The Impact of Elevated Soil pH Levels on Cranberry Growth, Physiology, and Metabolites
Abstract
1. Introduction
2. Results
2.1. Soil pH, Water pH, and Nutrients
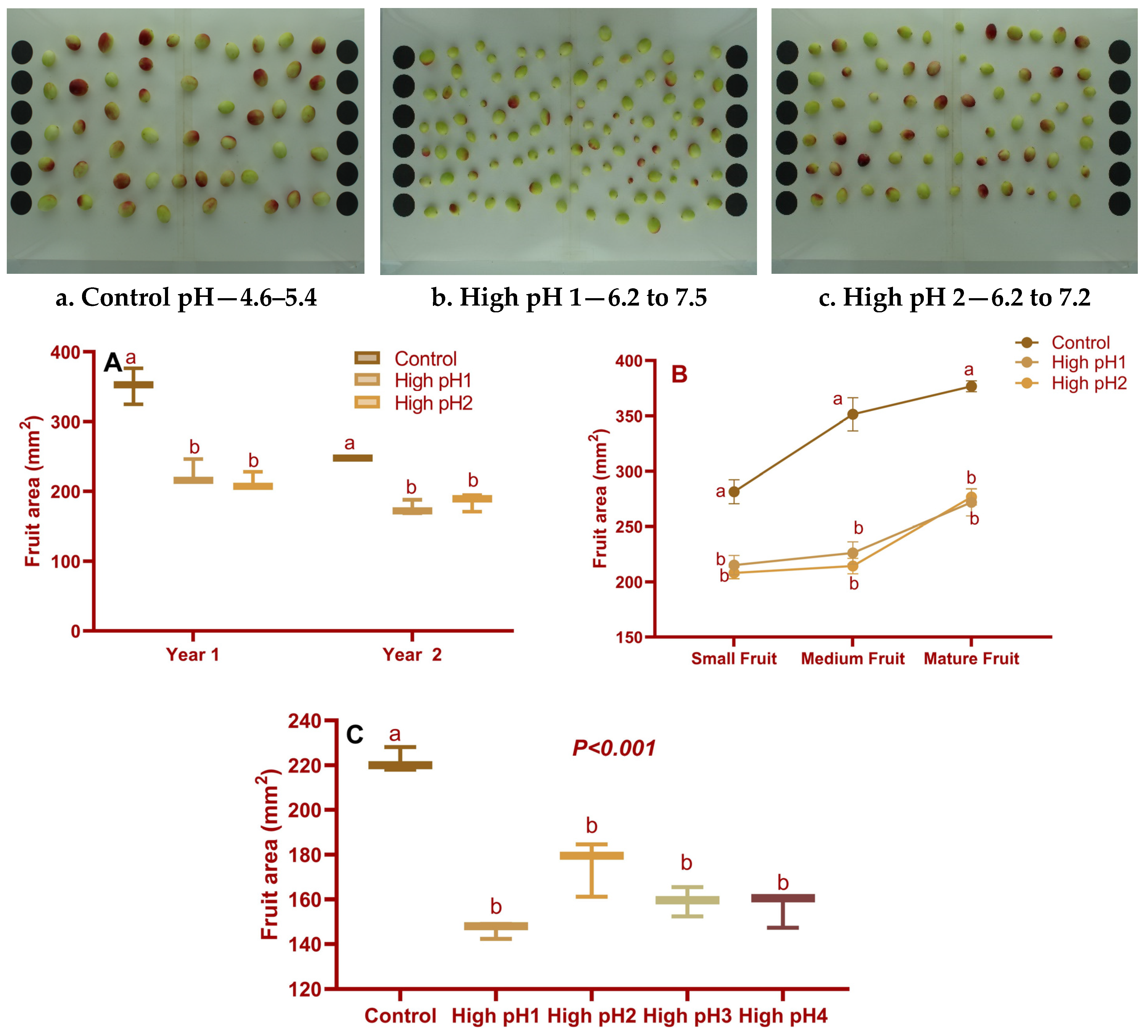
2.2. Fruit Traits
2.2.1. Fruit Size
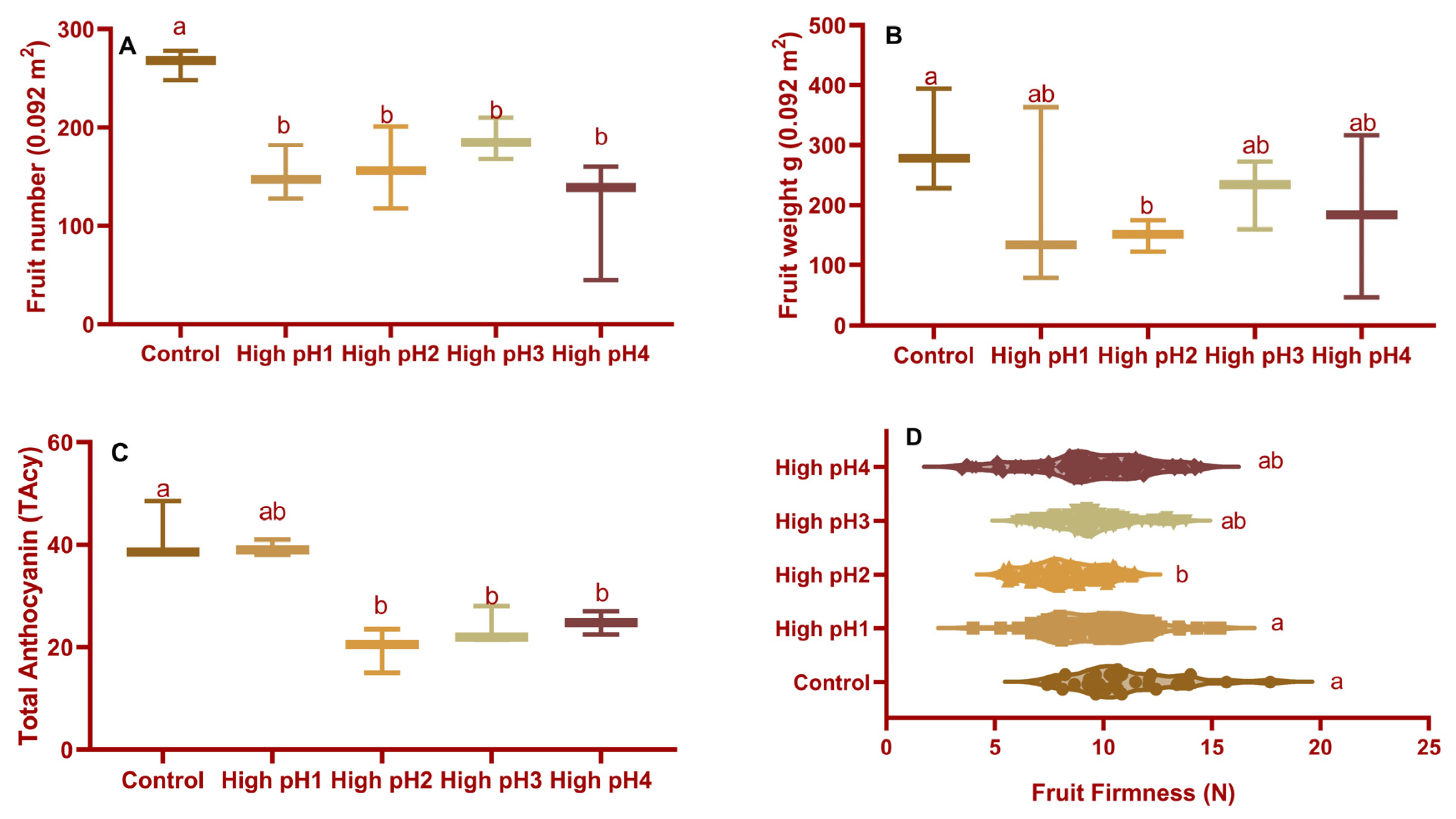
2.2.2. Fruit Yield and Weight
2.2.3. Fruit Color and Firmness
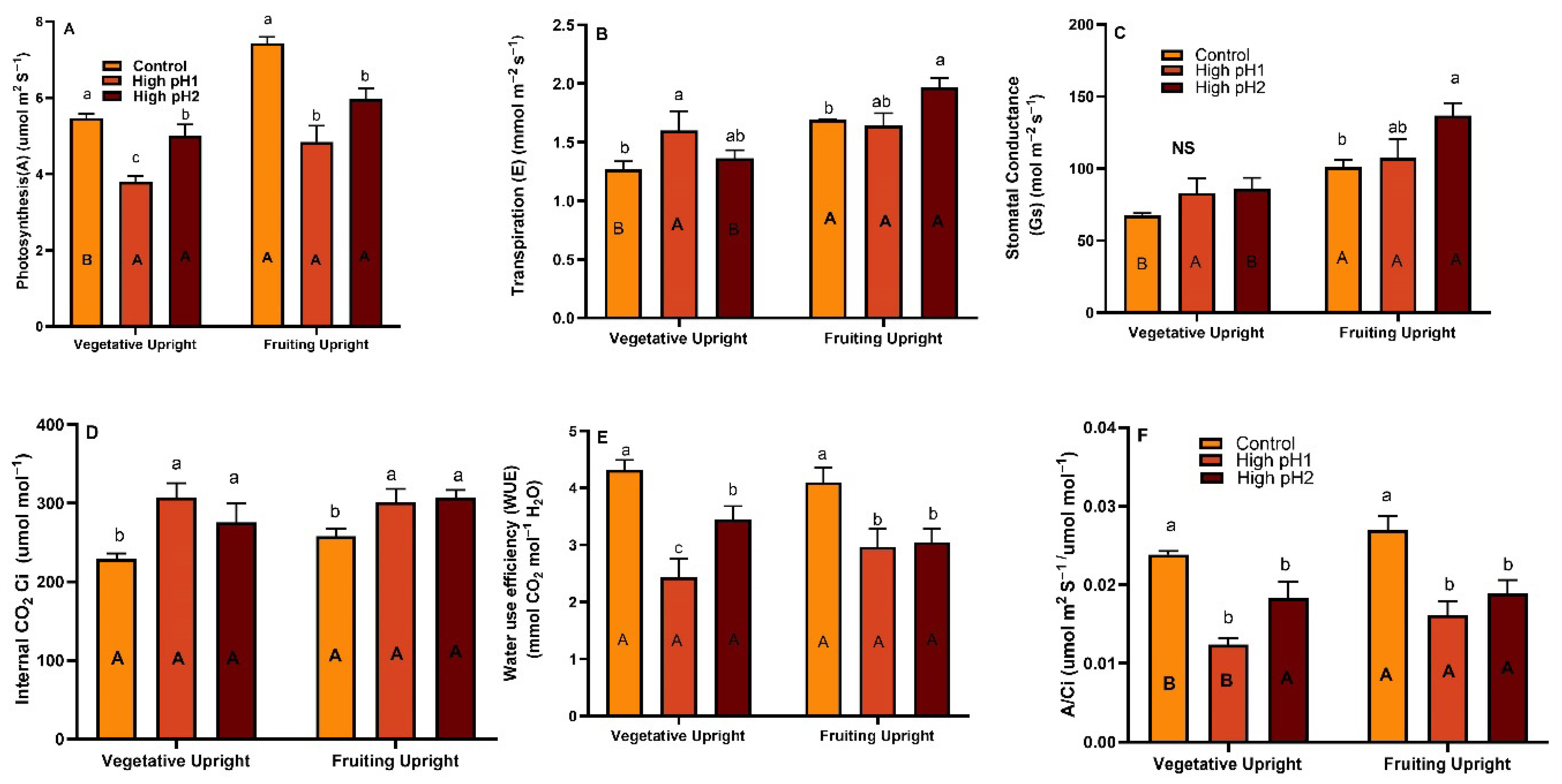
2.3. Gas Exchange Parameters
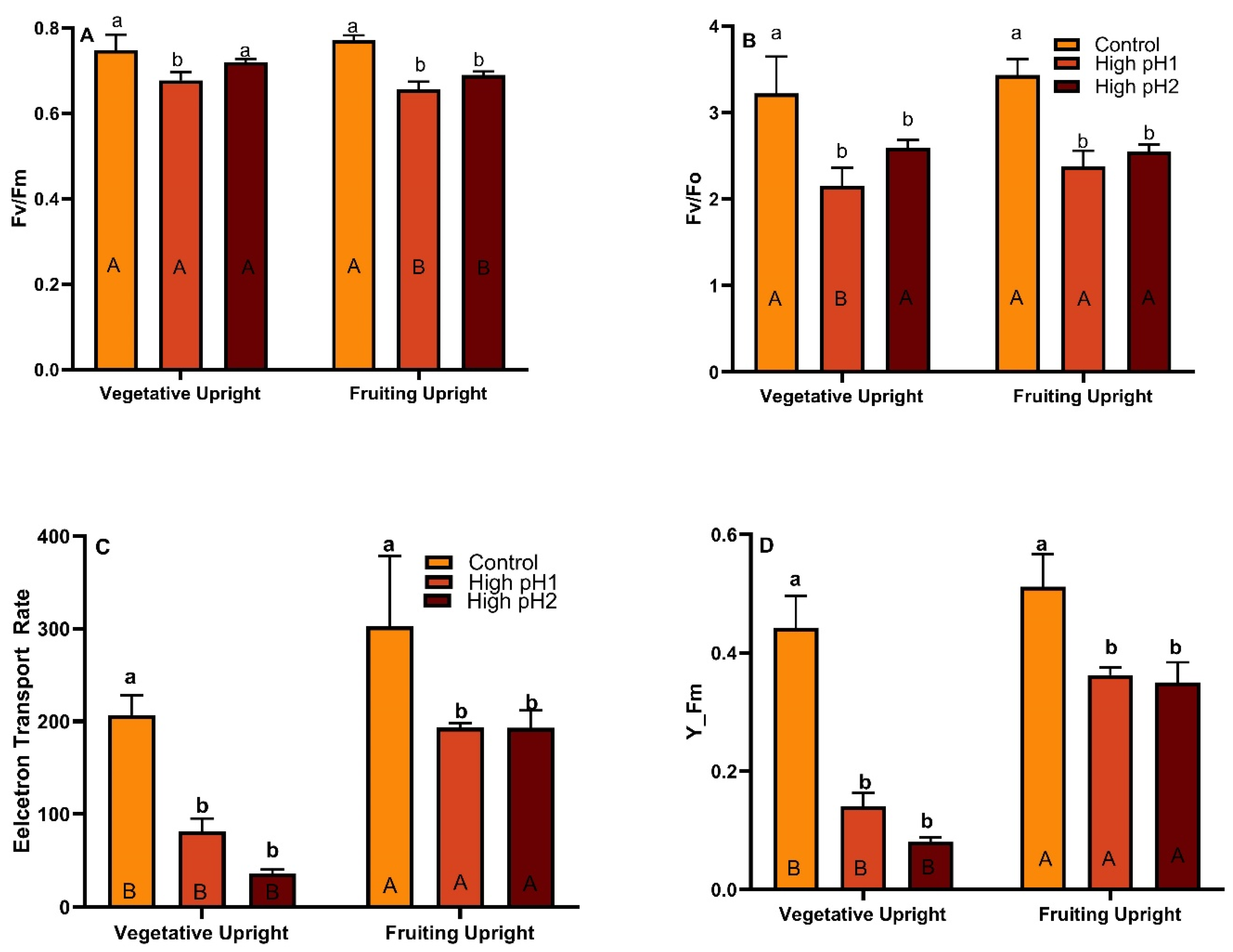
2.4. Chlorophyll Fluorescence
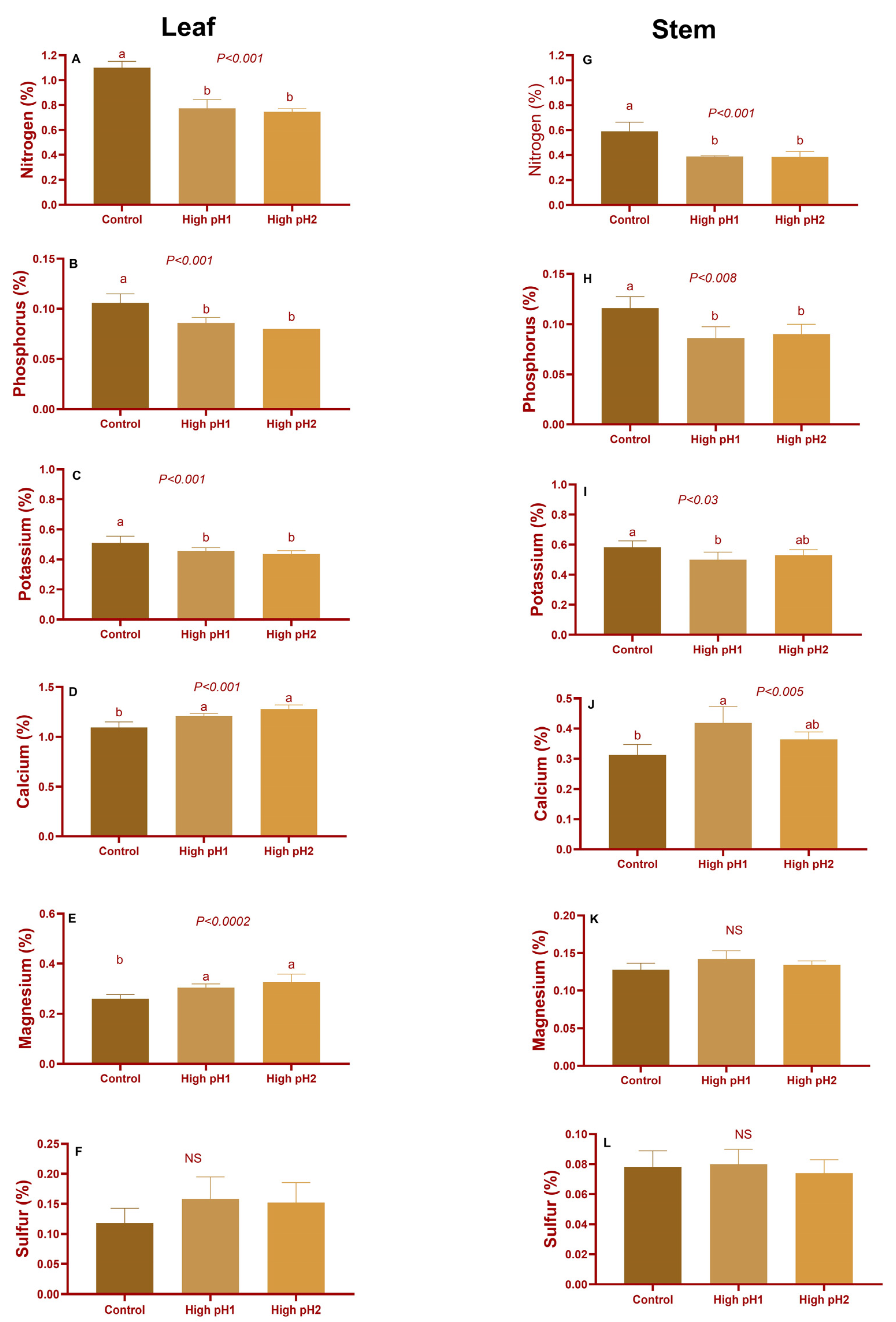
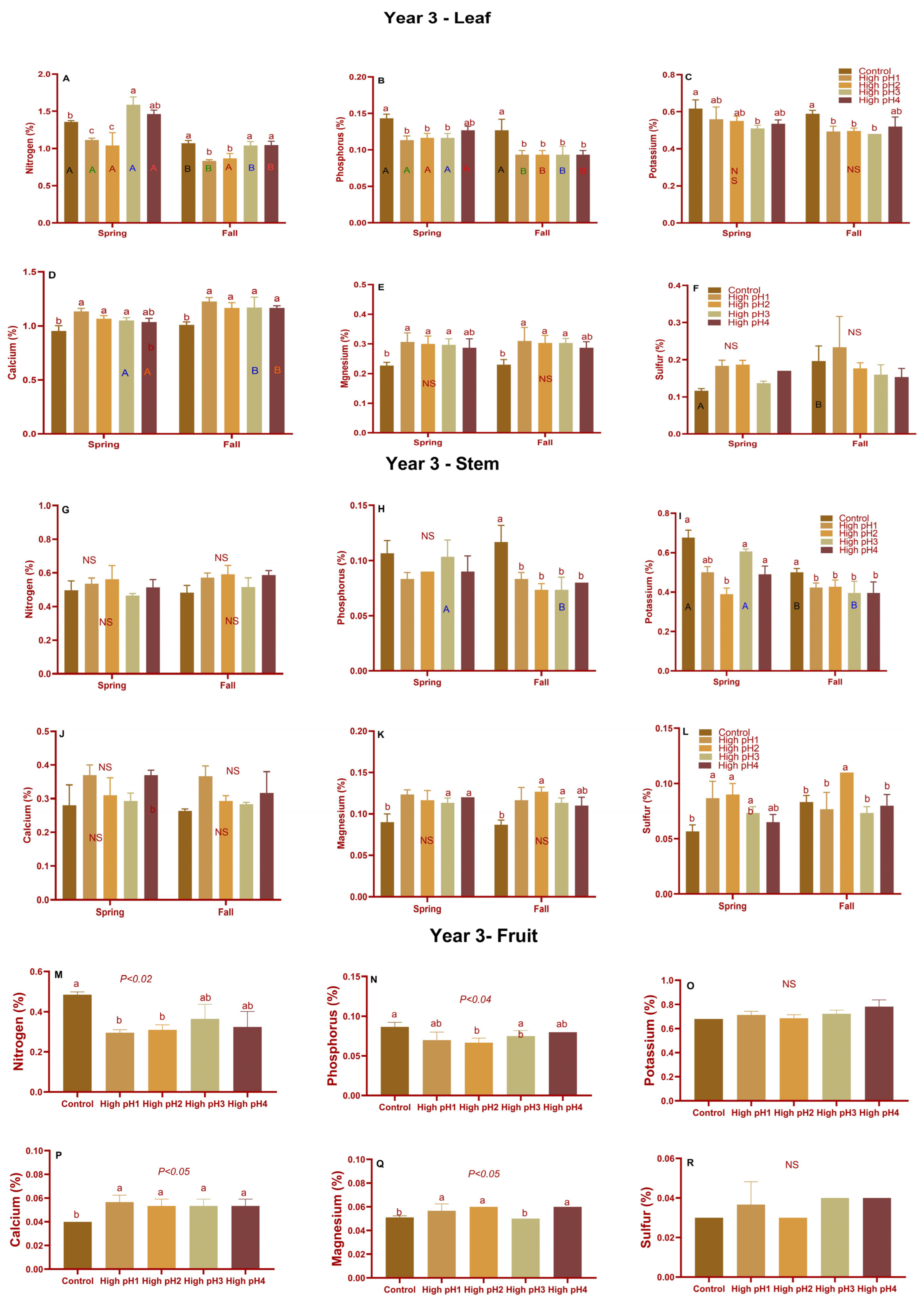
