Integrative Transcriptomic and Metabolomic Analyses Elucidate the Molecular Mechanisms Underlying Enhanced Yield and Bacterial Blight Resistance in the RXN2 Rice Cultivar
Abstract
1. Introduction
2. Results
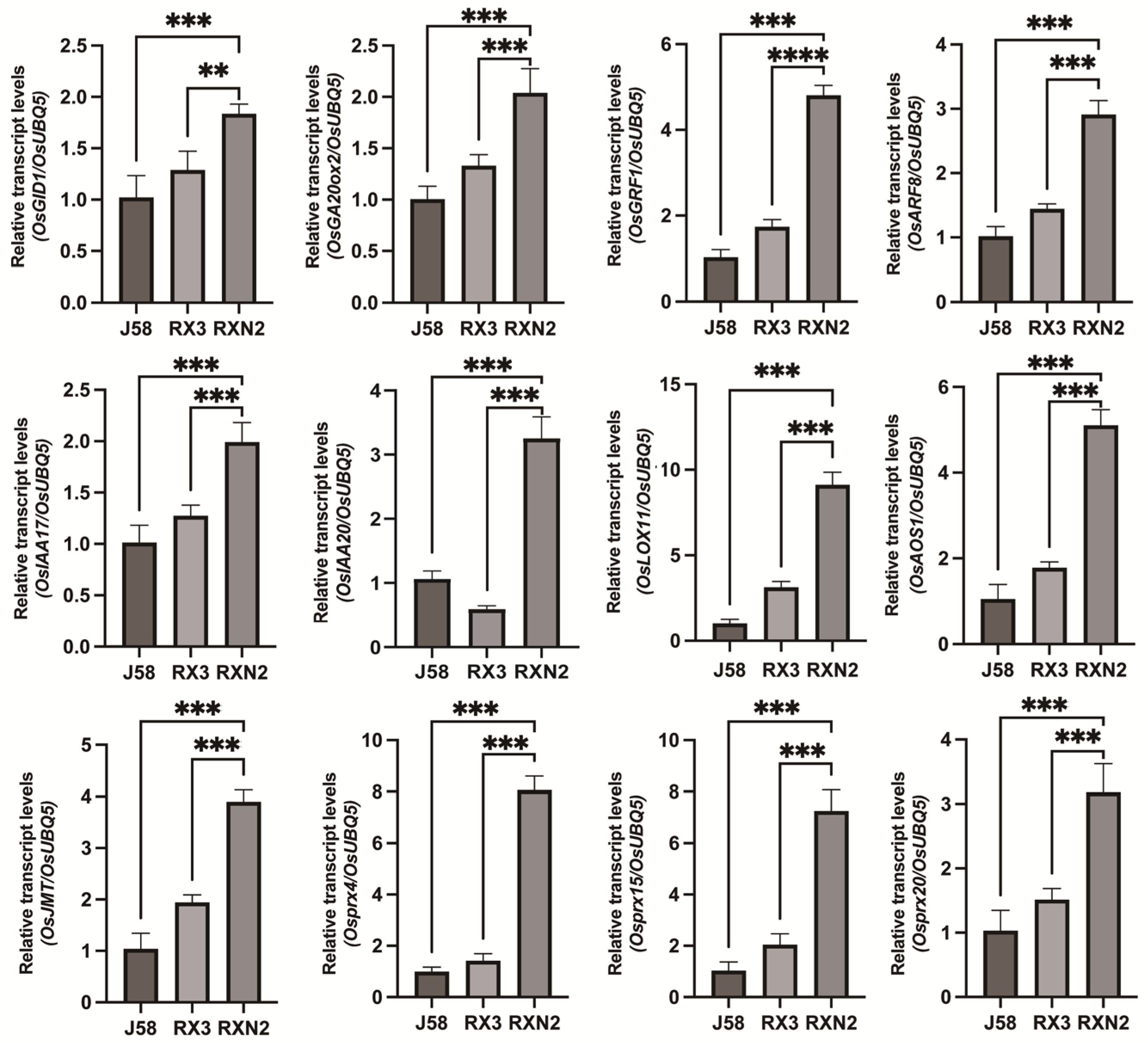
2.1. RXN2 Displayed Higher Grain Yield and Enhanced Bacterial Blight Resistance
2.2. Transcriptome Analysis
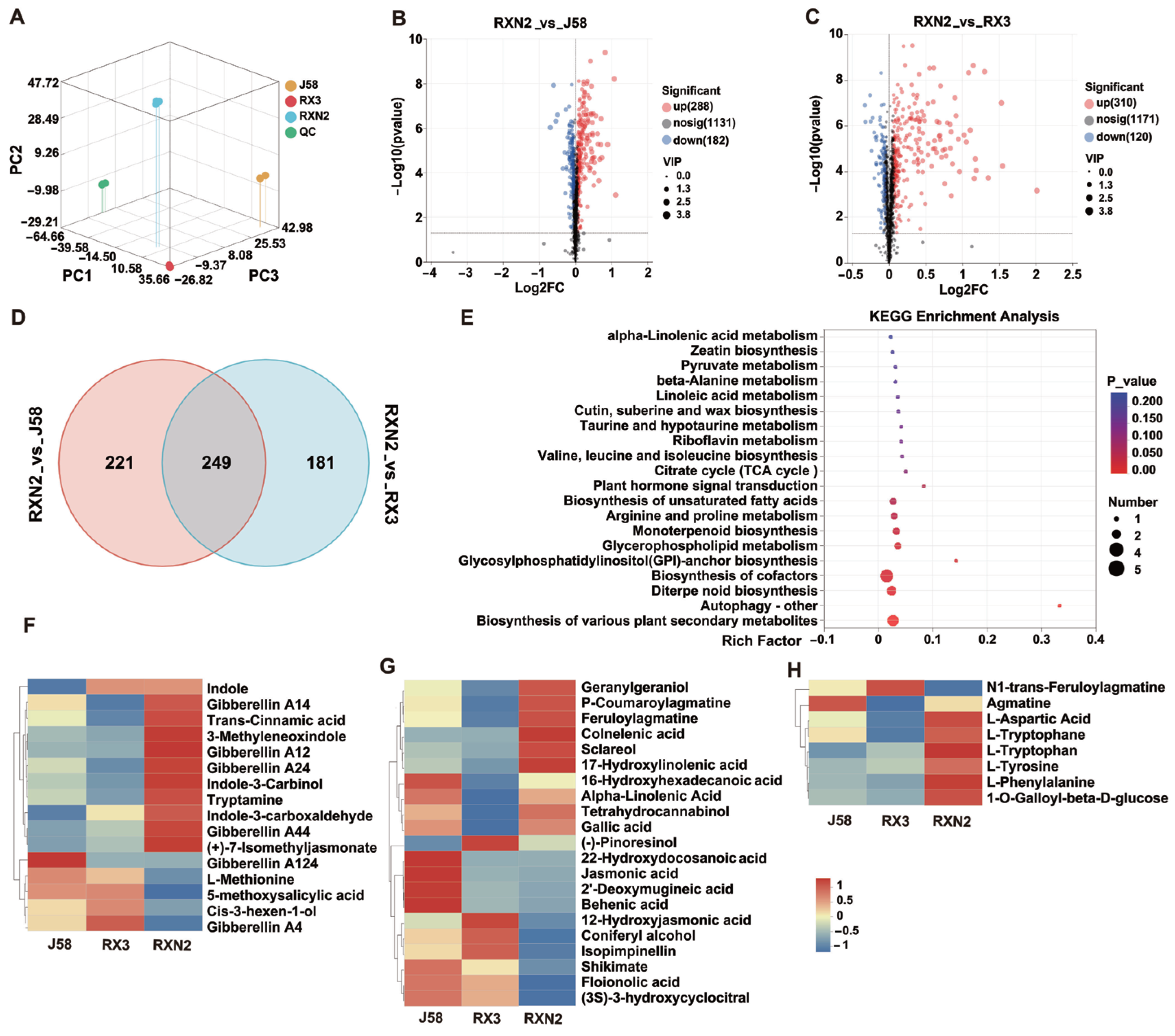
2.3. Metabolomics Analysis
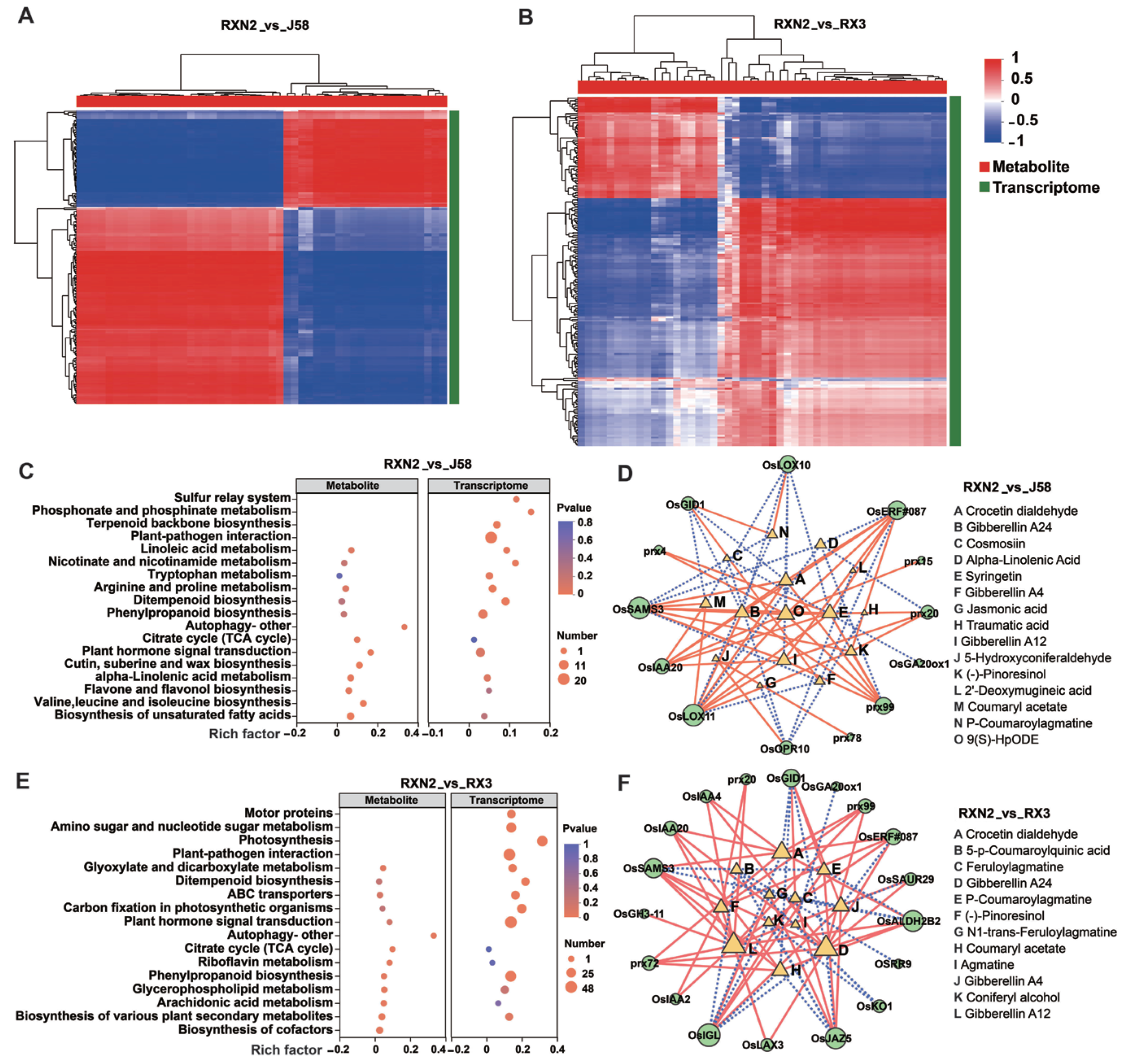
2.4. Joint Analysis of the Transcriptome and Metabolome
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growing Conditions
4.2. Pathogen Inoculation
4.3. RNA-Seq Library Preparation and Sequencing
4.4. Total RNA Extraction and RT-qPCR
4.5. Widely Targeted Metabolomics Assay and Statistical Analysis
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, H.; Liu, W.; Dou, Z.; Zhou, Q.; Chen, W.; Wang, S.; Ding, Y. Nitrogen fertilizer at heading stage effectively compensates for the deterioration of rice quality by affecting the starch-related properties under elevated temperatures. Food Chem. 2019, 277, 455–462. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, J.W.; Li, J.; Han, B. Designing future crops: Challenges and strategies for sustainable agriculture. Plant J. 2021, 105, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Khush, G.S.; Gupta, P. Strategies for increasing the yield potential of cereals: Case of rice as an example. Plant Breed. 2013, 132, 433–436. [Google Scholar] [CrossRef]
- Bhuyan, S.J.; Kumar, M.; Ramrao Devde, P.; Rai, A.C.; Mishra, A.K.; Singh, P.K.; Siddique, K.H.M. Progress in gene editing tools, implications and success in plants: A review. Front. Genome Ed. 2023, 5, 1272678. [Google Scholar] [CrossRef] [PubMed]
- Sao, R.; Sahu, P.K.; Patel, R.S.; Das, B.K.; Jankuloski, L.; Sharma, D. Genetic improvement in plant architecture, maturity duration and agronomic traits of three traditional rice landraces through gamma ray-based induced mutagenesis. Plants 2022, 11, 3448. [Google Scholar] [CrossRef]
- Mohsen, G.; Soliman, S.; Mahgoub, E.; Ismail, T.A.; Mansour, E.; Alwutayd, K.M.; Safhi, F.A.; ElMoneim, D.; Alshamrani, R.; Atallah, O.O.; et al. Gamma-rays induced mutations increase soybean oil and protein contents. PeerJ 2023, 11, e16395. [Google Scholar] [CrossRef]
