Pre-Chilling CGA Application Alleviates Chilling Injury in Tomato by Maintaining Photosynthetic Efficiency and Altering Phenylpropanoid Metabolism
Abstract
1. Introduction
2. Material and Methods
2.1. Experiment Set-Up
2.2. The Photosynthetic Capacity, Tissue Redox Homeostasis and Membrane Damage
2.3. Plant Biomass and Mineral Nutrients Accumulation
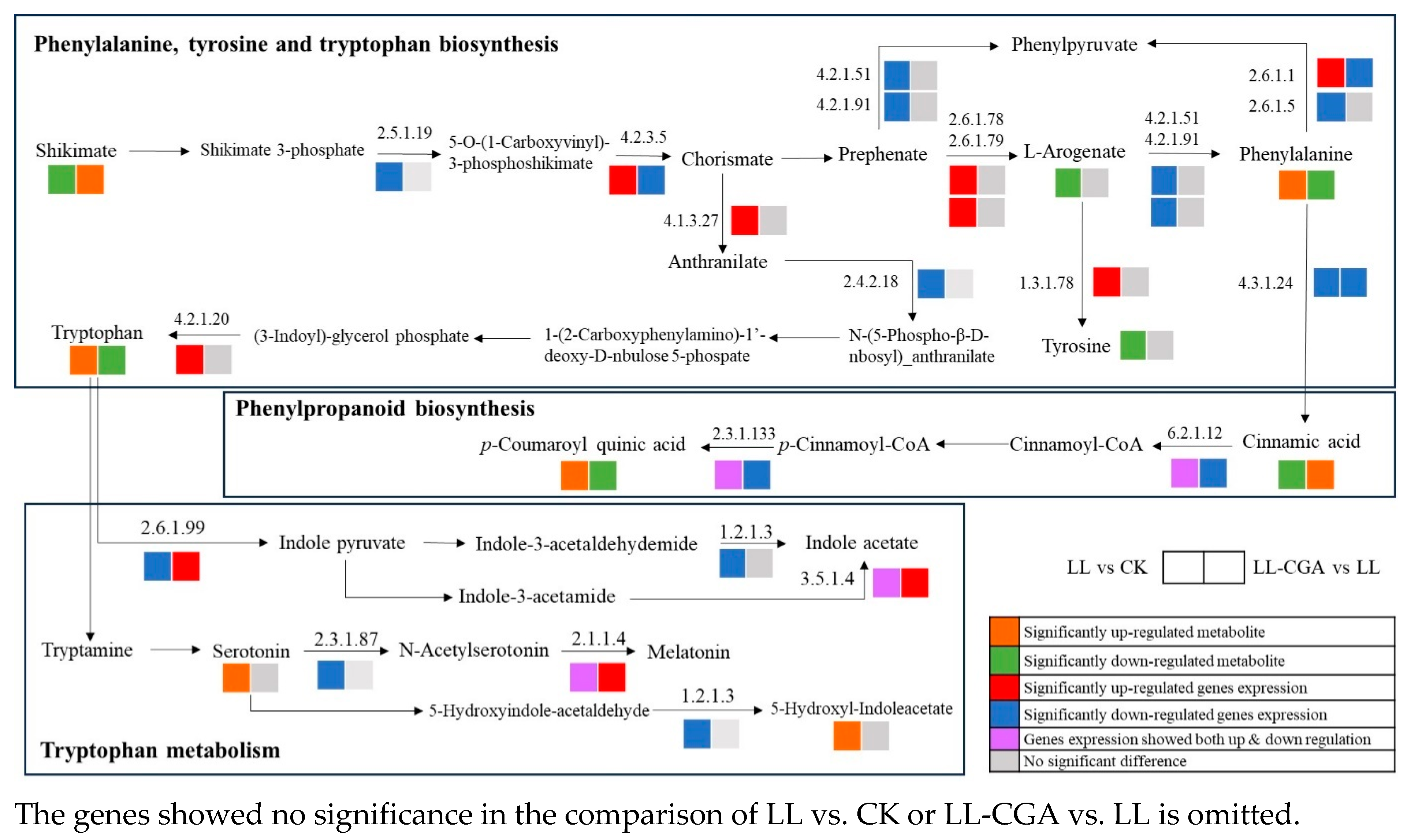
2.4. The Transcriptome and Metabolomic Analysis
2.5. Statistical and WGCNA Analysis
3. Results
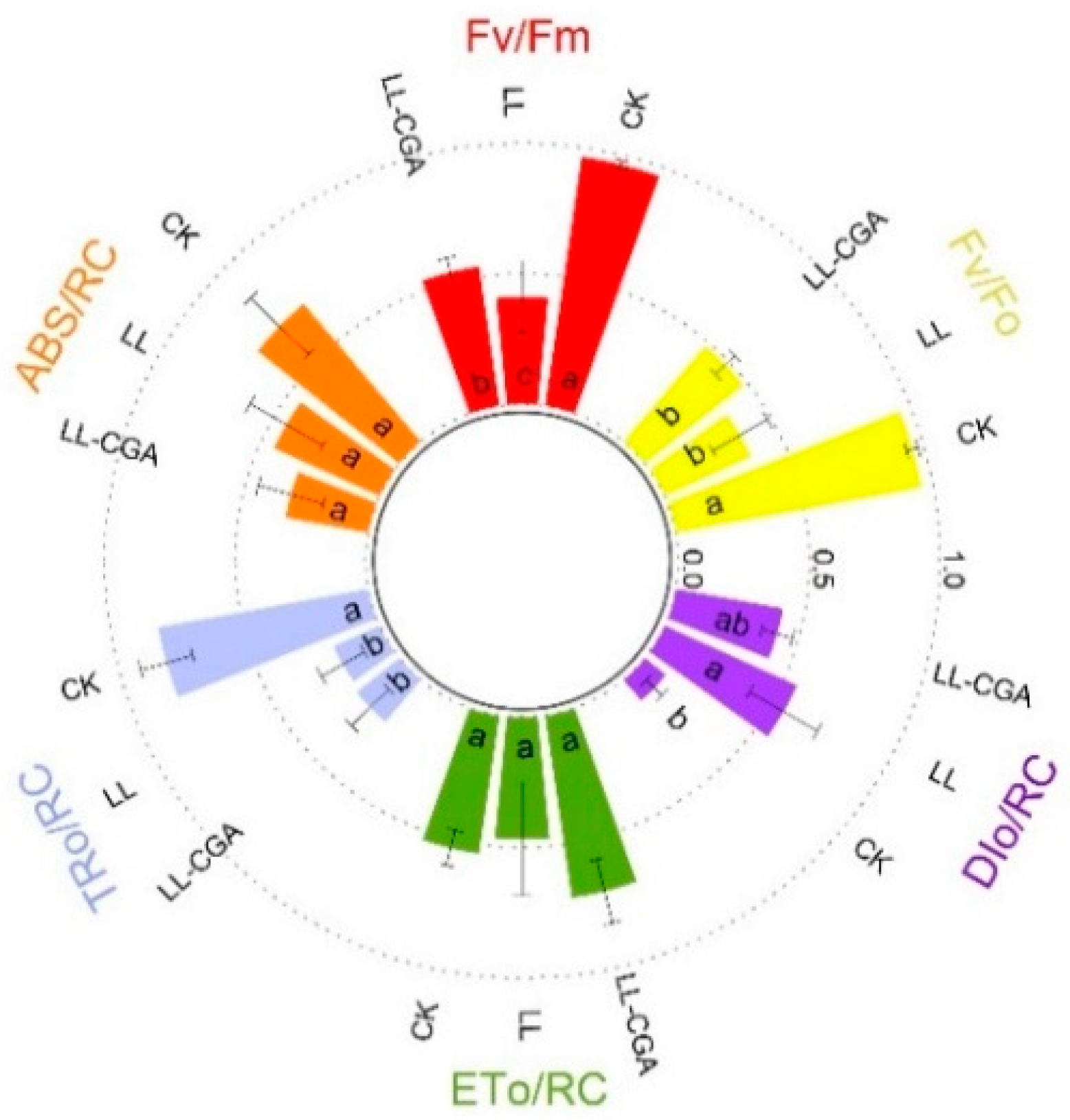
3.1. Assessing Cell Membrane Damage, Photosynthesis Capacity and Biomass with Chilling and CGA Effects
3.2. Chilling and CGA Effects on Nutrient/Mineral Accumulation and Translocation
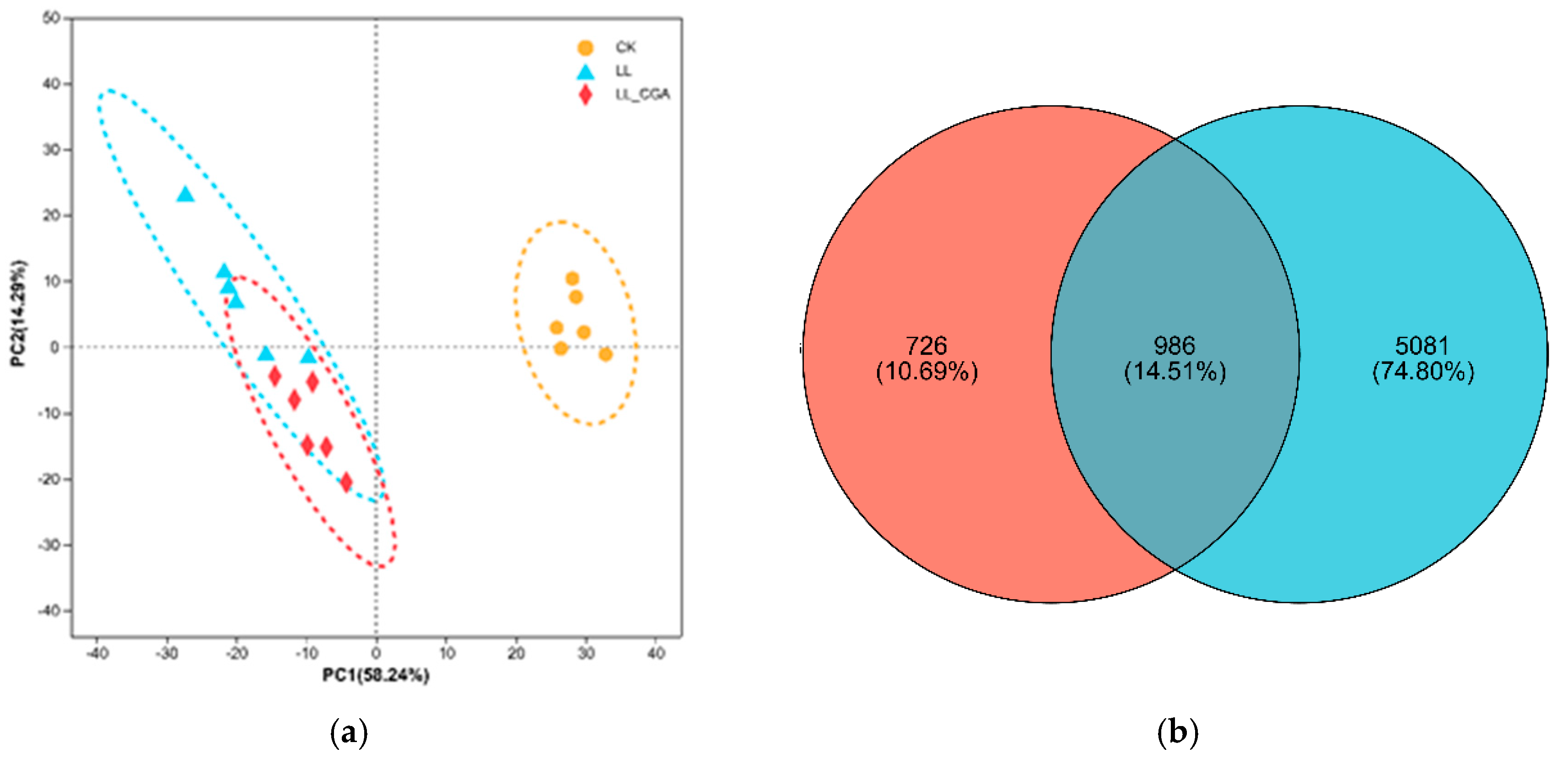
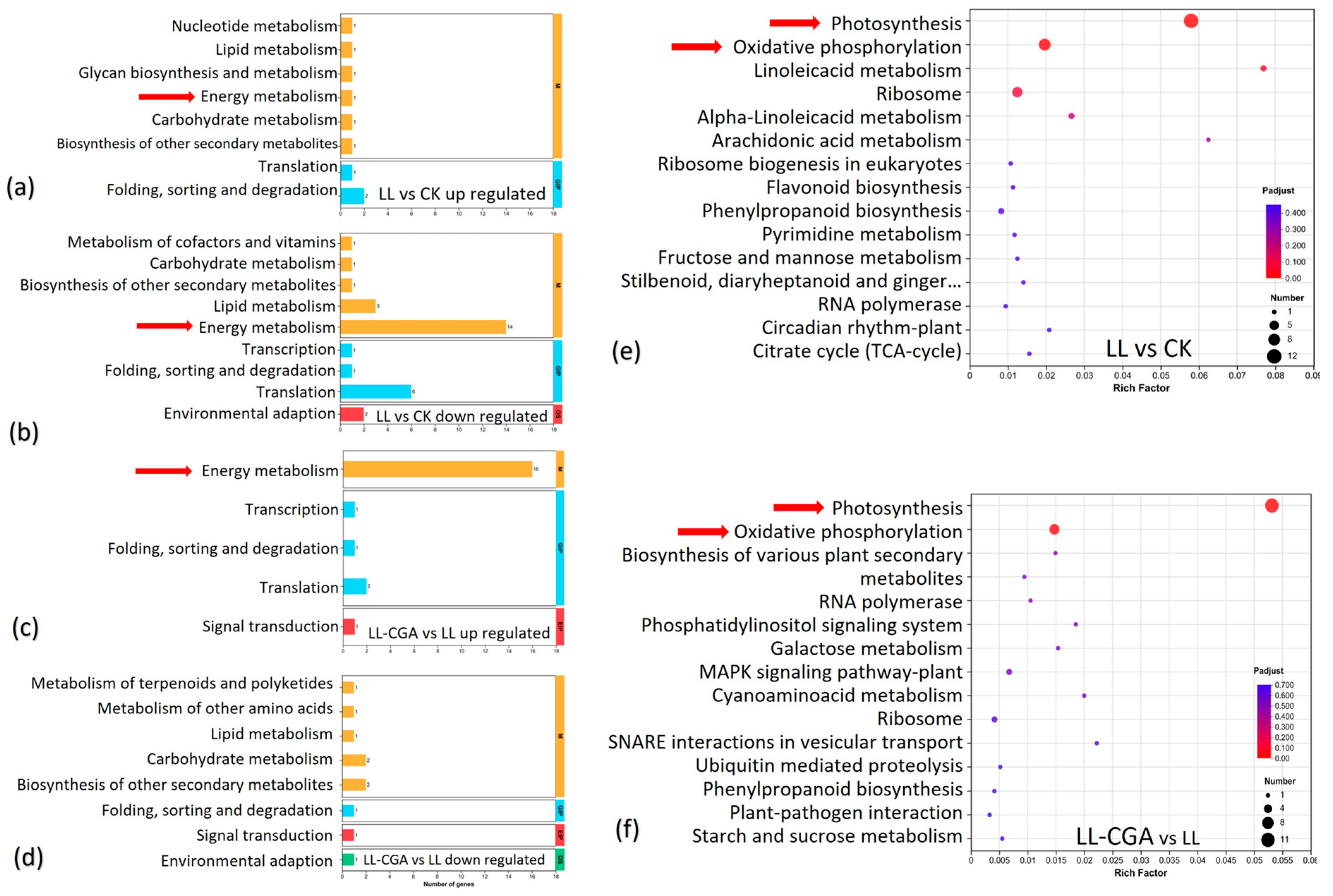
3.3. Leaf Transcriptome Analysis of Chilling and CGA Effects
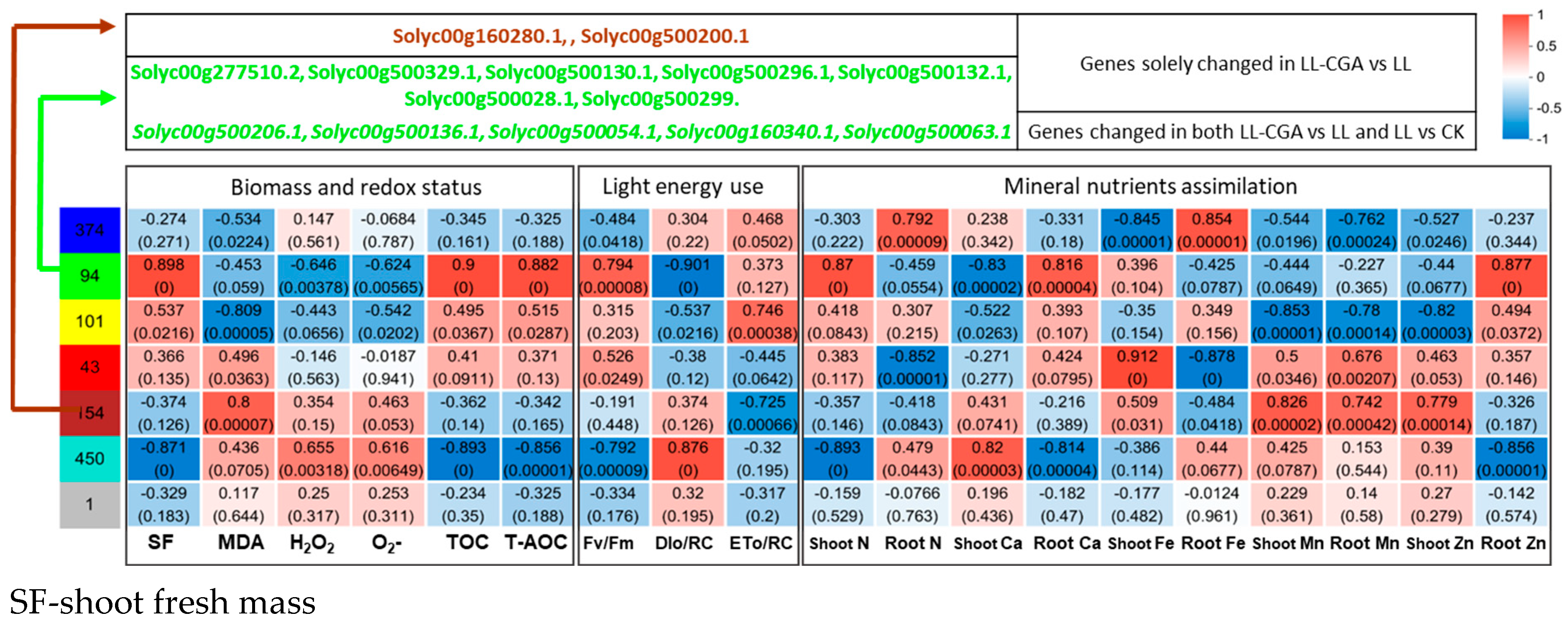
3.4. Leaf Transcriptomic and Metabolomic Co-Analysis of Chilling—CGA Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ding, F.; Wang, X.; Li, Z.; Wang, M. Jasmonate Positively Regulates Cold Tolerance by Promoting ABA Biosynthesis in Tomato. Plants 2022, 12, 60. [Google Scholar] [CrossRef]
- Cai, B.; Li, Q.; Liu, F.; Bi, H.; Ai, X. Decreasing fructose-1,6-bisphosphate aldolase activity reduces plant growth and tolerance to chilling stress in tomato seedlings. Physiol. Plant. 2018, 163, 247–258. [Google Scholar] [CrossRef]
