Seasonal Dynamics of Ecosystem Carbon Exchange and Their Influencing Factors in Grasslands of Different Degrees of Salinization in Northern China
Abstract
1. Introduction
2. Results
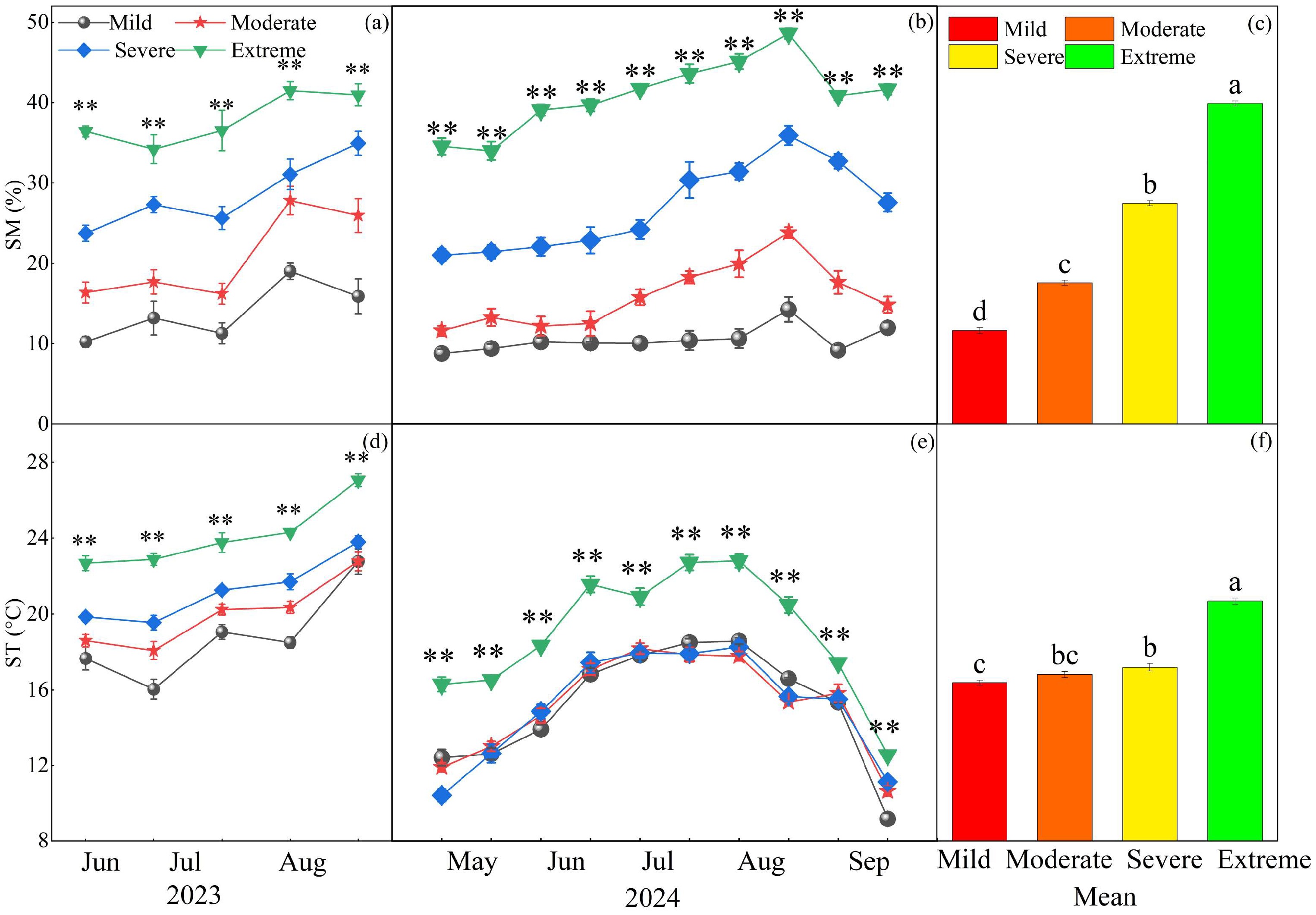
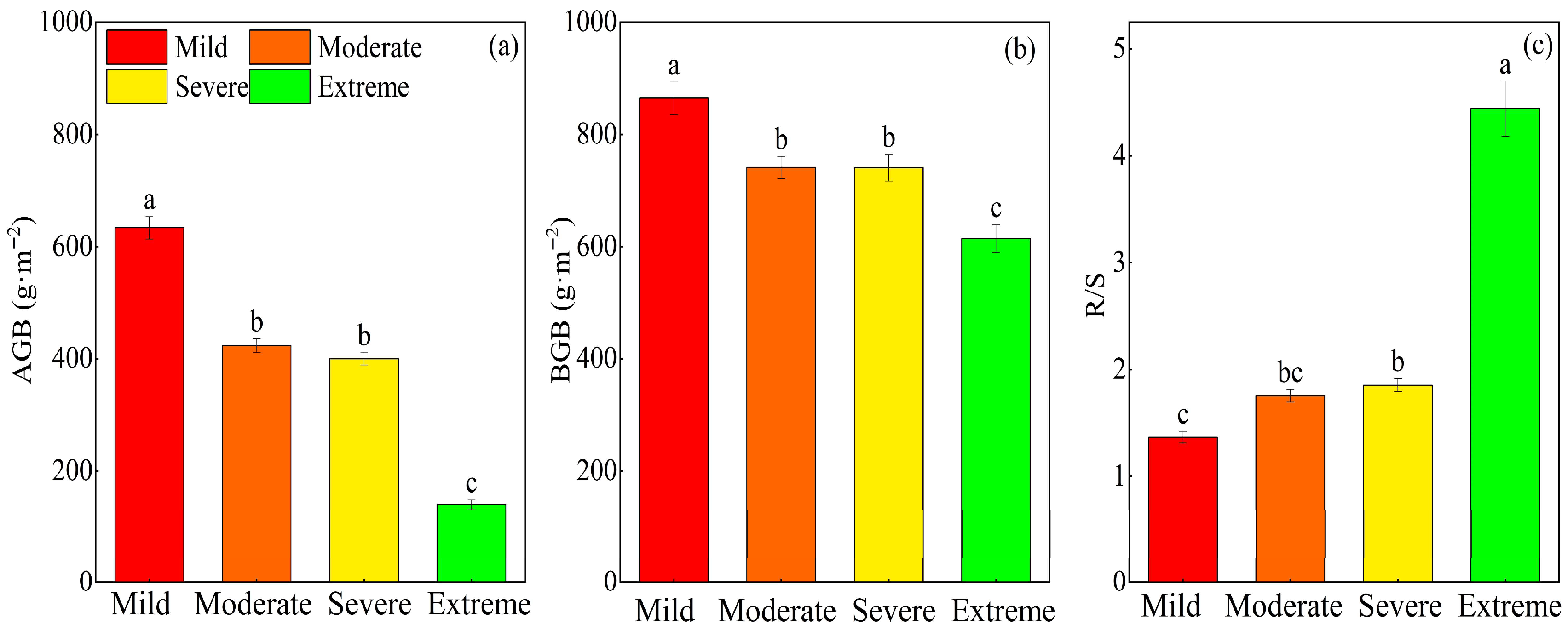
2.1. Effects of Different Degrees of Salinization on Soil Physicochemical Properties and Plant Productivity
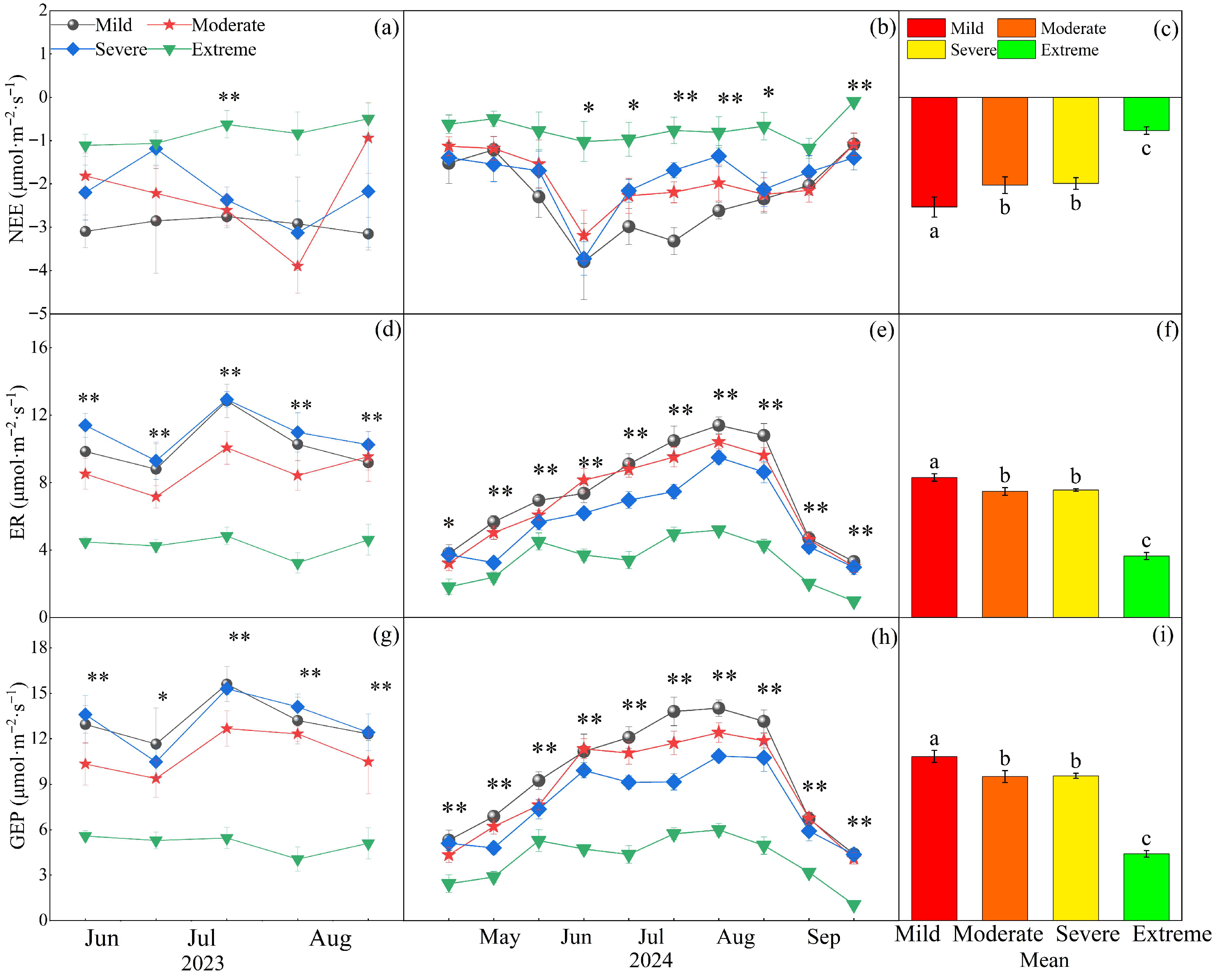
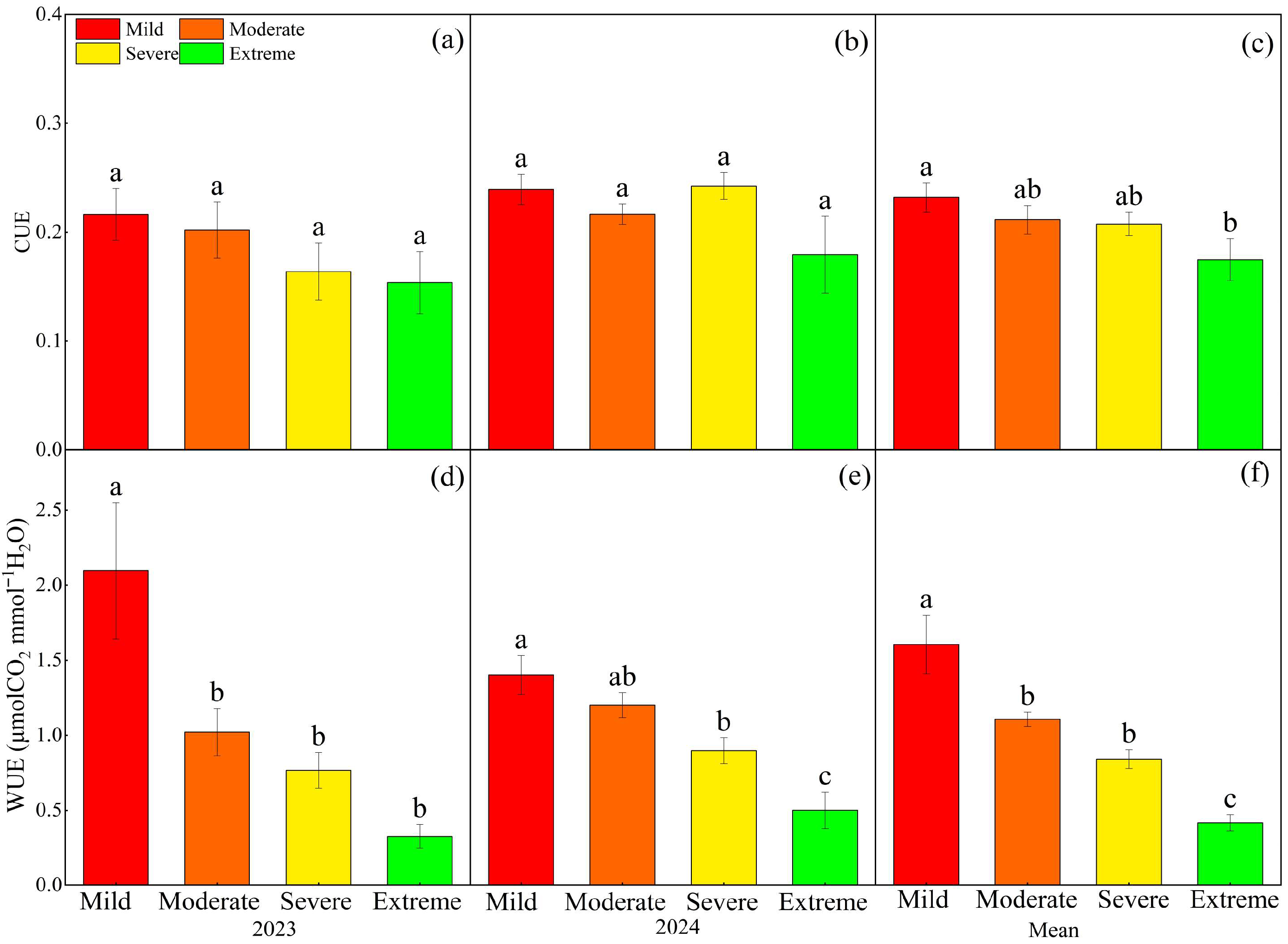
2.2. Effects of Different Degrees of Salinization on Ecosystem Carbon Exchange and Resource Use Efficiency
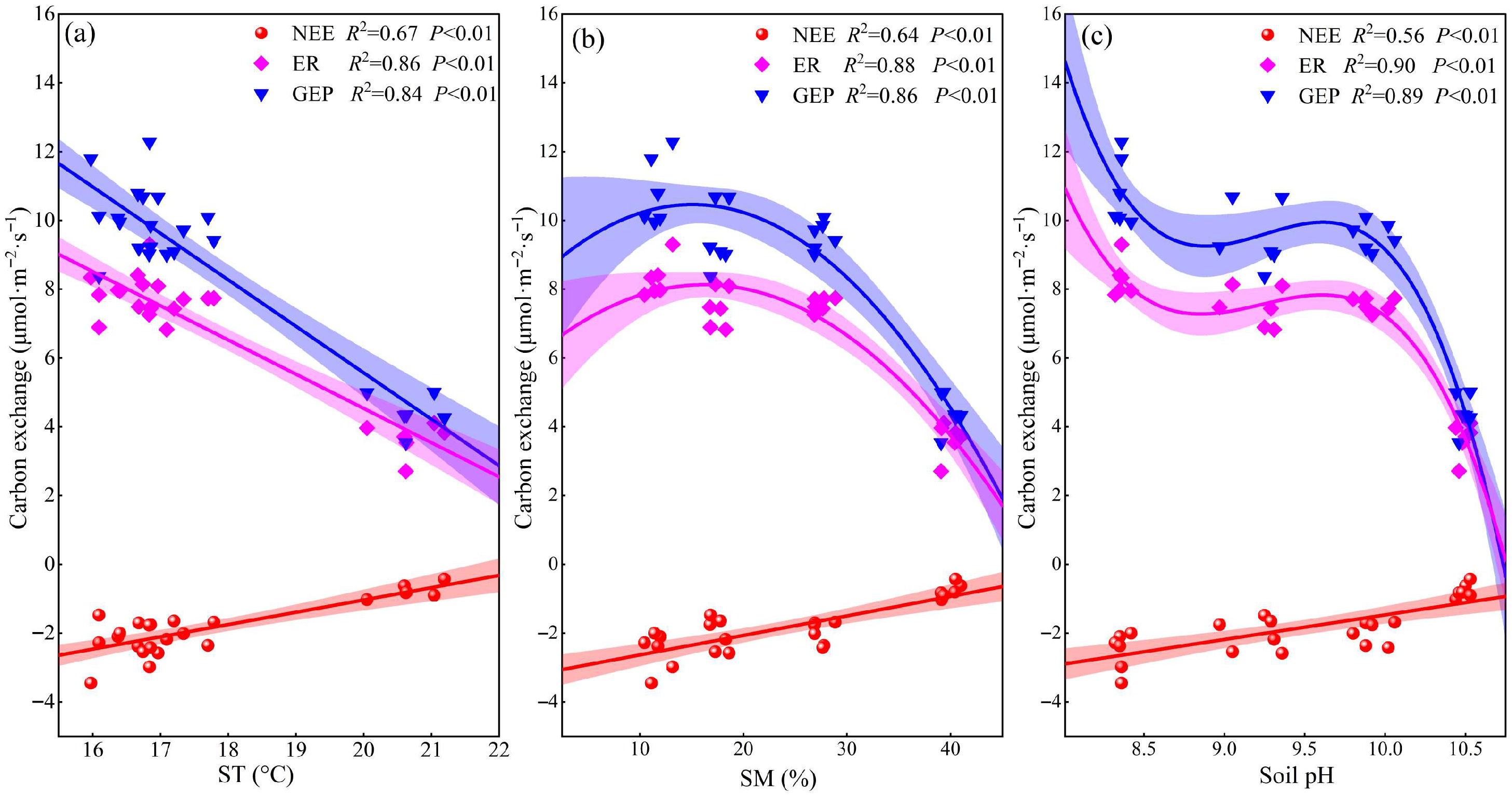
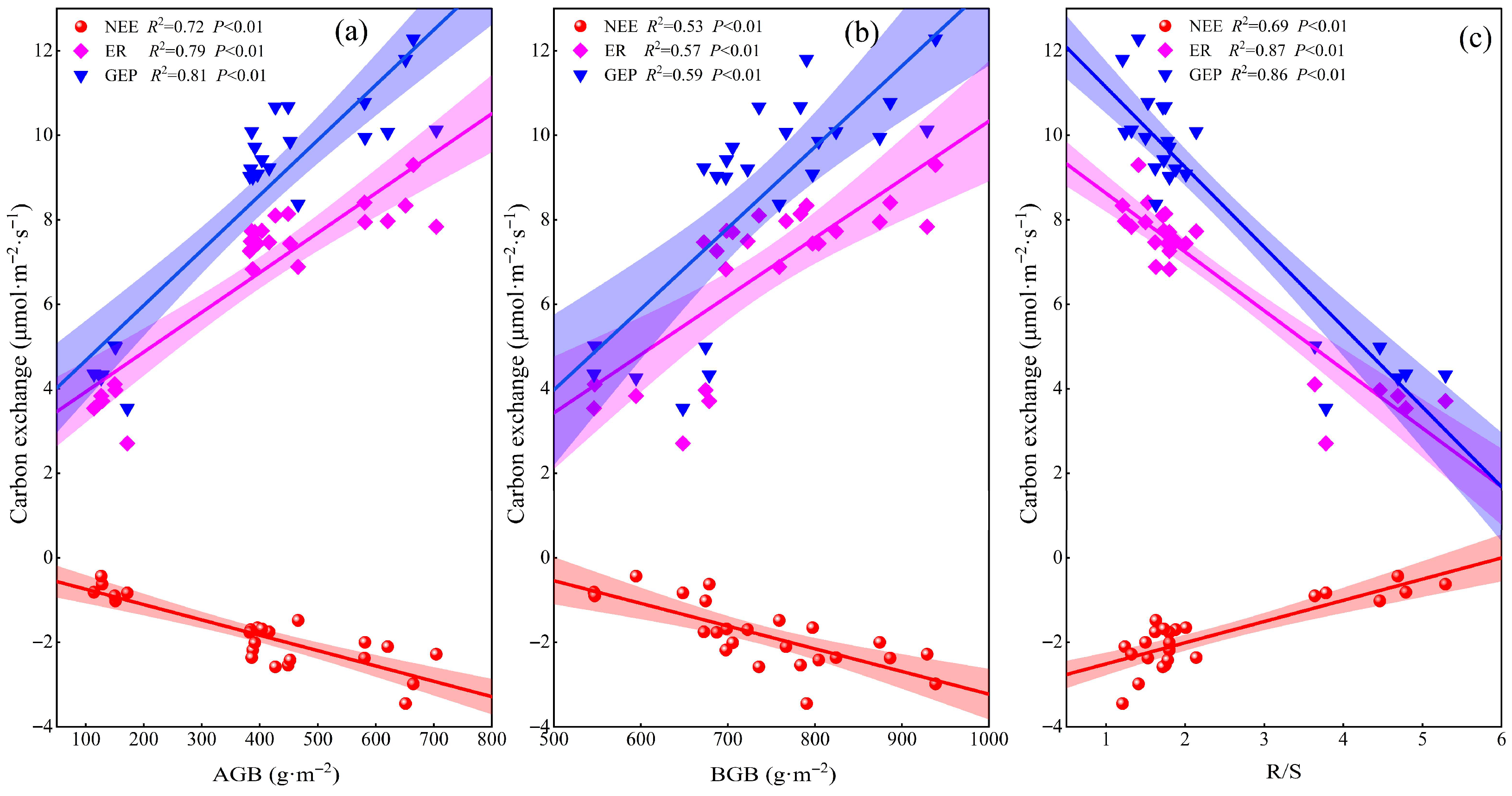
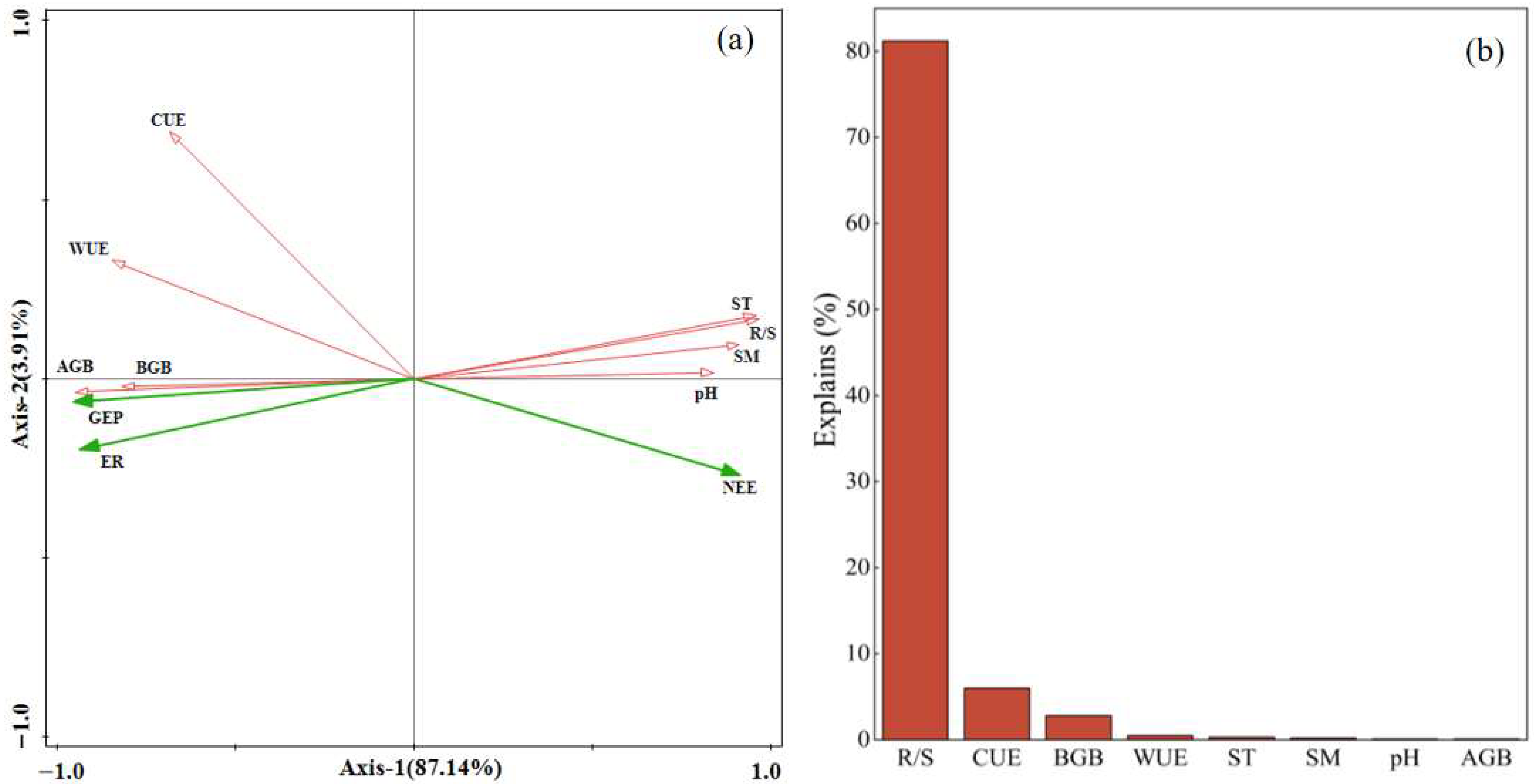
2.3. Factors Influencing Ecosystem Carbon Exchange
3. Discussion
3.1. Effects of Different Degrees of Salinization on Carbon Exchange in Grassland Ecosystems
3.2. Regulation of Carbon Exchange in Grassland Ecosystems
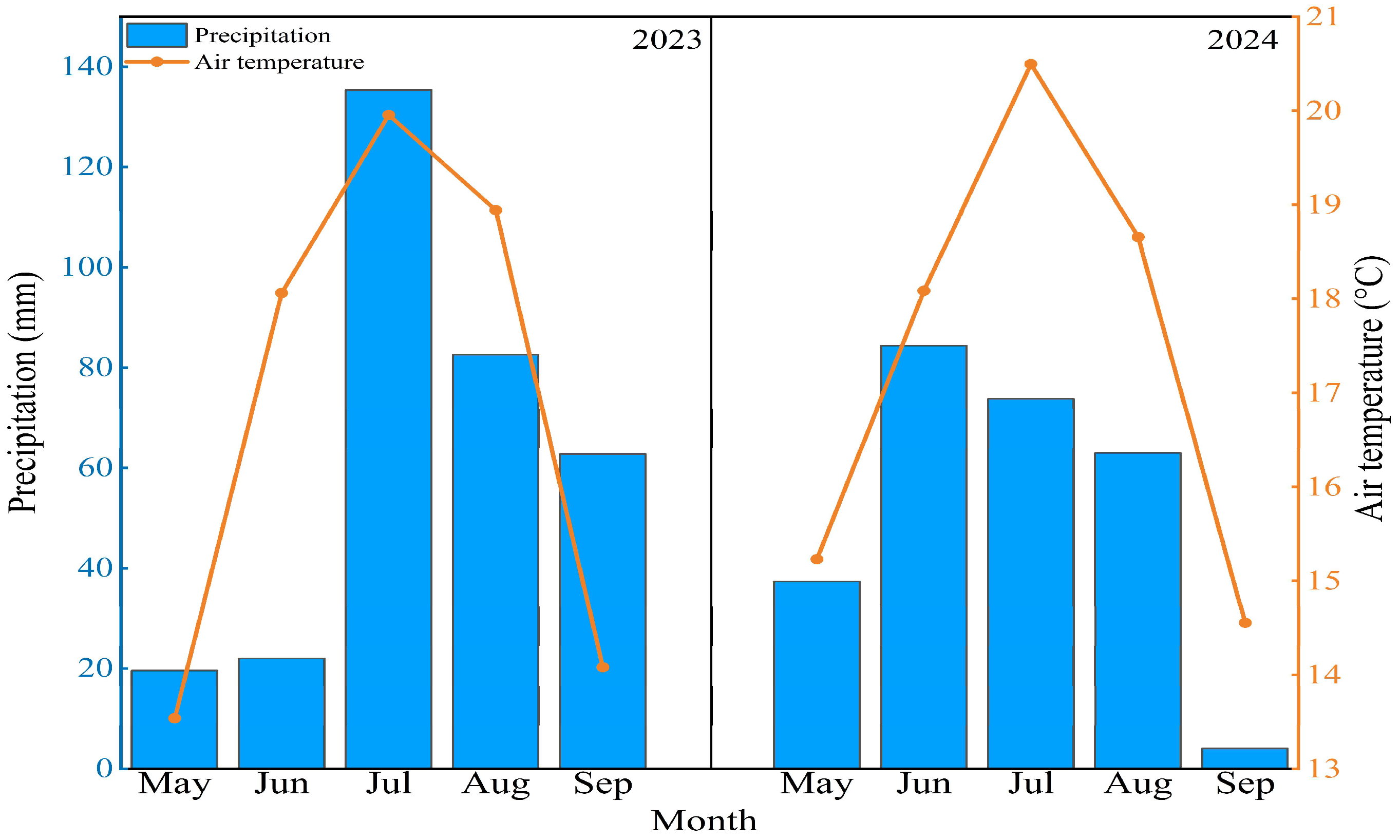
4. Materials and Methods
4.1. Study Site
4.2. Experimental Design
4.3. Measurement of Ecosystem Carbon Fluxes
4.4. Measurement of Plant Biomass and Soil Physicochemical Properties
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, L.; Zhang, X.; Liu, Y.; Zhang, A.; Song, W.; Li, L.; Zhao, J.; Pang, Q. Salt-alkali-tolerant growth-promoting Streptomyces sp. Jrh8-9 enhances alfalfa growth and resilience under saline-alkali stress through integrated modulation of photosynthesis, antioxidant defense, and hormone signaling. Microbiol. Res. 2025, 296, 128158. [Google Scholar]
- Baloch, M.; Zhang, W.; Sultana, T.; Akram, M.; Shoumik, B.; Khan, B.; Farooq, M. Utilization of sewage sludge to manage saline–alkali soil and increase crop production: Is it safe or not? Environ. Technol. Innov. 2023, 32, 103266. [Google Scholar] [CrossRef]