- Deng, D.; Sun, S.; Wu, W.; Xiang, C.; Duan, C.; Yu, D.; Wu, X.; Zhu, Z. Disease resistance and molecular variations in irradiation-induced mutants of two pea cultivars. Int. J. Mol. Sci. 2022, 23, 8793. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Luo, K. Molecular mechanisms of heterosis and its applications in tree breeding: Progress and perspectives. Int. J. Mol. Sci. 2024, 25, 12344. [Google Scholar] [CrossRef]
- Awan, M.J.A.; Farooq, M.A.; Buzdar, M.I.; Zia, A.; Ehsan, A.; Waqas, M.A.B.; Hensel, G.; Amin, I.; Mansoor, S. Advances in gene editing-led route for hybrid breeding in crops. Biotechnol. Adv. 2025, 81, 108569. [Google Scholar] [CrossRef] [PubMed]
- Hochholdinger, F.; Yu, P. Molecular concepts to explain heterosis in crops. Trends Plant Sci. 2025, 30, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Han, B. Unlocking the mystery of heterosis opens the era of intelligent rice breeding. Plant Physiol. 2024, 196, 735–744. [Google Scholar] [CrossRef]
- Ma, X.; Jia, Q.; Li, S.; Chen, Z.; Ming, X.; Zhao, Y.; Zhou, D.X. An enhanced network of energy metabolism, lysine acetylation, and growth-promoting protein accumulation is associated with heterosis in elite hybrid rice. Plant Commun. 2023, 4, 100560. [Google Scholar] [CrossRef]
- Ma, M.; Shen, S.Y.; Bai, C.; Wang, W.Q.; Feng, X.H.; Ying, J.Z.; Song, X.J. Control of grain size in rice by TGW3 phosphorylation of OsIAA10 through potentiation of OsIAA10-OsARF4-mediated auxin signaling. Cell Rep. 2023, 42, 112187. [Google Scholar] [CrossRef]
- Cui, L.; Song, Y.; Zhao, Y.; Gao, R.; Wang, Y.; Lin, Q.; Jiang, J.; Xie, H.; Cai, Q.; Zhu, Y.; et al. Nei 6 You 7075, a hybrid rice cultivar, exhibits enhanced disease resistance and drought tolerance traits. BMC Plant Biol. 2024, 24, 1252. [Google Scholar] [CrossRef] [PubMed]
