Jasmonate-Mediated Mitigation of Salinity Stress During Germination and Early Vegetative Development in Hemp
Abstract
1. Introduction
2. Results
2.1. Effectiveness of Salt Stress on Hemp Development
2.2. Modulation of Hemp Seed Germination and Early Growth by MeJA and MEF Under Salt Stress
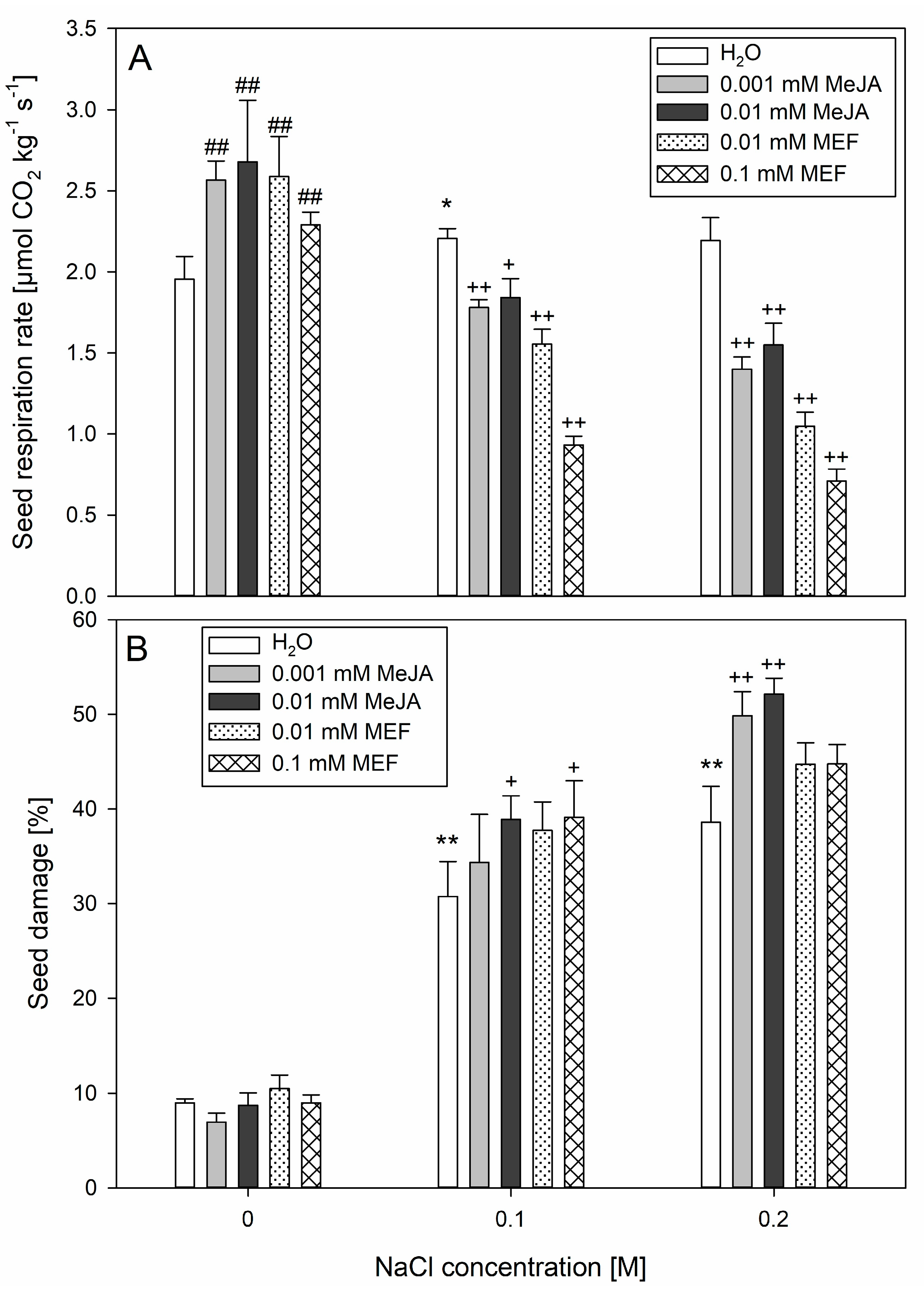
2.3. Jasmonate Modulation Alters Seed Damage and Respiration Caused by Salt Stress
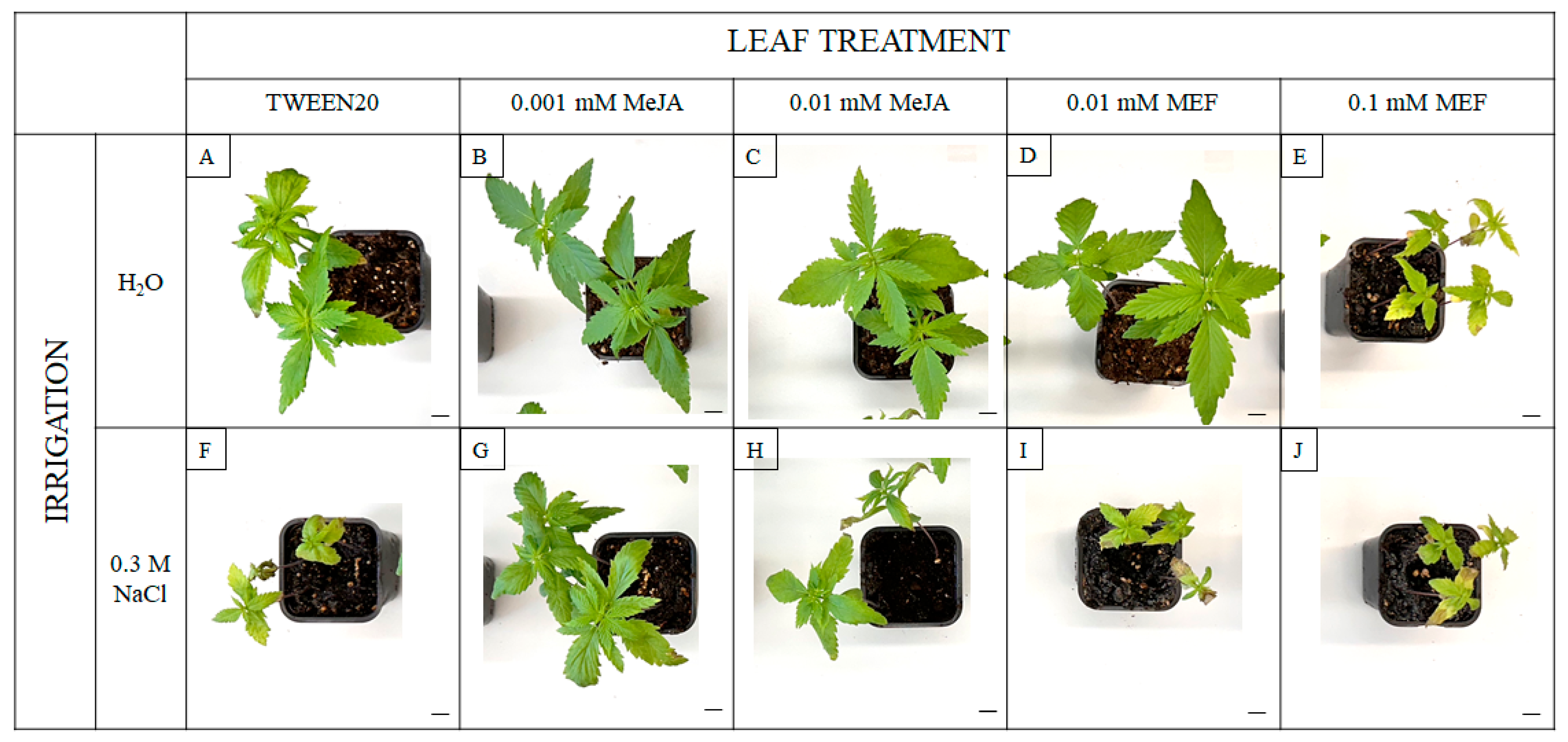
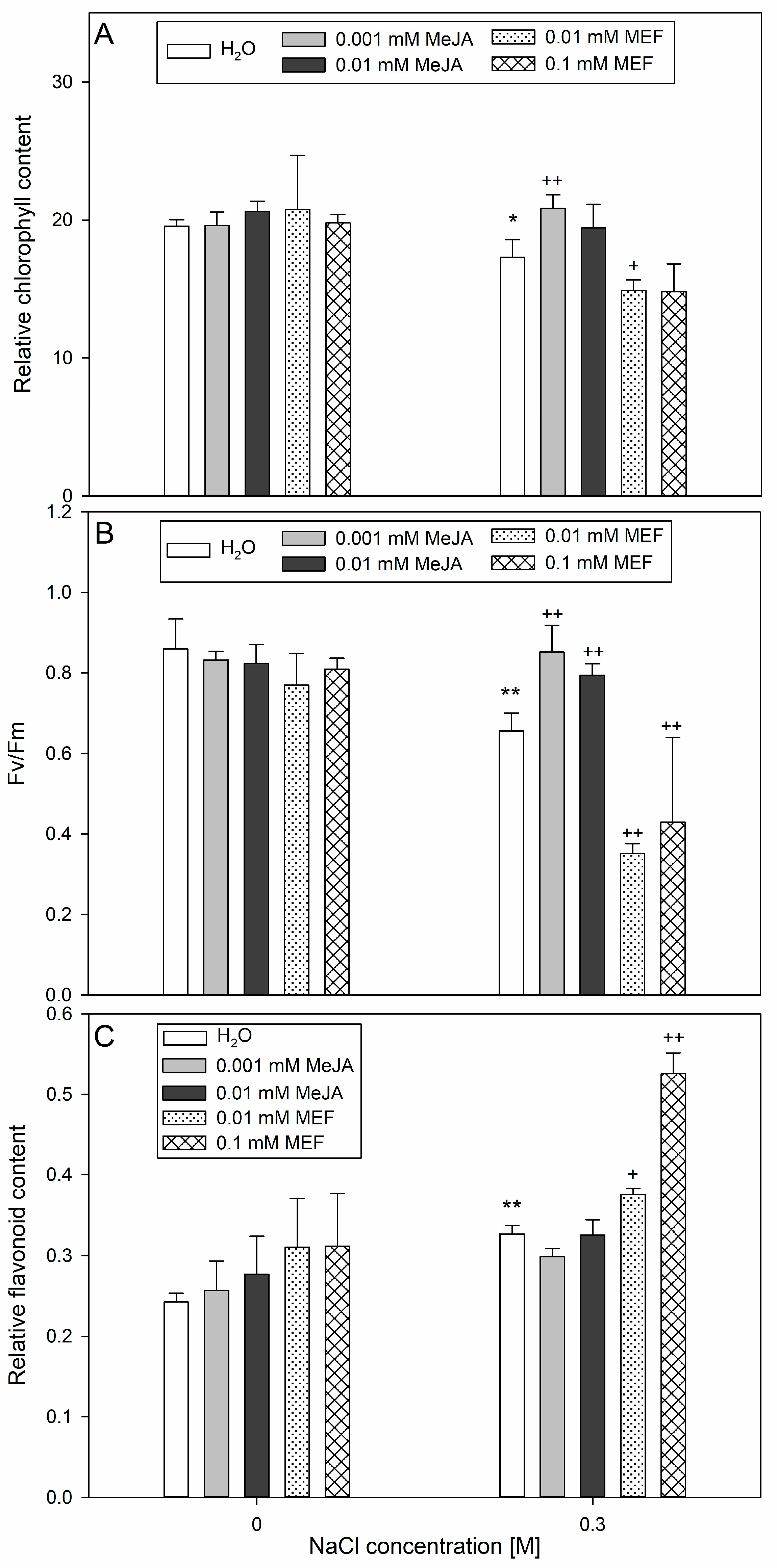
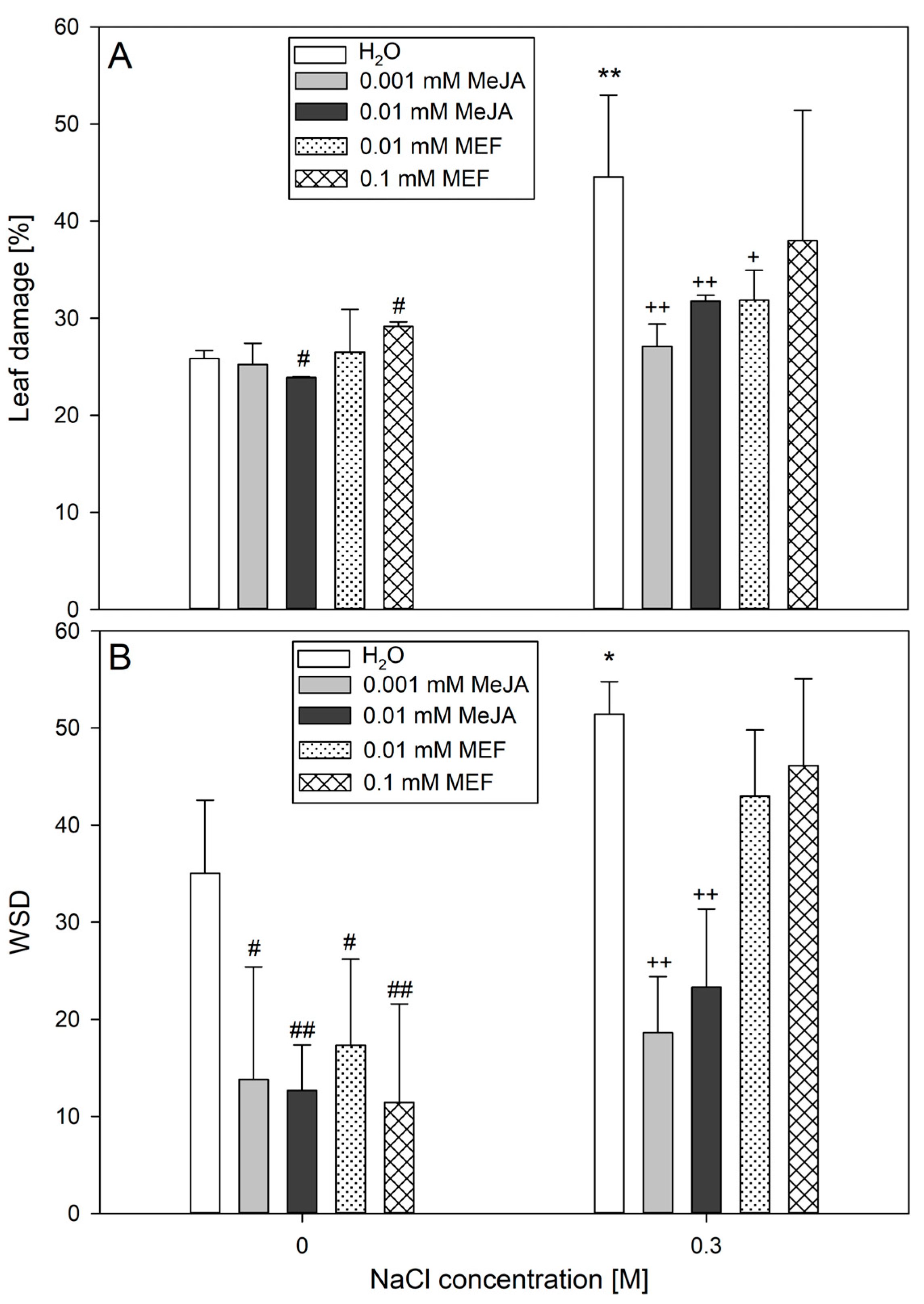
2.4. The Multifaced Influence of MeJA on Hemp Involves Modulation of Plant Morphology, Biometric Traits, and Photosynthesis-Related Parameters Under Salinity Stress
3. Discussion
4. Material and Methods
4.1. Plant Material and Experimental Design
4.2. Germination Test
4.3. Seed Respiration Rate
4.4. Hemp Cultivation and Plant Treatments
4.5. Photosynthetic-Related Parameters
4.6. Water Saturation Deficit
4.7. Tissue Damage
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. Global Status of Salt-Affected Soils—Main Report; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Waheed, A.; Zhuo, L.; Wang, M.; Xu, H.; Tong, Z.; Wang, C.; Aili, A. Integrative mechanisms of plant salt tolerance: Biological pathways, phytohormonal regulation, and technological innovations. Plant Stress 2024, 14, 100652. [Google Scholar] [CrossRef]
- Li, R.; Shi, F.; Fukuda, K.; Yang, Y. Effects of salt and alkali stresses on germination, growth, photosynthesis and ion accumulation in alfalfa (Medicago sativa L.). Soil Sci. Plant Nutr. 2010, 56, 725–733. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Atta, K.; Mondal, S.; Gorai, S.; Singh, A.P.; Kumari, A.; Ghosh, T.; Roy, A.; Hembram, S.; Gaikwad, D.J.; Mondal, S.; et al. Impacts of salinity stress on crop plants: Improving salt tolerance through genetic and molecular dissection. Front. Plant Sci. 2023, 14, 1241736. [Google Scholar] [CrossRef]
- Riemann, M.; Dhakarey, R.; Hazman, M.; Miro, B.; Kohli, A.; Nick, P. Exploring Jasmonates in the Hormonal Network of Drought and Salinity Responses. Front. Plant Sci. 2015, 6, 1077. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.T.M.F.; Islam, M.Z.; Mahmud, M.S.; Sarker, M.E.; Islam, M.R. Hemp as a potential raw material toward a sustainable world: A review. Heliyon 2022, 8, e08753. [Google Scholar] [CrossRef] [PubMed]
- Karche, T.; Singh, M.R. The application of hemp Cannabis sativa L. for a green economy: A review. Turk. J. Bot. 2019, 43, 710–723. [Google Scholar] [CrossRef]
