Cultivar-Specific Differences in C6 and C7 Sugar Metabolism During Avocado Ripening: Comparative Insights from Bacon, Fuerte, and Hass
Abstract
1. Introduction
2. Results and Discussion
2.1. Quantitative Profiles and Fluctuation Trends of Individual Sugars During Ripening
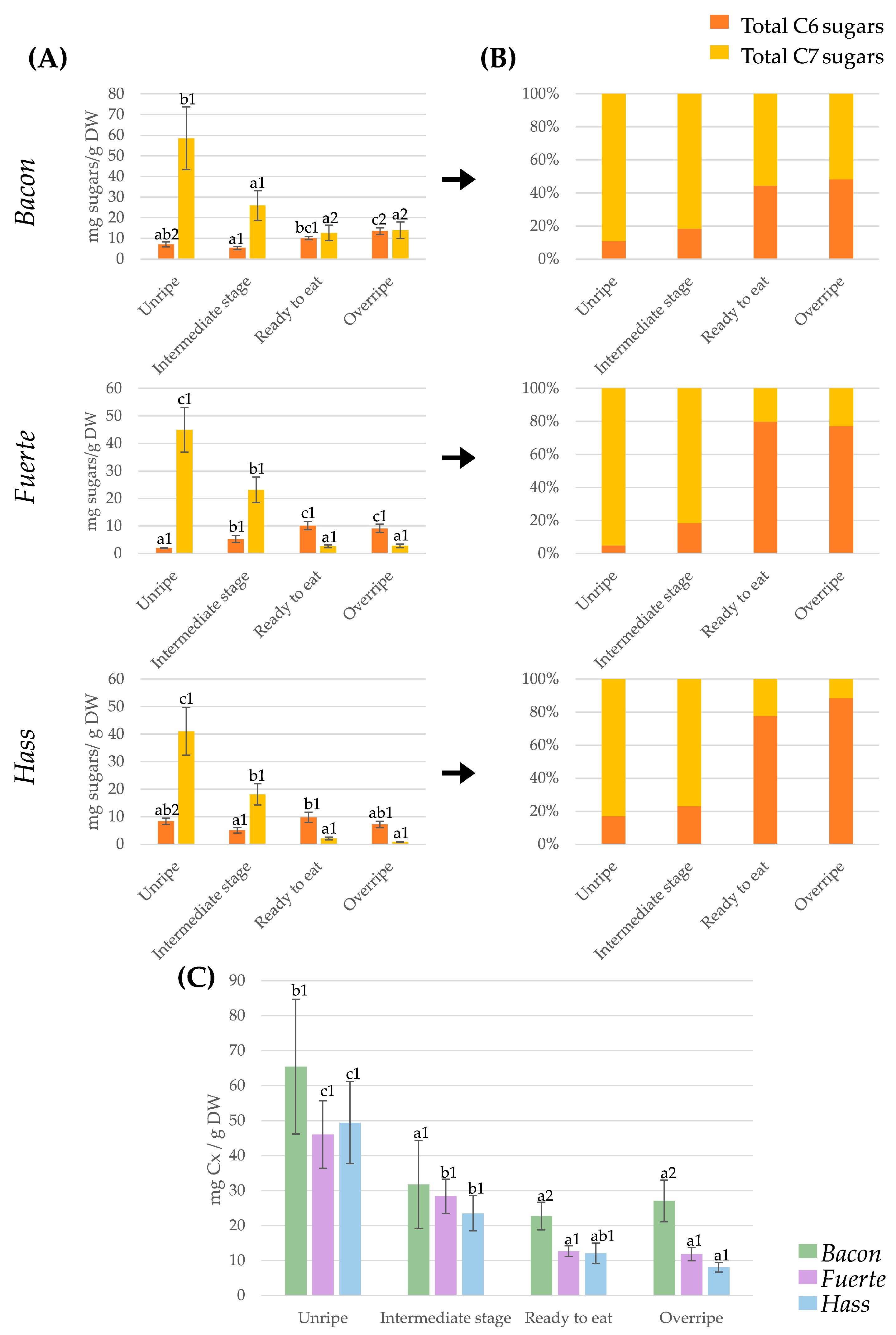
2.2. Dynamic Changes in Total C6 and C7 Sugar and Total Carbohydrate Content During Avocado Ripening
2.3. Multivariate Analysis Highlights the Predominant Effect of Variety on the Sugar Content of Avocado Fruits
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Samples
3.3. Carbohydrates Extraction Procedure and HILIC-MS Analyses
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- FAO Statistics Division of Food and Agriculture Organization of the United Nations (FAOSTAT). Available online: https://www.fao.org/faostat/es/#data/QCL (accessed on 26 July 2024).