- Waadt, R.; Seller, C.; Hsu, P.; Takahashi, Y.; Munemasa, S.; Schroeder, J. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, J.; Qi, J.; Zhao, D.; Jin, M.; Wang, T.; Yang, Y.; Shi, H.; Guo, L.; Zhang, H. Crosstalk among plant hormone regulates the root development. Plant Signal. Behav. 2024, 19, 2404807. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Hao, Z.; Ning, Y.; He, Z. Revisiting growth-defence trade-offs and breeding strategies in crops. Plant Biotechnol. J. 2024, 22, 1198–1205. [Google Scholar] [CrossRef]
- Ali, J.; Mukarram, M.; Ojo, J.; Dawam, N.; Riyazuddin, R.; Ghramh, H.A.; Khan, K.A.; Chen, R.; Kurjak, D.; Bayram, A. Harnessing phytohormones: Advancing plant growth and defense strategies for sustainable agriculture. Physiol. Plant. 2024, 176, e14307. [Google Scholar] [CrossRef]
- Sasaki, A.; Ashikari, M.; Ueguchi-Tanaka, M.; Itoh, H.; Nishimura, A.; Swapan, D.; Ishiyama, K.; Saito, T.; Kobayashi, M.; Khush, G.S.; et al. Green revolution: A mutant gibberellin-synthesis gene in rice. Nature 2002, 416, 701–702. [Google Scholar] [CrossRef]
- Ashikari, M.; Sasaki, A.; Ueguchi-Tanaka, M.; Itoh, H.; Nishimura, A.; Datta, S.; Ishiyama, K.; Saito, T.; Kobayashi, M.; Khush, G.S.; et al. Loss-of-function of a rice gibberellin biosynthetic gene, GA20 oxidase (GA20ox-20), led to the rice “green revolution”. Breed. Sci. 2002, 52, 143–150. [Google Scholar] [CrossRef]
- Xian, F.; Liu, S.; Huang, J.; Xie, B.; Zhu, L.; Zhang, Q.; Lv, C.; Xu, Y.; Zhang, X.; Hu, J. The OsIAA3-OsARF16-OsBUL1 auxin signaling module regulates grain size in rice. Plant Physiol. 2025, 197, kiaf122. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Xiao, J.; Ding, X.; Xiong, M.; Cai, M.; Cao, Y.; Li, X.; Xu, C.; Wang, S. OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol. Plant Microbe Interact. 2007, 20, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Caarls, L.; Elberse, J.; Awwanah, M.; Ludwig, N.R.; de Vries, M.; Zeilmaker, T.; Van Wees, S.; Schuurink, R.C.; Van den Ackerveken, G. Arabidopsis JASMONATE-INDUCED OXYGENASES down-regulate plant immunity by hydroxylation and inactivation of the hormone jasmonic acid. Proc. Natl. Acad. Sci. USA 2017, 114, 6388–6393. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Wang, G.; Chen, X.; Li, L.; Zhang, X.; Chen, S.; He, Y.; Hong, G. OsPHR2 modulates phosphate starvation-induced OsMYC2 signaling and resistance to Xanthomonas oryzae pv. oryzae. Plant Cell Environ. 2021, 44, 3432–3444. [Google Scholar] [CrossRef]