- Wang, L.; Seki, K.; Miyazaki, T.; Ishihama, Y. The causes of soil alkalinization in the Songnen Plain of Northeast China. Paddy Water Environ. 2009, 7, 259–270. [Google Scholar] [CrossRef]
- Gong, H.; Li, Y.; Li, S. Effects of the interaction between biochar and nutrients on soil organic carbon sequestration in soda saline-alkali grassland: A review. Glob. Ecol. Conserv. 2021, 26, e01449. [Google Scholar] [CrossRef]
- Muhammad, M.; Waheed, A.; Wahab, A.; Majeed, M.; Nazim, M.; Liu, Y.; Li, L.; Li, W. Soil salinity and drought tolerance: An evaluation of plant growth, productivity, microbial diversity, and amelioration strategies. Plant Stress 2024, 11, 100319. [Google Scholar]
- Chen, J.; Shiyomi, M.; Wu, Y.; Hori, Y.; Yamamura, Y. Vegetation and its spatial pattern analysis on salinized grasslands in the semiarid Inner Mongolia steppe. Grassl. Sci. 2015, 61, 121–130. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Van der Tol, C.; Luo, G.; Su, Z. Growing season net ecosystem CO2 exchange of two desert ecosystems with alkaline soils in Kazakhstan. Ecol. Evol. 2014, 4, 14–26. [Google Scholar]
- Han, L.; Ganjurjav, H.; Hu, G.; Wu, J.; Yan, Y.; Danjiu, L.; He, S.; Xie, W.; Yan, J.; Cao, Q. Nitrogen addition affects ecosystem carbon exchange by regulating plant community assembly and altering soil properties in an alpine meadow on the Qinghai–Tibetan Plateau. Front. Plant Sci. 2022, 13, 900722. [Google Scholar] [CrossRef]
- Hasi, M.; Zhang, X.; Niu, G.; Wang, Y.; Geng, Q.; Quan, Q.; Chen, S.; Han, X.; Huang, J. Soil moisture, temperature and nitrogen availability interactively regulate carbon exchange in a meadow steppe ecosystem. Agric. For. Meteorol. 2021, 304, 108389. [Google Scholar]
