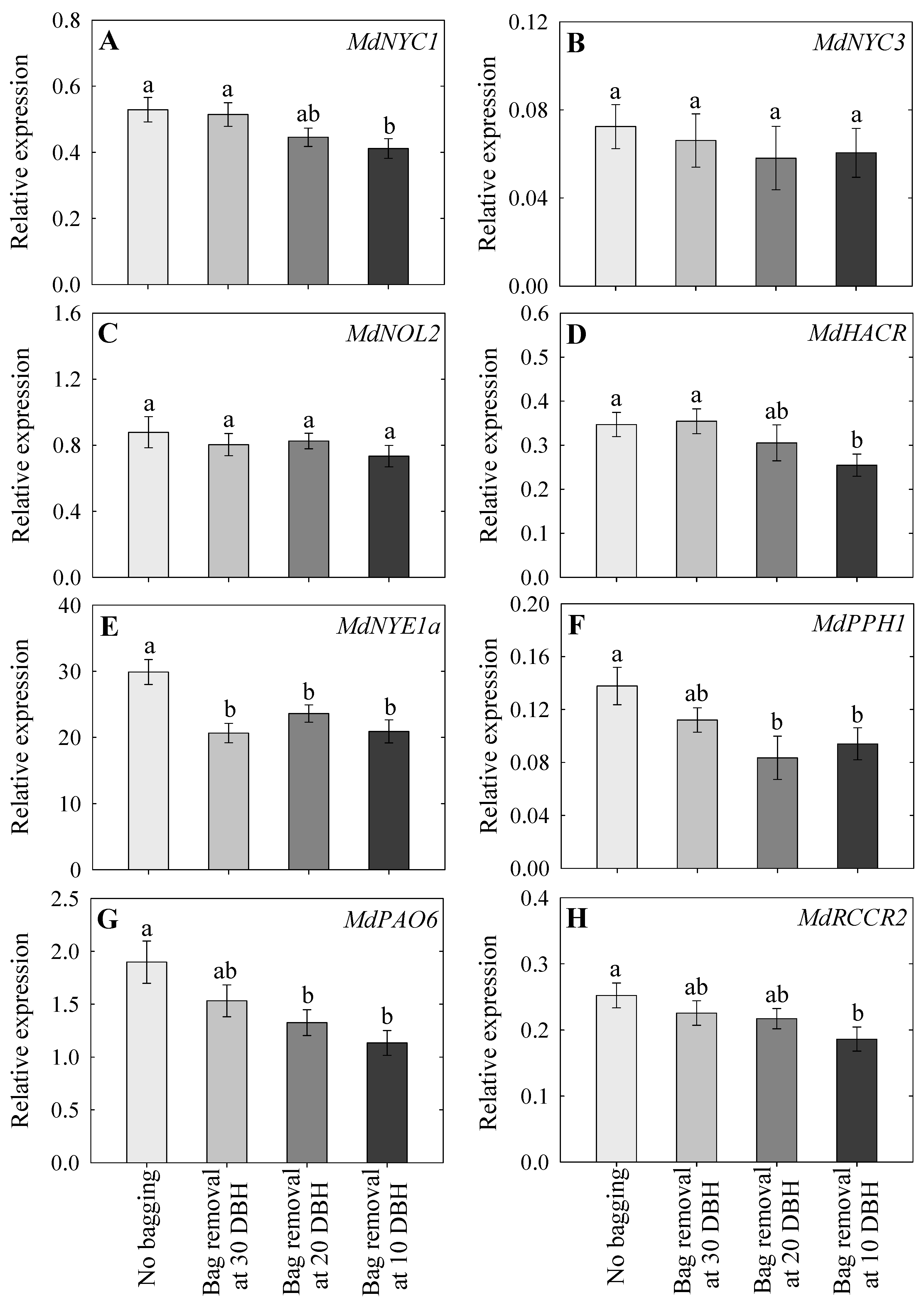
2.5. Expression Analysis of Chlorophyll Degradation-Associated Genes
2.6. Expression Analysis of Anthocyanin-Associated Genes
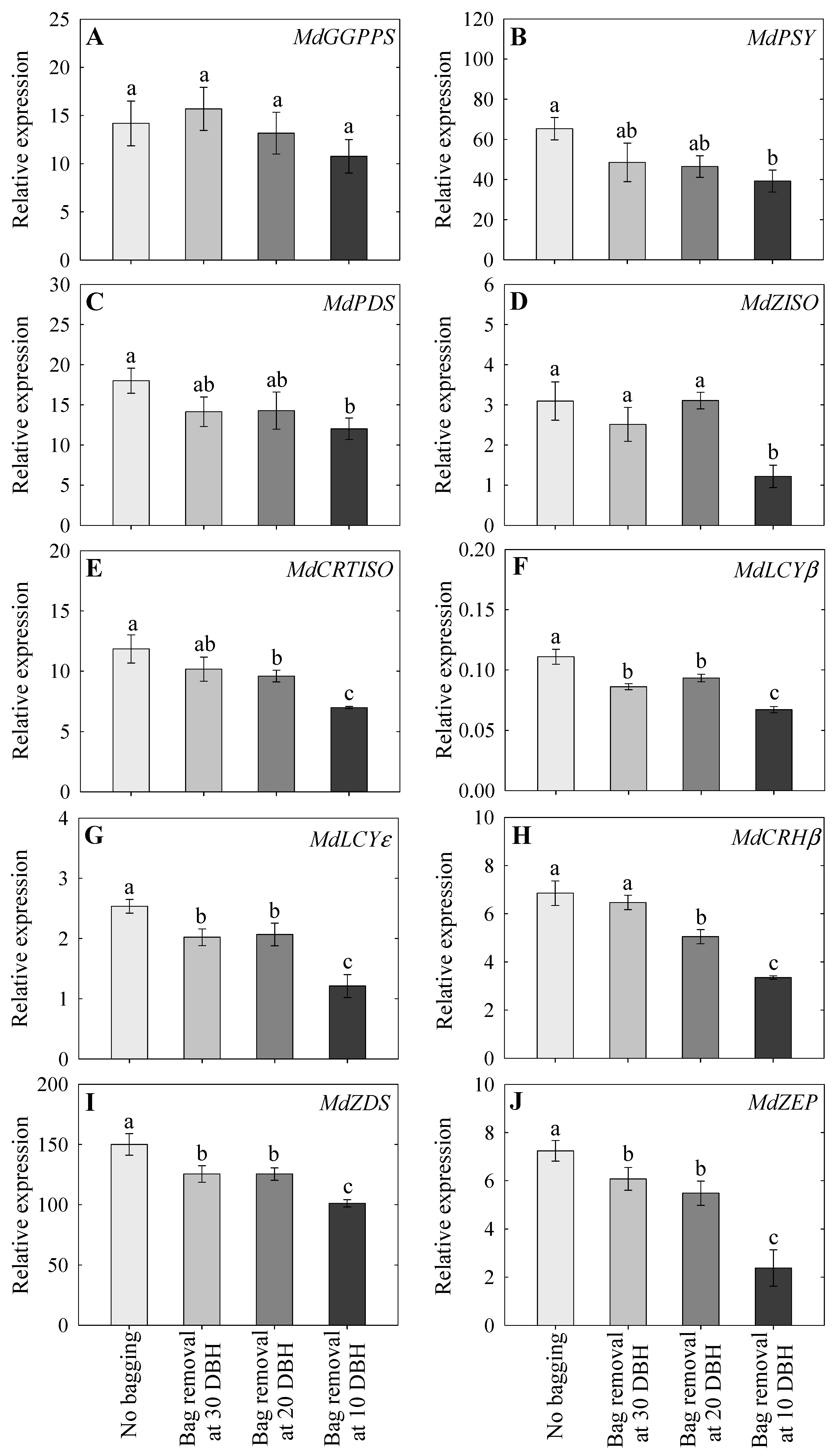
2.7. Expression Analysis of Carotenoid-Associated Genes
2.8. PCA Score and Loading Plots
3. Discussion
4. Materials and Methods
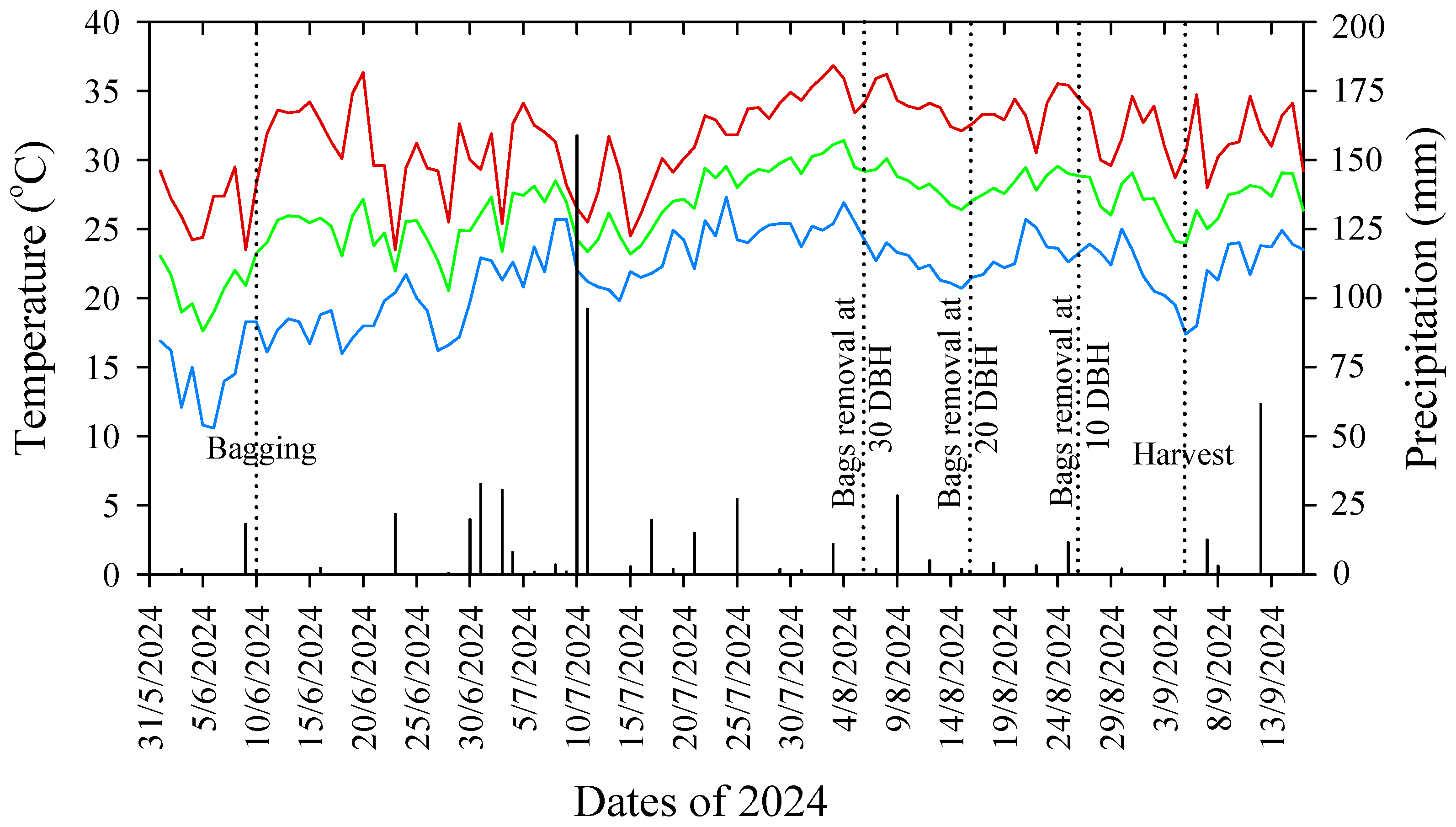
4.1. Experimental Orchard and Tree Selection
4.2. Fruit Bagging Treatments
4.3. Determination of Fruit Cracking, Sunburn, and Infection Incidence
4.4. Determination of Fruit Surface Temperature and Fruit Quality Characteristics
4.5. Determination of Chlorophyll, Anthocyanin, and Carotenoid Contents
4.6. RNA Extraction and qRT-PCR Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Djekic, I.; Radivojevic, D.; Milivojevic, J. Quality perception throughout the apple fruit chain. J. Food Meas. Charact. 2019, 13, 3106–3118. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Kwon, S.I.; Kim, J.H.; Park, M.Y.; Park, J.T.; Lee, J. ‘Arisoo’, a midseason apple. HortScience 2021, 56, 1139–1141. [Google Scholar] [CrossRef]
- Dalhaus, T.; Schlenker, W.; Blanke, M.M.; Bravin, E.; Finger, R. The effects of extreme weather on apple quality. Sci. Rep. 2020, 10, 7919. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Ogawa, H.; Fukuda, N.; Moriguchi, T. Changes in the taste and textural attributes of apples in response to climate change. Sci. Rep. 2013, 3, 2418. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.R.; Arakawa, O.; Nissato, K.; Ito, D. Changes in the harvesting window and quality of apple fruit cultivated under long-term high temperature and CO2. Sci. Hortic. 2024, 338, 113611. [Google Scholar] [CrossRef]
- Park, Y.S.; Kim, M.; Yun, S.K.; Kim, S.S.; Joa, J. A simple model for predicting sunburn on satsuma mandarin fruit. Sci. Hortic. 2022, 27, 110658. [Google Scholar] [CrossRef]
