Synergistic Interactions Between Leaf Traits and Photosynthetic Performance in Young Pinus tabuliformis and Robinia pseudoacacia Trees Under Drought and Shade
Abstract
1. Introduction
2. Results
2.1. Effects of Drought and Shade on Leaf Traits of P. tabuliformis and R. pseudoacacia
2.1.1. Specific Leaf Area
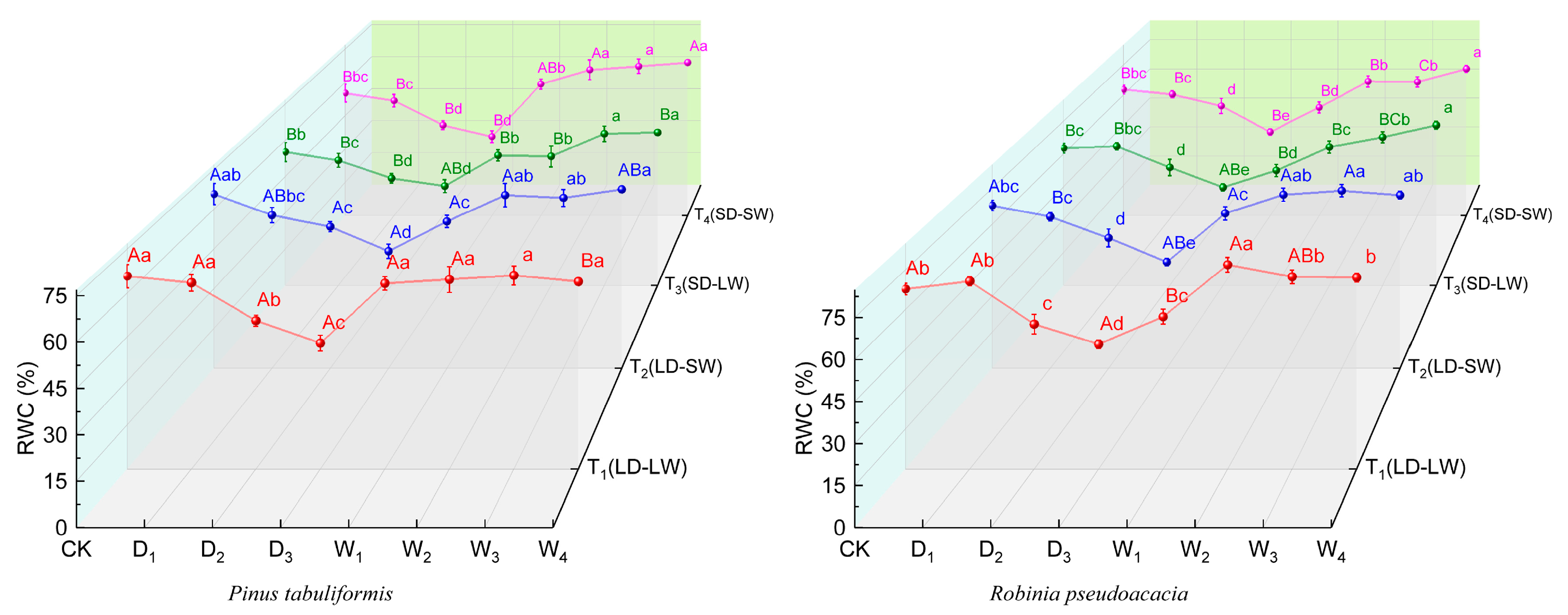
2.1.2. Relative Leaf Water Content
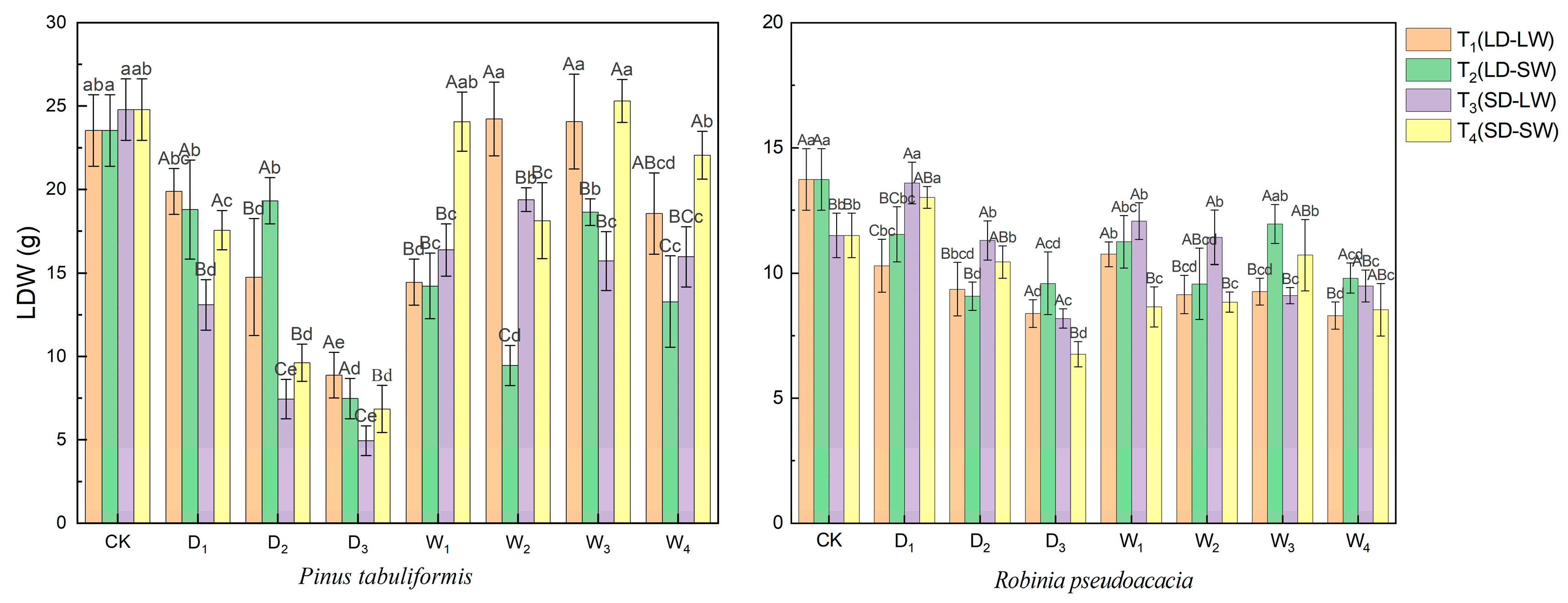
2.1.3. Leaf Dry Weight
2.2. Effects of Drought and Shade on Photosynthetic Parameters of P. tabuliformis and R. pseudoacacia
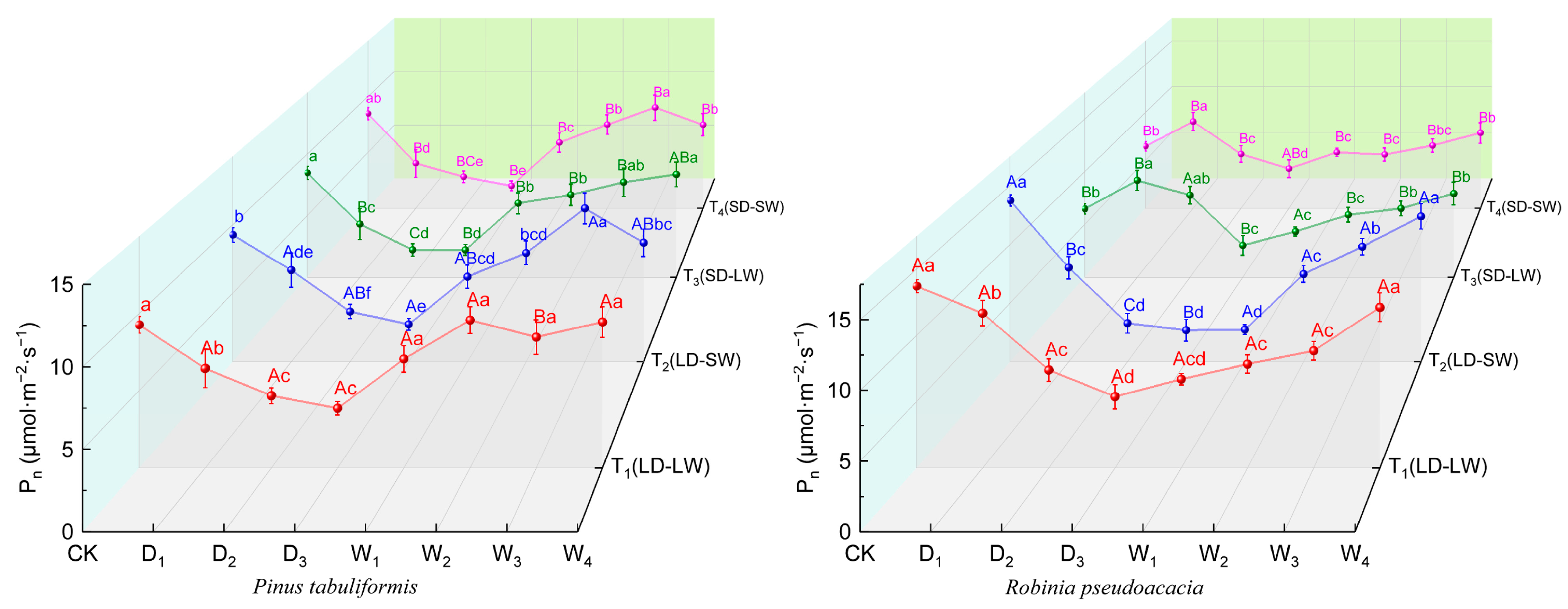
2.2.1. Photosynthetic Rate
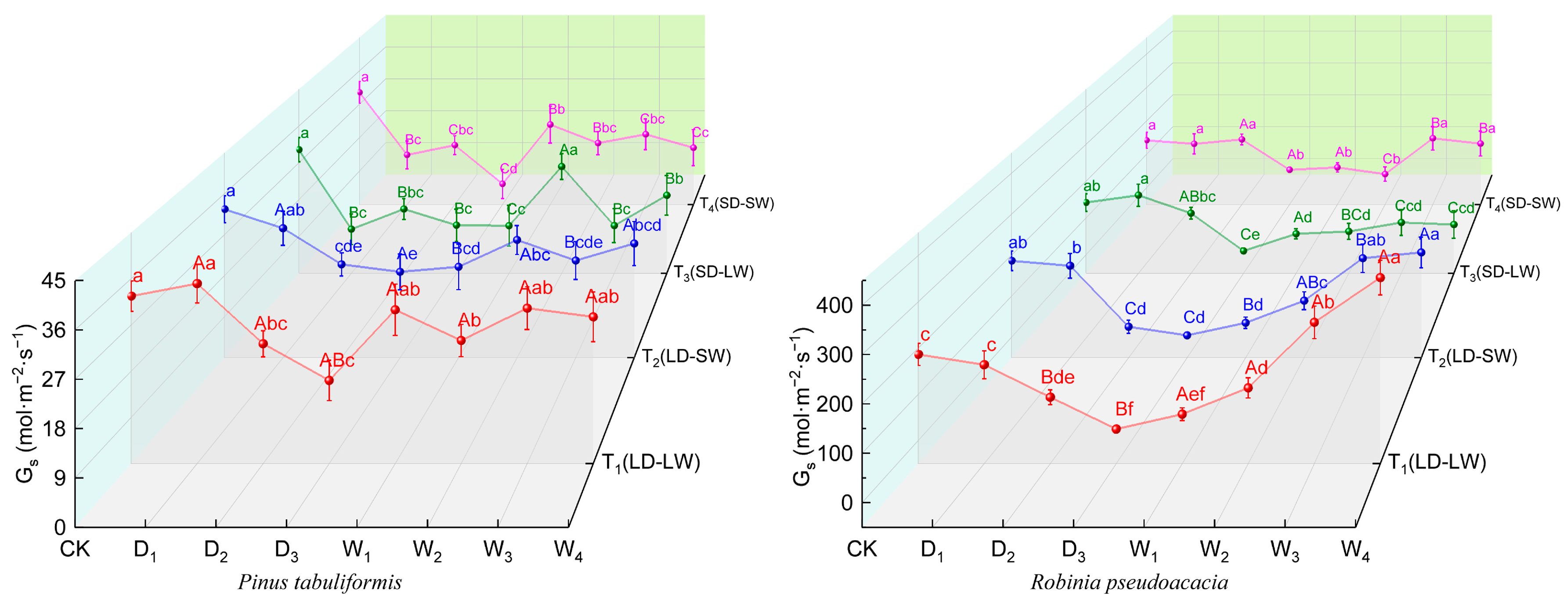
2.2.2. Stomatal Conductance
2.2.3. Transpiration Rate
2.3. Effects of Drought and Shade on Fluorescence Parameters of P. tabuliformis and R. pseudoacacia
2.4. Synergistic Effects of Drought and Shade on Plant Performance—Interaction Analysis
2.5. Correlations Between Drought-Restoration Duration and Plant Physiological Indicators
2.5.1. Correlations Between Drought-Rehydration Duration and Physiological Indicators in P. tabuliformis
2.5.2. Correlations Between Drought-Rehydration Duration and Physiological Indicators in R. pseudoacacia
2.6. Analysis of Synergistic Effects Between Leaf Traits, Photosynthesis, and Fluorescence Parameters in P. tabuliformis and R. pseudoacacia
2.6.1. Correlation of Parameters of P. tabuliformis in Four Treatment Groups
2.6.2. Correlation of Parameters of R. pseudoacacia in Four Treatment Groups
3. Discussion
3.1. Differential Effects of Full-Light/Shade Drought and Rehydration on Leaf Traits and Photosynthetic and Fluorescence Parameters
3.2. Correlation of Full-Light/Shade Drought and Rehydration Time Dynamics with Physiological Response
3.3. Synergistic Mechanisms of Leaf Traits and Photosynthetic Performance Under Full-Light/Shade Drought and Recurring Water
4. Materials and Methods
4.1. Materials
4.2. Treatment Design
4.3. Indicator Measurement
4.4. Data Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2021: The Physical Science Basis; IPCC: Geneva, Switzerland, 2021. [Google Scholar]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How tree roots respond to drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef]
- Nguyen, H.; Vanclay, J.; Herbohn, J.; Firn, J. Drivers of tree growth, mortality and harvest preferences in species-rich plantations for smallholders and communities in the tropics. PLoS ONE 2016, 11, e0164957. [Google Scholar] [CrossRef] [PubMed]
- Sevanto, S.; Mcdowell, N.G.; Dickman, L.T.; Pangle, R.; Pockman, W.T. How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant Cell Environ. 2014, 37, 153–161. [Google Scholar] [CrossRef]
- Fu, C.; Liu, J.; Wang, H.; Xiang, C.; Fu, X.; Luan, Q. Urban Storm Flooding: Characteristics and Management in Beijing. MATEC Web Conf. 2018, 246, 01042. [Google Scholar] [CrossRef]
- Kwon, M.Y.; Woo, S.Y. Plants’ responses to drought and shade environments. Afr. J. Biotechnol. 2016, 15, 29–31. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Van Hees, A.F.M. Growth and morphology of pedunculate oak (Quercus robur L.) and beech (Fagus sylvatica L.) seedlings in relation to shading and drought. Ann. Sci. For. 1997, 54, 9–18. [Google Scholar] [CrossRef]