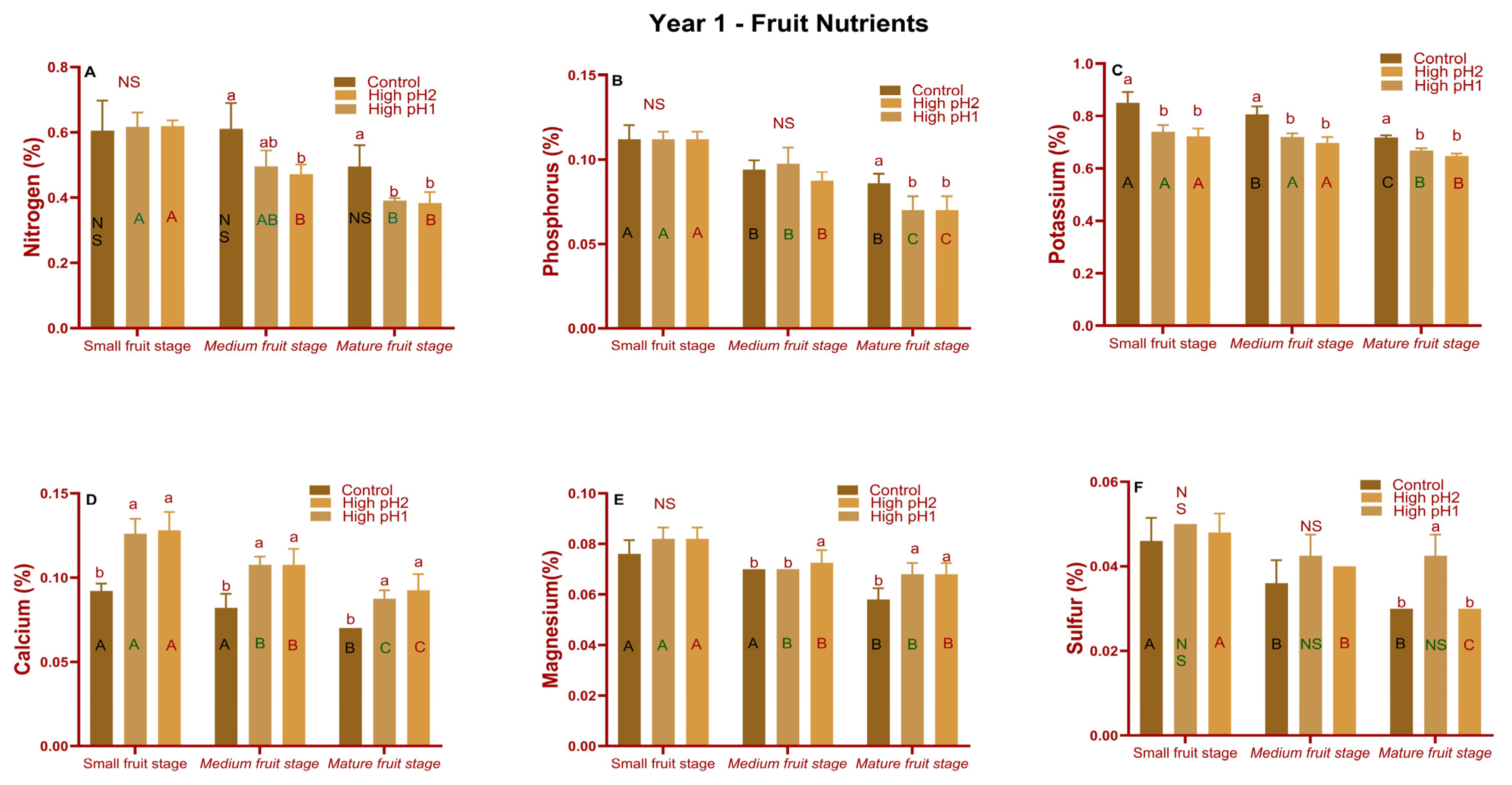
2.5. Stem, Leaf, and Fruit Nutrients
2.5.1. Leaves, Stems, and Shoots Macro and Micronutrients
2.5.2. Fruit Macro and Micronutrients
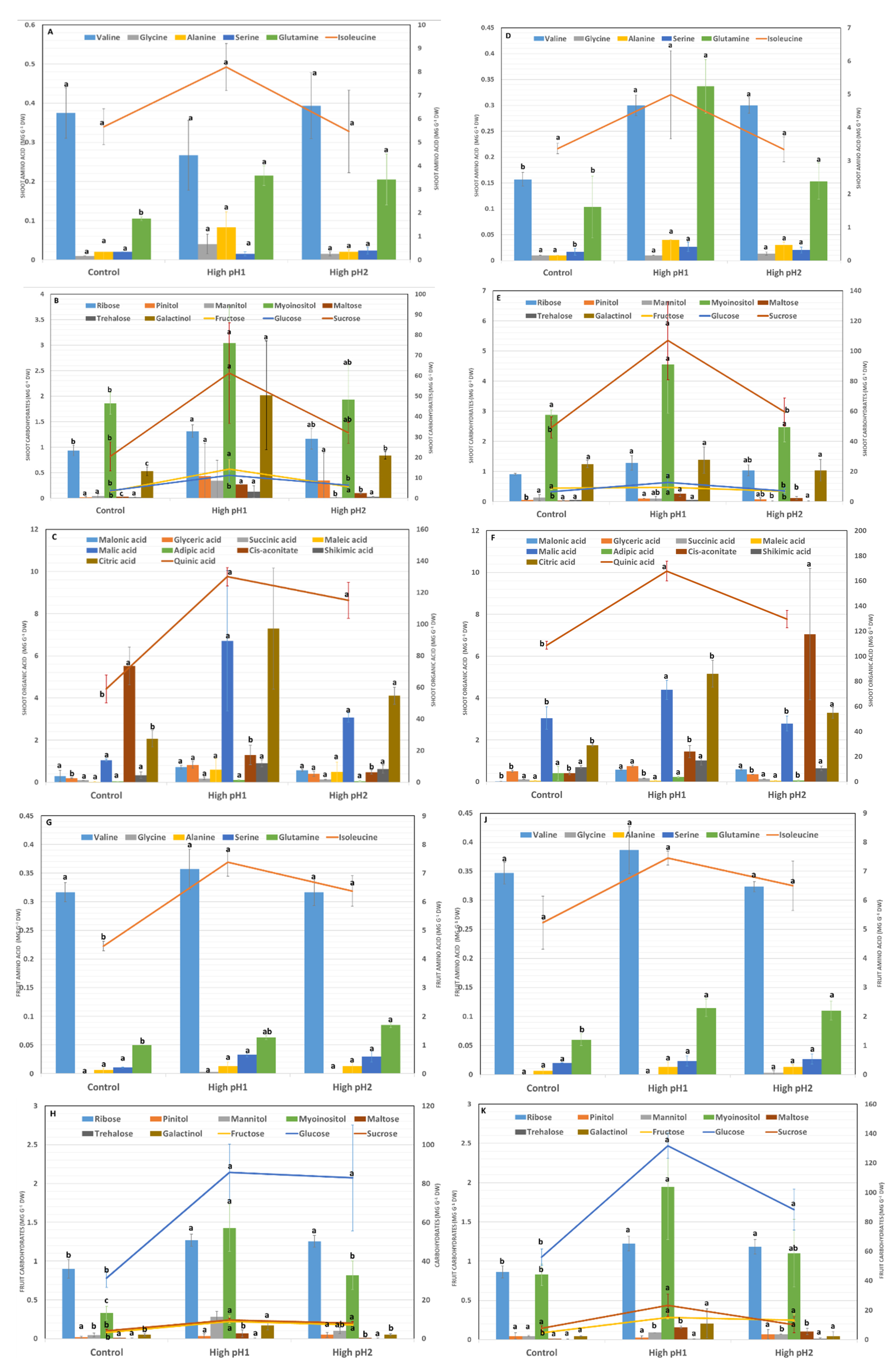
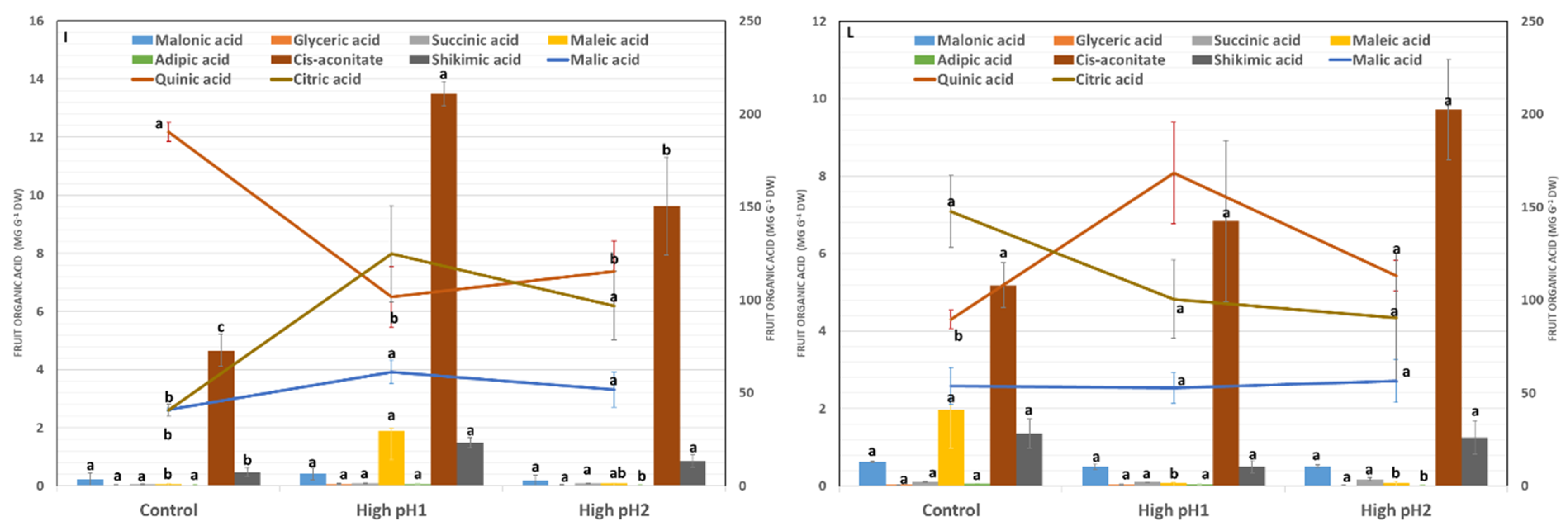
2.6. Shoot and Fruit Metabolite Responses to High Soil pH
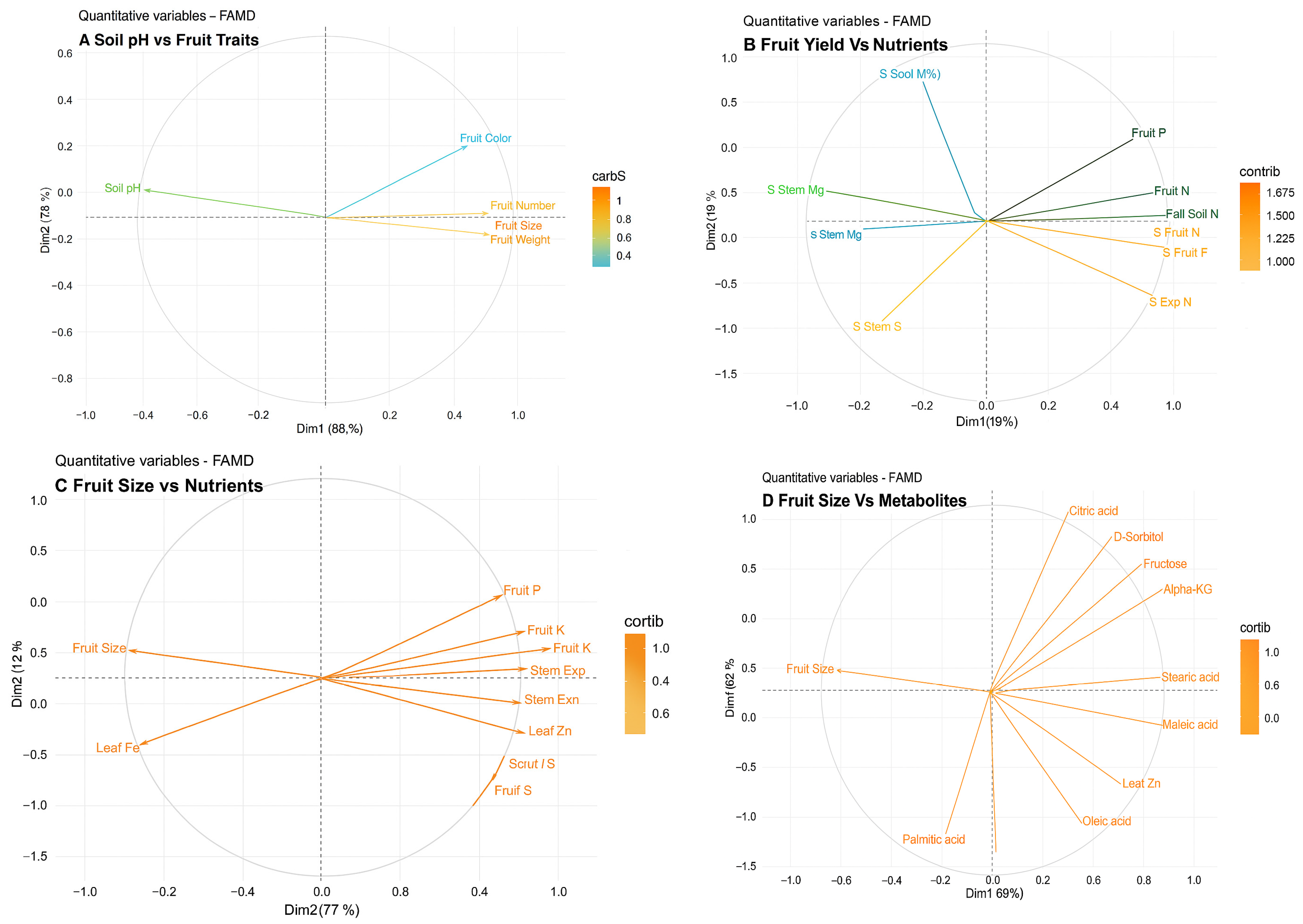
2.7. Soil pH vs. Fruit Traits vs. Fruit Nutrients vs. Fruit Metabolites
3. Discussion
3.1. Effect of Soil pH on Soil Nutrient Availability and Plant Nutrient Absorption in Cranberries
3.2. Photosynthesis and Chlorophyll Fluorescence Were Affected by Soil pH in Cranberries