- Rodríguez-López, C.E.; Hernández-Brenes, C.; Treviño, V.; Díaz de la Garza, R.I. Avocado Fruit Maturation and Ripening: Dynamics of Aliphatic Acetogenins and Lipidomic Profiles from Mesocarp, Idioblasts and Seed. BMC Plant Biol. 2017, 17, 9–12. [Google Scholar] [CrossRef]
- Shezi, S.; Magwaza, L.S.; Tesfay, S.Z.; Mditshwa, A. Biochemical Changes in Response to Canopy Position of Avocado Fruit (Cv. ‘Carmen’ and ‘Hass’) during Growth and Development and Relationship with Maturity. Sci. Hortic. 2020, 265, 109227. [Google Scholar] [CrossRef]
- Bower, J.P.; Cutting, J.G. Avocado Fruit Development and Ripening Physiology. Hortic. Rev. (Am. Soc. Hortic. Sci.) 1988, 10, 229–271. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Y.; Khuong, T.; Lovatt, C.J. Effect of Harvest Date on the Nutritional Quality and Antioxidant Capacity in “Hass” Avocado during Storage. Food Chem. 2012, 135, 694–698. [Google Scholar] [CrossRef]
- Roch, L.; Prigent, S.; Klose, H.; Cakpo, C.B.; Beauvoit, B.; Deborde, C.; Fouillen, L.; Van Delft, P.; Jacob, D.; Usadel, B.; et al. Biomass Composition Explains Fruit Relative Growth Rate and Discriminates Climacteric from Non-Climacteric Species. J. Exp. Bot. 2020, 71, 5823–5836. [Google Scholar] [CrossRef]
- Yahia, E.M.; Carrillo-López, A.; Bello-Perez, L.A. Carbohydrates; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128132784. [Google Scholar]
- Durán-Soria, S.; Pott, D.M.; Osorio, S.; Vallarino, J.G. Sugar Signaling During Fruit Ripening. Front. Plant Sci. 2020, 11, 564917. [Google Scholar] [CrossRef]
- Jia, H.; Wang, Y.; Sun, M.; Li, B.; Han, Y.; Zhao, Y.; Li, X.; Ding, N.; Li, C.; Ji, W.; et al. Sucrose Functions as a Signal Involved in the Regulation of Strawberry Fruit Development and Ripening. New Phytol. 2013, 198, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa-Yokoi, A.; Yabuta, Y.; Shigeoka, S. The Contribution of Carbohydrates Including Raffinose Family Oligosaccharides and Sugar Alcohols to Protection of Plant Cells from Oxidative Damage. Plant Signal. Behav. 2008, 3, 1016–1018. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.D.; Terry, L.A. Fatty Acid and Sugar Composition of Avocado, Cv. Hass, in Response to Treatment with an Ethylene Scavenger or 1-Methylcyclopropene to Extend Storage Life. Food Chem. 2010, 121, 1203–1210. [Google Scholar] [CrossRef]
- Liu, X.; Robinson, P.W.; Madore, M.A.; Witney, G.W.; Arpaia, M.L. “Hass” Avocado Carbohydrate Fluctuations. I. Growth and Phenology. J. Am. Soc. Hortic. Sci. 1999, 124, 671–675. [Google Scholar] [CrossRef]
- Pedreschi, R.; Muñoz, P.; Robledo, P.; Becerra, C.; Defilippi, B.G.; van Eekelen, H.; Mumm, R.; Westra, E.; De Vos, R.C.H. Metabolomics Analysis of Postharvest Ripening Heterogeneity of “Hass” Avocadoes. Postharvest Biol. Technol. 2014, 92, 172–179. [Google Scholar] [CrossRef]
- Tesfay, S.Z.; Bertling, I.; Bower, J.P. D-Mannoheptulose and Perseitol in “Hass” Avocado: Metabolism in Seed and Mesocarp Tissue. S. Afr. J. Bot. 2012, 79, 159–165. [Google Scholar] [CrossRef][Green Version]
