Fig Macula as a Key Multifunctional Structure Mediating the Fig–Fig Wasp Mutualism
Abstract
1. Introduction
2. Results
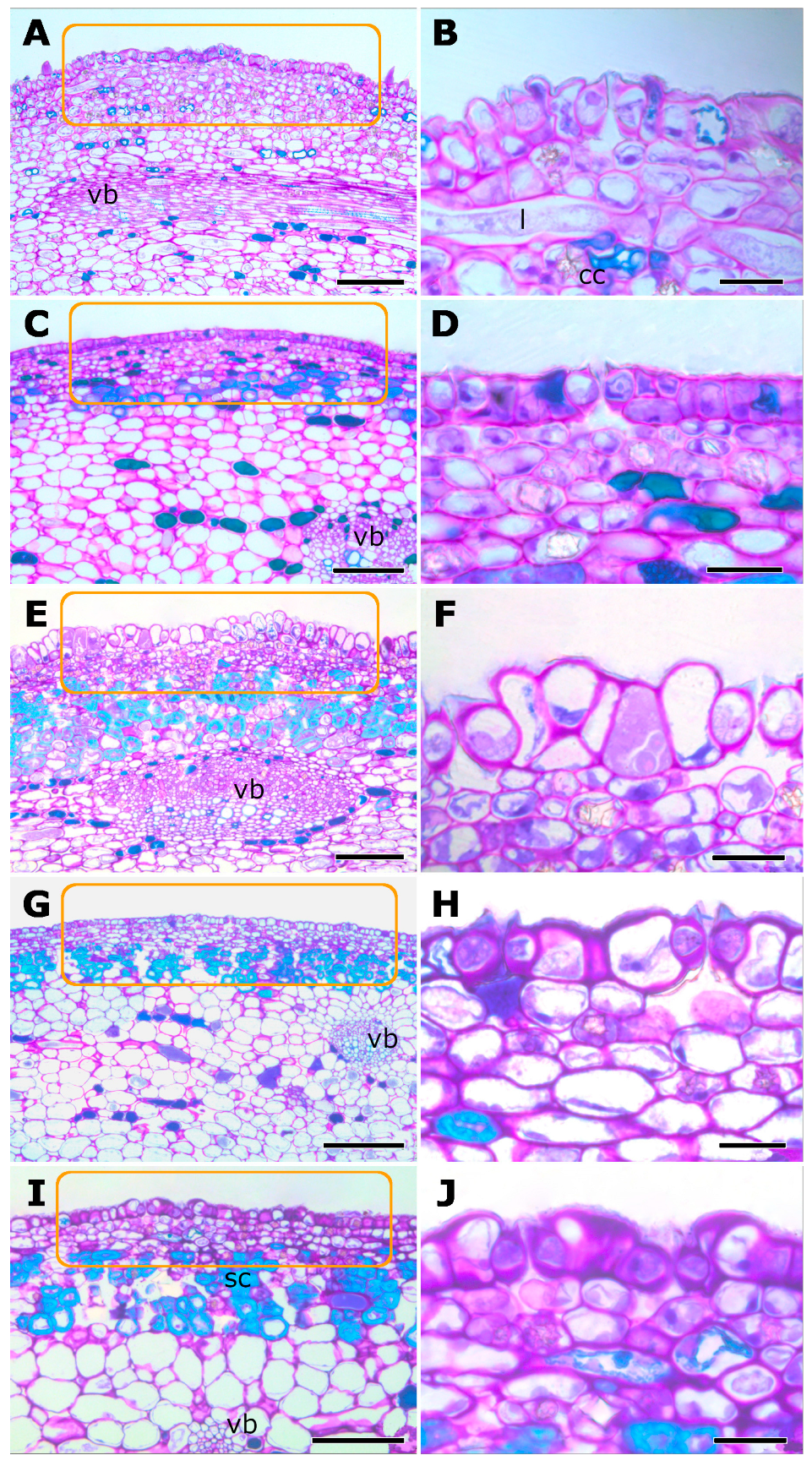
2.1. Morphological Aspects
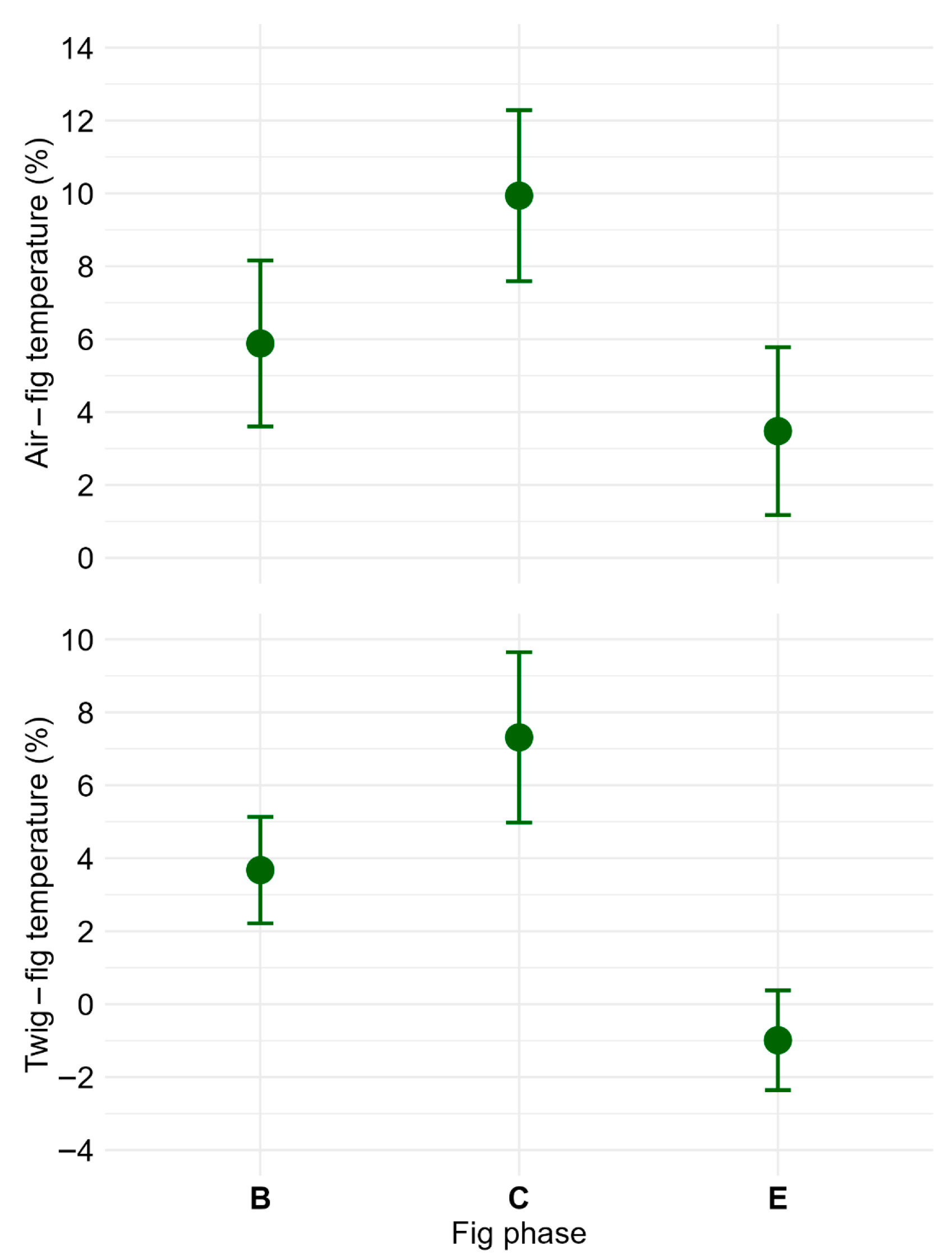
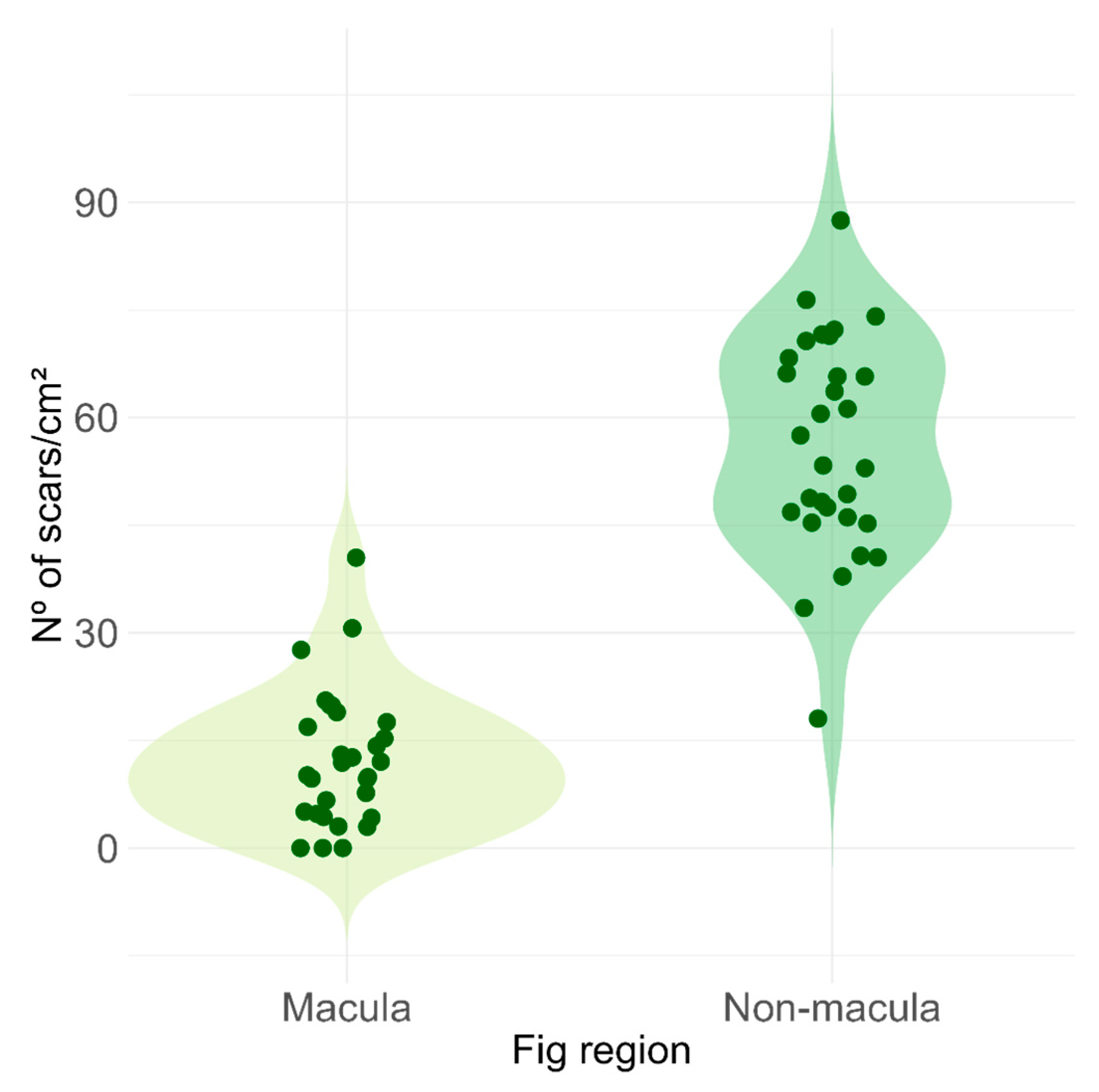
2.2. Ecological Aspects
3. Discussion
4. Materials and Methods
4.1. Study Species and Site
4.2. Morphological Aspects
4.3. Ecological Aspects
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NPFW | Non-Pollinating Fig Wasp |
| SEM | Scanning Electron Microscope |
| TEM | Transmission Electron Microscope |
| EFN | Extrafloral Nectarie |
| VOC | Volatile Organic Compound |
| SPFR | Herbário do Departamento de Biologia da Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto. Universidade de São Paulo |
References
- Fordyce, J.A. Host Shifts and Evolutionary Radiations of Butterflies. Proc. R. Soc. B. 2010, 277, 3735–3743. [Google Scholar] [CrossRef]
- Futuyma, D.J.; Agrawal, A.A. Macroevolution and the Biological Diversity of Plants and Herbivores. Proc. Natl. Acad. Sci. USA 2009, 106, 18054–18061. [Google Scholar] [CrossRef]
- Janz, N.; Nylin, S.; Wahlberg, N. Diversity Begets Diversity: Host Expansions and the Diversification of Plant-Feeding Insects. BMC Evol. Biol. 2006, 6, 4. [Google Scholar] [CrossRef]