- Amissah, L.; Mohren, G.M.; Kyereh, B.; Poorter, L. The effects of drought and shade on the performance, morphology and physiology of Ghanaian tree species. PLoS ONE 2015, 10, e0121004. [Google Scholar] [CrossRef]
- Holmgren, M.; Scheffer, M.; Huston, A.M. The interplay of facilitation and competition in plant communities. Ecology 1997, 78, 1966–1975. [Google Scholar] [CrossRef]
- Smith, T.; Huston, M. A theory of the spatial and temporal dynamics of plant communities. Vegetatio 1989, 83, 49–69. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Quero, J.L.; Villar, R.; Maraón, T.; Zamora, R. Interactions of drought and shade effects on seedlings of four Quercus species: Physiological and structural leaf responses. New Phytol. 2006, 170, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, M. Combined effects of shade and drought on tulip poplar seedlings: Trade-off in tolerance or facilitation? Oikos 2000, 90, 67–78. [Google Scholar] [CrossRef]
- Naseer, M.A.; Hussain, S.; Mukhtar, A.; Rui, Q.; Ru, G.; Ahmad, H.; Zhang, Z.Q.; Shi, L.B.; Asad, M.S.; Chen, X.; et al. Chlorophyll fluorescence, physiology, and yield of winter wheat under different irrigation and shade durations during the grain-filling stage. Front. Plant Sci. 2024, 15, 1396929. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, Z.; Xie, Y.; Su, L.; Zhang, R.; Wang, H.; Li, C.; Long, S. Drought resistance mechanisms of Phedimus aizoon L. Sci. Rep. 2021, 11, 13600. [Google Scholar] [CrossRef]
- Yavas, I.; Jamal, M.A.; Ul Din, K.; Ali, S.; Hussain, S.; Farooq, M. Drought-Induced Changes in Leaf Morphology and Anatomy: Overview, Implications and Perspectives. Pol. J. Environ. Stud. 2024, 33, 1517–1530. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Dong, B.; Wang, Y.; Qiao, Y.; Shi, C.; Jin, L.; Liu, M. Photosynthetic base of reduced grain yield by shading stress during the early reproductive stage of two wheat cultivars. Sci. Rep. 2020, 10, 14353. [Google Scholar] [CrossRef]
- Chen, X.; Ye, K.; Xu, Y.; Zhao, Y.; Zhao, D. Effect of shading on the morphological, physiological, and biochemical characteristics as well as the transcriptome of matcha green tea. Int. J. Mol. Sci. 2022, 23, 14169. [Google Scholar] [CrossRef]
- Prerostova, S.; Dobrev, P.I.; Knirsch, V.; Jarosova, J.; Gaudinova, A.; Zupkova, B.; Prášil, I.T.; Janda, T.; Brzobohatý, B.; Skalák, J.; et al. Light quality and intensity modulate cold acclimation in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 2736. [Google Scholar] [CrossRef]
- Shafiq, I.; Hussain, S.; Hassan, B.; Raza, A.; Ahmad, I.; Asghar, M.A.; Wang, Z.; Tan, T.; Li, S.; Tan, X.; et al. Crop responses and management strategies under shade and drought stress. Photosynthetica 2021, 59, 664–682. [Google Scholar] [CrossRef]
- Maguire, A.J.; Kobe, R.K. Drought and shade deplete nonstructural carbohydrate reserves in seedlings of five temperate tree species. Ecol. Evol. 2015, 5, 5711–5721. [Google Scholar] [CrossRef] [PubMed]