- Wu, S.; Su, Y.; Wang, G.; Hao, J.; Ju, X.; Diao, H.; Dong, K.; Wang, H.; Zhao, X. Ecosystem multifunctionality enhancement by short-term nitrogen addition in semi-arid saline–alkaline grassland of northern China. Sci. Total Environ. 2025, 972, 179151. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Zhao, F.; Chen, J.; Qu, L.; Jiang, S.; Chen, J.; Xin, X.; Shao, C. Land uses changed the dynamics and controls of carbon-water exchanges in alkali-saline Song nen Plain of Northeast China. Ecol. Indic. 2021, 133, 108353. [Google Scholar] [CrossRef]
- Diao, H.; Yang, J.; Hao, J.; Yan, X.; Dong, K.; Wang, C. Seasonal precipitation regulates magnitude and direction of the effect of nitrogen addition on net ecosystem CO2 exchange in saline-alkaline grassland of northern China. Sci. Total Environ. 2023, 877, 162907. [Google Scholar] [CrossRef]
- Xu, D.; Mou, W.; Wang, X.; Zhang, R.; Gao, T.; Ai, D.; Yuan, J.; Zhang, R.; Fang, X. Consistent responses of ecosystem CO2 exchange to grassland degradation in alpine meadow of the Qinghai-Tibetan Plateau. Ecol. Indic. 2022, 141, 109036. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Z.; Zhou, N.; Shi, X.; Lock, T.; Kallenbach, R.; Yuan, Z. Diversity-stability relationships in temperate grasslands as a function of soil pH. Land Degrad. Dev. 2022, 33, 1704–1717. [Google Scholar] [CrossRef]
- Diao, H.; Chen, X.; Zhao, X.; Dong, K.; Wang, C. Effects of nitrogen addition and precipitation alteration on soil respiration and its components in a saline-alkaline grassland. Geoderma 2022, 406, 115541. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Miao, F.; Li, Z.; Tang, W.; Sun, J. Assessing the effect of soil salinization on soil microbial respiration and diversities under incubation conditions. Appl. Soil Ecol. 2020, 155, 103671. [Google Scholar] [CrossRef]