- Jousselin, E.; Rasplus, J.Y.; Kjellberg, F. Convergence and Coevolution in a Mutualism: Evidence from a Molecular Phylogeny of Ficus. Evolution 2003, 57, 1255–1269. [Google Scholar] [CrossRef]
- Heil, M. Extrafloral Nectar at the Plant-Insect Interface: A Spotlight on Chemical Ecology, Phenotypic Plasticity, and Food Webs. Annu. Rev. Entomol. 2015, 60, 213–232. [Google Scholar] [CrossRef]
- Barônio, G.J.; Maciel, A.A.; Oliveira, A.C.; Kobal, R.O.A.C.; Meireles, D.A.L.; Brito, V.L.G.; Rech, A.R. Plantas, Polinizadores e Algumas Articulações da Biologia da Polinização com a Teoria Ecológica. Rodriguésia 2016, 67, 275–293. [Google Scholar] [CrossRef][Green Version]
- Torezan-Silingardi, H.M.; Silberbauer-Gottsberger, I.; Gottsberger, G. Pollination Ecology: Natural History, Perspectives and Future Directions. In Plant-Animal Interactions; Del-Claro, K., Torezan-Silingardi, H.M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 119–174. ISBN 978-3-030-66876-1. [Google Scholar][Green Version]
- Pereira, R.A.S. Wasp Pollination: Mechanisms, Evolution and Ecological Significance in Neglected Pollinator Groups. J Appl. Entomol. 2025, 149, 866–881. [Google Scholar] [CrossRef]
- Chomicki, G.; Thorogood, C.J.; Naikatini, A.; Renner, S.S. Squamellaria: Plants Domesticated by Ants. Plants People Planet 2019, 1, 302–305. [Google Scholar] [CrossRef]