- Sack, L.; Grubb, P.J. The combined impacts of deep shade and drought on the growth and biomass allocation of shade-tolerant woody seedlings. Oecologia 2002, 131, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Guo, W.; Luo, Y.; Tan, X.; Du, N.; Wang, R. Morphological and biomass characteristic acclimation of trident maple (Acer buergerianum Miq.) in response to light and water stress. Acta Physiol. Plant. 2013, 35, 1149–1159. [Google Scholar] [CrossRef]
- Guo, K.; Werger, M.J.A. Different responses to shade of evergreen and deciduous oak seedlings and the effect of acorn size. Acta Oecologica 1999, 20, 579–586. [Google Scholar] [CrossRef]
- Cailleret, M.; Nourtier, M.; Amm, A.; Durand-Gillmann, M.; Davi, H. Drought-induced decline and mortality of silver fir differ among three sites in Southern France. Ann. For. Sci. 2014, 71, 643–657. [Google Scholar] [CrossRef]
- Sperry, J.S.; Hacke, U.G.; Oren, R.; Comstock, J.P. Water deficits and hydraulic limits to leaf water supply. Plant Cell Environ. 2002, 25, 251–263. [Google Scholar] [CrossRef]
- Maggard, A.; Will, R.; Wilson, D.; Meek, C. Response of mid-rotation loblolly pine (Pinus taeda L.) physiology and productivity to sustained, moderate drought on the western edge of the range. Forests 2016, 7, 203. [Google Scholar] [CrossRef]
- Eziz, A.; Yan, Z.; Tian, D.; Han, W.; Tang, Z.; Fang, J. Drought effect on plant biomass allocation: A meta-analysis. Ecol. Evol. 2017, 7, 11002–11010. [Google Scholar] [CrossRef]
- Hartmann, H.; Bahn, M.; Carbone, M.; Richardson, A.D. Plant carbon allocation in a changing world—Challenges and progress. New Phytol. 2020, 227, 981–988. [Google Scholar] [CrossRef]
- Wegener, F.; Beyschlag, W.; Werner, C. High intraspecific ability to adjust both carbon uptake and allocation under light and nutrient reduction in Halimium halimifolium L. Front. Plant Sci. 2015, 6, 609. [Google Scholar] [CrossRef]
- Russo, S.E.; Ledder, G.; Muller, E.B.; Nisbet, R.M. Dynamic Energy Budget models: Fertile ground for understanding resource allocation in plants in a changing world. Conserv. Physiol. 2022, 10, coac061. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Kuronuma, T.; Wang, Q.; Ando, M.; Watanabe, H. Effects of Different Light Intensities on the Growth and Accumulation of Photosynthetic Products in Panax ginseng C.A. Meyers. Environ. Control Biol. 2020, 58, 131–135. [Google Scholar] [CrossRef]
- Eamus, D.; Boulain, N.; Cleverly, J.; Breshears, D.D. Global change-type drought-induced tree mortality: Vapor pressure deficit is more important than temperature per se in causing decline in tree health. Ecol. Evol. 2013, 3, 2711–2729. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhong, Y.; Shangguan, Z. A meta-analysis of leaf gas exchange and water status responses to drought. Sci. Rep. 2016, 6, 20917. [Google Scholar] [CrossRef]
- Yao, K.; Wang, Y.; Wu, Y. Competition and niche differentiation of water and nutrients between Broussonetia papyrifera and Platycladus orientalis under prolonged drought stress. Agronomy 2022, 12, 1489. [Google Scholar] [CrossRef]