- Lee, J.C.; Park, Y.S.; Jeong, H.N.; Kim, J.H.; Heo, J.Y. Temperature changes affected spring phenology and fruit quality of apples grown in high-latitude region of South Korea. Horticulturae 2023, 9, 794. [Google Scholar] [CrossRef]
- Racsko, J.; Schrader, L.E. Sunburn of apple fruit: Historical background, recent advances and future perspectives. Crit. Rev. Plant Sci. 2012, 31, 455–504. [Google Scholar] [CrossRef]
- Morales-Quintana, L.; Waite, J.M.; Kalcsits, L.; Torres, C.A.; Ramos, P. Sun injury on apple fruit: Physiological, biochemical and molecular advances, and future challenges. Sci. Hortic. 2020, 260, 108866. [Google Scholar] [CrossRef]
- Xue, X.; Duan, Y.; Wang, J.; Ma, F.; Li, P. Nighttime temperatures and sunlight intensities interact to influence anthocyanin biosynthesis and photooxidative sunburn in ‘Fuji’ apple. Front. Plant Sci. 2021, 12, 694954. [Google Scholar] [CrossRef]
- Fisher, G.; Balaguera-López, H.E.; Álvarez-Herrera, J. Causes of fruit cracking in the era of climate change. A review. Agron. Colomb. 2021, 39, 196–207. [Google Scholar] [CrossRef]
- Ali, M.M.; Anwar, R.; Yousef, A.F.; Li, B.; Luvisi, A.; De Bellis, L.; Aprile, A.; Chen, F. Influence of bagging on the development and quality of fruits. Plants 2021, 10, 358. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.L. Evaluation of fruit bagging as a pest management option for direct pests of apple. Insects 2018, 9, 178. [Google Scholar] [CrossRef]
- Xu, G.; Nie, J.; Wu, Y.; Yan, Z.; Ye, M. The effects of fruit bagging on residue behavior and dietary risk for four pesticides in apple. Sci. Rep. 2018, 8, 14348. [Google Scholar] [CrossRef]
- Buthelezi, N.M.D.; Mafeo, T.P.; Mathaba, N. Preharvest bagging as an alternative technique for enhancing fruit quality: A review. HortTechnology 2021, 31, 4–13. [Google Scholar] [CrossRef]
