Non-CG DNA Methylation Regulates Root Stem Cell Niche Maintenance, Auxin Signaling, and ROS Homeostasis in Arabidopsis Under Cadmium Stress
Abstract
1. Introduction
2. Results
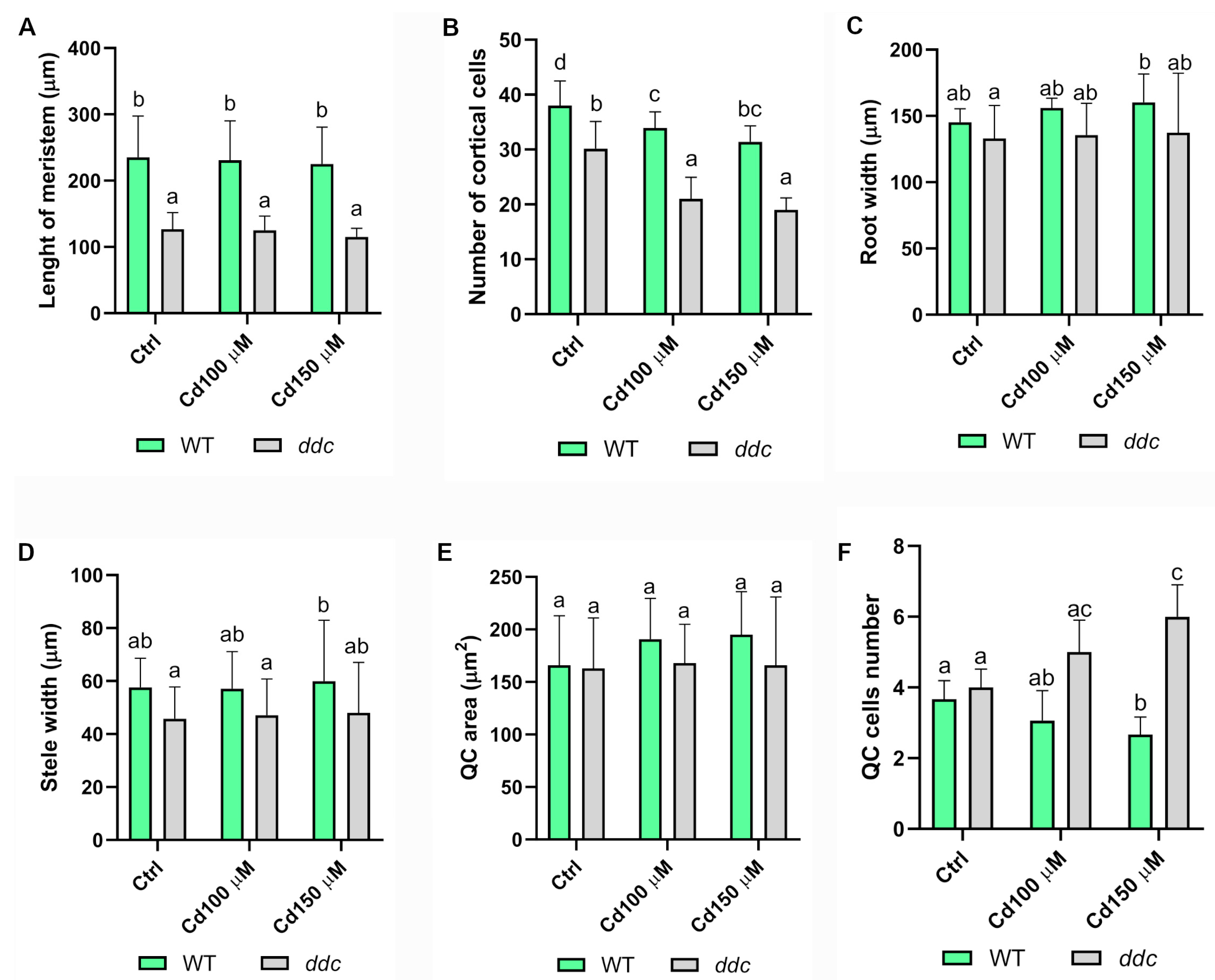
2.1. Cadmium Exposure Inhibits Primary Root Growth in Both WT and ddc Seedlings
2.2. Non-CG DNA Methylation Affects Quiescent Center Dynamics Under Cadmium Stress
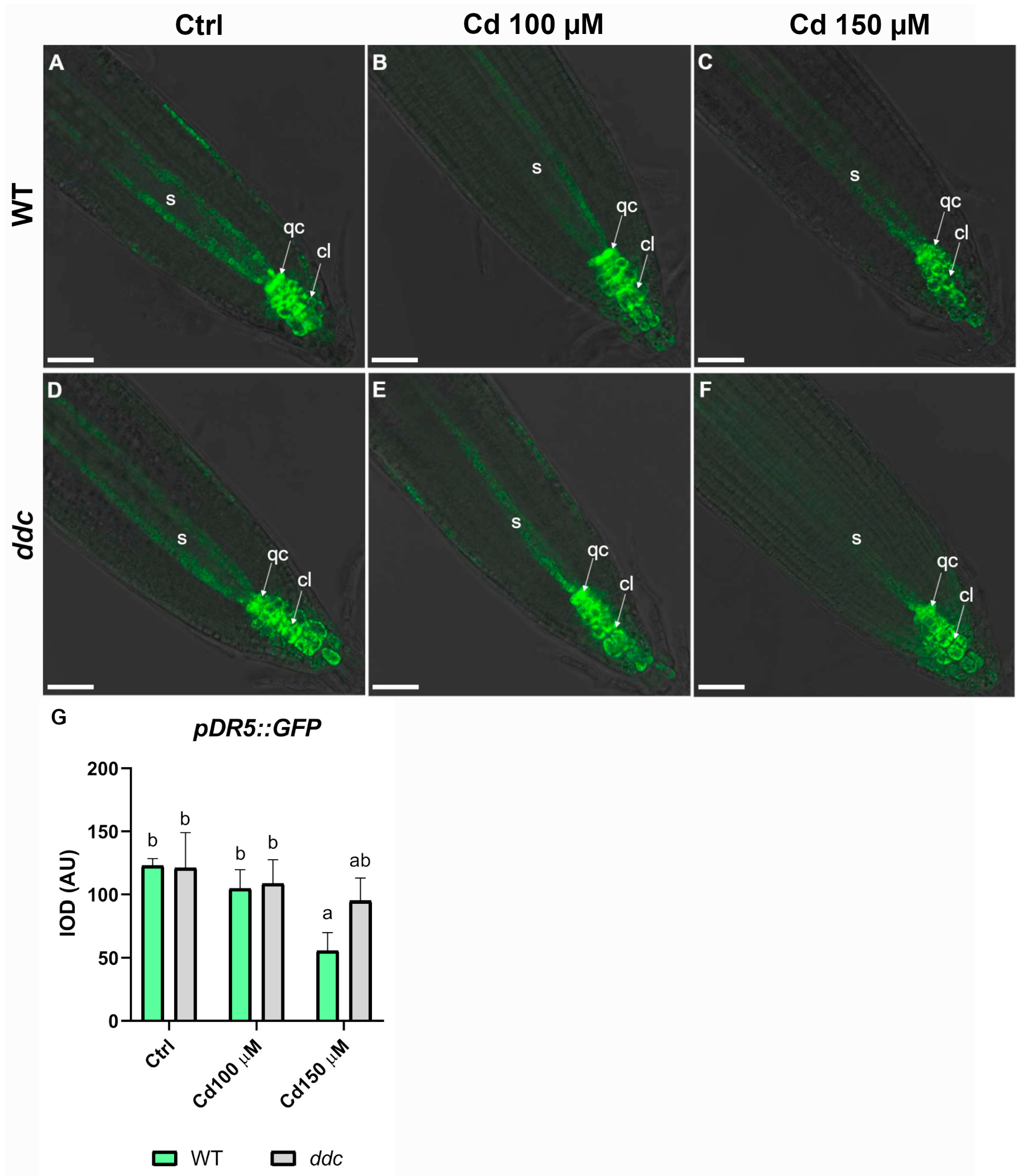
2.3. Differential Auxin Signaling Response to Cadmium Stress in Wild Type and ddc Mutant Roots
2.4. ROS Differentially Accumulate in Wild Type and ddc Mutant Roots Under Cadmium Stress
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Analysis of Root Growth Parameters
4.3. Histochemical Staining (mPS-PI Staining)
4.4. Confocal Microscopy Analysis of GFP Signal Localization
4.5. Reactive Oxygen Species Detection
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| APX | Ascorbate peroxidase |
| CAT | Catalase |
| Cd2+ | Cadmium |
| CdCl2 | Cadmium chloride |
| CMT3 | CHROMOMETHYLASE 3 |
| Ctrl | Control |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| ddc | drm1 drm2 cmt3 triple mutant |
| DRM1/DRM2 | DOMAINS REARRANGED METHYLTRANSFERASE 1/2 |
| GFP | Green fluorescent protein |
| GPX | Glutathione peroxidase |
| H2DCFDA | 2′,7′-Dichlorodihydrofluorescein diacetate |
| H2O2 | Hydrogen peroxide |
| IAA | Indole-3-acetic acid |
| IOD | Integrated optical density |
| JA | Jasmonic acid |
| MAPKs | Mitogen-activated protein kinases |
| MET1 | METHYLTRANSFERASE 1 |
| NO | Nitric oxide |
| O2•− | Superoxide radical |
| PIN | PIN-FORMED proteins |
| PLT | PLETHORA |
| QC | Quiescent center |
| RAM | Root apical meristem |
| RBOHD | Respiratory burst oxidase homolog D |
| ROS | Reactive oxygen species |
| SA | Salicylic acid |
| SCR | SCARECROW |
| WT | Wild type |
| WOX5 | WUSCHEL-RELATED HOMEOBOX 5 |
| WUS | WUSCHEL |
References
- Khan, Z.; Elahi, A.; Bukhari, D.A.; Rehman, A. Cadmium sources, toxicity, resistance and removal by microorganisms-A potential strategy for cadmium eradication. J. Saudi Chem. Soc. 2022, 26, 101569. [Google Scholar] [CrossRef]
- Sharma, R.K.; Archana, G. Cadmium minimization in food crops by cadmium resistant plant growth promoting rhizobacteria. Appl. Soil Ecol. 2016, 107, 66–78. [Google Scholar] [CrossRef]
