A Preliminary Insight into Under-Researched Plants from the Asteraceae Family in the Balkan Peninsula: Bioactive Compound Diversity and Antioxidant Potential
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction Yield
2.2. Classes and Subclasses of Bioactive Compounds
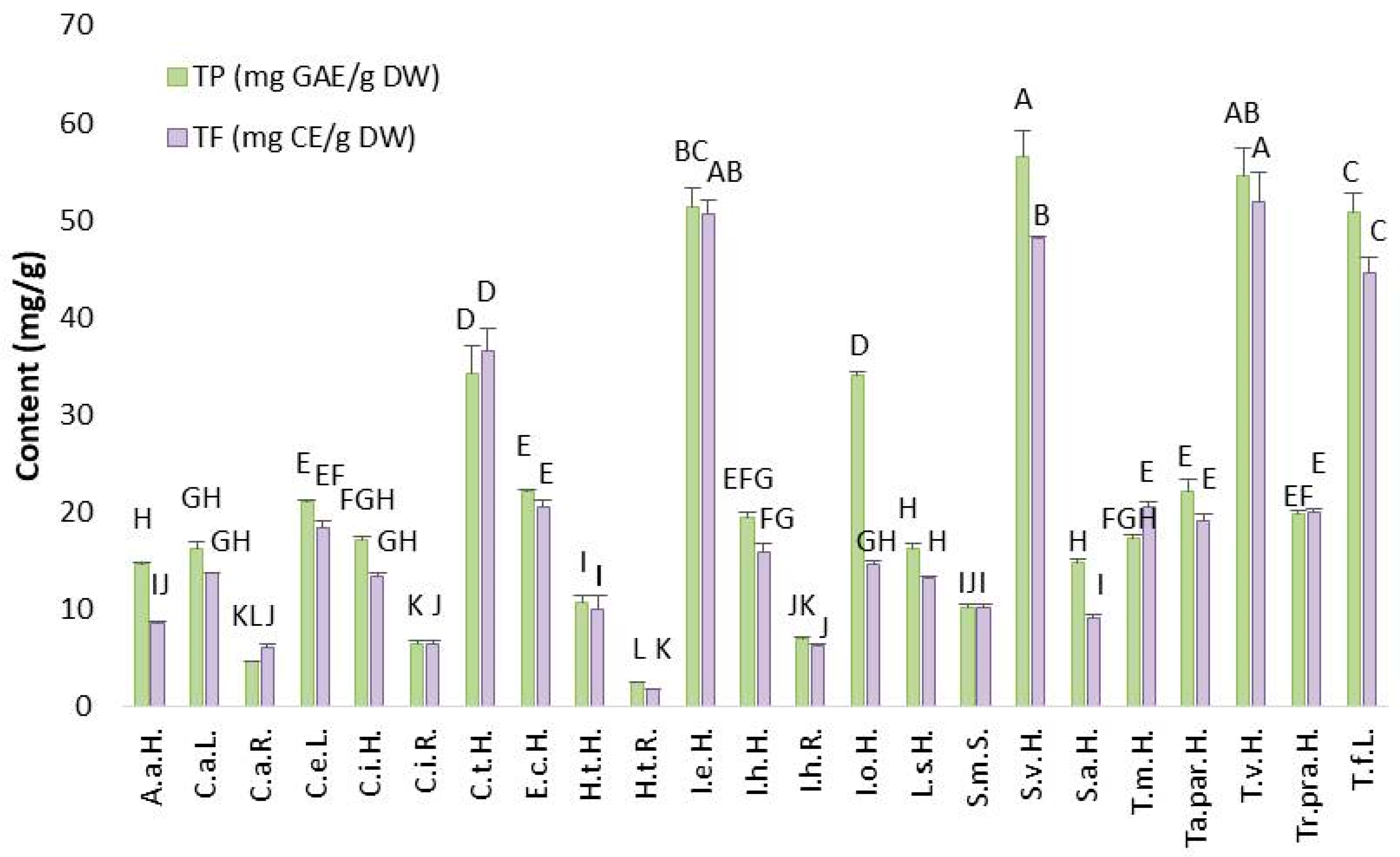
2.2.1. Total Phenolic (TP) and Flavonoid (TF) Content in Asteraceae Plant’s Extracts
2.2.2. Total Content of Hydroxycinnamic Acids (HCA), Flavones (FL) and Condensed Tannins (CT) in Asteraceae Plant’s Extracts
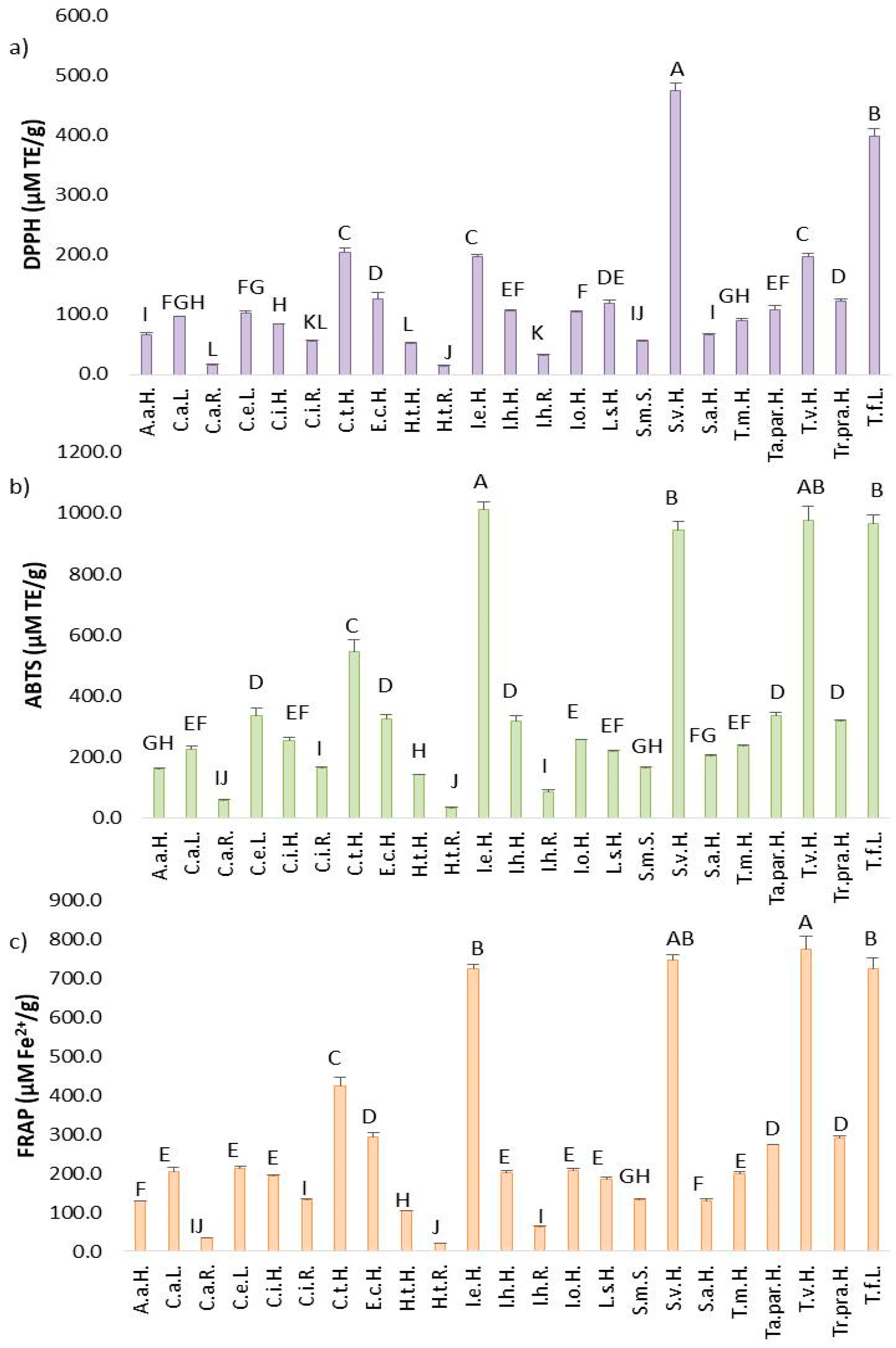
2.3. In Vitro Antioxidant Activity of Asteraceae Plant’s Extracts
2.4. Correlation Between TP, TF, HCA, FL, CT and DPPH, ABTS, FRAP
2.5. Chemical Profile of Asteraceae Plant’s Extracts by LC-MS
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals, Reagents and Standards
3.3. Experimental Design
3.4. Conventional Solid/Liquid Extraction Technique
3.5. Classes and Subclasses of Bioactive Compounds
3.5.1. Total Phenolic (TP) and Flavonoid Content (TF)
3.5.2. Total Content of Hydroxycinnamic Acids (HCA), Flavonols (FL) and Condensed Tannins (CT)
3.6. In Vitro Antioxidant Activity
3.6.1. DPPH Assay
3.6.2. ABTS Assay
3.6.3. FRAP Assay
3.7. Chemical Profile of Asteraceae Plant’s Extracts by LC-MS
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arora, R.K. Diversity in Underutilized Plant Species: An Asia-Pacific Perspective; Bioversity International: Rome, Italy, 2014; ISBN 9292550071. [Google Scholar]
- Olennikov, D.N.; Chirikova, N.K. Phenolic compounds of six unexplored Asteraceae species from asia: Comparison of wild and cultivated plants. Horticulturae 2024, 10, 486. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. The plants of the Asteraceae family as agents in the protection of human health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef]
- Achika, J.I.; Arthur, D.E.; Gerald, I.; Adedayo, A. A review on the phytoconstituents and related medicinal properties of plants in the Asteraceae family. IOSR J. Appl. Chem. 2014, 7, 1–8. [Google Scholar] [CrossRef]
- Kenny, O.; Smyth, T.J.; Walsh, D.; Kelleher, C.T.; Hewage, C.M.; Brunton, N.P. Investigating the potential of under-utilised plants from the Asteraceae family as a source of natural antimicrobial and antioxidant extracts. Food Chem. 2014, 161, 79–86. [Google Scholar] [CrossRef]
- Rolnik, A.; Soluch, A.; Kowalska, I.; Olas, B. Antioxidant and hemostatic properties of preparations from Asteraceae family and their chemical composition—Comparative studies. Biomed. Pharmacother. 2021, 142, 111982. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Janaćković, P.; Kolašinac, S.M.; Dajić Stevanović, Z.P. Balkans’ Asteraceae species as a source of biologically active compounds for the pharmaceutical and food industry. Chem. Biodivers. 2020, 17, e2000097. [Google Scholar] [CrossRef]