- Tao, Z.; Liu, H.; Qiu, D.; Zhou, Y.; Li, X.; Xu, C.; Wang, S. A pair of allelic WRKY genes play opposite roles in rice-bacteria interactions. Plant Physiol. 2009, 151, 936–948. [Google Scholar] [CrossRef]
- Uji, Y.; Taniguchi, S.; Tamaoki, D.; Shishido, H.; Akimitsu, K.; Gomi, K. Overexpression of OsMYC2 results in the up-regulation of early JA-responsive genes and bacterial blight resistance in rice. Plant Cell Physiol. 2016, 57, 1814–1827. [Google Scholar] [CrossRef]
- He, Y.; Zhang, H.; Sun, Z.; Li, J.; Hong, G.; Zhu, Q.; Zhou, X.; MacFarlane, S.; Yan, F.; Chen, J. Jasmonic acid-mediated defense suppresses brassinosteroid-mediated susceptibility to Rice black streaked dwarf virus infection in rice. New Phytol. 2017, 214, 388–399. [Google Scholar] [CrossRef]
- He, Y.; Zhao, Y.; Hu, J.; Wang, L.; Li, L.; Zhang, X.; Zhou, Z.; Chen, L.; Wang, H.; Wang, J.; et al. The OsBZR1-OsSPX1/2 module fine-tunes the growth-immunity trade-off in adaptation to phosphate availability in rice. Mol. Plant 2024, 17, 258–276. [Google Scholar] [CrossRef]
- Xie, K.; Li, L.; Zhang, H.; Wang, R.; Tan, X.; He, Y.; Hong, G.; Li, J.; Ming, F.; Yao, X.; et al. Abscisic acid negatively modulates plant defence against rice black-streaked dwarf virus infection by suppressing the jasmonate pathway and regulating reactive oxygen species levels in rice. Plant Cell Environ. 2018, 41, 2504–2514. [Google Scholar] [CrossRef]
- Jin, G.; Qi, J.; Zu, H.; Liu, S.; Gershenzon, J.; Lou, Y.; Baldwin, I.T.; Li, R. Jasmonate-mediated gibberellin catabolism constrains growth during herbivore attack in rice. Plant Cell 2023, 35, 3828–3844. [Google Scholar] [CrossRef]
- Yuan, H.; Cheng, M.; Fan, F.; Zheng, X.; Wang, R.; Si, F.; Luo, X.; Li, N.; Li, S. OsGRF6-OsYUCCA1/OsWRKY82 signaling cascade upgrade grain yield and bacterial blight resistance in rice. Adv. Sci. 2024, 11, e2407733. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.Q.; Chen, Y.X.; Ju, Y.L.; Pan, C.Y.; Shan, J.X.; Ye, W.W.; Dong, N.Q.; Kan, Y.; Yang, Y.B.; Zhao, H.Y.; et al. Fine-tuning gibberellin improves rice alkali-thermal tolerance and yield. Nature 2025, 639, 162–171. [Google Scholar] [CrossRef]