- Gill, S.S.; Hasanuzzaman, M.; Nahar, K.; Macovei, A.; Tuteja, N. Importance of nitric oxide in cadmium stress tolerance in crop plants. Plant Physiol. Biochem. 2013, 63, 254–261. [Google Scholar] [CrossRef]
- Bolan, N.; Mahimairaja, S.; Kunhikrishnan, A.; Naidu, R. Sorption–bioavailability nexus of arsenic and cadmium in variable-charge soils. J. Hazard. Mater. 2013, 261, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhao, C.; Zhang, Y.; Wang, X.; Wang, X.; Wang, J.; Wang, F.; Bi, Y. Calcium alleviates cadmium-induced inhibition on root growth by maintaining auxin homeostasis in Arabidopsis seedlings. Protoplasma 2016, 253, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Bruno, L.; Pacenza, M.; Forgione, I.; Lamerton, L.R.; Greco, M.; Chiappetta, A.; Bitonti, M.B. In Arabidopsis thaliana cadmium impact on the growth of primary root by altering SCR expression and auxin-cytokinin cross-talk. Front. Plant Sci. 2017, 8, 1323. [Google Scholar] [CrossRef] [PubMed]
- Bruno, L.; Talarico, E.; Madeo, M.L.; Muto, A.; Minervino, M.; Araniti, F.; Bitonti, M.B.; Chiappetta, A. Cadmium affects cell niches maintenance in Arabidopsis thaliana post-embryonic shoot and root apical meristem by altering the expression of WUS/WOX homolog genes and cytokinin accumulation. Plant Physiol. Biochem. 2021, 167, 785–794. [Google Scholar] [CrossRef]
- Lee, J.Y.; Benfey, P.N. Root apical meristems. In Handbook of Plant Science, 2 Volume Set; Roberts, K., Ed.; John Wiley & Sons: Chichester, UK, 2007; Volume 1, pp. 47–53. [Google Scholar] [CrossRef]
- López-González, D.; Bruno, L.; Díaz-Tielas, C.; Lupini, A.; Aci, M.M.; Talarico, E.; Madeo, M.L.; Muto, A.; Sánchez-Moreiras, A.M.; Araniti, F. Short-Term Effects of Trans-Cinnamic Acid on the Metabolism of Zea mays L. Roots. Plants 2023, 12, 189. [Google Scholar] [CrossRef]
- Lukačová, Z.; Švubová, R.; Kohanová, J.; Lux, A. Silicon mitigates the Cd toxicity in maize in relation to cadmium translocation, cell distribution, antioxidant enzymes stimulation and enhanced endodermal apoplasmic barrier development. Plant Growth Regul. 2013, 70, 89–103. [Google Scholar] [CrossRef]