- Feng, F.; Li, M.; Ma, F.; Cheng, L. The effects of bagging and debagging on external fruit quality, metabolites, and the expression of anthocyanin biosynthetic genes in ‘Jonagold’ apple (Malus domestica Borkh.). Sci. Hortic. 2014, 165, 123–131. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, Z.; Ye, Z. Effect of bagging duration on peach fruit peel color and key protein changes based on iTRAQ quantitation. Sci. Hortic. 2019, 246, 217–226. [Google Scholar] [CrossRef]
- Li, Y.; Qi, X.; Cui, W.; Lin, M.; Qiao, C.; Zhong, Y.; Fang, J.; Hu, C. Restraint of bagging on fruit skin coloration in on-tree kiwifruit (Actinidia arguta). J. Plant Growth Regul. 2021, 40, 603–616. [Google Scholar] [CrossRef]
- Sarkomi, F.H.; Moradinezhad, F.; Khayyat, M. Pre-harvest bagging influences sunburn, cracking and quality of pomegranate fruits. J. Hortic. Postharvest Res. 2019, 2, 131–142. [Google Scholar] [CrossRef]
- Guo, S.H.; Xu, T.F.; Shi, T.C.; Jin, X.Q.; Feng, M.X.; Zhao, X.H.; Zhang, Z.W.; Meng, J.F. Cluster bagging promotes melatonin biosynthesis in the berry skins of Vitis vinifera cv. Cabernet Sauvignon and Carignan during development and ripening. Food Chem. 2020, 305, 125502. [Google Scholar] [CrossRef]
- Do, V.G.; Kim, S.; Lee, Y.; Yang, S.; Kim, J.H.; Win, N.M.; Kwon, Y.S.; Park, J.; Park, J.T. Differential coloration, pigment biosynthesis-related gene expression, and accumulation according to developmental stage in the ‘Enbu’ apple. Horticulturae 2023, 9, 1072. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, M.; Bai, L.; Han, X.; Ge, Y.; Wang, W.; Li, J. Effects of 1-methylcyclopropene (1-MCP) on the expression of genes involved in the chlorophyll degradation pathway of apple fruit during storage. Food Chem. 2020, 308, 125707. [Google Scholar] [CrossRef]
- Meng, R.; Qu, D.; Liu, Y.; Gao, Z.; Yang, H.; Shi, X.; Zhao, Z. Anthocyanin accumulation and related gene family expression in the skin of dark-grown red and non-red apples (Malus domestica Borkh.) in response to sunlight. Sci. Hortic. 2015, 189, 66–73. [Google Scholar] [CrossRef]