- Hadjichambis, A.C.H.; Paraskeva-Hadjichambi, D.; Della, A.; Elena Giusti, M.; De Pasquale, C.; Lenzarini, C.; Censorii, E.; Reyes Gonzales-Tejero, M.; Patricia Sanchez-Rojas, C.; Ramiro-Gutierrez, J.M. Wild and semi-domesticated food plant consumption in seven circum-Mediterranean areas. Int. J. Food Sci. Nutr. 2008, 59, 383–414. [Google Scholar] [CrossRef]
- Pieroni, A.; Quave, C.L. Ethnobotany and Biocultural Diversities in the Balkans: Perspectives on Sustainable Rural Development and Reconciliation; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 1493914928. [Google Scholar]
- Žnidarčič, D.; Ban, D.; Šircelj, H. Carotenoid and chlorophyll composition of commonly consumed leafy vegetables in Mediterranean countries. Food Chem. 2011, 129, 1164–1168. [Google Scholar] [CrossRef]
- Stevanović, Z.D.; Petrović, M.; Aćić, S.; Dajić Stevanović, Z.; Petrović, M.; Aćić, S. Ethnobotanical knowledge and traditional use of plants in Serbia in relation to sustainable rural development. In Ethnobotany and Biocultural Diversities in the Balkans: Perspectives on Sustainable Rural Development and Reconciliation; Springer: Berlin/Heidelberg, Germany, 2014; pp. 229–252. [Google Scholar] [CrossRef]
- Cvetanović, A.; Švarc-Gajić, J.; Mašković, P.; Savić, S.; Nikolić, L. Antioxidant and biological activity of chamomile extracts obtained by different techniques: Perspective of using superheated water for isolation of biologically active compounds. Ind. Crops Prod. 2015, 65, 582–591. [Google Scholar] [CrossRef]
- Sun, Z.; Su, R.; Qiao, J.; Zhao, Z.; Wang, X. Flavonoids extraction from Taraxacum officinale (Dandelion): Optimisation using response surface methodology and antioxidant activity. J. Chem. 2014, 2014, 956278. [Google Scholar] [CrossRef]
- Ijaz, A.; Ahmad, S.; Gul, H.; Jabeen, N.; Gulfraz, M. Antioxidant and antimicrobial activities of root leaves and flowers of Cichorium intybus. Int. J. Pharmacogn. 2017, 4, 23–32. [Google Scholar]
- Predescu, N.C.; Papuc, C.; Nicorescu, V.; Gajaila, I.; Goran, G.V.; Petcu, C.D.; Stefan, G. The influence of solid-to-solvent ratio and extraction method on total phenolic content, flavonoid content and antioxidant properties of some ethanolic plant extracts. Rev. Chim. 2016, 67, 1922–1927. [Google Scholar]
- Devi, E.L.; Kumar, S.; Singh, T.B.; Sharma, S.K.; Beemrote, A.; Devi, C.P.; Chongtham, S.K.; Singh, C.H.; Yumlembam, R.A.; Haribhushan, A. Adaptation strategies and defence mechanisms of plants during environmental stress. In Medicinal Plants and Environmental Challenges; Springer: Berlin/Heidelberg, Germany, 2017; pp. 359–413. [Google Scholar]