- Cai, B.; Ning, Y.; Li, Q.; Li, Q.; Ai, X. Effects of the Chloroplast Fructose-1,6-Bisphosphate Aldolase Gene on Growth and Low-Temperature Tolerance of Tomato. Int. J. Mol. Sci. 2022, 23, 728. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, B.; Chen, G.; Chen, H.; Peng, Y.; Sohail, H.; Geng, S.; Luo, G.; Xu, D.; Ouyang, B.; et al. The essential role of jasmonate signaling in Solanum habrochaites rootstock-mediated cold tolerance in tomato grafts. Hortic. Res. 2023, 10, uhac227. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xu, J.; Zhang, J.; Li, J.; Hu, X. Exogenous 5-aminolevulinic acid pretreatment ameliorates oxidative stress triggered by low-temperature stress of Solanum lycopersicum. Acta Physiol. Plant 2018, 40, 210. [Google Scholar] [CrossRef]
- Rajametov, S.N.; Lee, K.; Jeong, H.; Cho, M.; Nam, C.; Yang, E. Physiological Traits of Thirty-Five Tomato Accessions in Response to Low Temperature. Agriculture 2021, 11, 792. [Google Scholar] [CrossRef]
- Kale, R.; Hebert, A.E.; Frankel, L.K.; Sallans, L.; Bricker, T.M.; Pospíšil, P. Amino acid oxidation of the D1 and D2 proteins by oxygen radicals during photoinhibition of Photosystem II. Proc. Natl. Acad. Sci. USA 2017, 114, 2988–2993. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Moustaka, J.; Sperdouli, I. Hormesis in photosystem II: A mechanistic understanding. Curr. Opin. Toxicol. 2022, 29, 57–64. [Google Scholar] [CrossRef]
- Lu, T.; Song, Y.; Yu, H.; Li, Q.; Xu, J.; Qin, Y.; Zhang, G.; Liu, Y.; Jiang, W. Cold Stress Resistance of Tomato (Solanum lycopersicum) Seedlings Is Enhanced by Light Supplementation From Underneath the Canopy. Front. Plant Sci. 2022, 13, 831314. [Google Scholar] [CrossRef]
- Ihtisham, M.; Hasanuzzaman, M.; El-Sappah, A.H.; Zaman, F.; Khan, N.; Raza, A.; Sarraf, M.; Khan, S.; Abbas, M.; Hassan, M.J.; et al. Primary plant nutrients modulate the reactive oxygen species metabolism and mitigate the impact of cold stress in overseeded perennial ryegrass. Front. Plant Sci. 2023, 14, 1149832. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.A.; Sohail, H.; Hassan, M.A.; Sajad, S.; Xing, Y.; Song, J. Cold stress in Brassica vegetables: Morpho-physiological and molecular responses underlying adaptive mechanism. Sci. Hortic. 2024, 329, 113002. [Google Scholar] [CrossRef]
- Jia, Y.; Zou, D.; Wang, J.; Liu, H.; Inayat, M.A.; Sha, H.; Zheng, H.; Sun, J.; Zhao, H. Effect of low water temperature at reproductive stage on yield and glutamate metabolism of rice (Oryza sativa L.) in China. Field Crop Res. 2015, 175, 16–25. [Google Scholar] [CrossRef]