- Seymour, R.S.; Gibernau, M.; Ito, K. Thermogenesis and Respiration of Inflorescences of the Dead Horse Arum Helicodiceros muscivorus, a Pseudo-thermoregulatory Aroid Associated with Fly Pollination. Funct. Ecol. 2003, 17, 886–894. [Google Scholar] [CrossRef]
- Patiño, S.; Aalto, T.; Edwards, A.A.; Grace, J. Is Rafflesia an Endothermic Flower? New Phytol. 2002, 154, 429–437. [Google Scholar] [CrossRef]
- Seymour, R.S. Scaling of Heat Production by Thermogenic Flowers: Limits to Floral Size and Maximum Rate of Respiration: Scaling of Thermogenic Flowers. Plant Cell Environ. 2010, 33, 1474–1485. [Google Scholar] [CrossRef]
- Seymour, R.S.; Ito, K.; Umekawa, Y.; Matthews, P.D.G.; Pirintsos, S.A. The Oxygen Supply to Thermogenic Flowers. Plant Cell Environ. 2015, 38, 827–837. [Google Scholar] [CrossRef]
- Kjellberg, F.; Jousselin, E.; Hossaert-Mckey, M.; Rasplus, J.Y. Biology, Ecology and Evolution of Fig-Pollinating Wasps (Chalcidoidea, Agaonidae). In Biology, Ecology and Evolution of Gall-Inducing Arthropods; Raman, A., Schaefer, C.W., Withers, T.M., Eds.; Science Publishers, Inc.: Enfield, NH, USA, 2005; pp. 539–571. [Google Scholar]
- Galil, J.; Eisikowitch, D. On the Pollination Ecology of Ficus sycomorus in East Africa. Ecology 1968, 49, 259–269. [Google Scholar] [CrossRef]