- Yan, W.; Zheng, S.; Zhong, Y.; Shangguan, Z. Contrasting dynamics of leaf potential and gas exchange during progressive drought cycles and recovery in Amorpha fruticosa and Robinia pseudoacacia. Sci. Rep. 2017, 7, 4470. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G.; Shimizu, H. Are plant growth and photosynthesis limited by pre-drought following rewatering in grass? J. Exp. Bot. 2009, 60, 3737–3749. [Google Scholar] [CrossRef]
- Li, X.; Bao, J.; Wang, J.; Blackman, C.; Tissue, D. Antecedent drought condition affects responses of plant physiology and growth to drought and post-drought recovery. Front. For. Glob. Change 2021, 4, 704470. [Google Scholar] [CrossRef]
- Zhou, H.; Hou, L.; Lv, X.; Yang, G.; Wang, Y.; Wang, X. Compensatory growth as a response to post-drought in grassland. Front. Plant Sci. 2022, 13, 1004553. [Google Scholar] [CrossRef]
- Aidar, S.D.T.; Meirelles, S.T.; Oliveira, R.F.D.; Chaves, A.D.M.; Fernandes-Júnior, P.I. Photosynthetic response of poikilochlorophyllous desiccation-tolerant Pleurostima purpurea (Velloziaceae) to dehydration and rehydration. Photosynthetica 2014, 52, 124–133. [Google Scholar] [CrossRef]
- Yamazaki, J.Y.; Kamata, K.; Maruta, E. Seasonal changes in the excess energy dissipation from Photosystem II antennae in overwintering evergreen broad-leaved trees Quercus myrsinaefolia and Machilus thunbergia. J. Photochem. Photobiol. B 2011, 104, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, U.; Field, B. Molecular mechanisms controlling plant growth during abiotic stress. J. Exp. Bot. 2018, 69, 2753–2758. [Google Scholar] [CrossRef] [PubMed]
- Montanaro, G.; Dichio, B.; Xiloyannis, C. Shade mitigates photoinhibition and enhances water use efficiency in kiwifruit under drought. Photosynthetica 2009, 47, 363–371. [Google Scholar] [CrossRef]
- Georgieva, K.; Solti, Á.; Mészáros, I.; Keresztes, Á.; Sárvári, É. Light sensitivity of Haberlea rhodopensis shade adapted phenotype under drought stress. Acta Physiol. Plant. 2017, 39, 164. [Google Scholar] [CrossRef]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The impact of drought in plant metabolism: How to exploit tolerance mechanisms to increase crop production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Tezara, W.; Marín, O.; Rengifo, E.; Martínez, D.; Herrera, A. Photosynthesis and photoinhibition in two xerophytic shrubs during drought. Photosynthetica 2005, 43, 37–45. [Google Scholar] [CrossRef]
- Bortolheiro, F.P.; Silva, M.A. Physiological response and productivity of safflower lines under water deficit and rehydration. An. Acad. Bras. Ciênc. 2017, 89, 3051–3066. [Google Scholar] [CrossRef]
- Diawara, S.; Savadogo, P.; Ouedraogo, A.; Lamien, N.; Bouda, H.N. Water stress responses of Saba senegalensis provenances during the seedling stage. Bois For. Trop. 2023, 355, 5–19. [Google Scholar] [CrossRef]
- Nawaz, M.; Li, L.; Azeem, F.; Shabbir, S.; Zohaib, A.; Ashraf, U.; Yang, H.; Wang, Z. Insight of transcriptional regulators reveals the tolerance mechanism of carpet-grass (Axonopus compressus) against drought. BMC Plant Biol. 2021, 21, 71. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, S.; Wang, Y. Physiological and proteomic changes of Castanopsis fissa in response to drought stress. Sci. Rep. 2023, 13, 12567. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, C. Individual and interactive responses of woody plants’ biomass and leaf traits to drought and shade. Glob. Ecol. Biogeogr. 2023, 32, 35–48. [Google Scholar] [CrossRef]