- Gao, H.N.; Jiang, H.; Cui, J.Y.; You, C.X.; Li, Y.Y. Review: The effects of hormones and environmental factors on anthocyanin biosynthesis in apple. Plant Sci. 2021, 312, 111024. [Google Scholar] [CrossRef] [PubMed]
- Espley, R.V.; Hellens, R.P.; Putterill, J.; Stevenson, D.E.; Kutty-Amma, S.; Allan, A.C. Red colouration in apple fruit is due to the activity of the MYB transcription factor, MYB10. Plant J. 2007, 49, 414–427. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Meng, J.; Li, A.; Duan, W.; Sun, S.; Pan, L.; Zeng, W.; Wang, Z.; Niu, L. Expression analysis of chlorophyll-degradation-related genes in Prunus persica L. peel and the functional verification of key genes. Plants 2025, 14, 312. [Google Scholar] [CrossRef]
- Han, Z.; Hu, Y.; Lv, Y.; Rose, J.K.C.; Sun, Y.; Shen, F.; Wang, Y.; Zhang, X.; Xu, X.; Wu, T.; et al. Natural variation underlines differences in ETHYLENE RESPONSE FACTOR17 activity in fruit peel degreening. Plant Physiol. 2018, 176, 2292–2304. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, X.; Liu, Y.; Shi, X.; Wang, Y.; Zhang, C.; Zhao, Z. The effect of fruit bagging on the color, phenolic compounds and expression of the anthocyanin biosynthesis and regulatory genes on the ‘Granny Smith’ apples. Eur. Food Res. Technol. 2013, 237, 875–885. [Google Scholar] [CrossRef]
- Ma, C.; Liang, B.; Chang, B.; Yan, J.; Liu, L.; Wang, Y.; Yang, Y.; Zhao, Z. Transcriptome profiling of anthocyanin biosynthesis in the peel of ‘Granny Smith’ apples (Malus domestica) after bag removal. BMC Genom. 2019, 20, 353. [Google Scholar] [CrossRef]
- Santos, M.; Egea-Cortines, M.; Gonçalves, B.; Matos, M. Molecular mechanism involved in fruit cracking: A review. Front. Plant Sci. 2023, 14, 1130857. [Google Scholar] [CrossRef]
- La Spada, P.; Dominguez, E.; Continella, A.; Heredia, A.; Gentile, A. Factors influencing fruit cracking: An environmental and agronomic perspective. Front. Plant Sci. 2024, 15, 1343452. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, L.; Zhao, X.; Zhao, Y.; Hao, Z.; Luo, H.; Yuan, Z. Advances in mechanisms and omics pertaining to fruit cracking in horticultural plants. Agronomy 2021, 11, 1045. [Google Scholar] [CrossRef]