- Zampori, L.; Dotelli, G.; Vernelli, V. Life cycle assessment of hemp cultivation and use of hemp-based thermal insulator materials in buildings. Environ. Sci. Technol. 2013, 47, 7413–7420. [Google Scholar] [CrossRef]
- Cerino, P.; Buonerba, C.; Cannazza, G.; D’Auria, J.; Ottoni, E.; Fulgione, A.; Di Stasio, A.; Pierri, B.; Gallo, A.A. Review of Hemp as Food and Nutritional Supplement. Cannabis Cannabinoid Res. 2021, 6, 19–27. [Google Scholar] [CrossRef]
- Mistry, M.; Turumella, S.; Prajapati, V.; Dholakiya, B.Z. Harnessing hemp seed oil for a circular bioeconomy: A data-driven exploration of sustainable applications for next-generation industries. Bioresour. Technol. Rep. 2025, 30, 102126. [Google Scholar] [CrossRef]
- Rheay, H.T.; Omondi, E.C.; Brewer, C.E. Potential of hemp (Cannabis sativa L.) for paired phytoremediation and bioenergy production. GCB Bioenergy 2021, 13, 525–536. [Google Scholar] [CrossRef]
- Hakami, A.Y.; Alshehri, F.S. Therapeutic potential of cannabinoids in neurological conditions: A systematic review of clinical trials. Front. Pharmacol. 2025, 16, 1521792. [Google Scholar] [CrossRef] [PubMed]
- Michels, M.; Brinkmann, A.; Mußhoff, O. Economic, ecological and social perspectives of industrial hemp cultivation in Germany: A qualitative analysis. J. Environ. Manag. 2025, 389, 126117. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Wang, Y.; Yan, Y.; Liu, Y.; Wang, J.; Chen, S. A Review on Plant Responses to Salt Stress and Their Mechanisms of Salt Resistance. Horticulturae 2021, 7, 132. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Shao, H.; Qi, W.; Daly, P.; Sharma, A.; Shaghaleh, H.; Hamoud, Y.A.; El-Esawi, M.A.; Pan, R.; Wan, Q.; et al. Seed priming and foliar application with jasmonic acid enhance salinity stress tolerance of soybean (Glycine max L.) seedlings. J. Sci. Food Agric. 2021, 101, 2027–2041. [Google Scholar] [CrossRef]
- Huang, H.; Liu, B.; Liu, L.; Song, S. Jasmonate action in plant growth and development. J. Exp. Bot. 2017, 68, 1349–1359. [Google Scholar] [CrossRef]
- Adams, E.; Turner, J. COI1 a jasmonate receptor, is involved in ethylene-induced inhibition of Arabidopsis root growth in the light. J. Exp. Bot. 2010, 61, 4373–4386. [Google Scholar] [CrossRef]
- Ismail, A.; Seo, M.; Takebayashi, Y.; Kamiya, Y.; Eiche, E.; Nick, P. Salt adaptation requires efficient fine-tuning of jasmonate signalling. Protoplasma 2014, 251, 881–898. [Google Scholar] [CrossRef]
