Multidisciplinary Bioanalytical Approach to Assess the Anti-Aging Properties of Flower Petals—A Promising Sustainable Cosmetic Ingredient
Abstract
1. Introduction
2. Results and Discussion
2.1. HPTLC Phenolic Profiles of PEs
2.2. UHPLC-DAD-MS/MS
2.3. Skin-Antiaging Assays
2.3.1. Tyrosinase and Elastase Inhibition Assays
2.3.2. Radical Scavenging (RS) Capacity
2.3.3. Determination of the Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.4. In Silico Docking Assessment
2.4.1. Tyrosinase Docking
2.4.2. Elastase Docking
2.5. Toxicity of PEs
2.5.1. Cytotoxic Activity Against HaCaT Cells
2.5.2. Developmental Toxicity of Evaluated PEs in Zebrafish Embryos
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Petal Material and Preparation of Extracts
3.2.1. Petal Sampling
3.2.2. Petal Material Extraction
3.3. HPTLC Analysis
Derivatization with Natural Product Reagent (NPR)
3.4. UHPLC-DAD-MS
3.5. Spectrophotometric Assays
3.5.1. Radical Scavenging Capacity Assays
(DPPH● Radical Scavenging Assay)
(ABTS●+ Radical Scavenging Assay)
3.5.2. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
3.5.3. Inhibition of Tyrosinase (Anti-Tyrosinase Assay)
- A = Absorbance of the enzyme with the substrate (positive control)
- B = Absorbance of the blank (substrate without enzyme)
- C = Absorbance of the sample with the enzyme and substrate
- D = Absorbance of the sample blank (sample without enzyme)
3.5.4. Inhibition of Elastase (Anti-Elastase Assay)
- A = Absorbance of the positive control (enzyme with substrate)
- B = Absorbance of the sample (enzyme with substrate and sample)
3.6. Docking Studies
3.7. Evaluation of PEs’ Toxicity
3.7.1. HaCaT Viability Assay
3.7.2. In Vivo Toxicity of PEs
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AES | Aesculetin |
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| AST | Astragalin |
| CA | Chlorogenic acid |
| CAFFA | Caffeic acid |
| CTRL | Control |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DMSO | Dimethyl sulfoxide |
| EC | Epicatechin |
| ECM | Extracellular matrix |
| EGCG | Epigallocatechin gallate |
| EI | Elastase inhibition |
| FET | Fish Embryo Toxicity |
| FBS | Fetal Bovine Serum |
| GA | Gallic acid |
| HaCaT | Human Immortalized Keratinocytes |
| Hpf | Hours post fertilization |
| HPTLC | High-performance thin-layer chromatography |
| IC50 | Half maximal inhibitory concentration |
| ISOQ | Isoquercetin |
| ISORH | Isorhamnetin |
| ISORH-3-O-G | Isorhamnetin-3-O-glucoside |
| ISORH-3-O-R | Isorhamnetin-3-O-rutinoside |
| K | Kaempferol |
| L-DOPA | 3,4-Dihydroxy-L-phenylalanine |
| LU | Luteolin |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NAR | Naringin |
| NEAA | Non-essential amino acids |
| NMR | Nuclear Magnetic Resonance |
| NPR | Natural Product Reagent |
| PEs | Petal extracts |
| PPE | Porcine pancreatic elastase |
| PEG | Polyethylene Glycol |
| Q | Quercetin |
| Q-3-O-G | Quercetin-3-O-rhamnoside |
| Q-3-O-R | Quercetin-3-O-glucoside |
| RS | Radical scavenging |
| RU | Rutin |
| TE | Trolox equivalents |
| TFC | Total Flavonoid Content |
| TI | Tyrosinase inhibition |
| TPC | Total phenolic content |
| UHPLC- | Ultra-High-Performance Liquid Chromatography coupled with Tandem |
| MS/MS | Mass Spectrometry |
References
- Future Market Insights. Herbal Beauty Products Market Set to Reach USD 135,897.65 Million by 2034, Driven by Growing Demand for Natural & Sustainable Skincare Solutions. Available online: https://www.globenewswire.com/news-release/2024/12/18/2998692/0/en/Herbal-Beauty-Products-Market-Set-to-Reach-USD-135-897-65-Million-by-2034-Driven-by-Growing-Demand-for-Natural-Sustainable-Skincare-Solutions-Future-Market-Insights-Inc.html (accessed on 19 July 2025).
- Food and Agriculture Organization of the United Nations (FAO). Impact of Cultivation and Gathering of Medicinal Plants on Biodiversity: Global Trends and Issues. Available online: https://www.fao.org/4/aa010e/AA010E00.pdf (accessed on 26 August 2025).