- Shi, C.H.; Qi, B.; Wang, X.Q.; Shen, L.Y.; Luo, J.; Zhang, Y.X. Proteomic analysis ofthe key mechanism of exocarp russet pigmentation of semi-russet pear under rainwater condition. Sci. Hortic. 2019, 254, 178–186. [Google Scholar] [CrossRef]
- Kasai, S.; Hayama, H.; Kashimura, Y.; Kudo, S.; Osanai, Y. Relationship between fruit cracking and expression of the expansion gene MdEXPA3 in ‘Fuji’ apples (Malus domestica Borkh.). Sci. Hortic. 2008, 116, 194–198. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Vincent, C. Physiological mechanism underlying fruit sunburn. Crit. Rev. Plant Sci. 2019, 38, 140–157. [Google Scholar] [CrossRef]
- Felicetti, D.A.; Schrader, L.E. Photooxidative sunburn of apples: Characterization of a third type of apple sunburn. Int. J. Fruit Sci. 2008, 8, 160–172. [Google Scholar] [CrossRef]
- Lal, N.; Sahu, N. Management strategies of sunburn in fruit crops: A review. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1126–1138. [Google Scholar] [CrossRef]
- Sharma, R.R.; Pal, R.K.; Sagar, V.R.; Parmanick, K.K.; Paul, V.; Gupta, V.K.; Kumar, K.; Rana, M.R. Impact of pre-harvest fruit-bagging with different coloured bags on peel colour and the incidence of insect pests, disease and storage disorders in ‘Royal Delicious’ apple. J. Hortic. Sci. Biotechnol. 2014, 89, 613–618. [Google Scholar] [CrossRef]
- Griñán, I.; Morales, D.; Galindo, A.; Torrecillas, A.; Pérez-López, D.; Moriana, A.; Collado-González, J.; Carbobell-Barrachina, A.A.; Hernandez, F. Effect of preharvest fruit bagging on fruit quality characteristics and incidence of fruit physiopathies in fully irrigated and water stressed pomegranate trees. J. Sci. Food Agric. 2019, 99, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Rajametov, S.; Nurbekov, A. Effects of different types of paper bags on ‘Golden Delicious’ apple fruits. Acta Hortic. 2020, 1275, 415–418. [Google Scholar] [CrossRef]