- Choppala, G.; Saifullah, B.N.; Bibi, S.; Iqbal, M.; Rengel, Z.; Kunhikrishnan, A.; Ashwath, N.; Ok, Y.S. Cellular mechanisms in higher plants governing tolerance to cadmium toxicity. Crit. Rev. Plant Sci. 2014, 33, 374–391. [Google Scholar] [CrossRef]
- Shahid, M.; Pourrut, B.; Dumat, C.; Nadeem, M.; Aslam, M.; Pinelli, E. Heavy-metal-induced reactive oxygen species: Phytotoxicity and physicochemical changes in plants. Rev. Environ. Contam. Toxicol. 2014, 232, 1–44. [Google Scholar] [CrossRef]
- Benavides, M.P.; Gallego, S.M.; Tomaro, M.L. Cadmium toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 21–34. [Google Scholar] [CrossRef]
- Araniti, F.; Talarico, E.; Madeo, M.L.; Greco, E.; Minervino, M.; Álvarez-Rodríguez, S.; Muto, A.; Ferrari, M.; Chiappetta, A.; Bruno, L. Short-term exposition to acute cadmium toxicity induces the loss of root gravitropic stimuli perception through PIN2-mediated auxin redistribution in Arabidopsis thaliana (L.) Heynh. Plant Sci. 2023, 332, 111726. [Google Scholar] [CrossRef]
- Bruno, L.; Talarico, E.; Cabeiras-Freijanes, L.; Madeo, M.L.; Muto, A.; Minervino, M.; Lucini, L.; Miras-Moreno, B.; Sofo, A.; Araniti, F. Coumarin Interferes with Polar Auxin Transport Altering Microtubule Cortical Array Organization in Arabidopsis thaliana (L.) Heynh Root Apical Meristem. Int. J. Mol. Sci. 2021, 22, 7305. [Google Scholar] [CrossRef]
- Haghpanah, M.; Namdari, A.; Kaleji, M.K.; Nikbakht-dehkordi, A.; Arzani, A.; Araniti, F. Interplay Between ROS and Hormones in Plant Defense Against Pathogens. Plants 2025, 14, 1297. [Google Scholar] [CrossRef] [PubMed]
- Novikova, O. Chromodomains and LTR retrotransposons in plants. Commun. Integr. Biol. 2009, 2, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Talarico, E.; Zambelli, A.; Araniti, F.; Greco, E.; Chiappetta, A.; Bruno, L. Unravelling the epigenetic code: DNA methylation in plants and its role in stress response. Epigenomes 2024, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Garro, M.; Greco, E.; Vannay, G.J.; Leonova, A.; Bruno, L.; Capella, M. Non-CG DNA methylation represses SDC expression to modulate hypocotyl elongation during thermormorphogenesis in Arabidopsis. J. Exp. Bot. 2025, 76, 2517–2534. [Google Scholar] [CrossRef]