- Yang, W.; Liu, F.; Wu, G.; Liang, S.; Bai, X.; Liu, B.; Zhang, B.; Chen, H.; Yang, J. Widely Targeted Metabolomics Analysis of the Roots, Stems, Leaves, Flowers, and Fruits of Camellia luteoflora, a Species with an Extremely Small Population. Molecules 2024, 29, 4754. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, X.; Zhou, Y.; Yang, H.; Wang, Y.; Chen, T.; Chen, Q.; Deng, Y. Metabolite Profiling of External and Internal Petals in Three Different Colors of Tea Flowers (Camellia sinensis) Using Widely Targeted Metabolomics. Metabolites 2023, 13, 784. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. The Role of Transcription Factor Nrf2 in Skin Cells Metabolism. Arch. Dermatol. Res. 2015, 307, 385–396. [Google Scholar] [CrossRef]
- Cruz, A.M.; Gonçalves, M.C.; Marques, M.S.; Veiga, F.; Paiva-Santos, A.C.; Pires, P.C. In Vitro Models for Anti-Aging Efficacy Assessment: A Critical Update in Dermocosmetic Research. Cosmetics 2023, 10, 66. [Google Scholar] [CrossRef]
- Ersoy, E.; Eroglu Ozkan, E.; Boga, M.; Yilmaz, M.; Mat, A. Anti-Aging Potential and Anti-Tyrosinase Activity of Three Hypericum Species with Focus on Phytochemical Composition by LC–MS/MS. Ind. Crops Prod. 2019, 141, 111735. [Google Scholar] [CrossRef]
- Salem, M.A.; Radwan, R.; Mostafa, E.; Alseekh, S.; Fernie, A.; Ezzat, S. Using a UPLC/MS-Based Untargeted Metabolomics Approach for Assessing the Antioxidant Capacity and Anti-Aging Potential of Selected Herbs. RSC Adv. 2020, 10, 44263–44275. [Google Scholar] [CrossRef]
- Hussin, M.; Abdul Hamid, A.; Abas, F.; Ramli, N.S.; Jaafar, A.; Roowi, S.; Abdul Majid, N.; Pak Dek, M.S. NMR-Based Metabolomics Profiling for Radical Scavenging and Anti-Aging Properties of Selected Herbs. Molecules 2019, 24, 3208. [Google Scholar] [CrossRef]
- Ivković, Đ.; Andrić, F.; Senćanski, M.; Stević, T.; Krstić Ristivojević, M.; Ristivojević, P. Innovative Analytical Methodology for Skin Anti-Aging Compounds Discovery from Plant Extracts: Integration of High-Performance Thin-Layer Chromatography-in Vitro Spectrophotometry Bioassays with Multivariate Modeling and Molecular Docking. J. Chromatogr. A 2025, 1742, 465640. [Google Scholar] [CrossRef]
- Stanojević, L.P.; Marković, J.M.; Catapano, A.; Santoso, E.F.; Cvetković, D.; Zeković, Z. Chemical Profile, Cytotoxic Activity and Oxidative Stress Reduction of Different Syringa vulgaris L. Extracts. Molecules 2021, 26, 3104. [Google Scholar] [CrossRef] [PubMed]
- Radenković, M.; Duletić-Laušević, S.; Čabarkapa, I.; Jovanović, S.; Veličković, L.; Tešević, V.; Todorović, V.; Gajić, I.; Mišić, D. Chemical Profile and Skin-Beneficial Activities of the Petal Extracts of Paeonia tenuifolia L. from Serbia. Pharmaceuticals 2022, 15, 1537. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Kwon, Y.S.; Son, K.H.; Kim, H.P.; Heo, M.Y. Antioxidative Constituents from Paeonia lactiflora. Arch. Pharm. Res. 2005, 28, 775–783. [Google Scholar] [CrossRef]
- Stoycheva, C.; Batovska, D.; Malfa, G.A.; Acquaviva, R.; Statti, G.; Kozuharova, E. Prospective Approaches to the Sustainable Use of Peonies in Bulgaria. Plants 2025, 14, 969. [Google Scholar] [CrossRef]
- Lv, M.; Yang, Y.; Choisy, P.; Xu, T.; Pays, K.; Zhang, L.; Zhu, J.; Wang, Q.; Li, S.; Wang, L. Flavonoid Components and Anti-Photoaging Activity of Flower Extracts from Six Paeonia Cultivars. Ind. Crops Prod. 2023, 200, 116707. [Google Scholar] [CrossRef]
- Dudek-Makuch, M.; Matławska, I. Flavonoids from the Flowers of Aesculus Hippocastanum. Acta Pol. Pharm. 2011, 68, 403–408. [Google Scholar] [PubMed]
- Mosleh, G.; Azadi, A.; Khademian, S.; Heidari, R.; Mohagheghzadeh, A. Anti-Inflammatory Activity and Quality Control of Erysimum cheiri (L.) Crantz. BioMed Res. Int. 2021, 2021, 5526644. [Google Scholar] [CrossRef]
- Yüksel, M.; Yüksel, A.K.; Topdaş, E.F. Phytochemical Contents and In Vitro Antioxidant and Antibacterial Activities of Root Extracts from Pelargonium Species. Chem. Biodivers. 2025, 22, e202402382. [Google Scholar] [CrossRef]
- Pető, Á.; Kósa, D.; Haimhoffer, Á.; Nemes, D.; Fehér, P.; Ujhelyi, Z.; Vecsernyés, M.; Váradi, J.; Fenyvesi, F.; Frum, A.; et al. Topical Dosage Formulation of Lyophilized Philadelphus coronarius L. Leaf and Flower: Antimicrobial, Antioxidant and Anti-Inflammatory Assessment of the Plant. Molecules 2022, 27, 2652. [Google Scholar] [CrossRef]
- Klečáková, J.; Chobot, V.; Jahodář, L.; Laakso, I.; Víchová, P. Antiradical Activity of Petals of Philadelphus coronarius L. Cent. Eur. J. Public Health 2004, 12, S39–S40. [Google Scholar]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible Flowers as Sources of Phenolic Compounds with Bioactive Potential. Food Res. Int. 2018, 105, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Gąsecka, M.; Krzymińska-Bródka, A.; Magdziak, Z.; Czuchaj, P.; Bykowska, J. Phenolic Compounds and Organic Acid Composition of Syringa vulgaris L. Flowers and Infusions. Molecules 2023, 28, 5159. [Google Scholar] [CrossRef] [PubMed]
- Tamfu, A.N.; Kucukaydin, S.; Quradha, M.M.; Ceylan, O.; Ugur, A.; Duru, M.E. Ultrasound-Assisted Extraction of Syringa vulgaris Mill., Citrus sinensis L. and Hypericum perforatum L.: Phenolic Composition, Enzyme Inhibition and Anti-Quorum Sensing Activities. Chem. Afr. 2022, 5, 237–249. [Google Scholar] [CrossRef]
- Hyun, H.B.; Hyeon, H.J.; Kim, S.C.; Go, B.; Yoon, S.-A.; Jung, Y.-H.; Ham, Y.-M. Anti-Melanogenesis Effects of Schizophragma hydrangeoides Leaf Ethanol Extracts via Downregulation of Tyrosinase Activity. Korean J. Plant Resour. 2021, 34, 510–516. [Google Scholar] [CrossRef]