- Ali, J.; Mukarram, M.; Ojo, J.; Dawam, N.; Riyazuddin, R.; Ghramh, H.A.; Khan, K.A.; Chen, R.; Kurjak, D.; Bayram, A. Harnessing Phytohormones: Advancing Plant Growth and Defence Strategies for Sustainable Agriculture. Physiol. Plant 2024, 176, e14307. [Google Scholar] [CrossRef]
- Doares, S.H.; Narvaez-Vasquez, J.; Conconi, A.; Ryan, C.A. Salicylic Acid Inhibits Synthesis of Proteinase Inhibitors in Tomato Leaves Induced by Systemin and Jasmonic Acid. Plant Physiol. 1995, 108, 1741–1746. [Google Scholar] [CrossRef]
- Shan, X.; Wang, J.; Chua, L.; Jiang, D.; Peng, W.; Xie, D. The role of Arabidopsis Rubisco activase in jasmonate-induced leaf senescence. Plant Physiol. 2011, 155, 751–764. [Google Scholar] [CrossRef]
- Uppalapati, S.R.; Ayoubi, P.; Weng, H.; Palmer, D.A.; Mitchell, R.E.; Jones, W.; Bender, C.L. The phytotoxin coronatine and methyl jasmonate impact multiple phytohormone pathways in tomato. Plant J. 2005, 42, 201–217. [Google Scholar] [CrossRef]
- Jiang, Y.; Ye, J.; Li, S.; Niinemets, Ü. Methyl jasmonate-induced emission of biogenic volatiles is biphasic in cucumber: A high-resolution analysis of dose dependence. J. Exp. Bot. 2017, 68, 4679–4694. [Google Scholar] [CrossRef]
- Wang, Y.; Mopper, S.; Hasenstein, K.H. Effects of salinity on endogenous ABA, IAA, JA, and SA in Iris hexagona. J. Chem. Ecol. 2001, 27, 327–342. [Google Scholar] [CrossRef]
- Trifunović-Momčilov, M.; Motyka, V.; Dobrev, P.I.; Marković, M.; Milošević, S.; Jevremović, S.; Dragićević, I.Č.; Subotić, A. Phytohormone profiles in non-transformed and AtCKX transgenic centaury (Centaurium erythraea Rafn) shoots and roots in response to salinity stress in vitro. Sci. Rep. 2021, 11, 21471. [Google Scholar] [CrossRef]
- Gharbi, E.; Martínez, J.P.; Benahmed, H.; Hichri, I.; Dobrev, P.I.; Motyka, V.; Quinet, M.; Lutts, S. Phytohormone profiling in relation to osmotic adjustment in NaCl-treated plants of the halophyte tomato wild relative species Solanum chilense comparatively to the cultivated glycophyte Solanum lycopersicum. Plant Sci. 2017, 258, 77–89. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Shi, L.; Lv, G.; Li, X.; Liu, Y.; Jia, X.; Liu, J.; Chen, Y.; Zhu, L.; et al. Jasmonate signaling pathway confers salt tolerance through a NUCLEAR FACTOR-Y trimeric transcription factor complex in Arabidopsis. Cell Rep. 2024, 43, 113825. [Google Scholar] [CrossRef]
- Yuan, F.; Liang, X.; Li, Y.; Yin, S.; Wang, B. Methyl jasmonate improves tolerance to high salt stress in the recretohalophyte Limonium bicolor. Funct. Plant Biol. 2018, 46, 82–92. [Google Scholar] [CrossRef]