- Mammen, D. Chemical perspective and drawbacks in flavonoid estimation assays. In Frontiers in Natural Product Chemistry; Bentham Science Publishers: Sharjah, United Arab Emirates, 2022; pp. 189–228. [Google Scholar]
- Güçlü, G.; İnanır, M.; Ucar, E.; Eruygur, N.; Ataş, M.; Uskutoğlu, T.; Şenkal, B.C. Biological activities of different plant species belonging to the Asteraceae family. Int. J. Second. Metab. 2023, 10, 11–22. [Google Scholar] [CrossRef]
- Barral-Martinez, M.; Garcia-Oliveira, P.; Nuñez-Estevez, B.; Silva, A.; Finimundy, T.C.; Calhelha, R.; Nenadic, M.; Sokovic, M.; Barroso, F.; Simal-Gandara, J. Plants of the family Asteraceae: Evaluation of biological properties and identification of phenolic compounds. Chem. Proc. 2021, 5, 51. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, G.; Yue, J.; Qian, B.; Liu, Z.; Wang, D.; Zhong, Y.; Zhao, Y. Influences of ripening stages and extracting solvents on the polyphenolic compounds, antimicrobial and antioxidant activities of blueberry leaf extracts. Food Control 2014, 38, 184–191. [Google Scholar] [CrossRef]
- Tušek, A.J.; Benković, M.; Cvitanović, A.B.; Valinger, D.; Jurina, T.; Kljusurić, J.G. Kinetics and thermodynamics of the solid-liquid extraction process of total polyphenols, antioxidants and extraction yield from Asteraceae plants. Ind. Crops Prod. 2016, 91, 205–214. [Google Scholar] [CrossRef]
- Rosłon, W.; Osińska, E.; Mazur, K.; Geszprych, A. Chemical characteristics of European goldenrod (Solidago virgaurea L. subsp. virgaurea) from natural sites in Central and Eastern Poland. Acta Sci. Pol. Hortorum Cultus 2014, 13, 55–65. [Google Scholar]
- Šukele, R.; Lauberte, L.; Kovalcuka, L.; Logviss, K.; Bārzdiņa, A.; Brangule, A.; Horváth, Z.M.; Bandere, D. Chemical profiling and antioxidant activity of Tanacetum vulgare L. wild-growing in Latvia. Plants 2023, 12, 1968. [Google Scholar] [CrossRef]
- Ivănescu, B.; Tuchiluș, C.; Corciovă, A.; Lungu, C.; Mihai, C.T.; Gheldiu, A.-M.; Vlase, L. Antioxidant, antimicrobial and cytotoxic activity of Tanacetum vulgare, Tanacetum corymbosum and Tanacetum macrophyllum extracts. Farmacia 2018, 66, 282–288. [Google Scholar]
- Bessada, S.M.F.; Barreira, J.C.M.; Oliveira, M.B.P.P. Asteraceae species with most prominent bioactivity and their potential applications: A review. Ind. Crops Prod. 2015, 76, 604–615. [Google Scholar] [CrossRef]
- Villalva, M.; Santoyo, S.; Salas-Pérez, L.; Siles-Sánchez, M.; de las, N.; Rodriguez Garcia-Risco, M.; Fornari, T.; Reglero, G.; Jaime, L. Sustainable extraction techniques for obtaining antioxidant and anti-inflammatory compounds from the Lamiaceae and Asteraceae Species. Foods 2021, 10, 2067. [Google Scholar] [CrossRef]