- Ullah, A.; Tariq, A.; Sardans, J.; Graciano, C.; Zeng, F.; Noor, J.; Zhang, Z.; Chai, X.; Ahmed, Z.; Peñuelas, J. Calligonum mongolicum employs a variety of physiological and biochemical strategies to acclimatize to hyperarid saline deserts. Acta Physiol. Plant. 2025, 47, 9. [Google Scholar] [CrossRef]
- Diao, H.; Hao, J.; Yang, Q.; Gao, Y.; Ma, T.; Han, F.; Liang, W.; Chang, J.; Yi, L.; Pang, G.; et al. Soil environment and annual rainfall co-regulate the response of soil respiration to different grazing intensities in saline-alkaline grassland. Catena 2024, 236, 107709. [Google Scholar] [CrossRef]
- Liu, H.; Yu, B.; Sun, Z.; He, P.; Dong, Y.; Yang, H. Spatial variability and driving factors of soil pH in the desert grasslands of northern Xinjiang. Environ. Res. 2025, 276, 121489. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, J.; Wang, Y.; Kang, H.; Zang, J. The tolerance to saline–alkaline stress was dependent on the roots in wheat. Physiol. Mol. Biol. Plants 2020, 26, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Guo, C.; He, F.; Zhang, Q.; Ren, G. Soil Stoichiometric Characteristics and its Relationship with Plant Diversity in Saline-Alkali Grassland of Northern Shanxi. Acta Agrestia Sin. 2021, 29, 2800. [Google Scholar]
- Wen, B.; Li, X.; Yang, F.; Lu, X.; Li, X.; Yang, F. Growth and physiology responses of Phragmites australis to combined drought-flooding condition in inland saline-alkaline marsh, Northeast China. Ecol. Eng. 2017, 108, 234–239. [Google Scholar]
- Zhang, J.; Wu, X.; Shi, Y.; Jin, C.; Yang, Y.; Wei, X.; Mu, C.; Wang, J. A slight increase in soil pH benefits soil organic carbon and nitrogen storage in a semi-arid grassland. Ecol. Indic. 2021, 130, 108037. [Google Scholar]