- Hussain, S.; Zhang, R.; Liu, S.; Li, R.; Wang, Y.; Chen, Y.; Hou, H.; Dai, Q. Methyl Jasmonate Alleviates the Deleterious Effects of Salinity Stress by Augmenting Antioxidant Enzyme Activity and Ion Homeostasis in Rice (Oryza sativa L.). Agronomy 2022, 12, 2343. [Google Scholar] [CrossRef]
- Qiu, Z.; Guo, J.; Zhu, A.; Zhang, L.; Zhang, M. Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotoxicol. Environ. Saf. 2014, 104, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Manan, A.; Ayyub, C.M.; Pervez, M.A.; Ahmad, R. Methyl jasmonate brings about resistance against salinity stressed tomato plants by altering biochemical and physiological processes. Pak. J. Agric. Sci. 2016, 53, 35–41. [Google Scholar] [CrossRef]
- Mulaudzi, T.; Sias, G.; Nkuna, M.; Ndou, N.; Hendricks, K.; Ikebudu, V.; Koo, A.J.; Ajayi, R.F.; Iwuoha, E. Seed Priming with MeJa Prevents Salt-Induced Growth Inhibition and Oxidative Damage in Sorghum bicolor by Inducing the Expression of Jasmonic Acid Biosynthesis Genes. Int. J. Mol. Sci. 2023, 24, 10368. [Google Scholar] [CrossRef]
- Jiang, Y.; Deyholos, M.K. Comprehensive transcriptional profiling of NaCl-stressed Arabidopsis roots reveals novel classes of responsive genes. BMC Plant Biol. 2006, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Gong, Q.; Bohnert, H.J. Dissecting salt stress pathways. J. Exp. Bot. 2006, 57, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-J.; Seo, Y.-J.; Lee, J.-D.; Ishii, R.; Kim, K.U.; Shin, D.H.; Park, S.K.; Jang, S.W.; Lee, I.-J. Jasmonic acid differentially affects growth, ion uptake and abscisic acid concentration in salt-tolerant and salt-sensitive rice cultivars. J. Agron. Crop Sci. 2005, 191, 273–282. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Q.; Zhai, H.; Li, Y.; Wang, X.; Liu, Q.; He, S. Transcript profile analysis reveals important roles of jasmonic acid signalling pathway in the response of sweet potato to salt stress. Sci. Rep. 2017, 7, 40819. [Google Scholar] [CrossRef]
- Valenzuela, C.E.; Acevedo-Acevedo, O.; Miranda, G.S.; Vergara-Barros, P.; Holuigue, L.; Figueroa, C.R.; Figueroa, P.M. Salt stress response triggers activation of the jasmonate signaling pathway leading to inhibition of cell elongation in Arabidopsis primary root. J. Exp. Bot. 2016, 67, 4209–4220. [Google Scholar] [CrossRef]
- Tamogami, S.; Noge, K.; Abe, M.; Agrawal, G.K.; Rakwal, R. Methyl jasmonate is transported to distal leaves via vascular process metabolizing itself into JA-Ile and triggering VOCs emission as defensive metabolites. Plant Signal. Behav. 2012, 11, 1378–1381. [Google Scholar] [CrossRef][Green Version]