- Michel, J.; Abd Rani, N.Z.; Husain, K. A review on the potential use of medicinal plants from Asteraceae and Lamiaceae plant family in cardiovascular diseases. Front. Pharmacol. 2020, 11, 852. [Google Scholar] [CrossRef]
- Piątkowska, E.; Biel, W.; Witkowicz, R.; Kępińska-Pacelik, J. Chemical composition and antioxidant activity of Asteraceae family plants. Appl. Sci. 2022, 12, 12293. [Google Scholar] [CrossRef]
- Koc, S.; Isgor, B.S.; Isgor, Y.G.; Shomali Moghaddam, N.; Yildirim, O. The potential medicinal value of plants from Asteraceae family with antioxidant defense enzymes as biological targets. Pharm. Biol. 2015, 53, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Paun, G.; Neagu, E.; Albu, C.; Alecu, A.; Seciu-Grama, A.; Radu, G. Evaluation of the Antioxidant, and Antidiabetic Properties of Flavonoids and Isoflavonoids-Rich Extracts of Medicago sativa and Solidago virgaurea. Preprints 2023. [Google Scholar] [CrossRef]
- Demir, H.; Acik, L.; Bali, E.B.; Koç, L.Y.; Kaynak, G. Antioxidant and antimicrobial activities of Solidago virgaurea extracts. Afr. J. Biotechnol. 2009, 8, 274–279. [Google Scholar]
- Juan-Badaturuge, M.; Habtemariam, S.; Jackson, C.; Thomas, M.J.K. Antioxidant principles of Tanacetum vulgare L. aerial parts. Nat. Prod. Commun. 2009, 4, 1934578X0900401121. [Google Scholar] [CrossRef]
- Ak, G.; Gevrenova, R.; Sinan, K.I.; Zengin, G.; Zheleva, D.; Mahomoodally, M.F.; Senkardes, I.; Brunetti, L.; Leone, S.; Di Simone, S.C. Tanacetum vulgare L. (Tansy) as an effective bioresource with promising pharmacological effects from natural arsenal. Food Chem. Toxicol. 2021, 153, 112268. [Google Scholar] [CrossRef] [PubMed]
- Bota, V.B.; Neamtu, A.-A.; Olah, N.-K.; Chișe, E.; Burtescu, R.F.; Pripon Furtuna, F.R.; Nicula, A.-S.; Neamtu, C.; Maghiar, A.-M.; Ivănescu, L.-C. A Comparative analysis of the anatomy, phenolic profile, and antioxidant capacity of Tussilago farfara L. vegetative organs. Plants 2022, 11, 1663. [Google Scholar] [CrossRef]
- Uysal, S.; Senkardes, I.; Mollica, A.; Zengin, G.; Bulut, G.; Dogan, A.; Glamočlija, J.; Soković, M.; Lobine, D.; Mahomoodally, F.M. Biologically active compounds from two members of the Asteraceae family: Tragopogon dubius Scop. and Tussilago farfara L. J. Biomol. Struct. Dyn. 2019, 37, 3269–3281. [Google Scholar] [CrossRef]
- Ferrer, D.B.; Venskutonis, P.R.; Talou, T.; Zebib, B.; Ferrer, J.M.B.; Merah, O. Identification and in vitro activity of bioactive compounds extracted from Tussilago farfara (L.) plant grown in Lithuania and France. Free Radic. Antioxid. 2018, 8, 40–47. [Google Scholar]