- Giap, D.V.; Kim, S.; Lee, Y.; Kweon, H.J. Effect of reflected sunlight on differential expression of anthocyanin synthesis-related genes in young apple fruit. Int. J. Fruit Sci. 2021, 21, 440–455. [Google Scholar] [CrossRef]
- Win, N.M.; Yoo, J.; Do, V.G.; Yang, S.; Kwon, S.I.; Kweon, H.J.; Kim, S.; Lee, Y.; Kang, I.K.; Park, J. Effects of pneumatic defoliation on fruit quality and skin coloration in ‘Fuji’ apples. Agriculture 2024, 14, 1582. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, Y.; Wang, H.; Liu, X.; Zhao, Z. Effects of different pre-harvest bagging times on fruit quality of apple. Foods 2024, 13, 1243. [Google Scholar] [CrossRef] [PubMed]
- Ubi, B.E.; Honda, C.; Bessho, H.; Kondo, S.; Wada, M.; Kobayashi, S.; Moriguchi, T. Expression analysis of anthocyanin biosynthetic genes in apple skin: Effect of UV-B and temperature. Plant Sci. 2006, 170, 571–578. [Google Scholar] [CrossRef]
- Wang, H.; Tian, Y.; Li, Y.; Wei, J.; Ma, F.; Liang, W.; Li, C. Analysis of carotenoid and gene expression in apple germplasm resources reveals the role of MdCRTISO and MdLCYE in the accumulation of carotenoids. J. Agric. Food Chem. 2023, 71, 15121–15131. [Google Scholar] [CrossRef] [PubMed]
- Do, V.G.; Lee, Y.; Kweon, H.; Kim, S. Light induces carotenoid biosynthesis-related gene expression, accumulation of pigment content, and expression of the small heat shock protein in apple fruit. Int. J. Mol. Sci. 2022, 23, 6153. [Google Scholar] [CrossRef]
- Wang, T.; Tan, L.; Chen, Z.; Yang, Y.; Yuan, Y.; Zheng, Z.; Deng, L.; Zhang, M.; Sun, G.; He, S.; et al. Mitigating citrus fruit cracking: The efficacy of chelated calcium or silicon foliar fertilizers in ‘Okitsu no. 58’ citrus fruit. Front. Plant Sci. 2024, 15, 1402945. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Win, N.M.; Kang, I.K. Changes in the environmental conditions of an ‘Arisoo’ apple orchard with a shading net system. Korean J. Agric. Sci. 2022, 49, 561–570. [Google Scholar] [CrossRef]