- Wei, H.; Wang, J.; Wang, Q.; He, W.; Liao, S.; Huang, J.; Hu, W.; Tang, M.; Chen, H. Role of melatonin in enhancing arbuscular mycorrhizal symbiosis and mitigating cold stress in perennial ryegrass (Lolium perenne L.). Front. Microbiol. 2023, 14, 1123632. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Y.; Pan, X.; Xi, Z. Amelioration of cold-induced oxidative stress by exogenous 24-epibrassinolide treatment in grapevine seedlings: Toward regulating the ascorbate–glutathione cycle. Sci. Hortic. 2019, 244, 379–387. [Google Scholar] [CrossRef]
- Liu, T.; Jiao, X.; Yang, S.; Zhang, Z.; Ye, X.; Li, J.; Qi, H.; Hu, X. Crosstalk between GABA and ALA to improve antioxidation and cell expansion of tomato seedling under cold stress. Environ. Exp. Bot. 2020, 180, 104228. [Google Scholar] [CrossRef]
- Song, W.; Zhang, P.; Zhang, H.; Xue, Y.A.; Zhang, Q.; Ning, M.; Zhao, X.; Cai, W.; Liu, X.; Zhang, X.; et al. A low concentration of exogenous salicylic acid enhances cold tolerance in Hami melons (Cucumis melo var. saccharinus) by modulating salicylic acid-response CmGST genes. Postharvest Biol. Technol. 2022, 193, 112034. [Google Scholar] [CrossRef]
- Xin, J.; Che, T.; Huang, X.; Yan, H.; Jiang, S. A comprehensive view of metabolic responses to CYP98 perturbation in ancestral plants. Plant Physiol. Biochem. 2023, 201, 107793. [Google Scholar] [CrossRef]