- Cheong, J.J.; Choi, Y.D. Methyl jasmonate as a vital substance in plants. Trends Genet. 2003, 19, 409–413. [Google Scholar] [CrossRef]
- Jang, G.; Shim, J.S.; Jung, C.; Song, J.T.; Lee, H.Y.; Chung, P.J.; Kim, J.-K.; Choi, Y.D. Volatile methyl jasmonate is a transmissible form of jasmonate and its biosynthesis is involved in systemic jasmonate response in wounding. Plant Biotechnol. Rep. 2014, 8, 409–419. [Google Scholar] [CrossRef]
- Zuñiga, P.E.; Castañeda, Y.; Arrey-Salas, O.; Fuentes, L.; Aburto, F.; Figueroa, C.R. Methyl Jasmonate Applications From Flowering to Ripe Fruit Stages of Strawberry (Fragaria × ananassa ‘Camarosa’) Reinforce the Fruit Antioxidant Response at Post-harvest. Front. Plant Sci. 2020, 11, 538. [Google Scholar] [CrossRef]
- Jung, S. Effect of chlorophyll reduction in Arabidopsis thaliana by methyl jasmonate or norflurazon on antioxidant systems. Plant Physiol. Biochem. 2004, 42, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.I.; Karimi, K.; Struik, P.C. Effect of exogenous application of methyl jasmonate on physiological and biochemical characteristics of Brassica napus L. cv. Talaye under salinity stress. S. Afr. J. Bot. 2018, 115, 5–11. [Google Scholar] [CrossRef]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Liu, H.; Liu, F. Seed germination of hemp (Cannabis sativa L.) cultivars responds differently to the stress of salt type and concentration. Ind. Crop. Prod. 2018, 123, 254–261. [Google Scholar] [CrossRef]
- Mbarki, S.; Skalicky, M.; Vachova, P.; Hajihashemi, S.; Jouini, L.; Zivcak, M.; Tlustos, P.; Brestic, M.; Hejnak, V.; Khelil, A.Z. Comparing Salt Tolerance at Seedling and Germination Stages in Local Populations of Medicago ciliaris L. to Medicago intertexta L. and Medicago scutellata L. Plants 2020, 9, 526. [Google Scholar]
- Preston, C.A.; Betts, H.; Baldwin, I.T. Methyl Jasmonate as an Allelopathic Agent: Sagebrush Inhibits Germination of a Neighboring Tobacco, Nicotiana Attenuata. J. Chem. Ecol. 2002, 28, 2343–2369. [Google Scholar] [CrossRef]
- Kępczyński, J.; Białecka, B. Stimulatory effect of ethephon, ACC, gibberellin A3 and A4+7 on germination of methyl jasmonate inhibited Amaranthus caudatus L. seeds. Plant Growth Regul. 1994, 14, 211–216. [Google Scholar] [CrossRef]
- Corbineau, F.; Rudnicki, R.M.; Côme, D. The effects of methyl jasmonate on sunflower (Helianthus annuus L.) seed germination and seedling development. Plant Growth Regul. 1988, 7, 157–169. [Google Scholar] [CrossRef]