- Deng, X.; Xiao, W.; Shi, Z.; Zeng, L.; Lei, L. Combined effects of drought and shading on growth and non-structural carbohydrates in Pinus massoniana Lamb. seedlings. Forests 2019, 11, 18. [Google Scholar] [CrossRef]
- Visser, E.J.W.; Zhang, Q.; De Gruyter, F.; Martens, S.; Huber, H. Shade affects responses to drought and flooding–acclimation to multiple stresses in bittersweet (Solanum dulcamara L.). Plant Biol. 2016, 18, 112–119. [Google Scholar] [CrossRef]
- Gao, S.; Wang, G. The enhancement of cyclic electron flow around photosystem I improves the recovery of severely desiccated Porphyra yezoensis (Bangiales, Rhodophyta). J. Exp. Bot. 2012, 63, 4349–4358. [Google Scholar] [CrossRef]
- Cai, J.G.; Wei, M.Q.; Zhang, Y.; Wei, Y.L. Effects of shading on photosynthetic characteristics and chlorophyll fluorescence parameters in leaves of Hydrangea macrophylla. Chin. J. Plant Ecol. 2017, 41, 570. [Google Scholar] [CrossRef][Green Version]
- Dai, Y.M.; Li, M.L.; Xu, M.Z.; Tian, Y.; Zhao, H.X.; Gao, S.J.; Hao, S.R.; Liu, P.; Jia, X.; Zha, T.S. Leaf traits of Artemisia ordosica at different dune fixation stages in Mau Us Sandy Land. Chin. J. Plant Ecol. 2022, 46, 1376. [Google Scholar] [CrossRef]
- Li, J.; Bai, X.; Ran, F.; Li, P.; Sadiq, M.; Chen, H. Photosynthetic and physiological responses to combined drought and low-temperature stress in Poa annua seedlings from different provenances. Agriculture 2023, 13, 1781. [Google Scholar] [CrossRef]
- Galdina, T.; Khazova, E. Adaptability of Pinus sylvestris L. to various environmental conditions. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 316, p. 012002. [Google Scholar] [CrossRef]
- Rawat, M.; Arunachalam, K.; Arunachalam, A.; Alatalo, J.M.; Pandey, R. Assessment of leaf morphological, physiological, chemical and stoichiometry functional traits for understanding the functioning of Himalayan temperate forest ecosystem. Sci. Rep. 2021, 11, 23807. [Google Scholar] [CrossRef]
- Kruijt, B.; Barton, C.; Rey, A.; Jarvis, P.G. The sensitivity of stand-scale photosynthesis and transpiration to changes in atmospheric CO2 concentration and climate. Hydrol. Earth Syst. Sci. 1999, 3, 55–69. [Google Scholar] [CrossRef]
- Chen, J.; Li, T.; Cai, J.; Yu, P.; Guo, Y. Physiological and molecular response of Liriodendron chinense to varying stand density. Plants 2024, 13, 508. [Google Scholar] [CrossRef]
- Moore, C.E.; Meacham-Hensold, K.; Lemonnier, P.; Slattery, R.A.; Benjamin, C.; Bernacchi, C.J.; Lawson, T.; Cavanagh, A.P. The effect of increasing temperature on crop photosynthesis: From enzymes to ecosystems. J. Exp. Bot. 2021, 72, 2822–2844. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V. Plants in light. Commun. Integr. Biol. 2009, 2, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Niyogi, K.K. Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol. 2011, 155, 86–92. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Nishiyama, Y.; Takahashi, S.; Miyairi, S.; Suzuki, I.; Murata, N. Systematic analysis of the relation of electron transport and ATP synthesis to the photodamage and repair of photosystem II in Synechocystis. Plant Physiol. 2005, 137, 263–273. [Google Scholar] [CrossRef]