- Forgione, I.; Wołoszyńska, M.; Pacenza, M.; Chiappetta, A.; Greco, M.; Araniti, F.; Abenavoli, M.R.; Van Lijsebettens, M.; Bitonti, M.B.; Bruno, L. Hypomethylated drm1 drm2 cmt3 mutant phenotype of Arabidopsis thaliana is related to auxin pathway impairment. Plant Sci. 2019, 280, 383–396. [Google Scholar] [CrossRef]
- Forgione, I.; Muto, A.; Woloszynska, M.; Chiappetta, A.; Ferrari, M.; Van Lijsebettens, M.; Bitonti, M.B.; Bruno, L. Epigenetic mechanisms affect the curled leaf phenotype in the hypomethylated ddc mutant of Arabidopsis thaliana. Plant Sci. 2022, 319, 111254. [Google Scholar] [CrossRef]
- Pacenza, M.; Muto, A.; Chiappetta, A.; Mariotti, L.; Talarico, E.; Picciarelli, P.; Picardi, E.; Bruno, L.; Bitonti, M.B. In Arabidopsis thaliana Cd differentially impacts on hormone genetic pathways in the methylation defective ddc mutant compared to wild type. Sci. Rep. 2021, 11, 10965. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Peng, J.; Li, F.; Ali, F.; Wang, Z. Regulation of seed germination: ROS, epigenetic, and hormonal aspects. J. Adv. Res. 2025, 71, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Satoh-Nagasawa, N.; Mori, M.; Nakazawa, N.; Kawamoto, T.; Nagato, Y.; Sakurai, K.; Takahashi, H.; Watanabe, A.; Akagi, H. Mutations in rice (Oryza sativa) heavy metal ATPase 2 (OsHMA2) restrict the translocation of zinc and cadmium. Plant Cell Physiol. 2012, 53, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Ronzan, M.; Piacentini, D.; Fattorini, L.; Della Rovere, F.; Eiche, E.; Riemann, M.; Altamura, M.M.; Falasca, G. Cadmium and arsenic affect root development in Oryza sativa L. negatively interacting with auxin. Environ. Exp. Bot. 2018, 151, 64–75. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, W.S.; Kim, S.H. Hormonal regulation of stem cell maintenance in roots. J. Exp. Bot. 2013, 64, 1153–1165. [Google Scholar] [CrossRef]
- Heyman, J.; Kumpf, R.P.; De Veylder, L. A quiescent path to plant longevity. Trends Cell Biol. 2014, 24, 443–448. [Google Scholar] [CrossRef]
- Fattorini, L.; Ronzan, M.; Piacentini, D.; Della Rovere, F.; De Virgilio, C.; Sofo, A.; Altamura, M.M.; Falasca, G. Cadmium and arsenic affect quiescent centre formation and maintenance in Arabidopsis thaliana post-embryonic roots disrupting auxin biosynthesis and transport. Environ. Exp. Bot. 2017, 144, 37–48. [Google Scholar] [CrossRef]