- Dobravalskyte, D.; Venskutonis, P.R.; Talou, T.; Zebib, B.; Merah, O.; Ragazinskiene, O. Antioxidant properties and composition of deodorized extracts of Tussilago farfara L. Rec. Nat. Prod. 2013, 7, 201. [Google Scholar]
- Trendafilova, A.; Ivanova, V.; Rangelov, M.; Todorova, M.; Ozek, G.; Yur, S.; Ozek, T.; Aneva, I.; Veleva, R.; Moskova-Doumanova, V. Caffeoylquinic acids, cytotoxic, antioxidant, acetylcholinesterase and tyrosinase enzyme inhibitory activities of six Inula species from Bulgaria. Chem. Biodivers. 2020, 17, e2000051. [Google Scholar] [CrossRef]
- Sevindik, E.; Aydin, S.; Paksoy, M.Y.; Sokmen, B.B. Anti-urease, total phenolic content and antioxidant activities of some Inula L. (Asteraceae) taxa in Turkey. Genetika 2020, 52, 825–834. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Zengin, G.; Eskandani, M.; Zali, A.; Sadoughi, M.-M.; Ayatollahi, S.A. Determination of phenolics composition, antioxidant activity, and therapeutic potential of Golden marguerite (Cota tinctoria). J. Food Meas. Charact. 2021, 15, 3314–3322. [Google Scholar] [CrossRef]
- Meriç, Z.; Özdemir Nath, E.; DOĞAN, A.; BİTİŞ, L. Antioxidant, anti-tyrosinase activities and characterization of phenolic compounds for some plants from the Marmara Region, Türkiye. J. Res. Pharm. 2024, 28, 396–408. [Google Scholar] [CrossRef]
- Orlando, G.; Zengin, G.; Ferrante, C.; Ronci, M.; Recinella, L.; Senkardes, I.; Gevrenova, R.; Zheleva-Dimitrova, D.; Chiavaroli, A.; Leone, S. Comprehensive chemical profiling and multidirectional biological investigation of two wild Anthemis species (Anthemis tinctoria var. Pallida and A. cretica subsp. tenuiloba): Focus on neuroprotective effects. Molecules 2019, 24, 2582. [Google Scholar] [CrossRef]
- Fursenco, C.; Calalb, T.; Uncu, L.; Dinu, M.; Ancuceanu, R. Solidago virgaurea L.: A review of its ethnomedicinal uses, phytochemistry, and pharmacological activities. Biomolecules 2020, 10, 1619. [Google Scholar] [CrossRef]
- Abdel Motaal, A.; Ezzat, S.M.; Tadros, M.G.; El-Askary, H.I. In vivo anti-inflammatory activity of caffeoylquinic acid derivatives from Solidago virgaurea in rats. Pharm. Biol. 2016, 54, 2864–2870. [Google Scholar] [CrossRef]
- Aćimović, M.; Puvača, N. Tanacetum vulgare L.—A systematic review. J. Agron. Technol. Eng. Manag. 2020, 3, 416–422. [Google Scholar]
- Mishchenko, O.Y.; Yurchenko, K.Y.; Ostashko, V.F.; Khalieieva, O.L. COMPARATIVE STUDY OF THE EFFECT OF PHARMACEUTICAL COMPOSITIONS OF THICK EXTRACT OF COMMON TANSY FLOWERS (TANACETUM VULGARE L.) AND ESSENTIAL OILS AT THE BILIARY FUNCTION OF THE LIVER AND THE COMPOSITION OF BILE. Pharmacologyonline 2021, 3, 1502–1511. [Google Scholar]