- Do, V.G.; Lee, Y.; Park, J.; Win, N.M.; Kwon, S.I.; Yang, S.; Kim, S. Heat stress and water irrigation management effects on the fruit color and quality of ‘Hongro’ apples. Agriculture 2024, 14, 761. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophyll and carotenoids-pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Win, N.M.; Yoo, J.; Lwin, H.P.; Lee, E.J.; Kang, I.K.; Lee, J. Effects of 1-methylcyclopropene and amionoethoxyvinylglycine treatments on fruit quality and antioxidant metabolites in cold-stored ‘Sangjudungsi’ persimmons. Hortic. Environ. Biotechnol. 2021, 62, 891–905. [Google Scholar] [CrossRef]
- Chang, S.; Puryear, J.; Cairney, J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 1993, 11, 113–116. [Google Scholar] [CrossRef]
- Lwin, H.P.; Han, S.Y.; Byeon, S.E.; Lee, J.; Yoo, J.; Jung, H.I.; Lee, J. Differential responses of bulb quality attributes, mineral nutrient contents, and targeted major metabolites in onion bulbs after long-term commercial cold storage. Hortic. Environ. Biotechnol. 2023, 64, 627–642. [Google Scholar] [CrossRef]





| Treatments | Fruit Weight (g) | Fruit Size (mm) | ||
|---|---|---|---|---|
| Length (L) | Diameter (D) | L/D Ratio | ||
| No bagging (control) | 274.17 ± 3.93 z a y | 79.18 ± 1.68 a | 86.72 ± 1.27 a | 0.91 ± 0.01 a |
| Bag removal at 30 DBH | 260.68 ± 8.40 a | 77.35 ± 2.58 ab | 84.90 ± 1.26 ab | 0.90 ± 0.01 a |
| Bag removal at 20 DBH | 243.50 ± 5.32 b | 75.50 ± 2.00 ab | 83.69 ± 1.40 b | 0.91 ± 0.01 a |
| Bag removal at 10 DBH | 238.08 ± 5.43 b | 74.92 ± 1.89 b | 82.04 ± 1.09 b | 0.90 ± 0.01 a |
| Treatments | Flesh Firmness (N) | SSC (Brix) | TA (%) | SSC/TA Ratio | SPI (1–8) | Fruit Skin Color | ||
|---|---|---|---|---|---|---|---|---|