- Roychoudhry, S.; Kepinski, S. Auxin in root development. Cold Spring Harb. Perspect. Biol. 2022, 14, a039933. [Google Scholar] [CrossRef]
- Ding, Z.J.; Friml, J. Auxin regulates distal stem cell differentiation in Arabidopsis roots. Proc. Natl. Acad. Sci. USA 2010, 107, 12046–12051. [Google Scholar] [CrossRef]
- Aida, M.; Beis, D.; Heidstra, R.; Willemsen, V.; Blilou, I.; Galinha, C.; Nussaume, L.; Noh, Y.S.; Amasino, R.; Scheres, B. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 2004, 119, 109–120. [Google Scholar] [CrossRef]
- Eljebbawi, A.; Dolata, A.; Strotmann, V.I.; Stahl, Y. Stem cell quiescence and dormancy in plant meristems. J. Exp. Bot. 2024, 75, 6022–6036. [Google Scholar] [CrossRef]
- Sabatini, S.; Heidstra, R.; Wildwater, M.; Scheres, B. SCARECROW is involved in positioning the stem cell niche in the Arabidopsis root meristem. Genes Dev. 2003, 17, 354–358. [Google Scholar] [CrossRef]
- Zhai, H.; Zhang, X.; You, Y.; Lin, L.; Zhou, W.; Li, C. SEUSS integrates transcriptional and epigenetic control of root stem cell organizer specification. EMBO J. 2020, 39, e105047. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Heyno, E.; Klose, C.; Krieger-Liszkay, A. Origin of cadmium-induced reactive oxygen species production: Mitochondrial electron transfer versus plasma membrane NADPH oxidase. New Phytol. 2008, 179, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Cho, U.H.; Sohn, J.Y. Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and lipid peroxidation in Arabidopsis thaliana. J. Plant Biol. 2004, 47, 262–269. [Google Scholar] [CrossRef]
- Zhang, T.; Xiao, J.; Zhao, Y.; Zhang, Y.; Jie, Y.; Shen, D.; Yue, C.; Huang, J.; Hua, Y.; Zhou, T. Comparative physiological and transcriptomic analyses reveal ascorbate and glutathione coregulation of cadmium toxicity resistance in wheat genotypes. BMC Plant Biol. 2021, 21, 459. [Google Scholar] [CrossRef]
- Gutiérrez-Martínez, P.B.; Torres-Morán, M.I.; Romero-Puertas, M.C.; Casas-Solís, J.; Zarazúa-Villaseñor, P.; Sandoval-Pinto, E.; Ramírez-Hernández, B.C. Assessment of antioxidant enzymes in leaves and roots of Phaseolus vulgaris plants under cadmium stress. Biotecnia 2020, 22, 110–118. [Google Scholar] [CrossRef]