- Jacobsen, J.V.; Barrero, J.M.; Hughes, T.; Julkowska, M.; Taylor, J.M.; Xu, Q.; Gubler, F. Roles for blue light, jasmonate and nitric oxide in the regulation of dormancy and germination in wheat grain (Triticum aestivum L). Planta 2013, 238, 121–138. [Google Scholar] [CrossRef]
- Nagao, K.; Takahashi, T.; Yokoyama, R. Effect of environmental conditions on seed germination and seedling growth in Cuscuta campestris. Plant Growth Regul. 2025, 105, 1157–1167. [Google Scholar] [CrossRef]
- Enteshari, S.; Jafari, T. The effects of methyl jasmonate and salinity on germination and seedling growth in Ocimum basilicum L. Iranian J. Plant Phys. 2013, 3, 749–756. [Google Scholar]
- Wang, F.; Wan, C.; Wu, W.; Zhang, Y.; Pan, Y.; Chen, X.; Li, C.; Pi, J.; Wang, Z.; Ye, Y.; et al. Methyl jasmonate (MeJA) enhances salt tolerance of okra (Abelmoschus esculentus L.) plants by regulating ABA signaling, osmotic adjustment substances, photosynthesis and ROS metabolism. Sci. Hort. 2023, 319, 112145. [Google Scholar] [CrossRef]
- Nie, G.; Zhou, J.; Jiang, Y.; He, J.; Wang, Y.; Liao, Z.; Appiah, C.; Li, D.; Feng, G.; Huang, L.; et al. Transcriptome characterization of candidate genes for heat tolerance in perennial ryegrass after exogenous methyl Jasmonate application. BMC Plant Biol. 2022, 22, 68. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, Z.Q.; Zhang, L.T.; Sun, M.M.; Lin, T.B. Photosynthetic responses of wheat (Triticum aestivum L.) to combined effects of drought and exogenous methyl jasmonate. Photosynthetica 2014, 52, 377–385. [Google Scholar] [CrossRef]
- Kononenko, N.; Baranova, E.; Dilovarova, T.; Akanov, E.; Fedoreyeva, L. Oxidative Damage to Various Root and Shoot Tissues of Durum and Soft Wheat Seedlings during Salinity. Agriculture 2020, 10, 55. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, F.; Sun, L.; Yang, L.; Yang, Y.; Wang, Y.; Siddique, K.H.M.; Pang, J. Alkaline Salt Inhibits Seed Germination and Seedling Growth of Canola More Than Neutral Salt. Front. Plant Sci. 2022, 13, 814755. [Google Scholar] [CrossRef]
- Kazan, K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015, 4, 219–229. [Google Scholar] [CrossRef]
- Yin, Y.L.; Yang, T.H.; Li, S.; Li, X.; Wang, W.; Fan, S.G. Transcriptomic analysis reveals that methyl jasmonate confers salt tolerance in alfalfa by regulating antioxidant activity and ion homeostasis. Front. Plant Sci. 2023, 14, 1258498. [Google Scholar] [CrossRef]