- Stoyanova, M.A.; Perifanova-Nemska, M.N. Biologically active compounds from Tussilago farfara L. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1031, 012103. [Google Scholar] [CrossRef]
- Hleba, L.; Vuković, N.; Horská, E.; Petrová, J.; Sukdolak, S.; Kačániová, M. Phenolic profile and antimicrobial activities to selected microorganisms of some wild medical plant from Slovakia. Asian Pacific J. Trop. Dis. 2014, 4, 269–274. [Google Scholar] [CrossRef]
- Zhao, J.; Evangelopoulos, D.; Bhakta, S.; Gray, A.I.; Seidel, V. Antitubercular activity of Arctium lappa and Tussilago farfara extracts and constituents. J. Ethnopharmacol. 2014, 155, 796–800. [Google Scholar] [CrossRef]
- Wu, Q.; Zhao, D.; Xiang, J.; Zhang, M.; Zhang, C.; Xu, X. Antitussive, expectorant, and anti-inflammatory activities of four caffeoylquinic acids isolated from Tussilago farfara. Pharm. Biol. 2016, 54, 1117–1124. [Google Scholar] [CrossRef]
- Stojakowska, A.; Malarz, J.; Zubek, S.; Turnau, K.; Kisiel, W. Terpenoids and phenolics from Inula ensifolia. Biochem. Syst. Ecol. 2010, 38, 232–235. [Google Scholar] [CrossRef]
- Mrkonjić, Ž.; Rakić, D.; Olgun, E.O.; Canli, O.; Kaplan, M.; Teslić, N.; Zeković, Z.; Pavlić, B. Optimization of antioxidants recovery from wild thyme (Thymus serpyllum L.) by ultrasound-assisted extraction: Multi-response approach. J. Appl. Res. Med. Aromat. Plants 2021, 24, 100333. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A.J. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Harborne, J.B. Methods of Plant Analysis; Springer: Cham, Switzerland, 1984. [Google Scholar]
- Howard, L.R.; Clark, J.R.; Brownmiller, C. Antioxidant capacity and phenolic content in blueberries as affected by genotype and growing season. J. Sci. Food Agric. 2003, 83, 1238–1247. [Google Scholar] [CrossRef]
- Sun, B.; Ricardo-da-Silva, J.M.; Spranger, I. Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Stojković, D.; Gašić, U.; Uba, A.I.; Zengin, G.; Rajaković, M.; Stevanović, M.; Drakulić, D. Chemical profiling of Anthriscus cerefolium (L.) Hoffm., biological potential of the herbal extract, molecular modeling and KEGG pathway analysis. Fitoterapia 2024, 177, 106115. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Cvetanović, A.; Gašić, U.; Dragićević, M.; Stupar, A.; Uysal, A.; Şenkardes, I.; Sinan, K.I.; Picot-Allain, M.C.N.; Ak, G.; et al. UHPLC-LTQ OrbiTrap MS analysis and biological properties of Origanum vulgare subsp. viridulum obtained by different extraction methods. Ind. Crops Prod. 2020, 154, 112747. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Harnly, J.M. LC-PDA-ESI/MS identification of the phenolic components of three compositae spices: Chamomile, tarragon, and Mexican arnica. Nat. Prod. Commun. 2012, 7, 1934578X1200700615. [Google Scholar] [CrossRef]