- Liu, H.; Dong, S.; Li, M.; Gu, F.; Yang, G.; Guo, T.; Chen, Z.; Wang, J. The Class III peroxidase gene OsPrx30, transcriptionally modulated by the AT-hook protein OsATH1, mediates rice bacterial blight-induced ROS accumulation. J. Integr. Plant Biol. 2021, 63, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Uji, Y.; Suzuki, G.; Fujii, Y.; Kashihara, K.; Yamada, S.; Gomi, K. Jasmonic acid (JA)-mediating MYB transcription factor1, JMTF1, coordinates the balance between JA and auxin signaling in the rice defence response. Physiol. Plant 2024, 176, e14257. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, M.; Liu, X.; Zhang, B.; Cui, Y.; Cao, X.; Zhang, Z.; Shi, C.; Wei, H.; He, H.; et al. RBB1 negatively regulates rice disease resistance by modulating protein glycosylation. J. Integr. Plant Biol. 2025, 67, 391–407. [Google Scholar] [CrossRef]
- Zhang, H.; He, Y.; Tan, X.; Xie, K.; Li, L.; Hong, G.; Li, J.; Cheng, Y.; Yan, F.; Chen, J.; et al. The dual effect of the brassinosteroid pathway on rice black-streaked dwarf virus infection by modulating the peroxidase-mediated oxidative burst and plant defense. Mol. Plant Microbe Interact. 2019, 32, 685. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Y.; Lv, Y.; Pu, Q.; Li, J.; Zhang, Y.; Deng, X.; Wang, M.; Wang, J.; Tao, D. Interspecific hybridization is an important driving force for origin and diversification of Asian cultivated rice Oryza sativa L. Front. Plant Sci. 2022, 13, 932737. [Google Scholar] [CrossRef]
- Guo, S.; Wei, Y.; Li, X.; Liu, K.; Huang, F.; Chen, C.; Gao, G. Development and identification of introgression lines from cross of Oryza sativa and Oryza minuta. Rice Sci. 2013, 20, 95–102. [Google Scholar] [CrossRef]
- Gaikwad, K.B.; Singh, N.; Bhatia, D.; Kaur, R.; Bains, N.S.; Bharaj, T.S.; Singh, K. Yield-enhancing heterotic QTL transferred from wild species to cultivated rice Oryza sativa L. PLoS ONE 2014, 9, e96939. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; Sabatino, L.; Vetrano, F. Effect of gibberellic acid on growth, yield, and quality of leaf lettuce and rocket grown in a floating system. Agronomy 2019, 9, 382. [Google Scholar] [CrossRef]