- Akin, M.; Saki, N. Antimicrobial, DPPH Scavenging and Tyrosinase Inhibitory Activities of Thymus vulgaris, Helichrysum arenarium and Rosa damascena mill. Ethanol Extracts by Using TLC Bioautography and Chemical Screening Methods. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 204–216. [Google Scholar] [CrossRef]
- Sopharadee, S.; Kittipitchakul, J.; Srisawas, N.; Neimkhum, W.; Yawootti, A.; Rades, T.; Chaiyana, W. Green Approach for Rosa damascena Mill. Petal Extract: Insights into Phytochemical Composition, Anti-Aging Potential, and Stability. Antioxidants 2025, 14, 541. [Google Scholar] [CrossRef]
- Rainer, B.; Revoltella, S.; Mayr, F.; Moesslacher, J.; Scalfari, V.; Kohl, R.; Waltenberger, B.; Pagitz, K.; Siewert, B.; Schwaiger, S.; et al. From Bench to Counter: Discovery and Validation of a Peony Extract as Tyrosinase Inhibiting Cosmeceutical. Eur. J. Med. Chem. 2019, 184, 111738. [Google Scholar] [CrossRef] [PubMed]
- Dimitropoulou, E.; Graikou, K.; Klontza, V.; Chinou, I. Chemical Profiling on Bioactive Stilbenoids in the Seeds of Paeonia Species Growing Wild in Greece. Separations 2023, 10, 540. [Google Scholar] [CrossRef]
- Batinić, P.; Jovanović, A.; Stojković, D.; Zengin, G.; Cvijetić, I.; Gašić, U.; Čutović, N.; Pešić, M.B.; Milinčić, D.D.; Carević, T.; et al. Phytochemical Analysis, Biological Activities, and Molecular Docking Studies of Root Extracts from Paeonia Species in Serbia. Pharmaceuticals 2024, 17, 518. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Locatelli, M.; Tartaglia, A.; Ferrone, V.; Juszczak, A.M.; Ozer, M.S.; Tepe, B.; Tomczyk, M. Enzyme and Biological Activities of the Water Extracts from the Plants Aesculus hippocastanum, Olea europaea and Hypericum perforatum That Are Used as Folk Remedies in Turkey. Molecules 2020, 25, 1202. [Google Scholar] [CrossRef]
- El-Otmani, N.A.J.L.A.E.; Akoh, R.; Ouazzani, R.; Zeouk, I.; Loukili, A.; Bousta, D.; Zahidi, A. Assessment of Pelargonium graveolens Flower Essential Oil: Antimicrobial, Antioxidant, Enzyme Inhibition and in Vivo Topical Analgesic and Anti-Inflammatory Efficacy as Treatment for Atopic Dermatitis. F1000Research 2024, 13, 1366. [Google Scholar] [CrossRef]
- Ak, G.; Zengin, G.; Ceylan, R.; Fawzi Mahomoodally, M.; Jugreet, S.; Mollica, A.; Stefanucci, A. Chemical Composition and Biological Activities of Essential Oils from Calendula officinalis L. Flowers and Leaves. Flavour Fragr. J. 2021, 36, 554–563. [Google Scholar] [CrossRef]
- Boo, Y.C. p-Coumaric Acid as An Active Ingredient in Cosmetics: A Review Focusing on its Antimelanogenic Effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef]
- Si, Y.-X.; Yin, S.-J.; Oh, S.; Wang, Z.-J.; Ye, S.; Yan, L.; Yang, J.-M.; Park, Y.-D.; Lee, J.; Qian, G.-Y. An Integrated Study of Tyrosinase Inhibition by Rutin: Progress Using a Computational Simulation. J. Biomol. Struct. Dyn. 2012, 29, 999–1012. [Google Scholar] [CrossRef]
- Sklirou, A.D.; Angelopoulou, M.T.; Argyropoulou, A.; Chaita, E.; Boka, V.I.; Cheimonidi, C.; Niforou, K.; Mavrogonatou, E.; Pratsinis, H.; Kalpoutzakis, E.; et al. Phytochemical Study and In Vitro Screening Focusing on the Anti-Aging Features of Various Plants of the Greek Flora. Antioxidants 2021, 10, 1206. [Google Scholar] [CrossRef]
- Mawarni, E.; Ginting, C.N.; Chiuman, L.; Girsang, E.; Handayani, R.A.S.; Widowati, W. Antioxidant and Elastase Inhibitor Potential of Petals and Receptacle of Rose Flower (Rosa damascena). Pharm. Sci. Res. 2020, 7, 105–113. [Google Scholar] [CrossRef]
- Păcularu-Burada, B.; Cîrîc, A.-I.; Begea, M. Anti-Aging Effects of Flavonoids from Plant Extracts. Foods 2024, 13, 2441. [Google Scholar] [CrossRef]
- Kaur, M.; Malik, J.; Naura, A.S. Inhibitory effects of polyphenols from grape pomace extract on elastase and collagenase activities. Food Chem. Toxicol. 2015, 80, 179–187. [Google Scholar] [CrossRef]
- Jakimiuk, K.; Gesek, J.; Atanasov, A.G.; Tomczyk, M. Flavonoids as inhibitors of human neutrophil elastase. J. Enzym. Inhib. Med. Chem. 2021, 36, 1016–1028. [Google Scholar] [CrossRef] [PubMed]
- Dienaitė, L.; Pukalskienė, M.; Pukalskas, A.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Isolation of Strong Antioxidants from Paeonia Officinalis Roots and Leaves and Evaluation of Their Bioactivities. Antioxidants 2019, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- Dubois, C.; Plainfossé, H.; Delcroix, M.; Trinel, M.; Verger-Dubois, G.; Azoulay, S.; Burger, P.; Fernandez, X. Anti-Aging Potential of a Rosa centifolia Stem Extract with Focus on Phytochemical Composition by Bioguided Fractionation. Chem. Biodivers. 2022, 19, e202200158. [Google Scholar] [CrossRef]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.-C. Correlation Study of Antioxidant Activity with Phenolic and Flavonoid Compounds in 12 Indonesian Indigenous Herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef]
- Li, H.-B.; Wong, C.-C.; Cheng, K.-W.; Chen, F. Antioxidant Properties in Vitro and Total Phenolic Contents in Methanol Extracts from Medicinal Plants. LWT 2008, 41, 385–390. [Google Scholar] [CrossRef]
- Krzymińska, A.; Gąsecka, M.; Magdziak, Z. Content of Phenolic Compounds and Organic Acids in the Flowers of Selected Tulipa gesneriana Cultivars. Molecules 2020, 25, 5627. [Google Scholar] [CrossRef] [PubMed]
- Nouioua, W.; Gaamoune, S.; Kaabache, M. Evaluation of antimicrobial and antioxidant activity of areal part methanolic extract of Paeonia mascula (L.) Mill. Sch. J. Agric. Vet. Sci. 2019, 6, 109–114. Available online: https://www.saspublishers.com/article/17447/ (accessed on 10 September 2025).