| L* | C | ho | ||||||
| No bagging (control) | 58.90 ± 1.24 z b y | 12.85 ± 0.14 a | 0.36 ± 0.01 a | 35.38 ± 1.20 a | 7.87 ± 0.17 a | 57.02 ± 0.81 b | 28.10 ± 0.86 b | 42.77 ± 0.48 c |
| Bag removal at 30 DBH | 60.35 ± 1.45 ab | 12.54 ± 0.17 ab | 0.38 ± 0.02 a | 33.97 ± 1.59 ab | 7.80 ± 0.09 a | 57.71 ± 1.33 b | 28.96 ± 0.90 b | 48.72 ± 0.70 b |
| Bag removal at 20 DBH | 60.60 ± 0.75 ab | 12.47 ± 0.25 ab | 0.36 ± 0.01 a | 34.27 ± 0.79 ab | 7.88 ± 0.14 a | 59.39 ± 0.84 ab | 29.99 ± 0.98 b | 50.58 ± 0.98 b |
| Bag removal at 10 DBH | 62.25 ± 0.93 a | 12.29 ± 0.10 b | 0.40 ± 0.02 a | 30.74 ± 1.35 b | 7.50 ± 0.15 a | 61.28 ± 1.48 a | 53.52 ± 1.82 a | 71.83 ± 1.35 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Win, N.M.; Do, V.G.; Kwon, J.-G.; Park, J.-T.; Park, J.; Lee, Y.; Kweon, H.-J.; Kang, I.-K.; Kwon, S.-I.; Kim, S. Fruit Bag Removal Timing Influences Fruit Coloration, Quality, and Physiological Disorders in ‘Arisoo’ Apples. Plants 2025, 14, 2923. https://doi.org/10.3390/plants14182923
Win NM, Do VG, Kwon J-G, Park J-T, Park J, Lee Y, Kweon H-J, Kang I-K, Kwon S-I, Kim S. Fruit Bag Removal Timing Influences Fruit Coloration, Quality, and Physiological Disorders in ‘Arisoo’ Apples. Plants. 2025; 14(18):2923. https://doi.org/10.3390/plants14182923
Chicago/Turabian StyleWin, Nay Myo, Van Giap Do, Jung-Geun Kwon, Jong-Taek Park, Juhyeon Park, Youngsuk Lee, Hun-Joong Kweon, In-Kyu Kang, Soon-Il Kwon, and Seonae Kim. 2025. "Fruit Bag Removal Timing Influences Fruit Coloration, Quality, and Physiological Disorders in ‘Arisoo’ Apples" Plants 14, no. 18: 2923. https://doi.org/10.3390/plants14182923
APA StyleWin, N. M., Do, V. G., Kwon, J.-G., Park, J.-T., Park, J., Lee, Y., Kweon, H.-J., Kang, I.-K., Kwon, S.-I., & Kim, S. (2025). Fruit Bag Removal Timing Influences Fruit Coloration, Quality, and Physiological Disorders in ‘Arisoo’ Apples. Plants, 14(18), 2923. https://doi.org/10.3390/plants14182923