- Lopez-Cristoffanini, C.; Serrat, X.; Jauregui, O.; Nogues, S.; Lopez-Carbonell, M. Phytohormone profiling method for rice: Effects of GA20ox mutation on the gibberellin content of Japonica rice varieties. Front. Plant Sci. 2019, 10, 733. [Google Scholar] [CrossRef]
- Ito, S.; Yamagami, D.; Umehara, M.; Hanada, A.; Yoshida, S.; Sasaki, Y.; Yajima, S.; Kyozuka, J.; Ueguchi-Tanaka, M.; Matsuoka, M.; et al. Regulation of strigolactone biosynthesis by gibberellin signaling. Plant Physiol. 2017, 174, 1250–1259. [Google Scholar] [CrossRef]
- Ueguchi-Tanaka, M.; Ashikari, M.; Nakajima, M.; Itoh, H.; Katoh, E.; Kobayashi, M.; Chow, T.Y.; Hsing, Y.I.; Kitano, H.; Yamaguchi, I.; et al. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 2005, 437, 693–698. [Google Scholar] [CrossRef]
- van der Knaap, E.; Kim, J.H.; Kende, H. A novel gibberellin-induced gene from rice and its potential regulatory role in stem growth. Plant Physiol. 2000, 122, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; An, J.; Song, J.; Yan, T.; Li, J.; Zhao, X.; Shi, X.; Meng, Y.; Rong, C.; Li, G.; et al. OsGRF1 and OsGRF2 play unequal redundant roles in regulating leaf vascular bundle formation. J. Exp. Bot. 2025, 76, 4055–4070. [Google Scholar] [CrossRef]
- Giacomelli, L.; Rota-Stabelli, O.; Masuero, D.; Acheampong, A.K.; Moretto, M.; Caputi, L.; Vrhovsek, U.; Moser, C. Gibberellin metabolism in Vitis vinifera L. during bloom and fruit-set: Functional characterization and evolution of grapevine gibberellin oxidases. J. Exp. Bot. 2013, 64, 4403–4419. [Google Scholar] [CrossRef] [PubMed]
- Loque, D.; Tillard, P.; Gojon, A.; Lepetit, M. Gene expression of the NO3− transporter NRT1.1 and the nitrate reductase NIA1 is repressed in Arabidopsis roots by NO2−, the product of NO3− reduction. Plant Physiol. 2003, 132, 958–967. [Google Scholar] [CrossRef]
- Effmert, U.; Dinse, C.; Piechulla, B. Influence of green leaf herbivory by Manduca sexta on floral volatile emission by Nicotiana suaveolens. Plant Physiol. 2008, 146, 1996–2007. [Google Scholar] [CrossRef][Green Version]