- Chen, X.; Diao, H.; Wang, S.; Li, H.; Wang, Z.; Shen, Y.; Degen, A.; Dong, K.; Wang, C. Plant community mediated methane uptake in response to increasing nitrogen addition level in a saline-alkaline grassland by rhizospheric effects. Geoderma 2023, 429, 116235. [Google Scholar] [CrossRef]
- AbuQamar, S.; El-Saadony, M.; Saad, A.; Desoky, E.; Elrys, A.; El-Mageed, T.; Semida, W.; Abdelkhalik, A.; Mosa, W.; Kafaas, S.; et al. Halotolerant plant growth-promoting rhizobacteria improve soil fertility and plant salinity tolerance for sustainable agriculture—A review. Plant Stress 2024, 12, 100482. [Google Scholar]
- Diao, H.; Chen, X.; Wang, G.; Ning, Q.; Hu, S.; Wei, S.; Dong, K.; Wang, C. The response of soil respiration to different N compounds addition in a saline–alkaline grassland of northern China. J. Plant Ecol. 2022, 15, 897–910. [Google Scholar] [CrossRef]
- Qu, L.; Liu, J.; Yang, J.; Bai, L.; Huang, Y.; Lu, N.; Yu, H.; Wang, Z.; Li, Z. Soil saline-alkali heterogeneity is an important factor driving the spatial expansion of clonal plant in grassland. Front. Environ. Sci. 2023, 10, 1106825. [Google Scholar] [CrossRef]
- Aung, T.; Shi, F.; Zhai, Y.; Xue, J.; Wang, S.; Ren, X.; Zhang, X. Acidic and alkaline conditions affect the growth of tree peony plants via altering photosynthetic characteristics, limiting nutrient assimilation, and impairing ROS balance. Int. J. Mol. Sci. 2022, 23, 5094. [Google Scholar] [CrossRef]
- Rao, Y.; Peng, T.; Xue, S. Mechanisms of plant saline-alkaline tolerance. Plant Physiol. 2023, 281, 153916. [Google Scholar]
- Zhang, Z.; Zhou, Z.; Feng, S.; Guo, P.; Wang, Y.; Hao, B.; Guo, W.; Li, F. Synergistic effects of AMF and PGPR on improving saline-alkaline tolerance of Leymus chinensis by strengthening the link between rhizosphere metabolites and microbiomes. Environ. Technol. Innov. 2024, 36, 103900. [Google Scholar] [CrossRef]