- Liu, Y.T.; Chen, Z.S.; Hong, C.Y. Cadmium-induced physiological response and antioxidant enzyme changes in the novel cadmium accumulator, Tagetes patula. J. Hazard. Mater. 2011, 189, 724–731. [Google Scholar] [CrossRef]
- Pasternak, T.; Palme, K.; Pérez-Pérez, J.M. Role of reactive oxygen species in the modulation of auxin flux and root development in Arabidopsis thaliana. Plant J. 2023, 114, 83–95. [Google Scholar] [CrossRef]
- Bruno, L.; Araniti, F.; Talarico, E.; Greco, E.; Muto, A.; Pacenza, M.; Chiappetta, A.; Bitonti, M.B. Transcriptomic and metabolomics analysis of the main stress-related pathways in the DNA methylation-defective ddc mutant of Arabidopsis thaliana exposed to Cd. Plant Biosyst. 2025; in press. [Google Scholar] [CrossRef]
- Truernit, E.; Bauby, H.; Dubreucq, B.; Grandjean, O.; Runions, J.; Barthélémy, J.; Palauqui, J.C. High-resolution whole-mount imaging of three-dimensional tissue organization and gene expression enables the study of phloem development and structure in Arabidopsis. Plant Cell 2008, 20, 1494–1503. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talarico, E.; Greco, E.; Araniti, F.; Chiappetta, A.; Bruno, L. Non-CG DNA Methylation Regulates Root Stem Cell Niche Maintenance, Auxin Signaling, and ROS Homeostasis in Arabidopsis Under Cadmium Stress. Plants 2025, 14, 2838. https://doi.org/10.3390/plants14182838
Talarico E, Greco E, Araniti F, Chiappetta A, Bruno L. Non-CG DNA Methylation Regulates Root Stem Cell Niche Maintenance, Auxin Signaling, and ROS Homeostasis in Arabidopsis Under Cadmium Stress. Plants. 2025; 14(18):2838. https://doi.org/10.3390/plants14182838
Chicago/Turabian StyleTalarico, Emanuela, Eleonora Greco, Fabrizio Araniti, Adriana Chiappetta, and Leonardo Bruno. 2025. "Non-CG DNA Methylation Regulates Root Stem Cell Niche Maintenance, Auxin Signaling, and ROS Homeostasis in Arabidopsis Under Cadmium Stress" Plants 14, no. 18: 2838. https://doi.org/10.3390/plants14182838
APA StyleTalarico, E., Greco, E., Araniti, F., Chiappetta, A., & Bruno, L. (2025). Non-CG DNA Methylation Regulates Root Stem Cell Niche Maintenance, Auxin Signaling, and ROS Homeostasis in Arabidopsis Under Cadmium Stress. Plants, 14(18), 2838. https://doi.org/10.3390/plants14182838