- Abdelgawad, Z.A.; Khalafaallah, A.A.; Abdallah, M.M. Impact of methyl jasmonate on antioxidant activity and some biochemical aspects of maize plant grown under water stress condition. Agric. Sci. 2014, 5, 1077–1088. [Google Scholar] [CrossRef]
- Ahmad, P.; Rasool, S.; Gul, A.; Sheikh, S.A.; Akram, N.A.; Ashraf, M.; Kazi, A.M.; Gucel, S. Jasmonates: Multifunctional Roles in Stress Tolerance. Front. Plant Sci. 2016, 7, 813. [Google Scholar] [CrossRef] [PubMed]
- Taheri, Z.; Vatankhah, E.; Jafarian, V. Methyl jasmonate improves physiological and biochemical responses of Anchusa italica under salinity stress. S. Afr. J. Bot. 2020, 130, 375–382. [Google Scholar]
- Fatma, M.; Iqbal, N.; Sehar, Z.; Alyemeni, M.N.; Kaushik, P.; Khan, N.A.; Ahmad, P. Methyl Jasmonate Protects the PS II System by Maintaining the Stability of Chloroplast D1 Protein and Accelerating Enzymatic Antioxidants in Heat-Stressed Wheat Plants. Antioxidants 2021, 10, 1216. [Google Scholar] [CrossRef] [PubMed]
- Bethmann, S.; Melzer, M.; Schwarz, N.; Jahns, P. The zeaxanthin epoxidase is degraded along with the D1 protein during photoinhibition of photosystem II. Plant Direct 2019, 3, e00185. [Google Scholar] [CrossRef]
- Hamidian, M.; Kazemeini, S.A.; Movahhedi Dehnavi, M.; Ramezanian, A.; Mottaghi Jahromie, M.R.; Farsijani, P.; Iranshahi, R.; Mohebi, P.; Fereshteh Hekmat, M.; Hassani, M.; et al. Individual and Combined Exogenous Application of Melatonin and Methyl Jasmonate Confer Salinity Stress Tolerance in Tomato by Enhancing Antioxidants Defense System. Sci. Hortic. 2025, 342, 114040. [Google Scholar] [CrossRef]
- Farooq, M.A.; Gill, R.A.; Islam, F.; Ali, B.; Liu, H.; Xu, J.; He, S.; Zhou, W. Methyl Jasmonate Regulates Antioxidant Defense and Suppresses Arsenic Uptake in Brassica napus L. Front. Plant Sci. 2016, 7, 468. [Google Scholar]
- Hazman, M.; Hause, B.; Eiche, E.; Nick, P.; Riemann, M. Increased tolerance to salt stress in OPDA-deficient rice ALLENE OXIDE CYCLASE mutants is linked to an increased ROS-scavenging activity. J. Exp. Bot. 2015, 66, 3339–3352. [Google Scholar] [CrossRef]
- Ueda, J.; Saniewski, J. Methyl Jasmonate-Induced Stimulation of Chlorophyll Formation in the Basal Part of Tulip Bulbs Kept under Natural Light Conditions. J. Fruit. Ornam. Plant Res. 2006, 14, 199–210. [Google Scholar]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar]
- Yamane, K.; Kawasaki, M.; Taniguchi, M.; Miyake, H. Correlation between Chloroplast Ultrastructure and Chlorophyll Fluorescence Characteristics in the Leaves of Rice (Oryza sativa L.) Grown under Salinity. Plant Prod. Sci. 2008, 11, 139–145. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Yu, Z.M.; Zeng, D.Q.; Si, C.; Zhao, C.H.; Wang, H.B.; Li, C.M.; He, C.M.; Duan, J. Transcriptome and metabolome reveal salt-stress responses of leaf tissues from Dendrobium officinale. Biomolecules 2021, 11, 736. [Google Scholar] [CrossRef] [PubMed]