- Beiro-Valenzuela, M.G.; Serrano-García, I.; Monasterio, R.P.; Moreno-Tovar, M.V.; Hurtado-Fernández, E.; González-Fernández, J.J.; Hormaza, J.I.; Pedreschi, R.; Olmo-García, L.; Carrasco-Pancorbo, A. Characterization of the Polar Profile of Bacon and Fuerte Avocado Fruits by Hydrophilic Interaction Liquid Chromatography-Mass Spectrometry: Distribution of Non-Structural Carbohydrates, Quinic Acid, and Chlorogenic Acid between Seed, Mesocarp, and Exocar. J. Agric. Food Chem. 2022, 71, 5674–5685. [Google Scholar] [CrossRef]
- Tesfay, S.Z.; Bertling, I.; Bower, J.P.; Lovatt, C.J. The Quest for the Function of “Hass” Avocado Carbohydrates: Clues from Fruit and Seed Development as Well as Seed Germination. Aust. J. Bot. 2012, 60, 79–86. [Google Scholar] [CrossRef]
- Cowan, A.K. Occurrence, Metabolism, Transport and Function of Seven-Carbon Sugars. Phytochem. Rev. 2017, 16, 137–157. [Google Scholar] [CrossRef]
- Pedreschi, R.; Uarrota, V.; Fuentealba, C.; Alvaro, J.E.; Olmedo, P.; Defilippi, B.G.; Meneses, C.; Campos-Vargas, R. Primary Metabolism in Avocado Fruit. Front. Plant Sci. 2019, 10, 795. [Google Scholar] [CrossRef]
- Liu, X.; Sievert, J.; Lu Arpaia, M.; Madore, M.A. Postulated Physiological Roles of the Seven-Carbon Sugars, Mannoheptulose, and Perseitol in Avocado. J. Am. Soc. Hortic. Sci. 2002, 127, 108–114. [Google Scholar] [CrossRef]
- Landahl, S.; Meyer, M.D.; Terry, L.A. Spatial and Temporal Analysis of Textural and Biochemical Changes of Imported Avocado Cv. Hass during Fruit Ripening. J. Agric. Food Chem. 2009, 57, 7039–7047. [Google Scholar] [CrossRef]
- Bertling, I.; Bower, J.P. Sugars as Energy Sources—Is There a Link to Avocado Fruit Quality? South African Avocado Grow. Assoc. Yearb. 2005, 28, 24–27. [Google Scholar]
- Cowan, A.K. Metabolic Control of Avocado Fruit Growth: 3-Hydroxy-3-Methylglutaryl Coenzyme a Reductase, Active Oxygen Species and the Role of C7 Sugars. S. Afr. J. Bot. 2004, 70, 75–82. [Google Scholar] [CrossRef]
- Ward, J.M.; Kühn, C.; Tegeder, M.; Frommer, W.B. Sucrose Transport in Higher Plants. Int. Rev. Cytol. 1998, 178, 41–71. [Google Scholar] [CrossRef]
- Basciano, H.; Federico, L.; Adeli, K. Fructose, Insulin Resistance, and Metabolic Dyslipidemia. Nutr. Metab. 2005, 2, 5. [Google Scholar] [CrossRef]
- Nasri, C.; Halabi, Y.; Hajib, A.; Choukri, H.; Harhar, H.; Lee, L.H.; Mani, V.; Ming, L.C.; Goh, K.W.; Bouyahya, A.; et al. Proximate Composition, Lipid and Elemental Profiling of Eight Varieties of Avocado (Persea Americana). Sci. Rep. 2023, 13, 22767. [Google Scholar] [CrossRef]
- Silva, A.P.F.B.; Do Nascimento, J.R.O.; Lajolo, F.M.; Cordenunsi, B.R. Starch Mobilization and Sucrose Accumulation in the Pulp of Keitt Mangoes during Postharvest Ripening. J. Food Biochem. 2008, 32, 384–395. [Google Scholar] [CrossRef]
- Blakey, R.J.; Tesfay, S.Z.; Bertling, I.; Bower, J.P. Changes in Sugars, Total Protein, and Oil in “Hass” Avocado (Persea Americana Mill.) Fruit during Ripening. J. Hortic. Sci. Biotechnol. 2012, 87, 381–387. [Google Scholar] [CrossRef]
- Kader, A.A. Flavor Quality of Fruits and Vegetables. J. Sci. Food Agric. 2008, 88, 1863–1868. [Google Scholar] [CrossRef]