- Shimomura, M.; Yoshida, H.; Fujiuchi, N.; Ariizumi, T.; Ezura, H.; Fukuda, N. Continuous blue lighting and elevated carbon dioxide concentration rapidly increase chlorogenic acid content in young lettuce plants. Sci. Hortic. 2020, 272, 109550. [Google Scholar] [CrossRef]
- Endo, M.; Fukuda, N.; Yoshida, H.; Fujiuchi, N.; Yano, R.; Kusano, M. Effects of light quality, photoperiod, CO2 concentration, and air temperature on chlorogenic acid and rutin accumulation in young lettuce plants. Plant Physiol. Biochem. 2022, 186, 290–298. [Google Scholar] [CrossRef]
- Öztürk, F.S.; Gökbulut, İ.; Hayaloğlu, A.A. Evaluation of physicochemical characteristics, antioxidant activity and phenolic profile of Crataegus species in Malatya, Turkey. Emir. J. Food Agric. 2023, 35, 78–85. [Google Scholar]
- Singh, S.K.; Thakur, K.; Sharma, V.; Saini, M.; Sharma, D.; Vishwas, S.; Kakoty, V.; Pal, R.S.; Chaitanya, M.V.N.L.; Babu, M.R.; et al. Exploring the multifaceted potential of chlorogenic acid: Journey from nutraceutical to nanomedicine. S. Afr. J. Bot. 2023, 159, 658–677. [Google Scholar] [CrossRef]
- Meot-Duros, L.; Magné, C. Antioxidant activity and phenol content of Crithmum maritimum L. leaves. Plant Physiol. Biochem. 2009, 47, 37–41. [Google Scholar] [CrossRef]