- Baranauskienė, R.; Kazernavičiūtė, R.; Pukalskienė, M.; Maždžierienė, R.; Venskutonis, P.R. Agrorefinery of Tanacetum vulgare L. into valuable products and evaluation of their antioxidant properties and phytochemical composition. Ind. Crops Prod. 2014, 60, 113–122. [Google Scholar] [CrossRef]
- Kuroda, M.; Ohshima, T.; Kan, C.; Mimaki, Y. Chemical constituents of the leaves of Tussilago farfara and their aldose reductase inhibitory activity. Nat. Prod. Commun. 2016, 11, 1934578X1601101109. [Google Scholar] [CrossRef]
- Sharma, N.; Kumar, C.; Dutt, P.; Gupta, S.; Satti, N.K.; Chandra, S.; Kitchlu, S.; Paul, S.; Vishwakarma, R.A.; Verma, M.K. Isolation, chemical fingerprinting and simultaneous quantification of four compounds from tanacetum gracile using a validated HPLC–ESI-QTOF-mass spectrometry method. J. Chromatogr. Sci. 2016, 54, 796–804. [Google Scholar] [CrossRef]
- Ozek, G.; Özbek, M.U.; Arslan, M. Lipid and essential oil constituents of Cota hamzaoglui (Asteraceae). J. Turkish Chem. Soc. Sect. A Chem. 2018, 5, 1361–1370. [Google Scholar] [CrossRef]
- Zengin, G.; Cvetanović, A.; Gašić, U.; Stupar, A.; Bulut, G.; Şenkardes, I.; Dogan, A.; Sinan, K.I.; Uysal, S.; Aumeeruddy-Elalfi, Z. Modern and traditional extraction techniques affect chemical composition and bioactivity of Tanacetum parthenium (L.) Sch. Bip. Ind. Crops Prod. 2020, 146, 112202. [Google Scholar] [CrossRef]
- Herrera-Mayorga, V.; Guerrero-Sánchez, J.A.; Méndez-Álvarez, D.; Paredes-Sánchez, F.A.; Rodríguez-Duran, L.V.; Niño-García, N.; Paz-González, A.D.; Rivera, G. Insecticidal activity of organic extracts of Solidago graminifolia and its main metabolites (quercetin and chlorogenic acid) against Spodoptera frugiperda: An in vitro and in silico approach. Molecules 2022, 27, 3325. [Google Scholar] [CrossRef]
- Tsagkaris, A.S.; Louckova, A.; Jaegerova, T.; Tokarova, V.; Hajslova, J. The in vitro inhibitory effect of selected Asteraceae plants on pancreatic lipase followed by phenolic content identification through Liquid Chromatography High Resolution Mass Spectrometry (LC-HRMS). Int. J. Mol. Sci. 2022, 23, 11204. [Google Scholar] [CrossRef] [PubMed]
- Rechek, H.; Haouat, A.; Hamaidia, K.; Pinto, D.C.G.A.; Boudiar, T.; Válega, M.S.G.A.; Pereira, D.M.; Pereira, R.B.; Silva, A.M.S. Inula viscosa (L.) Aiton ethanolic extract inhibits the growth of human AGS and A549 cancer cell lines. Chem. Biodivers. 2023, 20, e202200890. [Google Scholar] [CrossRef] [PubMed]
- The CAS SciFinder Chemical Compound Database. Available online: https://scifinder-n.cas.org/ (accessed on 10 March 2025).