- Campos, D.; Teran-Hilares, F.; Chirinos, R.; Aguilar-Galvez, A.; García-Ríos, D.; Pacheco-Avalos, A.; Pedreschi, R. Bioactive Compounds and Antioxidant Activity from Harvest to Edible Ripeness of Avocado Cv. Hass (Persea Americana) throughout the Harvest Seasons. Int. J. Food Sci. Technol. 2020, 55, 2208–2218. [Google Scholar] [CrossRef]
- Meyer, M.D.; Terry, L.A.; Raymond Chia, T.W.; Dykes, G.A. Development of a Rapid Method for the Sequential Extraction and Subsequent Quantification of Fatty Acids and Sugars from Avocado Mesocarp Tissue. J. Agric. Food Chem. 2008, 56, 7439–7445. [Google Scholar] [CrossRef] [PubMed]
- Burdon, J.; Billing, D.; Bowen, J.; Boldingh, H. Non-Structural Carbohydrate Composition of ‘Hass’ Avocado Fruit Is Affected by Maturity, Storage, and Ripening. Horticulturae 2024, 10, 866. [Google Scholar] [CrossRef]
- Ramos-Aguilar, A.L.; Ornelas-Paz, J.; Tapia-Vargas, L.M.; Gardea-Béjar, A.A.; Yahia, E.M.; Ornelas-Paz, J.d.J.; Perez-Martinez, J.D.; Rios-Velasco, C.; Escalante-Minakata, P. Metabolomic Analysis and Physical Attributes of Ripe Fruits from Mexican Creole (Persea Americana Var. Drymifolia) and “Hass” Avocados. Food Chem. 2021, 354, 129571. [Google Scholar] [CrossRef]
- Olmedo, P.; Núñez-Lillo, G.; Ponce, E.; Alvaro, J.E.; Baños, J.; Carrera, E.; González-Fernández, J.J.; Hormaza, J.I.; Campos, D.; Chirinos, R.; et al. Metabolite Profiling and Hormone Analysis of the Synchronized Exocarp-Mesocarp Development during Ripening of Cv. ‘Fuerte’ and ‘Hass’ Avocado Fruits. Food Biosci. 2024, 60, 104454. [Google Scholar] [CrossRef]
- Blanke, M.M.; Whiley, A.W. Bioenergetics, Respiration Cost and Water Relations of Developing Avocado Fruit. J. Plant Physiol. 1995, 145, 87–92. [Google Scholar] [CrossRef]
- Kassim, A.; Workneh, T.S. Influence of Postharvest Treatments and Storage Conditions on the Quality of Hass Avocados. Heliyon 2020, 6, e04234. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Fernández, E.; Pacchiarotta, T.; Mayboroda, O.A.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Metabolomic Analysis of Avocado Fruits by GC-APCI-TOF MS: Effects of Ripening Degrees and Fruit Varieties. Anal. Bioanal. Chem. 2015, 407, 547–555. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International, 20th ed.; AOAC: Gaithersburg, MD, USA, 2019; ISBN 0935584870. [Google Scholar]
- Osuna-García, J.A.; Doyon, G.; Salazar-García, S.; Goenaga, R.; González-Durán, I.J.L. Effect of Harvest Date and Ripening Degree on Quality and Shelf Life of Hass Avocado in Mexico. Fruits 2010, 65, 367–375. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat, version 2017.1.2. Statistical Software. Grupo InfoStat, FCA, National University of Córdoba: Córdoba, Argentina, 2017.

| Compounds Order | Compound | Rt (min) | m/z Detected | Molecular Formula | Calibration Curve | r2 | Lineal Range (mg/L) |
|---|---|---|---|---|---|---|---|
| 1 | Fructose | 6.7 | 179 [M-H]− | C6H12O6 | y = 1210.9x + 4767 | 0.9927 | LOQ-220 |
| 2 | Glucose | 8.6 | 179 [M-H]− | C6H12O6 | y = 1597.3x + 2349.1 | 0.9978 | LOQ-220 |
| 3 | D-Mannoheptulose | 11.5 | 209 [M-H]− | C7H14O7 | y = 1663.4x + 1010 y = 1179.8x + 63000 | 0.9976 0.9929 | LOQ-51 51–815 |