- Mei, Y.; Sun, H.; Du, G.; Wang, X.; Lyu, D. Exogenous chlorogenic acid alleviates oxidative stress in apple leaves by enhancing antioxidant capacity. Sci. Hortic. 2020, 274, 109676. [Google Scholar] [CrossRef]
- You, W.; Wang, C.; Zhang, J.; Ru, X.; Xu, F.; Wu, Z.; Jin, P.; Zheng, Y.; Cao, S. Exogenous chlorogenic acid inhibits quality deterioration in fresh-cut potato slices. Food Chem. 2024, 446, 138866. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, N.; Mitra, R.; Dasgupta, D.; Ganguly, R.; Acharya, K.; Minkina, T.; Popova, V.; Churyukina, E.; Keswani, C. Unraveling lipid peroxidation-mediated regulation of redox homeostasis for sustaining plant health. Plant Physiol. Biochem. 2024, 206, 108272. [Google Scholar] [CrossRef]
- Ilea, M.I.M.; Zapata, P.J.; Fernández-Picazo, C.; Díaz-Mula, H.M.; Castillo, S.; Guillén, F. Chlorogenic Acid as a Promising Tool for Mitigating Chilling Injury: Cold Tolerance and the Ripening Effect on Tomato Fruit (Solanum lycopersicum L.). Plants 2024, 13, 2055. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis; 2000; pp. 445–483. [Google Scholar]