- Borges, R.M. How to Be a Fig Wasp Parasite on the Fig-Fig Wasp Mutualism. Curr. Opin. Insect Sci. 2015, 8, 34–40. [Google Scholar] [CrossRef]
- Elias, L.G.; Teixeira, S.P.; Kjellberg, F.; Pereira, R.A.S. Diversification in the Use of Resources by Idarnes Species: Bypassing Functional Constraints in the Fig–Fig Wasp Interaction. Biol. J. Linn. Soc. 2012, 106, 114–122. [Google Scholar] [CrossRef]
- Jansen-Gonzalez, S.; Teixeira, S.P.; Kjellberg, F.; Pereira, R.A.S. Same but Different: Larval Development and Gall-Inducing Process of a Non-Pollinating Fig Wasp Compared to That of Pollinating Fig-Wasps. Acta Oecol. 2014, 57, 44–50. [Google Scholar] [CrossRef]
- Barros, L.O.; Jansen-González, S.; Pereira, R.A.S. Drill on Drill: Adaptive Oviposition Strategies of Sycophila and Physothorax Wasps on Ficus citrifolia. Rev. Bras. Entomol. 2025, 69, e20240081. [Google Scholar] [CrossRef]
- Pederneiras, L.C.; da Costa, A.F.; de Araujo, D.S.D.; Carauta, J.P.P. Moraceae of Restingas of the State of Rio de Janeiro. Rodriguésia 2011, 62, 77–92. [Google Scholar] [CrossRef][Green Version]
- Pelissari, G.; Romaniuc Neto, S. Ficus (Moraceae) da Serra Da Mantiqueira, Brasil. Rodriguésia 2013, 64, 91–111. [Google Scholar] [CrossRef]
- Moraceae. Available online: https://web.archive.org/web/20250826143036/https://www.philippineplants.org/Families/Moraceae.html (accessed on 26 August 2025).