- Hu, W.H.; Zhang, S.S.; Xiao, Y.A.; Yan, X.H. Physiological responses and photo-protective mechanisms of two Rhododendron plants to natural sunlight after long-term shading. Chin. J. Plant Ecol. 2015, 39, 1093. [Google Scholar] [CrossRef]
- Lawson, T.; Matthews, J. Guard cell metabolism and stomatal function. Annu. Rev. Plant Biol. 2020, 71, 273–302. [Google Scholar] [CrossRef]
- Liu, M.X.; Liang, G.L. Research progress on leaf mass per area. Chin. J. Plant Ecol. 2016, 40, 847. [Google Scholar] [CrossRef]
- Wang, J.; Yao, R.; Sun, Z.; Wang, M.; Jiang, C.; Zhao, X.; Liu, X.; Zhong, C.; Zhang, H.; Zhao, S.; et al. Effects of shading on morphology, photosynthesis characteristics, and yield of different shade-tolerant peanut varieties at the flowering stage. Front. Plant Sci. 2024, 15, 1429800. [Google Scholar] [CrossRef]
- Hussain, S.; Iqbal, N.; Raza, M.A.; Khan, M.N.; Yang, W. Distribution and effects of ionic titanium application on energy partitioning and quantum yield of soybean under different light conditions. Photosynthetica 2019, 57, 572–580. [Google Scholar] [CrossRef]
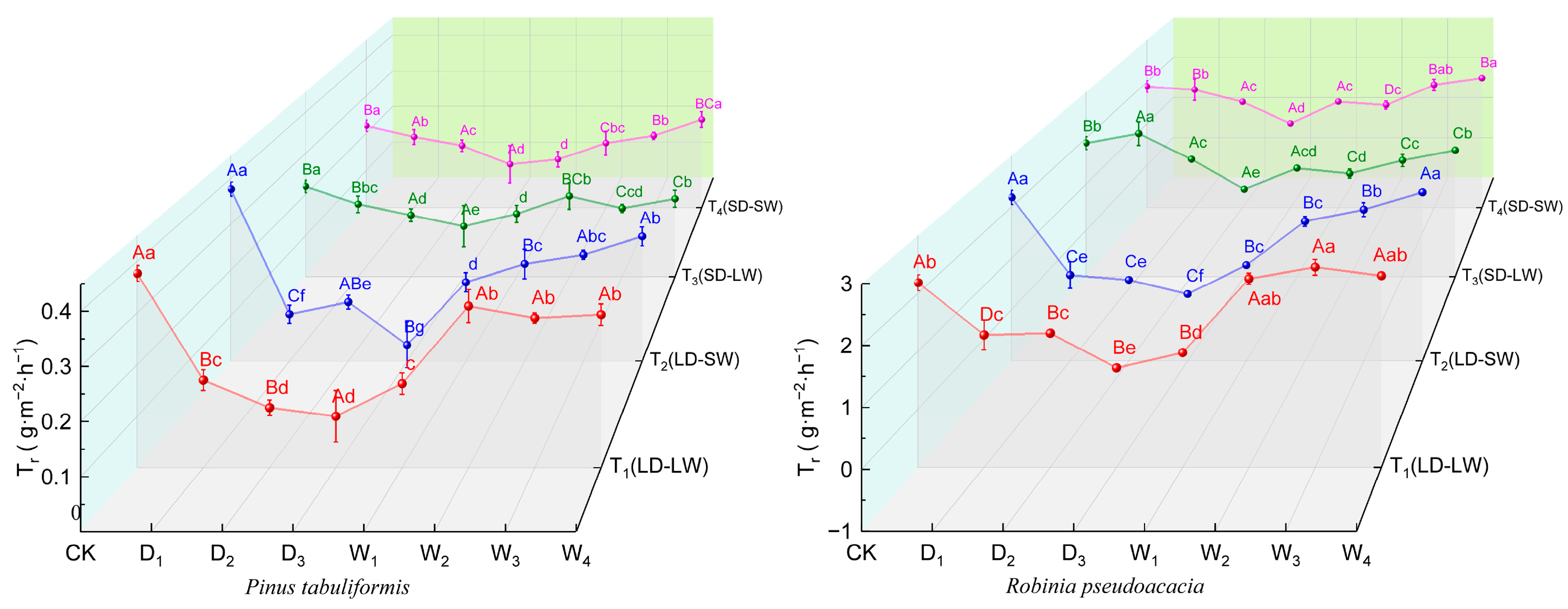
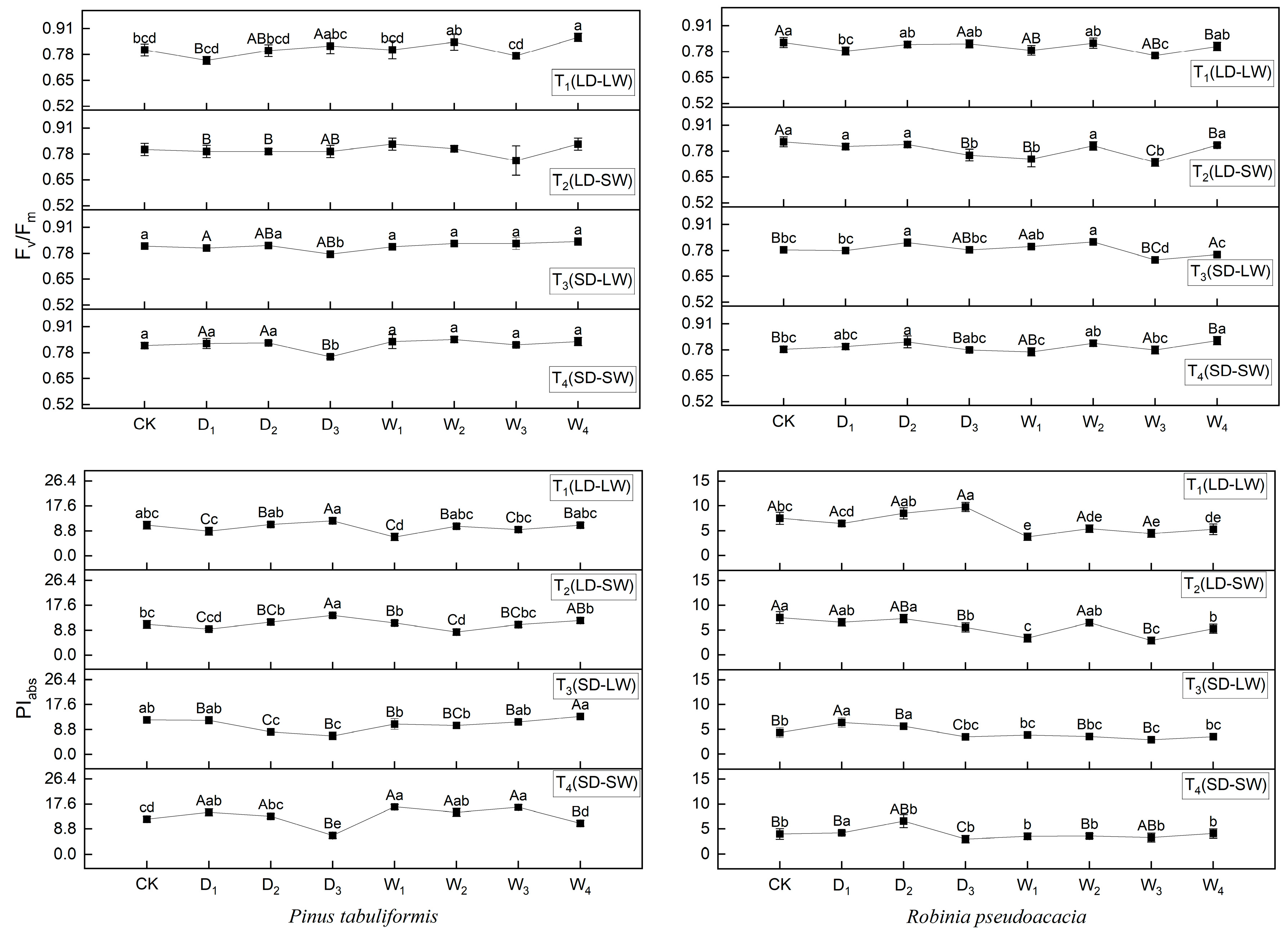
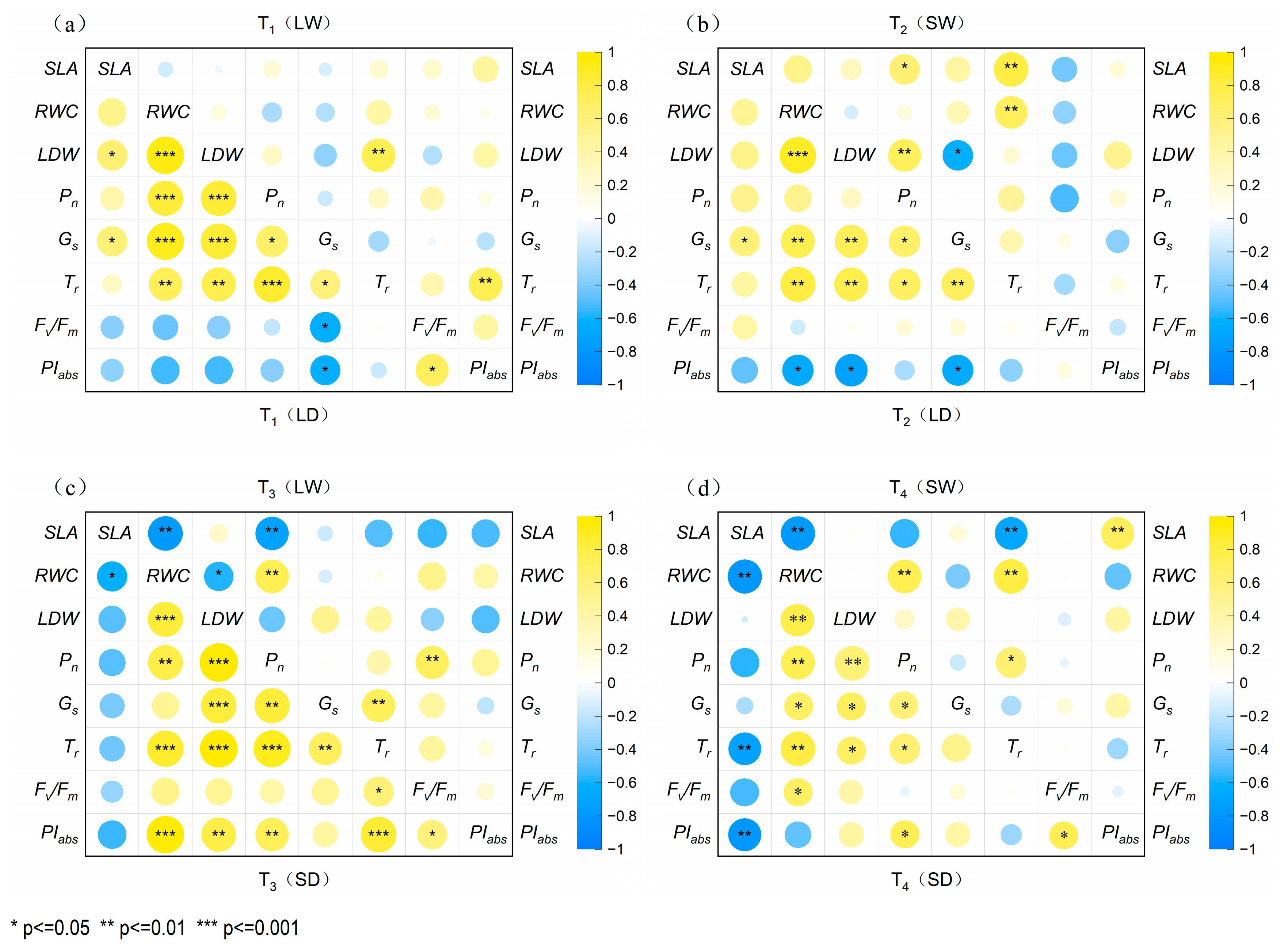
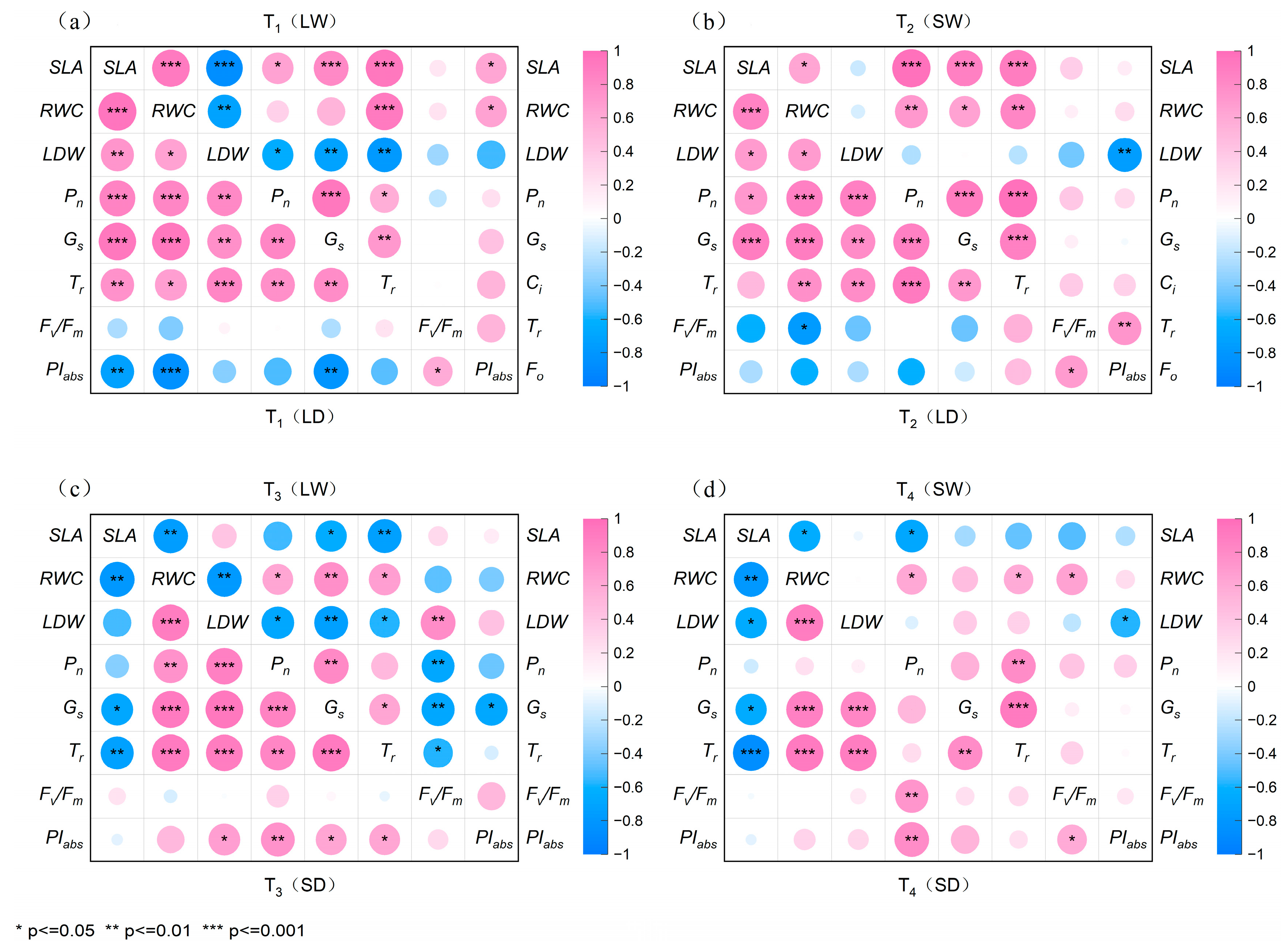
| Species | Stage | Specific Leaf Area (cm2/g) | |||
|---|---|---|---|---|---|
| T1 (LD-LW) | T2 (LD-SW) | T3 (SD-LW) | T4 (SD-SW) | ||
| P. tabuliformis | CK | 54 ± 5.51 a | 54 ± 5.51 a | 53 ± 5.29 d | 53 ± 5.29 bc |
| D1 | 54 ± 5.13 a | 54 ± 5.86 a | 55 ± 5.03 cd | 51 ± 3.21 c | |
| D2 | 53 ± 2.00 Aa | 48 ± 2.00 Cabcd | 57 ± 2.00 Abcd | 52 ± 2.00 Babc | |
| D3 | 38 ± 4.93 Cb | 45 ± 4.04 Bbc | 59 ± 1.53 Abc | 59 ± 2.08 Aa | |
| W1 | 37 ± 10.21 Cb | 41 ± 2.08 Cc | 74 ± 3.79 Aa | 57 ± 1.53 Bab | |
| W2 | 37 ± 2.65 Db | 45 ± 3.06 Cbc | 62 ± 2.08 Ab | 55 ± 1.00 Babc | |
| W3 | 42 ± 2.52 Bb | 52 ± 2.65 Aab | 52 ± 2.00 Ade | 53 ± 1.53 Abc | |
| W4 | 48 ± 5.69 b | 52 ± 4.58 ab | 47 ± 1.00 e | 51 ± 1.53 c | |
| R. pseudoacacia | CK | 155 ± 4.93 b | 155 ± 4.93 de | 159 ± 4.04 d | 159 ± 4.04 d |
| D1 | 157 ± 1.53 Cb | 165 ± 6.03 Ccd | 187 ± 6.81 Ac | 205 ± 6.66 Bc | |
| D2 | 134 ± 3.61 Cc | 137 ± 4.36 Cf | 194 ± 4.16 Ab | 224 ± 5.03 Bc | |
| D3 | 111 ± 1.00 Ce | 118 ± 4.16 Cg | 214 ± 1.53 Aa | 245 ± 8.14 Ba | |
| W1 | 125 ± 5.00 Cd | 152 ± 10.69 Bd | 222 ± 7.55 Ab | 229 ± 9.02 Aa | |
| W2 | 158 ± 4.73 Cb | 171 ± 3.51 Bc | 214 ± 4.58 Ab | 223 ± 7.02 Aa | |
| W3 | 157 ± 2.52 Cb | 192 ± 7.21 Bb | 215 ± 5.00 Ab | 221 ± 7.81 Aa | |
| W4 | 169 ± 2.52 Ba | 200 ± 10.58 Aa | 204 ± 6.66 Ac | 206 ± 3.21 Ab | |
| Species | Parameter | Drought × Light | Rewatering × Light | Species | Parameter | Drought × Light | Rewatering × Light |
|---|---|---|---|---|---|---|---|