- Amin, V.M.; Olson, N.F. Spectrophptometric Determination of Hydrogen Peroxide. J. Diary Sci. 1967, 50, 461–464. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Wang, X.; Huang, J. The Theory and Technology for Plant Physiology and Biochemistry Experiment 3rd Version; Higher Eucation Press: Beijing, China, 2015; ISBN 978-7-04-039646-1. [Google Scholar]
- Pellegrini, N.; Serafini, M.; Salvatore, S. Total antioxidant capacity of spices, dried fruits, nuts, pulses, cereals and sweets consumed in Italy assessed by three different in vitro assay. Mol. Nutr. Food Res. 2006, 50, 1030–1038. [Google Scholar] [CrossRef]
- Saez-Plaza, P.; Navas, M.I.J.; Wybraniec, S.; Michałowski, T.; Asuero, A.I.G.I. An Overview of the Kjeldahl Method of Nitrogen Determination. Part II. Sample Preparation, Working Scale, Instrumental Finish, and Quality Control. Crit. Rev. Anal. Chem. 2013, 43, 224–272. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, G.; Shi, C.; Liu, L.; Guo, Q.; Han, C.; Zhang, D.; Zhang, L.; Liu, B.; Gao, H.; et al. Majorbio Cloud: A one-stop, comprehensive bioinformatic platform for multiomics analyses. Imeta 2022, 1, e12. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Z.; Chong, K.; Xu, Y. Chilling tolerance in rice: Past and present. J. Plant Physiol. 2022, 268, 153576. [Google Scholar] [CrossRef]