- Garcia-Jimenez, A.; Teruel-Puche, J.A.; Garcia-Ruiz, P.A.; Saura-Sanmartin, A.; Berna, J.; Rodríguez-López, J.N.; Garcia-Canovas, F. Action of Tyrosinase on Caffeic Acid and Its N-Nonyl Ester. Catalysis and Suicide Inactivation. Int. J. Biol. Macromol. 2018, 107, 2650–2659. [Google Scholar] [CrossRef]
- Desmiaty, Y.; Mulatsari, E.; Chany Saputri, F.; Hanafi, M.; Prastiwi, R.; Elya, B. Inhibition of Pancreatic Elastase in Silico and in Vitro by Rubus rosifolius Leaves Extract and Its Constituents. J. Pharm. Bioallied Sci. 2020, 12, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Mechqoq, H.; Hourfane, S.; El Yaagoubi, M.; El Hamdaoui, A.; da Silva Almeida, J.R.G.; Rocha, J.M.; El Aouad, N. Molecular Docking, Tyrosinase, Collagenase, and Elastase Inhibition Activities of Argan By-Products. Cosmetics 2022, 9, 24. [Google Scholar] [CrossRef]
- Mohan, S.; Prabhakaran, V.-S.; Narayanaswamy, R. In Silico Analysis of Cissus Rotundifolia Constituents as Human Neutrophil Elastase (HNE), Matrix Metalloproteinases (MMP 2 and MMP 9), and Tyrosinase Inhibitors. Appl. Biochem. Biotechnol. 2022, 194, 232–245. [Google Scholar] [CrossRef]
- Deniz, F.S.S.; Salmas, R.E.; Emerce, E.; Cankaya, I.I.T.; Yusufoglu, H.S.; Orhan, I.E. Evaluation of Collagenase, Elastase and Tyrosinase Inhibitory Activities of Cotinus Coggygria Scop. through in Vitro and in Silico Approaches. South Afr. J. Bot. 2020, 132, 277–288. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Morlock, G.E.; Heil, J.; Bardot, V.; Lenoir, L.; Cotte, C.; Dubourdeaux, M. Effect-Directed Profiling of 17 Different Fortified Plant Extracts by High-Performance Thin-Layer Chromatography Combined with Six Planar Assays and High-Resolution Mass Spectrometry. Molecules 2021, 26, 1468. [Google Scholar] [CrossRef]
- Ivkovic, D.; Cvijetic, I.; Radoicic, A.; Stojkovic-Filipovic, J.; Trifkovic, J.; Krstic Ristivojevic, M.; Ristivojevic, P. NADES-Based Extracts of Selected Medicinal Herbs as Promising Formulations for Cosmetic Usage. Processes 2024, 12, 992. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal Structure of Agaricus bisporus Mushroom Tyrosinase: Identity of the Tetramer Subunits and Interaction with Tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef]