- Cao, C.; Tao, S.; Cui, Z.; Zhang, Y. Response of soil properties and microbial communities to increasing salinization in the meadow grassland of Northeast China. Microb. Ecol. 2021, 82, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Zhang, W.; Zheng, C.; Tang, G.; Adili, Y.; Ge, C.; Xu, W. Mechanisms of plant extracts in alleviating drought and saline-alkali stress in plants. Ind. Crops Prod. 2025, 233, 121346. [Google Scholar] [CrossRef]
- Wei, T.; Li, G.; Cui, Y.; Xie, J.; Gao, X.; Teng, X.; Zhao, X.; Guan, F.; Liang, Z. Variation Characteristics of Root Traits of Different Alfalfa Cultivars under Saline-Alkaline Stress and their Relationship with Soil Environmental Factors. Phyton-Int. J. Exp. Bot. 2024, 93, 29. [Google Scholar]
- Zhao, Y.; Wang, G.; Zhao, M.; Wang, M.; Liu, B.; Jiang, M. How soil salinization and alkalinization drive vegetation change in salt-affected inland wetlands. Plant Soil 2022, 480, 571–581. [Google Scholar] [CrossRef]
- Mu, C.; Zhang, T.; Zhao, Q.; Su, H.; Wang, S.; Cao, B.; Peng, X.; Wu, Q.; Wu, X. Permafrost affects carbon exchange and its response to experimental warming on the northern Qinghai-Tibetan Plateau. Agric. For. Meteorol. 2017, 247, 252–259. [Google Scholar] [CrossRef]
- Habtewold, J.; Helgason, B.; Yanni, S.; Janzen, H.; Ellert, B.; Gregorich, E. Warming effects on the structure of bacterial and fungal communities in diverse soils. Appl. Soil Ecol. 2021, 163, 103973. [Google Scholar] [CrossRef]
- Han, Y.; Qu, C.; Hu, X.; Wang, P.; Wan, D.; Cai, P.; Rong, X.; Chen, W.; Huang, Q. Warming and humidification mediated changes of DOM composition in an Alfisol. Sci. Total Environ. 2022, 805, 150198. [Google Scholar] [CrossRef]