- Karimi, R.; Gavili-Kilaneh, K.; Khadivi, A. Methyl jasmonate promotes salinity adaptation responses in two grapevine (Vitis vinifera L.) cultivars differing in salt tolerance. Food Chem. 2022, 375, 131667. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Ulhassan, Z.; Qi, W.; Lu, H.; AbdElgawad, H.; Minkina, T.; Sushkova, S.; Rajput, V.D.; El-Keblawy, A.; Jośko, I.; et al. Association of jasmonic acid priming with multiple defense mechanisms in wheat plants under high salt stress. Front. Plant Sci. 2022, 13, 886862. [Google Scholar] [CrossRef]
- Teleszko, M.; Zając, A.; Rusak, T. Hemp Seeds of the Polish ‘Bialobrzeskie’ and ‘Henola’ Varieties (Cannabis sativa L. var. sativa) as Prospective Plant Sources for Food Production. Molecules 2022, 27, 1448. [Google Scholar] [CrossRef]
- Wasternack, C.; Parthier, B. Jasmonate-signalled plant gene expression. Trends Plant Sci. 1997, 2, 1360–1385. [Google Scholar] [CrossRef]
- Wilmowicz, E.; Kućko, A.; Frankowski, K.; Świdziński, M.; Marciniak, K.; Kopcewicz, J. Methyl jasmonate-dependent senescence of cotyledons in Ipomoea nil. Acta Physiol. Plant. 2016, 38, 222. [Google Scholar] [CrossRef][Green Version]
- Turner, N.C. Techniques and Experimental Approaches for the Measurement of Plant Water Status. Plant Soil. 1981, 58, 339–366. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasprowiak, F.; Wilmowicz, E.; Kućko, A. Jasmonate-Mediated Mitigation of Salinity Stress During Germination and Early Vegetative Development in Hemp. Plants 2025, 14, 2864. https://doi.org/10.3390/plants14182864
Kasprowiak F, Wilmowicz E, Kućko A. Jasmonate-Mediated Mitigation of Salinity Stress During Germination and Early Vegetative Development in Hemp. Plants. 2025; 14(18):2864. https://doi.org/10.3390/plants14182864
Chicago/Turabian StyleKasprowiak, Franciszek, Emilia Wilmowicz, and Agata Kućko. 2025. "Jasmonate-Mediated Mitigation of Salinity Stress During Germination and Early Vegetative Development in Hemp" Plants 14, no. 18: 2864. https://doi.org/10.3390/plants14182864
APA StyleKasprowiak, F., Wilmowicz, E., & Kućko, A. (2025). Jasmonate-Mediated Mitigation of Salinity Stress During Germination and Early Vegetative Development in Hemp. Plants, 14(18), 2864. https://doi.org/10.3390/plants14182864