- Broad Institute. Morpheus Software 2025; Broad Institute: Cambridge, MA, USA, 2025. [Google Scholar]


| R | TP | TF | HCA | FL | CT | DPPH | ABTS | FRAP |
|---|---|---|---|---|---|---|---|---|
| TF | 0.960 0.000 | |||||||
| HCA | 0.952 0.000 | 0.980 0.000 | ||||||
| FL | 0.901 0.000 | 0.882 0.000 | 0.888 0.000 | |||||
| CT | −0.057 0.798 | −0.020 0.926 | −0.115 0.600 | −0.094 0.679 | ||||
| DPPH | 0.871 0.000 | 0.845 0.000 | 0.807 0.000 | 0.875 0.000 | 0.115 0.602 | |||
| ABTS | 0.968 0.000 | 0.984 0.000 | 0.974 0.000 | 0.874 0.000 | 0.028 0.897 | 0.864 0.000 | ||
| FRAP | 0.971 0.000 | 0.987 0.000 | 0.975 0.000 | 0.894 0.000 | −0.000 0.998 | 0.875 0.000 | 0.994 0.000 |
| Supplier | Chemicals | Analysis |
|---|---|---|
| Sigma-Aldrich Gmbh (Steinheim, Germany) | Folin–Ciocalteu reagent, 1,1-diphenyl-2-picryl-hydrazyl-hydrate (DPPH *), 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS *), 2,4,6-tris (2-pyridyl)-s-triazine (TPTZ, gallic acid, (±)-catechin, (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), catechin | TP test TF test DPPH assay ABTS assay FRAP assay CT test |
| Sani-Hem d.o.o. (Novi Bečej, Serbia) | ethanol 96% | ABTS assay |
| Acros Organics (Geel, Belgium) | potassium persulfate 99%, quercetin | ABTS assay, FL test |
| Carlo Erba (Emmendingen, Germany) | methanol 99.5% | DPPH assay |
| Carl ROTH GmbH + Co (Karlsruhe, Germany) | vanillin 99% | CT assay |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vojvodić, S.; Božović, D.; Aćimović, M.; Gašić, U.; Zeković, Z.; Bebek Markovinović, A.; Bursać Kovačević, D.; Zlatković, B.; Pavlić, B. A Preliminary Insight into Under-Researched Plants from the Asteraceae Family in the Balkan Peninsula: Bioactive Compound Diversity and Antioxidant Potential. Plants 2025, 14, 2904. https://doi.org/10.3390/plants14182904
Vojvodić S, Božović D, Aćimović M, Gašić U, Zeković Z, Bebek Markovinović A, Bursać Kovačević D, Zlatković B, Pavlić B. A Preliminary Insight into Under-Researched Plants from the Asteraceae Family in the Balkan Peninsula: Bioactive Compound Diversity and Antioxidant Potential. Plants. 2025; 14(18):2904. https://doi.org/10.3390/plants14182904
Chicago/Turabian StyleVojvodić, Sanja, Danica Božović, Milica Aćimović, Uroš Gašić, Zoran Zeković, Anica Bebek Markovinović, Danijela Bursać Kovačević, Bojan Zlatković, and Branimir Pavlić. 2025. "A Preliminary Insight into Under-Researched Plants from the Asteraceae Family in the Balkan Peninsula: Bioactive Compound Diversity and Antioxidant Potential" Plants 14, no. 18: 2904. https://doi.org/10.3390/plants14182904
APA StyleVojvodić, S., Božović, D., Aćimović, M., Gašić, U., Zeković, Z., Bebek Markovinović, A., Bursać Kovačević, D., Zlatković, B., & Pavlić, B. (2025). A Preliminary Insight into Under-Researched Plants from the Asteraceae Family in the Balkan Peninsula: Bioactive Compound Diversity and Antioxidant Potential. Plants, 14(18), 2904. https://doi.org/10.3390/plants14182904