- Section Sycidium—FigWeb. Available online: https://web.archive.org/web/20250826141526/https://www.figweb.org/Ficus/Subgenus_Sycidium/Section_Sycidium/index.htm (accessed on 26 August 2025).
- Verkerke, W. Structure and Function of the Fig. Experientia 1989, 45, 612–622. [Google Scholar] [CrossRef]
- Harrison, R.D. Ecology of a Fig Ant–Plant. Acta Oecol. 2014, 57, 88–96. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Chou, L.-S.; Bain, A. Sugar Secretion and Ant Protection in Ficus benguetensis: Toward a General Trend of Fig–Ant Interactions. Acta Oecol. 2018, 90, 168–172. [Google Scholar] [CrossRef]
- Patino, S.; Herre, E.A.; Tyree, M.T. Physiological Determinants of Ficus Fruit Temperature and Implications for Survival of Pollinator Wasp Species: Comparative Physiology through an Energy Budget Approach. Oecologia 1994, 100, 13–20. [Google Scholar] [CrossRef]
- Wist, T.J.; Davis, A.R. Floral Nectar Production and Nectary Anatomy and Ultrastructure of Echinacea purpurea (Asteraceae). Ann. Bot. 2006, 97, 177–193. [Google Scholar] [CrossRef]
- Rocha, J.F.; Machado, S.R. Anatomy, Ultrastructure and Secretion of Hibiscus pernambucensis Arruda (Malvaceae) Extrafloral Nectary. Rev. Bras. Bot. 2009, 32, 489–498. [Google Scholar] [CrossRef]
- Willmer, P.G.; Nuttman, C.V.; Raine, N.E.; Stone, G.N.; Pattrick, J.G.; Henson, K.; Stillman, P.; McIlroy, L.; Potts, S.G.; Knudsen, J.T. Floral Volatiles Controlling Ant Behaviour. Funct. Ecol. 2009, 23, 888–900. [Google Scholar] [CrossRef]
- Nicolson, S.W.; Nepi, M.; Pacini, E. (Eds.) Nectaries and Nectar; Springer: Dordrecht, The Netherlands, 2007; ISBN 978-1-4020-5936-0. [Google Scholar]
- Fahn, A. Secretory Tissues in Plants; Academic Press: New York, NY, USA, 1979. [Google Scholar]
- Roy, R.; Schmitt, A.J.; Thomas, J.B.; Carter, C.J. Review: Nectar Biology: From Molecules to Ecosystems. Plant Sci. 2017, 262, 148–164. [Google Scholar] [CrossRef]
- Silva, G.S.; Leite, V.G.; Falcão, M.J.A.; Paulino, J.V.; Teixeira, S.P.; Mansano, V.F. Ontogeny and Glandular Features of Alexa grandiflora Flowers Offer Evolutionary Insights into the Angylocalyx Clade: A Unique Non-Papilionaceous Lineage within Papilionoideae (Leguminosae). Preprint. Research Square. 2025. [Google Scholar] [CrossRef]
- Marinho, C.R.; Souza, C.D.; Barros, T.C.; Teixeira, S.P. Scent Glands in Legume Flowers. Plant Biol. 2014, 16, 215–226. [Google Scholar] [CrossRef]
- Souza, C.D.; Pereira, R.A.S.; Marinho, C.R.; Kjellberg, F.; Teixeira, S.P. Diversity of Fig Glands Is Associated with Nursery Mutualism in Fig Trees. Am. J. Bot. 2015, 102, 1564–1577. [Google Scholar] [CrossRef]
- Proffit, M.; Schatz, B.; Borges, R.M.; Hossaert-Mckey, M. Chemical Mediation and Niche Partitioning in Non-Pollinating Fig-Wasp Communities. J. Anim. Ecol. 2007, 76, 296–303. [Google Scholar] [CrossRef] [PubMed]
- González-Teuber, M.; Heil, M. Nectar Chemistry Is Tailored for Both Attraction of Mutualists and Protection from Exploiters. Plant Signal. Behav. 2009, 4, 809–813. [Google Scholar] [CrossRef]