- Klabunde, T.; Eicken, C.; Sacchettini, J.C.; Krebs, B. Crystal Structure of a Plant Catechol Oxidase Containing a Dicopper Center. Nat. Struct. Biol. 1998, 5, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Maria-Solano, M.A.; Ortiz-Ruiz, C.V.; Muñoz-Muñoz, J.L.; Teruel-Puche, J.A.; Berna, J.; Garcia-Ruiz, P.A.; Garcia-Canovas, F. Further Insight into the pH Effect on the Catalysis of Mushroom Tyrosinase. J. Mol. Catal. B Enzym. 2016, 125, 6–15. [Google Scholar] [CrossRef]
- Wilmouth, R.C.; Kassamally, S.; Westwood, N.J.; Sheppard, R.J.; Claridge, T.D.; Aplin, R.T.; Wright, P.A.; Pritchard, G.J.; Schofield, C.J. Mechanistic Insights into the Inhibition of Serine Proteases by Monocyclic Lactams. Biochemistry 1999, 38, 7989–7998. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminformatics 2012, 4, 17. [Google Scholar] [CrossRef]
- Halgren, T.A. Merck Molecular Force Field. I.; Basis, Form, Scope, Parameterization, and Performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods VI: More Modifications to the NDDO Approximations and Re-Optimization of Parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Santos-Martins, D.; Solis-Vasquez, L.; Tillack, A.F.; Sanner, M.F.; Koch, A.; Forli, S. Accelerating AutoDock4 with GPUs and Gradient-Based Local Search. J. Chem. Theory Comput. 2021, 17, 1060–1073. [Google Scholar] [CrossRef]
- Test No. 236: Fish Embryo Acute Toxicity (FET) Test. Available online: https://www.oecd.org/en/publications/test-no-236-fish-embryo-acute-toxicity-fet-test_9789264203709-en.html (accessed on 18 July 2025).
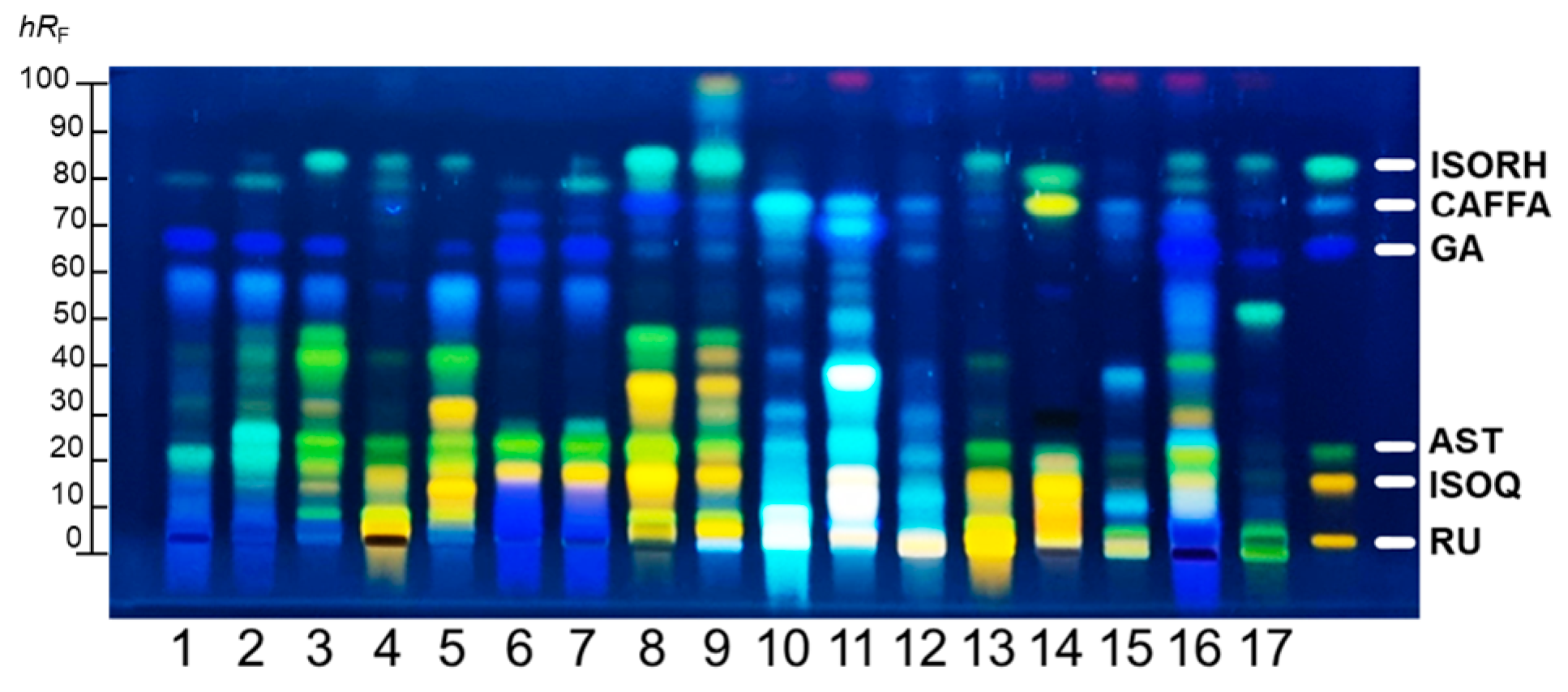
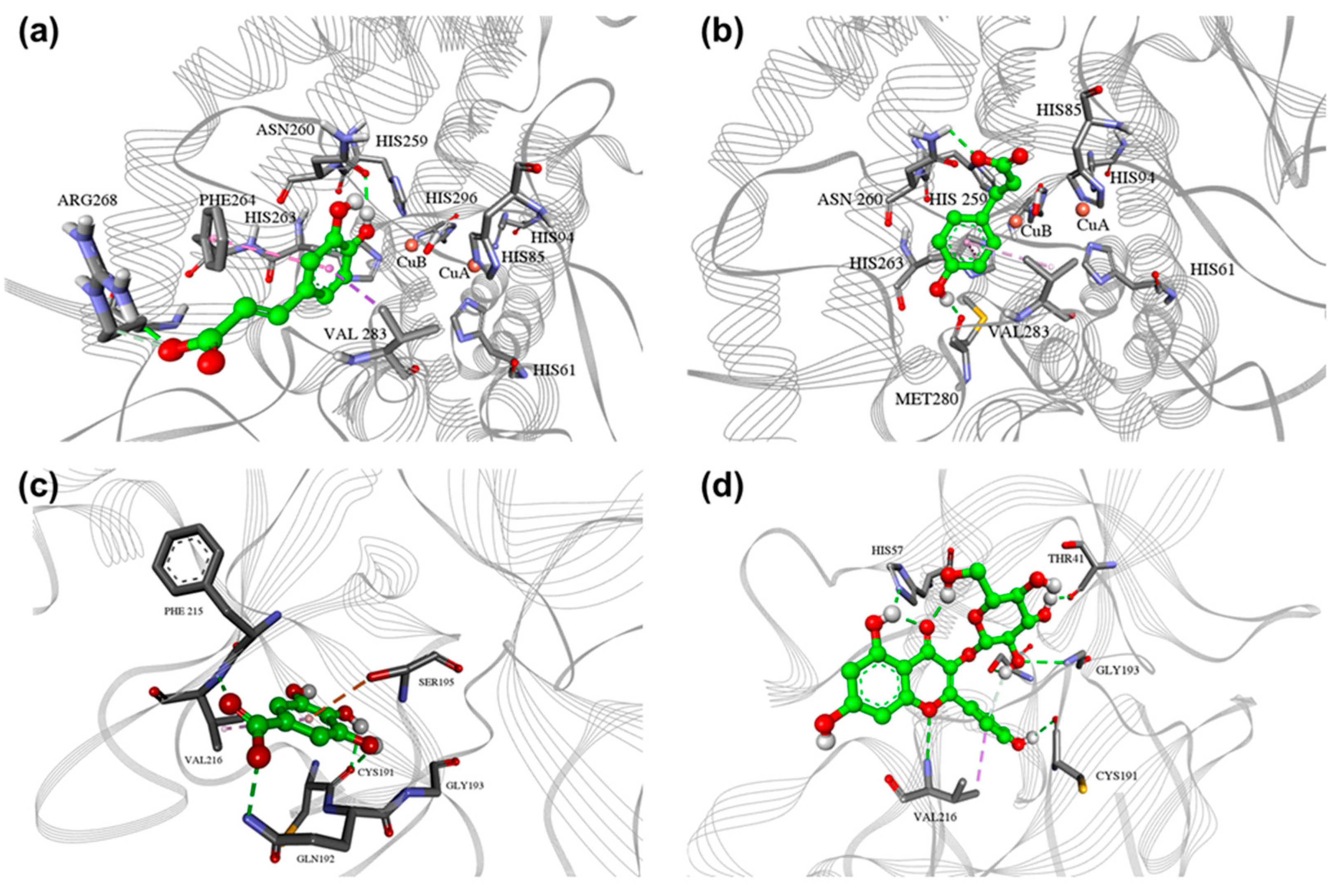
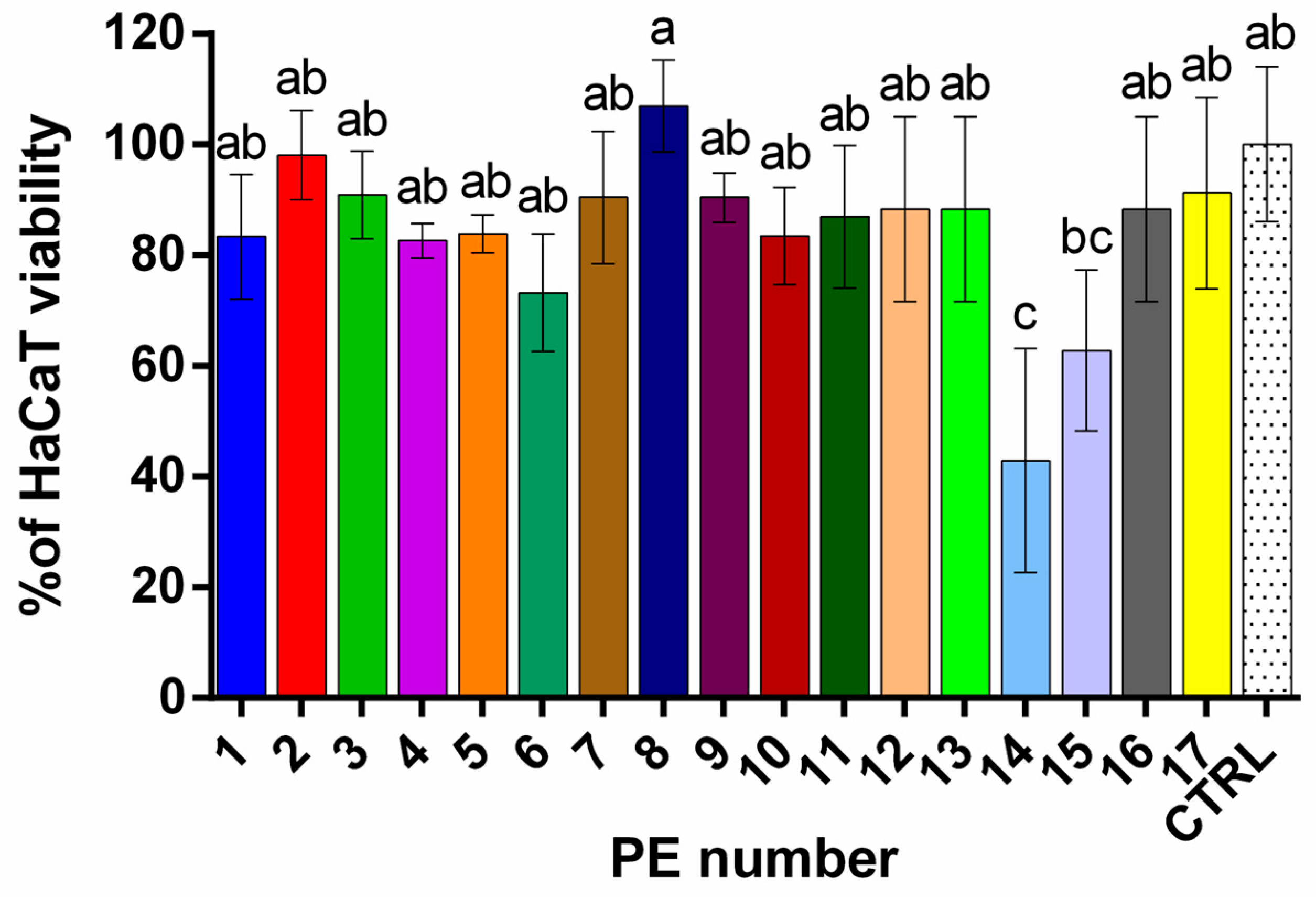
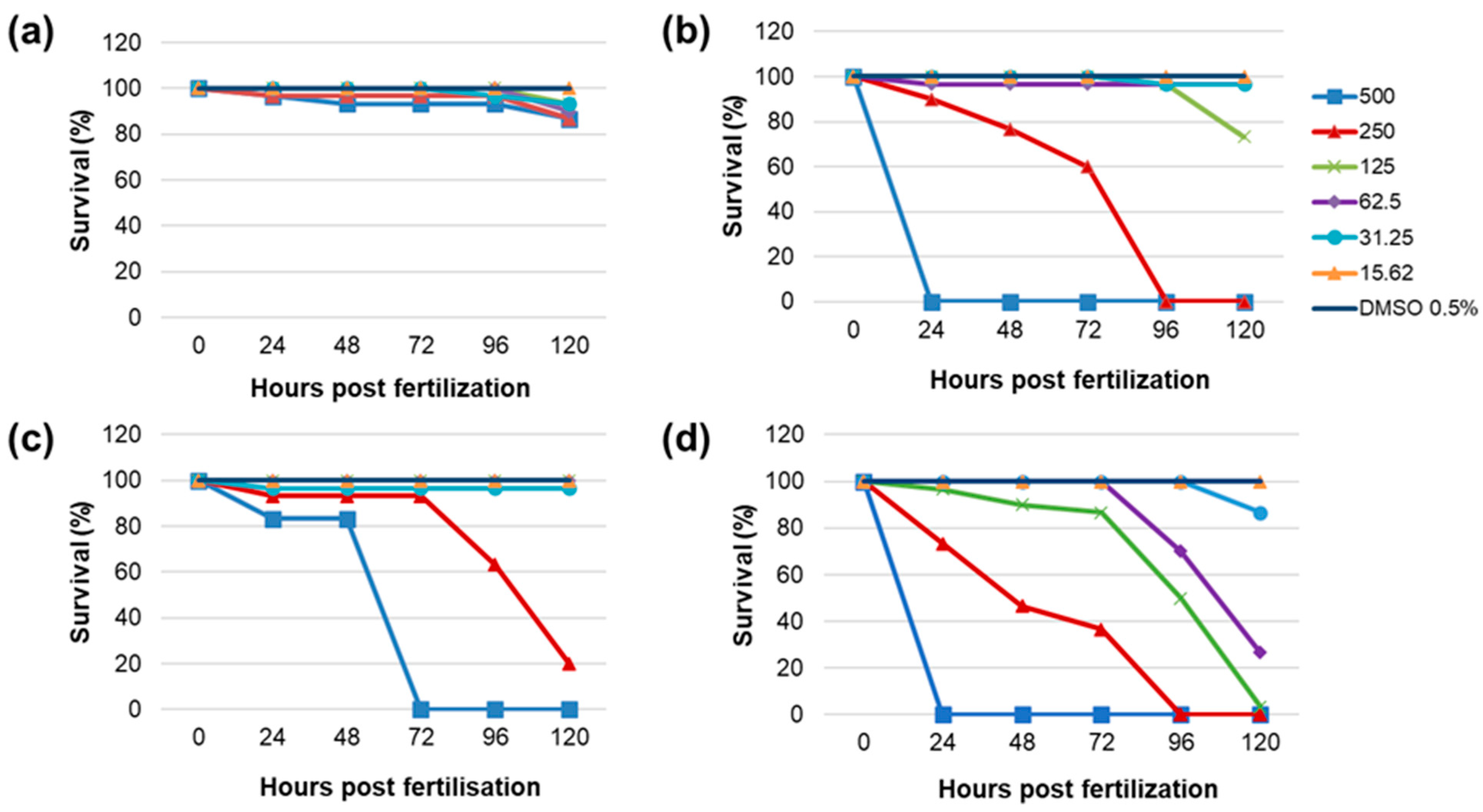

| Phenolics | Phenolic Acids | HQ | Flavonoid Glycosides | Flavonoids | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PEs | GA | CA | p-COUM | CAFFA | AES | RU | AST | HYP | NAR | ISORH-3-O-R | ISORH-3-O-G | Q-3-O-R | EC | Q | LU | K | ISORH |
| 1 | 25.77 ± 0.42 | 0.04 ± 0.01 | 0.20 ± 0.02 | 0.02 ± 0.01 | ND | 0.81 ± 0.01 | 1.06 ± 0.01 | 0.26 ± 0.01 | ND | ND | ND | 0.58 ± 0.06 | ND | 0.02 ± 0.01 | 0.22 ± 0.01 | 0.05 ± 0.01 | 0.06 ± 0.01 |