- Token, S.; Jiang, L.; Zhang, L.; Lv, G. Effects of plant diversity on primary productivity and community stability along soil water and salinity gradients. Glob. Ecol. Conserv. 2022, 36, e02095. [Google Scholar] [CrossRef]
- Su, B.; Su, Z.; Shangguan, Z. Trade-off analyses of plant biomass and soil moisture relations on the Loess Plateau. Catena 2021, 197, 104946. [Google Scholar] [CrossRef]
- Nie, X.; Jiao, P.; Yang, L.; Li, Z. Responses of microbial respiration rate and active bacterial communities to antecedent soil moisture on the Loess Plateau. Geoderma 2025, 458, 117348. [Google Scholar] [CrossRef]
- Song, W.; Tong, X.; Zhang, J.; Meng, P.; Li, J. How a root-microbial system regulates the response of soil respiration to temperature and moisture in a plantation. Pol. J. Environ. Stud. 2018, 27, 2749–2756. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.; Li, J.; Li, R.; Zhao, X.; Dong, K. Analysis of Soil Salinity and Phosphorous Availability of Grassland under Different Saline Spot Coverage. J. Shanxi Agric. Sci. 2020, 48, 1481–1486. [Google Scholar]
- Zhang, S.; Liu, T.; Duan, L.; Hao, L.; Tong, X.; Jia, T.; Lun, S. Characterization and drivers of water and carbon fluxes dynamics in dune ecosystems of the Horqin Sandy Land. Sci. Total Environ. 2024, 918, 170517. [Google Scholar] [CrossRef]
- Li, W.; He, Y.; Shen, R.; Hou, G.; Zheng, Z.; Zhao, B.; Zheng, J.; Jiang, Q.; Zhang, X.; Zhang, Y.; et al. Concurrent nitrogen and phosphorus enrichment increases ecosystem carbon use efficiency in an alpine grassland. Agric. Ecosyst. Environ. 2024, 375, 109182. [Google Scholar] [CrossRef]
- Ganjurjav, H.; Hu, G.; Zhang, Y.; Gornish, E.; Yu, T.; Gao, Q. Warming tends to decrease ecosystem carbon and water use efficiency in dissimilar ways in an alpine meadow and a cultivated grassland in the Tibetan Plateau. Agric. For. Meteorol. 2022, 323, 109079. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, J.; Li, M.; Chen, B.; Tilahun, M.; Zhang, X. Carbon use efficiency of alpine grasslands affected by grazing exclusion and local environmental context in Tibet, China. Glob. Ecol. Conserv. 2024, 56, e03275. [Google Scholar] [CrossRef]
| NEE | ER | GEP | CUE | WUE | ST | SM | |
|---|---|---|---|---|---|---|---|
| Year | 6.73 * | 93.90 ** | 82.41 ** | 4.55 * | 0.15 | 267.72 ** | 10.11 ** |
| Salinization | 31.60 ** | 67.97 ** | 82.52 ** | 2.40 | 16.85 ** | 45.92 ** | 483.79 ** |
| Year × Salinization | 0.65 | 10.25 ** | 7.71 ** | 0.77 | 2.47 | 2.81 * | 9.89 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, G.; Wang, J.; Wang, J.; Chen, Y.; Dong, K.; Diao, H. Seasonal Dynamics of Ecosystem Carbon Exchange and Their Influencing Factors in Grasslands of Different Degrees of Salinization in Northern China. Plants 2025, 14, 2854. https://doi.org/10.3390/plants14182854
Pang G, Wang J, Wang J, Chen Y, Dong K, Diao H. Seasonal Dynamics of Ecosystem Carbon Exchange and Their Influencing Factors in Grasslands of Different Degrees of Salinization in Northern China. Plants. 2025; 14(18):2854. https://doi.org/10.3390/plants14182854
Chicago/Turabian StylePang, Gaoliang, Jingjing Wang, Jianyu Wang, Yicong Chen, Kuanhu Dong, and Huajie Diao. 2025. "Seasonal Dynamics of Ecosystem Carbon Exchange and Their Influencing Factors in Grasslands of Different Degrees of Salinization in Northern China" Plants 14, no. 18: 2854. https://doi.org/10.3390/plants14182854
APA StylePang, G., Wang, J., Wang, J., Chen, Y., Dong, K., & Diao, H. (2025). Seasonal Dynamics of Ecosystem Carbon Exchange and Their Influencing Factors in Grasslands of Different Degrees of Salinization in Northern China. Plants, 14(18), 2854. https://doi.org/10.3390/plants14182854