- Wang, L.; Chen, Y.; Deng, F.; Jin, G.; Gong, X.; Wu, G.; Zhang, C.; Li, R.; Wang, H. A lipoxygenase gene modulates jasmonate biosynthesis to enhance blast resistance in rice. J. Exp. Bot. 2025, eraf026. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Chen, Y.; Feng, A.; Zou, W.; Wang, D.; Lin, P.; Chen, Y.; You, C.; Que, Y.; Su, Y. The allene oxide synthase gene family in sugarcane and its involvement in disease resistance. Ind. Crops Prod. 2023, 192, 116136. [Google Scholar] [CrossRef]
- Song, S.; Li, Y.; Zhang, Y.; Liu, F.; Zhu, Q.H.; Zhang, X.; Sun, J.; Li, Y. Transcriptome-based gene modules and soluble sugar content analyses reveal the defense response of cotton leaves to Verticillium dahliae. Int. J. Mol. Sci. 2024, 25, 13326. [Google Scholar] [CrossRef]
- Huan, C.; Yang, X.; Wang, L.; Kebbeh, M.; Wang, Y.; Dai, B.; Shen, S.; Zheng, X.; Zhou, H. Methyl jasmonate treatment regulates α-linolenic acid metabolism and jasmonate acid signaling pathway to improve chilling tolerance in both stony hard and melting flesh peaches. Postharvest Biol. Technol. 2022, 190, 111960. [Google Scholar] [CrossRef]
- Hong, Y.; Yu, Z.; Zhou, Q.; Chen, C.; Hao, Y.; Wang, Z.; Zhu, J.K.; Guo, H.; Huang, A.C. NAD+ deficiency primes defense metabolism via 1O2-escalated jasmonate biosynthesis in plants. Nat. Commun. 2024, 15, 6652. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Yuan, J.S.; Kollner, T.G.; Chen, Y.; Guo, Y.; Zhuang, X.; Chen, X.; Zhang, Y.; Fu, J.; et al. The rice terpene synthase gene OsTPS19 functions as an (S)-limonene synthase in planta, and its overexpression leads to enhanced resistance to the blast fungus Magnaporthe oryzae. Plant Biotechnol. J. 2018, 16, 1778–1787. [Google Scholar] [CrossRef]
- Yoshitomi, K.; Taniguchi, S.; Tanaka, K.; Uji, Y.; Akimitsu, K.; Gomi, K. Rice terpene synthase 24 (OsTPS24) encodes a jasmonate-responsive monoterpene synthase that produces an antibacterial gamma-terpinene against rice pathogen. J. Plant Physiol. 2016, 191, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.X.; Wang, Y.; Lin, J.S.; Chen, L.; Li, Y.J.; Liu, Q.; Wang, G.F.; Xu, F.; Liu, L.; Hou, B.K. The novel pathogen-responsive glycosyltransferase UGT73C7 mediates the redirection of phenylpropanoid metabolism and promotes SNC1-dependent Arabidopsis immunity. Plant J. 2021, 107, 149–165. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Webster, S.; He, S.Y. Growth-defense trade-offs in plants. Curr. Biol. 2022, 32, R634–R639. [Google Scholar] [CrossRef]