| 2 | 16.46 ± 0.48 | 0.15 ± 0.03 | 0.17 ± 0.02 | 0.02 ± 0.01 | ND | 0.70 ± 0.10 | 2.05 ± 0.11 | 1.36 ± 0.11 | ND | ND | 0.02 ± 0.01 | 0.46 ± 0.04 | 3.19 ± 0.11 | 0.13 ± 0.01 | 0.55 ± 0.08 | 0.74 ± 0.02 | 0.28 ± 0.02 |
| 3 | 8.39 ± 0.25 | 0.15 ± 0.03 | 0.04 ± 0.01 | 0.02 ± 0.01 | ND | 0.53 ± 0.03 | 4.55 ± 0.27 | 0.37 ± 0.04 | 0.08 ± 0.01 | ND | ND | 0.50 ± 0.04 | 0.05 ± 0.01 | 0.12 ± 0.01 | 1.80 ± 0.05 | 2.16 ± 0.20 | 0.14 ± 0.01 |
| 4 | 2.28 ± 0.09 | ND | 0.15 ± 0.02 | 0.03 ± 0.01 | ND | 16.96 ± 1.10 | 4.20 ± 0.26 | 0.33 ± 0.04 | 0.70 ± 0.05 | 0.26 ± 0.02 | 0.39 ± 0.03 | 0.26 ± 0.02 | 5.58 ± 0.21 | 0.41 ± 0.02 | 1.46 ± 0.05 | 1.28 ± 0.09 | 0.14 ± 0.01 |
| 5 | 7.35 ± 0.21 | 0.05 ± 0.01 | ND | ND | ND | 6.50 ± 0.20 | 4.35 ± 0.28 | 1.28 ± 0.18 | ND | ND | 0.01 ± 0.01 | 2.09 ± 0.05 | 1.85 ± 0.18 | 0.57 ± 0.02 | 1.58 ± 0.05 | 2.00 ± 0.18 | 0.22 ± 0.02 |
| 6 | 46.90 ± 1.20 | 0.07 ± 0.01 | ND | 0.03 ± 0.01 | ND | 0.15 ± 0.01 | 15.87 ± 1.77 | 2.00 ± 0.05 | ND | 0.02 ± 0.01 | 0.76 ± 0.09 | 0.07 ± 0.01 | 0.25 ± 0.02 | 0.08 ± 0.01 | 1.39 ± 0.06 | 1.76 ± 0.09 | 1.86 ± 0.09 |
| 7 | 39.71 ± 1.67 | 0.02 ± 0.01 | 0.13 ± 0.01 | ND | 0.07 ± 0.01 | 0.09 ± 0.01 | 9.12 ± 0.42 | 3.31 ± 0.25 | ND | ND | 1.29 ± 0.08 | 0.03 ± 0.01 | ND | 0.12 ± 0.01 | 1.28 ± 0.107 | 1.90 ± 0.05 | 2.20 ± 0.07 |
| 8 | 2.04 ± 0.05 | 0.05 ± 0.01 | 1.34 ± 0.15 | 0.16 ± 0.02 | 0.50 ± 0.04 | 14.57 ± 1.23 | 14.82 ± 1.64 | 3.29 ± 0.25 | ND | 0.12 ± 0.01 | 0.44 ± 0.05 | 0.81 ± 0.02 | 13.83 ± 0.56 | 1.26 ± 0.03 | 4.08 ± 0.11 | 5.67 ± 0.93 | 0.45 ± 0.05 |
| 9 | 0.03 ± 0.01 | 0.06 ± 0.01 | 0.25 ± 0.02 | 0.02 ± 0.01 | 0.30 ± 0.04 | 5.60 ± 0.55 | 3.50 ± 0.26 | 0.77 ± 0.09 | 0.05 ± 0.01 | 0.52 ± 0.04 | 0.51 ± 0.05 | 0.41 ± 0.04 | 2.11 ± 0.06 | 0.23 ± 0.02 | 1.20 ± 0.03 | 1.47 ± 0.16 | 0.39 ± 0.07 |
| 10 | 0.42 ± 0.09 | 0.13 ± 0.03 | 1.50 ± 0.02 | 2.68 ± 0.12 | 0.20 ± 0.03 | 1.93 ± 0.12 | 0.14 ± 0.10 | 0.11 ± 0.01 | ND | 0.53 ± 0.04 | 0.03 ± 0.01 | ND | ND | 0.08 ± 0.01 | 0.10 ± 0.01 | 0.06 ± 0.02 | 0.09 ± 0.02 |
| 11 | 0.44 ± 0.09 | 46.73 ± 2.13 | 0.14 ± 0.02 | 2.56 ± 0.14 | 0.41 ± 0.02 | 5.98 ± 0.23 | 1.97 ± 0.09 | 2.10 ± 0.08 | ND | 0.02 ± 0.01 | 0.01 ± 0.01 | ND | ND | 0.25 ± 0.02 | 0.14 ± 0.01 | 0.10 ± 0.01 | 0.05 ± 0.01 |
| 12 | ND | 1.71 ± 0.16 | ND | 0.27 ± 0.01 | 0.22 ± 0.01 | 1.29 ± 0.02 | 0.79 ± 0.08 | 0.19 ± 0.01 | ND | 0.19 ± 0.02 | 0.29 ± 0.02 | 0.03 ± 0.01 | 1.00 ± 0.05 | 0.15 ± 0.02 | 0.09 ± 0.02 | 0.03 ± 0.01 | 0.33 ± 0.04 |