- Kessler, D.; Gase, K.; Baldwin, I.T. Field Experiments with Transformed Plants Reveal the Sense of Floral Scents. Science 2008, 321, 1200–1202. [Google Scholar] [CrossRef]
- Heyneman, A.J.; Colwell, R.K.; Naeem, S.; Dobkin, D.S.; Hallet, B. Host Plant Discrimination: Experiments with Hummingbird Flower Mites. In Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions; Price, P.W., Lewinsohn, T.M., Fernandes, G.W., Benson, W.W., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 1991; pp. 455–485. [Google Scholar]
- Röse, U.S.R.; Lewis, J.; Tumlinson, J.H. Extrafloral Nectar from Cotton (Gossypium hirsutum) as a Food Source for Parasitic Wasps. Funct. Ecol. 2006, 20, 67–74. [Google Scholar] [CrossRef]
- Bain, A.; Harrison, R.D.; Schatz, B. How to Be an Ant on Figs. Acta Oecol. 2014, 57, 97–108. [Google Scholar] [CrossRef]
- Compton, S.G.; Robertson, H.G. Complex Interactions between Mutualisms: Ants Tending Homopterans Protect Fig Seeds and Pollinators. Ecology 1988, 69, 1302–1305. [Google Scholar] [CrossRef]
- Pereira, R.A.S.; Semir, J.; Menezes, A.O., Jr. Pollination and Other Biotic Interactions in Figs of Ficus eximia Schott (Moraceae). Braz. J. Bot. 2000, 23, 217–224. [Google Scholar] [CrossRef]
- Schatz, B.; Proffit, M.; Rakhi, B.V.; Borges, R.M.; Hossaert-McKey, M. Complex Interactions on Fig Trees: Ants Capturing Parasitic Wasps as Possible Indirect Mutualists of the Fig-Fig Wasp Interaction. OIKOS 2006, 113, 344–352. [Google Scholar] [CrossRef]
- Horn, M.H. Evidence for Dispersal of Fig Seeds by the Fruit-Eating Characid Fish Brycon guatemalensis Regan in a Costa Rican Tropical Rain Forest. Oecologia 1997, 109, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Banack, S.A.; Horn, M.H.; Gawlicka, A. Disperser- vs. Establishment-Limited Distribution of a Riparian Fig Tree (Ficus insipida) in a Costa Rican Tropical Rain Forest. Biotropica 2002, 34, 232–243. [Google Scholar]
- Berg, C.C.; Villavicencio, X. Taxonomic Studies on Ficus (Moraceae) in the West Indies, Extra-Amazonian Brazil and Bolivia. Ilicifolia 2004, 4, 1–132. [Google Scholar]
- Coelho, L.F.M.; Ribeiro, M.C.; Pereira, R.A.S. Water Availability Determines the Richness and Density of Fig Trees within Brazilian Semideciduous Forest Landscapes. Acta Oecol. 2014, 57, 109–116. [Google Scholar] [CrossRef]
- Compton, S.G.; Greeff, J.M. Few Figs for Frugivores: Riparian Fig Trees in Zimbabwe May Not Be a Dry Season Keystone Resource. Afr. J. Ecol. 2020, 58, 778–785. [Google Scholar] [CrossRef]
- Silman, M.R.; Krisel, C. Getting to the Root of Tree Neighbourhoods: Hectare-Scale Root Zones of a Neotropical Fig. J. Trop. Ecol. 2006, 22, 727–730. [Google Scholar] [CrossRef]
- Berg, C.C. Proposals for Treating Four Species Complexes in Ficus Subgenus Urostigma Section Americanae (Moraceae). Blumea 2007, 52, 295–312. [Google Scholar] [CrossRef]
- Proffit, M.; Lapeyre, B.; Buatois, B.; Deng, X.-X.; Arnal, P.; Gouzerh, F.; Carrasco, D.; Hossaert-McKey, M. Chemical Signal Is in the Blend: Bases of Plant-Pollinator Encounter in a Highly Specialized Interaction. Sci. Rep. 2020, 10, 10071. [Google Scholar] [CrossRef]