| 4 | Perseitol | 16.8 | 211 [M-H]− | C7H16O7 | y = 3984.1x + 7581.3 | 0.9933 | LOQ-200 |
| 5 | Sucrose | 18.3 | 341 [M-H]− | C12H22O11 | y = 1383.9x + 5001.8 | 0.9949 | LOQ-100 |
| Bacon | ||||
| Unripe | Intermediate ripening | Ready-to-eat | Overripe | |
| D-mannoheptulose | 49 ± 13 b2 | 20 ± 6 a2 | 10 ± 4 a1 | 13 ± 5 a1 |
| Fructose | 2 ± 1 a,b1 | 1.9 ± 0.4 a1 | 3.6 ± 0.5 b,c1 | 4.5 ± 0.9 c1 |
| Glucose | 2 ± 1 a1 | 2.3 ± 0.6 a1 | 4.7 ± 0.5 b1 | 5.3 ± 0.9 b2 |
| Perseitol | 10 ± 2 d1 | 6 ± 2 c1 | 2 ± 1 b1 | 0.5 ± 0.1 a1 |
| Sucrose | 2.5 ± 0.9 a2 | 1.7 ± 0.4 a1,2 | 1.8 ± 0.4 a1 | 3.2 ± 0.9 a1 |
| Fuerte | ||||
| Unripe | Intermediate ripening | Ready-to-eat | Overripe | |
| D-mannoheptulose | 20 ± 6 c1 | 8 ± 1 b1 | 0.8 ± 0.1 a2 | 0.8 ± 0.2 a2 |
| Fructose | 0.6 ± 0.1 a2 | 1.3 ± 0.7 ab1 | 4.2 ± 0.9 c1 | 3 ± 1 bc1 |
| Glucose | 0.8 ± 0.1 a2 | 1.5 ± 0.8 b1 | 4 ± 1 c1 | 3 ± 1 bc1 |
| Perseitol | 23 ± 5 b2 | 15 ± 4 b2 | 1.7 ± 0.5 a1 | 1.9 ± 0.7 a2 |
| Sucrose | 0.9 ± 0.3 a1,2 | 2 ± 1 b,c2 | 1.6 ± 0.6 a,b1 | 3.4 ± 0.8 c1 |
| Hass | ||||
| Unripe | Intermediate ripening | Ready-to-eat | Overripe | |
| D-mannoheptulose | 24 ± 11 d1,2 | 11 ± 4 c1,2 | 1.0 ± 0.5 b2 | 0.4 ± 0.1 a3 |
| Fructose | 3.9 ± 0.7 b3 | 2.2 ± 0.8 a1 | 2.7 ± 0.8 a,b1 | 1.6 ± 0.6 a1 |
| Glucose | 4.0 ± 0.8 b1 | 2.4 ± 0.8 a1 | 3 ± 1 a,b1 | 2.1 ± 0.7 a1 |
| Perseitol | 17 ± 2 d2 | 7 ± 2 c1 | 1.4 ± 0.5 b1 | 0.5 ± 0.2 a1 |
| Sucrose | 0.5 ± 0.1 a1 | 0.8 ± 0.2 a1 | 4 ± 1 b2 | 3.4 ± 0.9 b1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beiro-Valenzuela, M.G.; Monasterio, R.P.; Serrano-García, I.; Hurtado-Fernández, E.; Sánchez-Arévalo, C.M.; Fernández-Sánchez, J.F.; Pedreschi, R.; Olmo-García, L.; Carrasco-Pancorbo, A. Cultivar-Specific Differences in C6 and C7 Sugar Metabolism During Avocado Ripening: Comparative Insights from Bacon, Fuerte, and Hass. Plants 2025, 14, 2856. https://doi.org/10.3390/plants14182856
Beiro-Valenzuela MG, Monasterio RP, Serrano-García I, Hurtado-Fernández E, Sánchez-Arévalo CM, Fernández-Sánchez JF, Pedreschi R, Olmo-García L, Carrasco-Pancorbo A. Cultivar-Specific Differences in C6 and C7 Sugar Metabolism During Avocado Ripening: Comparative Insights from Bacon, Fuerte, and Hass. Plants. 2025; 14(18):2856. https://doi.org/10.3390/plants14182856
Chicago/Turabian StyleBeiro-Valenzuela, María Gemma, Romina P. Monasterio, Irene Serrano-García, Elena Hurtado-Fernández, Carmen María Sánchez-Arévalo, Jorge Fernando Fernández-Sánchez, Romina Pedreschi, Lucía Olmo-García, and Alegría Carrasco-Pancorbo. 2025. "Cultivar-Specific Differences in C6 and C7 Sugar Metabolism During Avocado Ripening: Comparative Insights from Bacon, Fuerte, and Hass" Plants 14, no. 18: 2856. https://doi.org/10.3390/plants14182856
APA StyleBeiro-Valenzuela, M. G., Monasterio, R. P., Serrano-García, I., Hurtado-Fernández, E., Sánchez-Arévalo, C. M., Fernández-Sánchez, J. F., Pedreschi, R., Olmo-García, L., & Carrasco-Pancorbo, A. (2025). Cultivar-Specific Differences in C6 and C7 Sugar Metabolism During Avocado Ripening: Comparative Insights from Bacon, Fuerte, and Hass. Plants, 14(18), 2856. https://doi.org/10.3390/plants14182856