- Qiao, S.; Sun, S.; Wang, L.; Wu, Z.; Li, C.; Li, X.; Wang, T.; Leng, L.; Tian, W.; Lu, T.; et al. The RLA1/SMOS1 transcription factor functions with OsBZR1 to regulate brassinosteroid signaling and rice architecture. Plant Cell 2017, 29, 292–309. [Google Scholar] [CrossRef]
- De Vleesschauwer, D.; Van Buyten, E.; Satoh, K.; Balidion, J.; Mauleon, R.; Choi, I.R.; Vera-Cruz, C.; Kikuchi, S.; Hofte, M. Brassinosteroids antagonize gibberellin- and salicylate-mediated root immunity in rice. Plant Physiol. 2012, 158, 1833–1846. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, J.; Wang, J.; Huang, X.; Jiang, J.; Shi, X.; Bao, G.; Meng, Q.; Yan, C. Integrative Transcriptomic and Metabolomic Analyses Elucidate the Molecular Mechanisms Underlying Enhanced Yield and Bacterial Blight Resistance in the RXN2 Rice Cultivar. Plants 2025, 14, 2921. https://doi.org/10.3390/plants14182921
Bi J, Wang J, Huang X, Jiang J, Shi X, Bao G, Meng Q, Yan C. Integrative Transcriptomic and Metabolomic Analyses Elucidate the Molecular Mechanisms Underlying Enhanced Yield and Bacterial Blight Resistance in the RXN2 Rice Cultivar. Plants. 2025; 14(18):2921. https://doi.org/10.3390/plants14182921
Chicago/Turabian StyleBi, Ji’an, Jingqi Wang, Xuan Huang, Jiefeng Jiang, Xianbo Shi, Genliang Bao, Qiufeng Meng, and Chengqi Yan. 2025. "Integrative Transcriptomic and Metabolomic Analyses Elucidate the Molecular Mechanisms Underlying Enhanced Yield and Bacterial Blight Resistance in the RXN2 Rice Cultivar" Plants 14, no. 18: 2921. https://doi.org/10.3390/plants14182921
APA StyleBi, J., Wang, J., Huang, X., Jiang, J., Shi, X., Bao, G., Meng, Q., & Yan, C. (2025). Integrative Transcriptomic and Metabolomic Analyses Elucidate the Molecular Mechanisms Underlying Enhanced Yield and Bacterial Blight Resistance in the RXN2 Rice Cultivar. Plants, 14(18), 2921. https://doi.org/10.3390/plants14182921