- Lillie, R.D. Histopathologic Technic and Practical Histochemistry; McGraw-Hill Book Co.: New York, NY, USA, 1954. [Google Scholar]
- Gerrits, P.O. The Application of Glycol Methacrylate in Histotechnology; Some Fundamental Principles; Department of Anatomy and Embriology, State University Groningen: Groningen, The Netherlands, 1991. [Google Scholar]
- O’Brien, T.P.; Feder, N.; Mccully, M.E. Polychromatic Staining of Plant Cell Walls by Toluidine Blue O. Protoplasma 1964, 59, 368–373. [Google Scholar] [CrossRef]
- Johansen, D.A. Plant Microtechnique; McGraw-Hill Book Company Inc.: New York, NY, USA, 1940. [Google Scholar]
- McManus, J.F.A. Histological and Histochemical Uses of Periodic Acid. Stain. Technol. 1948, 23, 97–98. [Google Scholar] [CrossRef]
- Kraus, J.; Arduin, M. Manual Básico de Métodos em Morfologia Vegetal; EDUR: Seropédica, Brazil, 1997. [Google Scholar]
- Vidal, B.C. Acid Glycosaminoglycans and Endochondral Ossification: Microespectrophotometric Evaluation and Macromolecular Orientation. Cell Mol. Biol. 1977, 22, 45–64. [Google Scholar]
- Sass, J.E. Botanical Microtechnique; The Iowa State College Press: Ames, IA, USA, 1951. [Google Scholar]
- David, R.; Carde, J.P. Coloration Différentielle des Inclusions Lipidiques et Terpéniques des Pseudophylles du Pin Maritime au Moyen du Réactif Nadi. Comptes Rendus Hebd. Séances L’académie Sci. Paris 1964, 258, 1338–1340. [Google Scholar]
- Karnovsky, M. A Formaldehyde-Glutaraldehyde Fixative of High Osmolarity for Use in Electron Microscopy. J. Cell Biol. 1965, 27, 137A–138A. [Google Scholar]
- Watson, M.L. Staining of Tissue Sections for Electron Microscopy with Heavy Metals. J. Biophys. Biochem. Cytol. 1958, 4, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.S. The Use of Lead Citrate at High pH as an Electron-Opaque Stain in Electron Microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, S.P.; Silva, J.V.; Santos, V.C.; Mazzeo, L.; Correa, R.C.C.; Pereira, R.A.S. Fig Macula as a Key Multifunctional Structure Mediating the Fig–Fig Wasp Mutualism. Plants 2025, 14, 2885. https://doi.org/10.3390/plants14182885
Teixeira SP, Silva JV, Santos VC, Mazzeo L, Correa RCC, Pereira RAS. Fig Macula as a Key Multifunctional Structure Mediating the Fig–Fig Wasp Mutualism. Plants. 2025; 14(18):2885. https://doi.org/10.3390/plants14182885
Chicago/Turabian StyleTeixeira, Simone Pádua, Jackeline Varanda Silva, Vitor Cassius Santos, Luan Mazzeo, Rayssa Conceição Coelho Correa, and Rodrigo Augusto Santinelo Pereira. 2025. "Fig Macula as a Key Multifunctional Structure Mediating the Fig–Fig Wasp Mutualism" Plants 14, no. 18: 2885. https://doi.org/10.3390/plants14182885
APA StyleTeixeira, S. P., Silva, J. V., Santos, V. C., Mazzeo, L., Correa, R. C. C., & Pereira, R. A. S. (2025). Fig Macula as a Key Multifunctional Structure Mediating the Fig–Fig Wasp Mutualism. Plants, 14(18), 2885. https://doi.org/10.3390/plants14182885