| P. tabuliformis | SLA | 0.000 | 0.550 | R. pseudoacacia | SLA | 0.581 | 0.922 |
| RWC | 0.344 | 0.150 | RWC | 0.593 | 0.575 | ||
| LDW | 0.025 | 0.007 | LDW | 0.000 | 0.001 | ||
| Pn | 0.235 | 0.015 | Pn | 0.452 | 0.235 | ||
| Gs | 0.000 | 0.868 | Gs | 0.222 | 0.844 | ||
| Tr | 0.887 | 0.675 | Tr | 0.125 | 0.855 | ||
| Fv/Fm | 0.000 | 0.518 | Fv/Fm | 0.029 | 0.006 | ||
| PIabs | 0.000 | 0.029 | PIabs | 0.295 | 0.549 |
| Pearson | P. tabuliformis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SLA | RWC | LDW | Pn | Gs | Tr | Fv/Fm | PIabs | ||
| T1 | Dn | −0.586 * | −0.941 ** | −0.943 ** | −0.929 ** | −0.852 ** | −0.875 ** | 0.332 | 0.476 |
| D1–2 | 0.464 | −0.956 ** | −0.762 | −0.751 | −0.913 * | −0.878 * | 0.768 | 0.778 | |
| D2–3 | −0.954 ** | −0.898 * | −0.805 | −0.722 | −0.797 | −0.266 | 0.373 | 0.646 | |
| Wn | 0.782 ** | −0.084 | −0.163 | 0.241 | 0.058 | 0.341 | 0.545 | 0.511 | |
| W0.25–0.5 | −0.027 | 0.226 | 0.956 ** | 0.545 | −0.560 | 0.959 ** | 0.497 | 0.900 * | |
| W1–7 | 0.845 * | −0.468 | −0.787 | 0.490 | −0.224 | 0.243 | 0.953 ** | 0.707 | |
| T2 | Dn | −0.672 * | −0.888 ** | −0.862 ** | −0.707 * | −0.873 ** | −0.859 ** | −0.146 | 0.703 * |
| D1–2 | 0.036 | −0.796 | −0.746 | −0.818 * | −0.536 | −0.996 ** | −0.197 | −0.708 | |
| D2–3 | −0.409 | −0.713 | −0.984 ** | 0.867 * | −0.355 | −0.962 ** | 0.000 | 0.859 * | |
| Wn | 0.428 | 0.480 | −0.187 | 0.054 | 0.553 | 0.658 * | 0.140 | 0.312 | |
| W0.25–0.5 | 0.601 | 0.802 | −0.322 | 0.105 | 0.394 | 0.915 * | −0.260 | −0.206 | |
| W1–7 | 0.000 | 0.433 | −0.852 * | −0.918 ** | 0.650 | 0.728 | 0.459 | 0.664 | |
| T3 | Dn | 0.608 * | −0.899 ** | −0.942 ** | −0.917 ** | −0.694 * | −0.951 ** | −0.578 * | −0.884 ** |
| D1–2 | 0.258 | −0.850 * | −0.931 ** | −0.959 ** | 0.758 | −0.784 | 0.535 | −0.916 * | |
| D2–3 | 0.676 | −0.590 | −0.824 * | −0.328 | −0.755 | −0.784 | −0.899 * | −0.614 | |
| Wn | −0.712 ** | 0.593 * | −0.291 | 0.592 * | 0.157 | 0.397 | 0.378 | 0.707 * | |
| W0.25–0.5 | −0.923 ** | −0.074 | 0.836 * | 0.060 | 0.955 ** | 0.891 * | 0.620 | −0.212 | |
| W1–7 | −0.889 * | 0.104 | 0.086 | 0.364 | 0.827 * | 0.868 * | 0.234 | 0.735 | |
| T4 | Dn | 0.532 | −0.907 ** | −0.913 ** | −0.906 ** | −0.860 ** | −0.841 ** | −0.595 * | −0.647 * |
| D1–2 | 0.151 | −0.848 * | −0.903 * | −0.751 | 0.443 | −0.626 | 0.106 | −0.619 | |
| D2–3 | 0.917 * | −0.804 | −0.348 | −0.884 * | −0.958 ** | −0.955 ** | −0.974 ** | −0.974 ** | |
| Wn | −0.720 ** | 0.541 | −0.033 | 0.096 | −0.455 | 0.454 | 0.013 | −0.871 ** | |
| W0.25–0.5 | −0.742 | 0.795 | −0.872 * | 0.814 * | −0.640 | 0.579 | 0.221 | −0.708 | |
| W1–7 | −0.626 | 0.357 | −0.825 * | −0.923 ** | −0.588 | 0.683 | 0.530 | −0.959 ** | |
| Pearson | R. pseudoacacia | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SLA | RWC | LDW | Pn | Gs | Tr | Fv/Fm | PIabs | ||
| T1 | Dn | −0.922 ** | −0.889 ** | −0.872 ** | −0.920 ** | −0.944 ** | −0.908 ** | 0.063 | 0.673 * |
| D1–2 | −0.982 ** | −0.959 ** | −0.479 | −0.946 ** | −0.872 * | 0.099 | 0.745 | 0.814 * | |
| D2–3 | −0.983 ** | −0.850 * | −0.573 | −0.014 | −0.958 ** | −0.993 ** | 0.114 | 0.606 | |
| Wn | 0.633 * | 0.236 | −0.650 * | 0.818 ** | 0.822 ** | 0.373 | 0.202 | 0.339 | |