3.3. Shoot and Fruit Metabolite Changes in Response to High pH Stress
3.4. Interactions of Plant Nutrients and Metabolites Under High Soil pH and Overall Reduction in Fruit Traits
4. Materials and Methods
4.1. Selection of High pH Soil and Control pH Soil Beds
4.2. Sample Collection
4.3. Sampling Times
4.4. Soil, Water, and Plant Nutrient Analysis
4.5. Gas Exchange Parameters and Chlorophyll Fluorescence
4.6. Fruit Traits
4.7. Metabolite Measurements
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Photosynthesis |
| ANOVA | Analysis of Variance |
| CIRAS 3 | CO2/H2O Gas Analyzer |
| E | Transpiration |
| ECe | Electrical Conductivity of the Saturation Extract |
| ETR | Electron Transport Rate |
| FAMD | Factor Analysis of Mixed Data |
| Fm | Maximum Fluorescence |
| Fo | Minimum Fluorescence |
| Fv/Fm | Maximum Photochemical Efficiency of Photosystem II (PSII) |
| Fv/Fo | Indicator of Plant Stress |
| Gs | Stomatal Conductance |
| ICP | Inductively Coupled Plasma |
| NDVI | Normalized Difference Vegetation Index |
| pH | Potential of Hydrogen |
| PSII | Photosystem II |
| TA.XTPlus | Texture Analyzer |
| TACy | Total Anthocyanin Content |
| WUE | Water Use efficiency |
| Y(II) | Effective Quantum Yield of PSII |
References
- Nemzer, B.V.; Al-Taher, F.; Yashin, A.; Revelsky, I.; Yashin, Y. Cranberry: Chemical Composition, Antioxidant Activity and Impact on Human Health: Overview. Molecules 2022, 27, 1503. [Google Scholar] [CrossRef]
- United States Department of Agriculture, National Agricultural Statistics Service. Noncitrus Fruits and Nuts 2023 Summary; USDA: Washington, DC, USA, 2024. Available online: https://www.nass.usda.gov/ (accessed on 25 July 2025).