| 13 | 0.03 ± 0.01 | ND | ND | 0.01 ± 0.01 | 0.02 ± 0.01 | 3.19 ± 0.26 | 0.77 ± 0.09 | 0.09 ± 0.01 | 0.10 ± 0.01 | 0.07 ± 0.01 | 0.12 ± 0.01 | 0.06 ± 0.01 | 1.10 ± 0.05 | 0.13 ± 0.02 | 0.17 ± 0.02 | 0.30 ± 0.02 | 0.06 ± 0.01 |
| 14 | 0.05 ± 0.01 | 0.03 ± 0.01 | 0.29 ± 0.02 | 0.07 ± 0.01 | 0.05 ± 0.01 | 1.29 ± 0.11 | 15.39 ± 0.92 | 0.76 ± 0.08 | 0.07 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 | ND | 0.05 ± 0.01 | 0.12 ± 0.02 | 0.43 ± 0.02 | 0.20 ± 0.02 | 0.14 ± 0.01 |
| 15 | 0.07 ± 0.01 | 4.68 ± 0.34 | 0.19 ± 0.02 | 1.59 ± 0.06 | 0.15 ± 0.02 | 1.61 ± 0.09 | 0.30 ± 0.04 | 0.48 ± 0.07 | ND | 5.07 ± 0.15 | 1.58 ± 0.05 | ND | 0.48 ± 0.03 | 0.06 ± 0.01 | 0.08 ± 0.02 | 0.04 ± 0.01 | 3.77 ± 0.11 |
| 16 | 50.55 ± 1.79 | 0.06 ± 0.01 | ND | 0.70 ± 0.05 | ND | 2.75 ± 0.21 | 4.89 ± 0.12 | 0.62 ± 0.10 | ND | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.28 ± 0.02 | 1.90 ± 0.01 | 0.13 ± 0.01 | 0.90 ± 0.09 | 1.09 ± 0.05 | 0.09 ± 0.01 |
| 17 | 0.07 ± 0.01 | ND | 0.46 ± 0.05 | 0.04 ± 0.01 | ND | 0.81 ± 0.05 | 1.20 ± 0.07 | 0.08 ± 0.01 | 0.06 ± 0.01 | 0.09 ± 0.01 | 0.05 ± 0.01 | 0.09 ± 0.01 | 3.59 ± 0.15 | 0.06 ± 0.01 | 0.69 ± 0.04 | 1.09 ± 0.04 | 0.20 ± 0.02 |
| Antioxidative Capacity | Bioactive Content | IC50 Values | ||||
|---|---|---|---|---|---|---|
| PEs | DPPH (μmol TE/g) | ABTS (μmol TE/g) | TPC (mg GAE/g) | TFC (mg RUE/g) | TI (µg/mL) | EI (µg/mL) |
| 1 | 571 ± 28 b | 577 ± 13 bc | 65 ± 3 ab | 2.1 ± 0.7 e | >4000 g | 222 ± 8 a |
| 2 | 220 ± 7 d | 213 ± 7 de | 26 ± 1 d | 18 ± 1 bc | 2812 ± 96 d | 554 ± 54 cd |
| 3 | 49 ± 3 g | 49 ± 1 ghi | 7.7 ± 0.1 fg | 3.6 ± 1.1 de | >4000 g | >1000 f |
| 4 | 36 ± 3 gh | 38 ± 1 hi | 12 ± 1 ef | 3.4 ± 0.4 de | >4000 g | >1000 f |
| 5 | 122 ± 10 ef | 146 ± 8 ef | 18 ± 1 de | 5.8 ± 0.5 d | >4000 g | 701 ± 6 de |
| 6 | 695 ± 12 a | 850 ± 20 a | 68 ± 2 a | 25 ± 2 b | / g | 230 ± 1 ab |
| 7 | 500 ± 29 bc | 640 ± 20 ab | 58 ± 1 bc | 285 ± 5 a | / g | 460 ± 22 bc |
| 8 | 197 ± 14 de | 246 ± 1 d | 40 ± 1 cd | 21 ± 6 b | 924 ± 4 b | 931 ± 3 e |
| 9 | 28 ± 2 hi | 21 ± 1 i | 4.4 ± 0.1 g | 5.9 ± 0.6 d | 1192 ± 19 bc | 553 ± 1 cd |
| 10 | 95 ± 7 ef | 97 ± 3 fgh | 21 ± 1 de | 80 ± 2 ab | 272 ± 28 a | >1000 f |