| W0.25–0.5 | 0.973 ** | 0.974 ** | −0.836 * | −0.673 | 0.887 * | 0.995 ** | 0.666 | 0.799 | |
| W1–7 | 0.946 ** | −0.071 | −0.736 | 0.911 * | 0.853 * | −0.668 | 0.868 * | 0.478 | |
| T2 | Dn | −0.847 ** | −0.944 ** | −0.819 ** | −0.941 ** | −0.922 ** | −0.870 ** | −0.686 * | −0.533 |
| D1–2 | −0.958 ** | −0.803 | −0.864 * | −0.923 ** | −0.984 ** | −0.806 | 0.372 | 0.489 | |
| D2–3 | −0.937 ** | −0.835 * | 0.307 | −0.726 | −0.961 ** | −0.966 ** | −0.808 | −0.777 | |
| Wn | 0.771 ** | 0.255 | −0.346 | 0.740 ** | 0.672 * | 0.590 * | 0.497 | 0.249 | |
| W0.25–0.5 | 0.830 * | 0.879 * | −0.637 | 0.954 ** | 0.975 ** | 0.987 ** | 0.788 | 0.934 ** | |
| W1–7 | 0.755 | −0.385 | −0.885 * | 0.845 * | 0.336 | 0.757 | 0.956 ** | 0.870 * | |
| T3 | Dn | 0.963 ** | −0.892 ** | −0.675 * | −0.531 | −0.806 ** | −0.853 ** | 0.216 | −0.285 |
| D1–2 | 0.892 * | −0.906 * | −0.867 * | −0.493 | −0.697 | −0.973 ** | 0.840 * | −0.491 | |
| D2–3 | 0.885 * | −0.868 * | −0.951 ** | −0.889 * | −0.952 ** | −0.982 ** | −0.784 | −0.904 * | |
| Wn | −0.830 ** | 0.731 ** | −0.501 | 0.577 * | 0.464 | 0.783 ** | −0.376 | 0.005 | |
| W0.25–0.5 | −0.414 | 0.940 ** | −0.392 | −0.218 | 0.436 | −0.480 | 0.742 | −0.347 | |
| W1–7 | −0.872 * | 0.873 * | 0.426 | 0.196 | −0.407 | 0.783 | 0.784 | 0.597 | |
| T4 | Dn | 0.958 ** | −0.902 ** | −0.793 ** | −0.043 | −0.714 ** | −0.927 ** | 0.067 | −0.053 |
| D1–2 | 0.605 | −0.656 | −0.946 ** | 0.948 ** | 0.499 | −0.826 * | 0.515 | 0.833 * | |
| D2–3 | 0.968 ** | −0.967 ** | −0.969 ** | −0.973 ** | −0.982 ** | −0.929 ** | −0.739 | −0.901 * | |
| Wn | −0.707 * | 0.675 * | −0.247 | 0.966 ** | 0.519 | 0.744 ** | 0.600 * | 0.384 | |
| W0.25–0.5 | −0.617 | 0.935 ** | 0.180 | −0.205 | −0.720 | −0.335 | 0.831 * | 0.066 | |
| W1–7 | −0.743 | 0.813 * | −0.729 | 0.974 ** | −0.468 | 0.758 | 0.814 * | 0.485 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Liu, C.; Li, S.; Xu, X.; Li, B.; Tian, M.; Lu, S.; Zhao, N. Synergistic Interactions Between Leaf Traits and Photosynthetic Performance in Young Pinus tabuliformis and Robinia pseudoacacia Trees Under Drought and Shade. Plants 2025, 14, 2825. https://doi.org/10.3390/plants14182825
Yang X, Liu C, Li S, Xu X, Li B, Tian M, Lu S, Zhao N. Synergistic Interactions Between Leaf Traits and Photosynthetic Performance in Young Pinus tabuliformis and Robinia pseudoacacia Trees Under Drought and Shade. Plants. 2025; 14(18):2825. https://doi.org/10.3390/plants14182825
Chicago/Turabian StyleYang, Xinbing, Chang Liu, Shaoning Li, Xiaotian Xu, Bin Li, Meng Tian, Shaowei Lu, and Na Zhao. 2025. "Synergistic Interactions Between Leaf Traits and Photosynthetic Performance in Young Pinus tabuliformis and Robinia pseudoacacia Trees Under Drought and Shade" Plants 14, no. 18: 2825. https://doi.org/10.3390/plants14182825
APA StyleYang, X., Liu, C., Li, S., Xu, X., Li, B., Tian, M., Lu, S., & Zhao, N. (2025). Synergistic Interactions Between Leaf Traits and Photosynthetic Performance in Young Pinus tabuliformis and Robinia pseudoacacia Trees Under Drought and Shade. Plants, 14(18), 2825. https://doi.org/10.3390/plants14182825


.png)