- Kramer, J. Cranberry Production in Top-Producing States to Increase Modestly in 2021. USDA Economic Research Service Newsletter, 24 November 2021. Available online: https://www.ers.usda.gov/ (accessed on 25 July 2025).
- Mitsuta, A.; Lourenço, K.S.; de Oliveira, B.G.; de Assis Costa, O.Y.; Cantarella, H.; Kuramae, E.E. Soil pH Determines the Shift of Key Microbial Energy Metabolic Pathways Associated with Soil Nutrient Cycle. Appl. Soil Ecol. 2025, 208, 105992. [Google Scholar] [CrossRef]
- Neina, D. The Role of Soil pH in Plant Nutrition and Soil Remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Davenport, J.R.; DeMoranville, C.J. A Survey of Several Soil Physical Characteristics of Cultivated Cranberry Bog Soils in North America. Commun. Soil Sci. Plant Anal. 1993, 24, 1769–1773. [Google Scholar] [CrossRef]
- Davenport, J.R.; DeMoranville, C.J.; Hart, J.; Kumidini, S.; Patten, K.; Poole, A.; Roper, T. Spatial and Temporal Variability of Cranberry Soil pH. Acta Hortic. 2002, 626, 315–327. [Google Scholar] [CrossRef]
- Hanson, E.J.; Stein, A. Effects of Water Use on Chemical Characteristics of Cranberry Soils. J. Plant Nutr. 1999, 22, 427–434. [Google Scholar] [CrossRef]
- Lončarić, Z.; Karalić, K.; Popović, B.; Rastija, D.; Vukobratović, M. Total and plant available micronutrients in acidic and calcareous soils in Croatia. Cereal Res. Commun. 2008, 36 (Suppl. S5), 331–334. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Zeng, Q.; Wei, J.; Yu, H. The Effect of Soil pH on Plant Growth, Leaf Chlorophyll Fluorescence and Mineral Element Content of Two Blueberries. Acta Hortic. 2016, 1180, 269–276. [Google Scholar] [CrossRef]
- Regasa, A.; Haile, W.; Abera, G. Effects of Lime and Vermicompost Application on Soil Physicochemical Properties and Phosphorus Availability in Acidic Soils. Sci. Rep. 2025, 15, 25544. [Google Scholar] [CrossRef]
- Rahman, S.U.; Han, J.C.; Ahmad, M.; Ashraf, M.N.; Khaliq, M.A.; Yousaf, M.; Wang, Y.; Yasin, G.; Nawaz, M.F.; Khan, K.A.; et al. Aluminum Phytotoxicity in Acidic Environments: A Comprehensive Review of Plant Tolerance and Adaptation Strategies. Ecotoxicol. Environ. Saf. 2024, 269, 115791. [Google Scholar] [CrossRef]
- Austin, M.; Bondari, K. Soil pH Effects on Yield and Fruit Size of Two Rabbiteye Blueberry Cultivars. J. Hortic. Sci. 1992, 67, 779–785. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, Q.; Wei, J.; Jiang, J.; Li, Y.; Chen, J.; Yu, H. Growth, Fruit Yield, Photosynthetic Characteristics, and Leaf Microelement Concentration of Two Blueberry Cultivars under Different Long-Term Soil pH Treatments. Agronomy 2019, 9, 357. [Google Scholar] [CrossRef]
- Yang, H.; Wu, Y.; Zhang, C.; Wu, W.; Lyu, L.; Li, W. Growth and Physiological Characteristics of Four Blueberry Cultivars under Different High Soil pH Treatments. Environ. Exp. Bot. 2022, 197, 104842. [Google Scholar] [CrossRef]
- Cen, H.; Weng, H.; Yao, J.; He, M.; Lv, J.; Hua, S.; Li, H.; He, Y. Chlorophyll Fluorescence Imaging Uncovers Photosynthetic Fingerprint of Citrus Huanglongbing. Front. Plant Sci. 2017, 8, 1509. [Google Scholar] [CrossRef]
- Salam, U.; Ullah, S.; Tang, Z.H.; Elateeq, A.A.; Khan, Y.; Khan, J.; Khan, A.; Ali, S. Plant Metabolomics: An Overview of the Role of Primary and Secondary Metabolites against Different Environmental Stress Factors. Life 2023, 13, 706. [Google Scholar] [CrossRef]
- Yan, G.; Shi, Y.; Chen, F.; Mu, C.; Wang, J. Physiological and Metabolic Responses of Leymus chinensis Seedlings to Alkali Stress. Plants 2022, 11, 1494. [Google Scholar] [CrossRef]
- Yang, H.; Wu, Y.; Zhang, C.; Wu, W.; Lyu, L.; Li, W. Comprehensive resistance evaluation of 15 blueberry cultivars under high soil pH stress based on growth phenotype and physiological traits. Front. Plant Sci. 2022, 13, 1072621. [Google Scholar] [CrossRef]
- Finn, C.E.; Rosen, C.J.; Luby, J.J. Nitrogen Form and Solution pH Effects on Root Anatomy of Cranberry. HortScience 1990, 25, 1419–1421. [Google Scholar] [CrossRef]
- Szwonek, E.; Maciorowski, R.; Smolarz, K.; Sas-Paszt, L.; Estabrooks, E. Initial Growth and Yield Structure of Selected Cultivars of Cranberry (Vaccinium macrocarpon Ait.) Cultivated on Mineral Soils. Folia Hortic. 2016, 28, 77–86. [Google Scholar] [CrossRef]
- Barrow, N.J.; Hartemink, A.E. The effects of pH on nutrient availability depend on both soils and plants. Plant Soil 2023, 487, 21–37. [Google Scholar] [CrossRef]
- Falchi, R.; Bonghi, C.; Drincovich, M.F.; Famiani, F.; Lara, M.V.; Walker, R.P.; Vizzotto, G. Sugar metabolism in stone fruit: Source-sink relationships and environmental and agronomical effects. Front. Plant Sci. 2020, 11, 573982. [Google Scholar] [CrossRef]
- Doyle, J.W.; Nambeesan, S.U.; Malladi, A. Physiology of nitrogen and calcium nutrition in blueberry (Vaccinium sp.). Agronomy 2021, 11, 765. [Google Scholar] [CrossRef]
- Rosen, C.J.; Allan, D.L.; Luby, J.J. Nitrogen form and solution pH influence growth and nutrition of two Vaccinium clones. J. Am. Soc. Hortic. Sci. 1990, 115, 83–89. [Google Scholar] [CrossRef]
- Barrow, N.J.; Debnath, A.; Sen, A. Effect of phosphate sorption on soil pH. Eur. J. Soil Sci. 2022, 73, e13172. [Google Scholar] [CrossRef]
- Ibrahim, M.; Iqbal, M.; Tang, Y.T.; Khan, S.; Guan, D.X.; Li, G. Phosphorus mobilization in plant–soil environments and inspired strategies for managing phosphorus: A review. Agronomy 2022, 12, 2539. [Google Scholar] [CrossRef]