| 11 | 105 ± 8 ef | 88 ± 3 gh | 20 ± 1 de | 52 ± 4 ab | 618 ± 51 ab | >1000 f |
| 12 | 7.1 ± 0.1 i | 5.3 ± 0.2 k | 1.1 ± 0.1 h | 1.5 ± 0.1 f | / g | >1000 f |
| 13 | 21 ± 1 hi | 11 ± 2 j | 12 ± 1 ef | 14 ± 1 c | / g | / f |
| 14 | 22 ± 6 hi | 32 ± 2 hi | 8.9 ± 0.2 f | 6.7 ± 0.1 d | 1204 ± 100 cd | >1000 f |
| 15 | 19.1 ± 0.1 hi | 16 ± 3 i | 3.6 ± 0.1 gh | 6.9 ± 0.8 d | 1090 ± 52 bc | >1000 f |
| 16 | 392 ± 6 c | 470 ± 3 c | 44 ± 1 c | 17 ± 1 bc | / g | 206 ± 3 a |
| 17 | 8.2 ± 0.6 i | 9.2 ± 1.6 j | 3.6 ± 0.1 gh | 0.7 ± 0.4 g | 3096 ± 26 de | >1000 f |
| KA | 50 ± 14 a | |||||
| EGCG | 348 ± 9 b | |||||
| Enzyme | Compound | Docking Energy, kcal/mol | Kd, μM |
|---|---|---|---|
| Tyrosinase | CAFFA | −4.14 | 916 |
| p-COUM | −4.38 | 611 | |
| Elastase | GA | −4.97 | 226 |
| AST | −6.52 | 16.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivković, Đ.; Senćanski, M.; Novković, M.; Stojković-Filipović, J.; Trifković, J.; Ristivojević, P.; Krstić Ristivojević, M. Multidisciplinary Bioanalytical Approach to Assess the Anti-Aging Properties of Flower Petals—A Promising Sustainable Cosmetic Ingredient. Plants 2025, 14, 2869. https://doi.org/10.3390/plants14182869
Ivković Đ, Senćanski M, Novković M, Stojković-Filipović J, Trifković J, Ristivojević P, Krstić Ristivojević M. Multidisciplinary Bioanalytical Approach to Assess the Anti-Aging Properties of Flower Petals—A Promising Sustainable Cosmetic Ingredient. Plants. 2025; 14(18):2869. https://doi.org/10.3390/plants14182869
Chicago/Turabian StyleIvković, Đurđa, Milan Senćanski, Mirjana Novković, Jelena Stojković-Filipović, Jelena Trifković, Petar Ristivojević, and Maja Krstić Ristivojević. 2025. "Multidisciplinary Bioanalytical Approach to Assess the Anti-Aging Properties of Flower Petals—A Promising Sustainable Cosmetic Ingredient" Plants 14, no. 18: 2869. https://doi.org/10.3390/plants14182869
APA StyleIvković, Đ., Senćanski, M., Novković, M., Stojković-Filipović, J., Trifković, J., Ristivojević, P., & Krstić Ristivojević, M. (2025). Multidisciplinary Bioanalytical Approach to Assess the Anti-Aging Properties of Flower Petals—A Promising Sustainable Cosmetic Ingredient. Plants, 14(18), 2869. https://doi.org/10.3390/plants14182869