- Harper, J.R.; Balke, N.E. Characterization of the inhibition of K absorption in oat roots by salicylic acid. Plant Physiol. 1981, 68, 1349–1353. [Google Scholar] [CrossRef]
- Maas, E.; Ogata, G. Absorption of magnesium and chloride by excised corn root. Plant Physiol. 1971, 47, 357–360. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, D.; Hang, H.; Chen, S.; Liu, H.; Su, J.; Lv, H.; Jia, H.; Zhao, G. Effects of Balancing Exchangeable Cations Ca, Mg, and K on the Growth of Tomato Seedlings (Solanum lycopersicum L.) Based on Increased Soil Cation Exchange Capacity. Agronomy 2024, 14, 629. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Crous, K.Y.; O’Sullivan, O.S.; Zaragoza-Castells, J.; Bloomfield, K.J.; Negrini, A.C.A.; Meir, P.; Turnbull, M.H.; Griffin, K.L.; Atkin, O.K. Nitrogen and phosphorus availabilities interact to modulate leaf trait scaling relationships across six plant functional types in a controlled-environment study. New Phytol. 2017, 215, 992–1008. [Google Scholar] [CrossRef]
- Adams, W.W., III; Demmig-Adams, B. Chlorophyll fluorescence as a tool to monitor plant response to the environment. In Chlorophyll a Fluorescence; Advances in Photosynthesis and Respiration; Springer: Cham, Switzerland, 2025; Volume 19. [Google Scholar]
- Pohland, A.C.; Bernát, G.; Geimer, S.; Schneider, D. Mg2+ Limitation Leads to a Decrease in Chlorophyll, Resulting in an Unbalanced Photosynthetic Apparatus in the Cyanobacterium Synechocytis sp. PCC6803. Photosynth. Res. 2024, 162, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, L.; Hao, F.; You, Y. Photosynthesis and chlorophyll-fluorescence of Magnolia grandiflora seedlings under low temperature stress. Acta Bot. Boreali-Occident. Sin. 2009, 29, 136–142. [Google Scholar]
- Ferrarezi, R.S.; Lin, X.; Gonzalez Neira, A.C.; Tabay Zambon, F.; Hu, H.; Wang, X.; Huang, J.-H.; Fan, G. Substrate pH influences the nutrient absorption and rhizosphere microbiome of Huanglongbing-affected grapefruit plants. Front. Plant Sci. 2022, 13, 856937. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Zahan, M.I.; Karim, M.M.; Imran, S.; Hunter, C.T.; Islam, M.S.; Mia, M.A.; Hannan, M.A.; Rhaman, M.S.; Hossain, M.A.; et al. Citric acid-mediated abiotic stress tolerance in plants. Int. J. Mol. Sci. 2021, 22, 7235. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, T.M. Synthesis versus Degradation: Directions of Amino Acid Metabolism during Arabidopsis Abiotic Stress Response. Plant Mol. Biol. 2018, 98, 121–135. [Google Scholar] [CrossRef]
- Obata, T.; Fernie, A.R. The Use of Metabolomics to Dissect Plant Responses to Abiotic Stresses. Cell. Mol. Life Sci. 2012, 69, 3225–3243. [Google Scholar] [CrossRef]
- Xu, Y.; Fu, X. Reprogramming of plant central metabolism in response to abiotic stresses: A metabolomics view. Int. J. Mol. Sci. 2022, 23, 5716. [Google Scholar] [CrossRef]
- Wen, G.; Cambouris, A.N.; Ziadi, N.; Bertrand, A.; Khelifi, M. Nitrogen rate and source effects on foliar sugar, glycoalkaloid, and amino acid composition of potato Russet Burbank. Can. J. Plant Sci. 2020, 101, 61–72. [Google Scholar] [CrossRef]
- Zhang, P.; Fu, J.; Hu, L. Effects of alkali stress on growth, free amino acids and carbohydrates metabolism in Kentucky bluegrass (Poa pratensis). Ecotoxicology 2012, 21, 1911–1918. [Google Scholar] [CrossRef]
- Fang, S.; Hou, X.; Liang, X. Response mechanisms of plants under saline-alkali stress. Front. Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Yang, F.; He, X.; Du, X.; Mu, P.; Ma, W. Advances in the functional study of glutamine synthetase in plant abiotic stress tolerance response. Crop J. 2022, 10, 917–923. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Nascimento, V.L.; Medeiros, D.B.; Nunes-Nesi, A.; Ribeiro, D.M.; Zsögön, A.; Araújo, W.L. Modifications in Organic Acid Profiles during Fruit Development and Ripening: Correlation or Causation? Front. Plant Sci. 2018, 9, 1689. [Google Scholar] [CrossRef] [PubMed]
- Tadych, M.; Vorsa, N.; Wang, Y.; Bergen, M.S.; Johnson-Cicalese, J.; Polashock, J.J.; White, J.F., Jr. Interactions between cranberries and fungi: The proposed function of organic acids in virulence suppression of fruit rot fungi. Front. Microbiol. 2015, 6, 835. [Google Scholar] [CrossRef] [PubMed]
- Si, P.; Shao, W.; Yu, H.; Xu, G.; Du, G. Differences in microbial communities stimulated by malic acid have the potential to improve nutrient absorption and fruit quality of grapes. Front. Microbiol. 2022, 13, 850807. [Google Scholar] [CrossRef]
- Farhad, M.; Rana, M.A.K.; Ahmad, R.; Virk, Z.A.; Iqbal, M.; Ilyas, M.F.; Gill, S.; Khan, S.A.; Ramzani, P.M.A.; Afzal, H.; et al. Roles of organic acids in plant stress tolerance, food security, and soil remediation. In Climate-Resilient Agriculture; Springer: Cham, Switzerland, 2023; Volume 1. [Google Scholar]
- Grayer, R.J.; Kokubun, T. Plant–fungal interactions: The search for phytoalexins and other antifungal compounds from higher plants. Phytochemistry 2001, 56, 253–263. [Google Scholar] [CrossRef]
- Diaz-Garcia, L.; Rodriguez-Bonilla, L.; Phillips, M.; Lopez-Hernandez, A.; Grygleski, E.; Atucha, A.; Zalapa, J. Comprehensive analysis of the internal structure and firmness in American cranberry (Vaccinium macrocarpon Ait.) fruit. PLoS ONE 2019, 14, e0222451. [Google Scholar] [CrossRef]
- Yun, Z.; Gao, H.; Jiang, Y. Insights into Metabolomics in Quality Attributes of Postharvest Fruit. Food Res. Int. 2021, 140, 110002. [Google Scholar] [CrossRef]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of Soluble Sugars in Reactive Oxygen Species Balance and Responses to Oxidative Stress in Plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef]
- Karimi, R.; Koulivand, M.; Ollat, N. Soluble sugars, phenolic acids and antioxidant capacity of grape berries as affected by iron and nitrogen. Acta Physiol. Plant. 2019, 41, 117. [Google Scholar] [CrossRef]
- Zahedifar, M.; Moosavi, A.A.; Gavili, E.; Ershadi, A. Tomato Fruit Quality and Nutrient Dynamics under Water Deficit Conditions: The Influence of an Organic Fertilizer. PLoS ONE 2025, 20, e0310916. [Google Scholar] [CrossRef]
- Sung, J.; Lee, S.; Lee, Y.; Ha, S.; Song, B.; Kim, T.; Waters, B.M.; Krishnan, H.B. Metabolomic profiling from leaves and roots of tomato (Solanum lycopersicum L.) plants grown under nitrogen, phosphorus or potassium-deficient condition. Plant Sci. 2015, 241, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Li, H.; Mu, X.; Gao, H.; Zhang, Y.; Li, R.; Cao, K.; Ye, L. Effects of organic fertilizer application on tomato yield and quality: A meta-analysis. Appl. Sci. 2023, 13, 2184. [Google Scholar] [CrossRef]
- Bao, J.; Xia, R.; Peng, S.; Li, G. Main soil nutrient status of Newhall orchards of Hubei province and its effect on fruit quality of Newhall orange. Soils 2006, 38, 75–80. [Google Scholar]
- Gerke, J. The central role of soil organic matter in soil fertility and carbon storage. Soil Syst. 2022, 6, 33. [Google Scholar] [CrossRef]
- Hocking, B.; Tyerman, S.D.; Burton, R.A.; Gilliham, M. Fruit calcium: Transport and physiology. Front. Plant Sci. 2016, 7, 569. [Google Scholar] [CrossRef]
- Hepler, P.K. Calcium: A central regulator of plant growth and development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fleisher, D.H.; Sicher, R.C.; Kim, J.; Baligar, V.C.; Reddy, V.R. Effects of CO2 enrichment and drought pretreatment on metabolite responses to water stress and subsequent rehydration using potato tubers from plants grown in sunlit chambers. J. Plant Physiol. 2015, 189, 126–136. [Google Scholar] [CrossRef] [PubMed]
| Control | High pH1 | High pH2 | High pH3 | High pH4 | |
|---|---|---|---|---|---|
| Water pH | 6.0 ± 0.05 b | 8.2 ± 0.07 a | 8.2 ± 0.07 a | 8.1 ± 0.09 a | 8.3 ± 0.04 a |
| Soil pH | 4.8 ± 0.04 b | 7.2 ± 0.09 a | 7.2 ± 0.09 a | 7.0 ± 0.07 a | 7.2 ± 0.06 a |
| Organic Matter (%) | 1.9 ± 0.002 a | 0.9 ± 0.001 b | 0.8 ± 0.002 b | 0.3 ± 0.0009 b | 0.5 ± 0.001 b |
| Available Phosphorus | 38 ± 4.0 a | 14 ± 2.8 b | 20 ± 2.2 b | - | - |
| Total Phosphorus | 40 ± 2.1 a | 22 ± 5.4 b | 33 ± 4.1 a | 37 ± 3.4 a | 30 ± 3.2 b |
| Potassium (ppm) | 41 ± 3.2 b | 24 ± 2.8 c | 26 ± 2.2 c | 54 ± 3.2 a | 34 ± 3.1 bc |
| Calcium (ppm) | 77 ± 12.1 c | 389 ± 14.5 ab | 515 ± 10.9 a | 258 ± 12.3 ab | 405 ± 11.9 ab |
| Magnesium (ppm) | 50 ± 1.5 b | 102 ± 3.1 ab | 155 ± 4.5 a | 78 ± 4.7 ab | 135 ± 2.1 a |
| Sodium (ppm) | 4.8 ± 0.5 b | 9.0 ± 2.2 a | 9.0 ± 2.1 a | 3.9 ± 0.5 b | 5.2 ± 0.9 ab |
| Sulfur (ppm) | 4.0 ± 0.20 b | 7.0 ± 0.50 a | 10.0 ± 1.90 a | 4.3 ± 0.20 b | 3.5 ± 0.50 b |
| Iron (ppm) | 65 ± 12.20 bc | 55 ± 10.05 bc | 42 ± 18.23 c | 87.2 ± 16.59 b | 160 ± 12.57 a |
| Manganese (ppm) | 6 ± 0.50 a | 2 ± 009 b | 3 ± 0.05 b | 0.8 ± 0.005 c | 7 ± 0.02 a |
| Zinc (ppm) | 1.0 ± 0.12 b | 0.6 ± 0.05 b | 0.6 ± 0.04 b | 1.4 ± 0.15 b | 4.4 ± 0.12 a |
| Copper (ppm) | 0.3 ± 0.001 b | 3.1 ± 0.001 a | 1.1 ± 0.005 b | 0.7 ± 0.03 b | 2.8 ± 0.02 b |
| Born (ppm) | 0.2 ± 0.005 a | 0.2 ± 0.001 a | 0.1 ± 0.00 a | 0.2 ± 0.005 a | 0.4 ± 0.008 a |
| Nitrogen (%) | 0.03 ± 0.009 a | 0.02 ±0.001 b | 0.01 ± 0.003 b | 0.03 ± 0.006 a | 0.03 ± 0.001 a |
| Carbon (%) | 1.09 ± 0.18 | 0.41 ± 0.11 | 0.37 ± 0.12 | 0.56 ± 0.16 | 0.57 ± 0.11 |
| Potassium (%) | 4.0 ± 0.15 ab | 2.1 ± 0.25 ab | 1.7 ± 0.09 b | 6.9 ± 0.40 a | 3.0 ± 0.20 ab |
| Magnesium (%) | 16 ± 1.85 b | 29.3 ± 2.25 b | 32.3 ± 0.86 a | 30 ± 1.22 b | 34 ± 1.47 a |
| Calcium (%) | 53 ± 2.58 b | 67.3 ± 6.30 a | 65 ± 1.28 a | 61 ± 1.18 a | 63 ± 1.75 a |
| Hydrogen (%) | 26.7 ± 2.50 a | 0 | 0 | 2.08 ± 0.54 b | 3.75 ± 0.74 b |
| Sodium (%) | - | 1.3 ± 0.005 a | 1.0 ± 0.00 a | - | 0.1 ± 0.00 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devi, M.J.; Barnaby, J.; Rohde, J.; Wang, Y.; Rodriguez-Bonilla, L.; Zalapa, J.; Atucha, A.; Mupambi, G. The Impact of Elevated Soil pH Levels on Cranberry Growth, Physiology, and Metabolites. Plants 2025, 14, 2833. https://doi.org/10.3390/plants14182833
Devi MJ, Barnaby J, Rohde J, Wang Y, Rodriguez-Bonilla L, Zalapa J, Atucha A, Mupambi G. The Impact of Elevated Soil pH Levels on Cranberry Growth, Physiology, and Metabolites. Plants. 2025; 14(18):2833. https://doi.org/10.3390/plants14182833
Chicago/Turabian StyleDevi, Mura Jyostna, Jinyoung Barnaby, Jessica Rohde, Yi Wang, Lorraine Rodriguez-Bonilla, Juan Zalapa, Amaya Atucha, and Giverson Mupambi. 2025. "The Impact of Elevated Soil pH Levels on Cranberry Growth, Physiology, and Metabolites" Plants 14, no. 18: 2833. https://doi.org/10.3390/plants14182833
APA StyleDevi, M. J., Barnaby, J., Rohde, J., Wang, Y., Rodriguez-Bonilla, L., Zalapa, J., Atucha, A., & Mupambi, G. (2025). The Impact of Elevated Soil pH Levels on Cranberry Growth, Physiology, and Metabolites. Plants, 14(18), 2833. https://doi.org/10.3390/plants14182833