- El-Mahdy, M.T.; Ali, M.; Pisam, W.M.M.; Abeed, A.H.A. Physiological and molecular analysis of pitaya (Hylocereus polyrhizus) reveal up-regulation of secondary metabolites, nitric oxide, antioxidant defense system, and expression of responsive genes under low-temperature stress by the pre-treatment of hydrogen peroxide. Plant Physiol. Biochem. 2024, 213, 108840. [Google Scholar] [CrossRef]
- Lei, S.; Rossi, S.; Huang, B. Metabolic and Physiological Regulation of Aspartic Acid-Mediated Enhancement of Heat Stress Tolerance in Perennial Ryegrass. Plants 2022, 11, 199. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, J.; Cao, J.; Cai, Q.; Li, X.; Sun, Y.; Li, S.; Li, Y.; Hu, G.; Cao, S.; et al. Leaf transcriptomic response mediated by cold stress in two maize inbred lines with contrasting tolerance levels. Genomics 2021, 113, 782–794. [Google Scholar] [CrossRef]
- Barbato, R.; Tadini, L.; Cannata, R.; Peracchio, C.; Jeran, N.; Alboresi, A.; Morosinotto, T.; Bajwa, A.A.; Paakkarinen, V.; Suorsa, M.; et al. Higher order photoprotection mutants reveal the importance of ΔpH-dependent photosynthesis-control in preventing light induced damage to both photosystem II and photosystem I. Sci. Rep. 2020, 10, 6770. [Google Scholar] [CrossRef]
- Saito, K.; Kikuchi, T.; Nakayama, M.; Mukai, K.; Sumi, H. A single chlorophyll in each of the core antennas CP43 and CP47 transferring excitation energies to the reaction center in Photosystem II of photosynthesis. J. Photochem. Photobiol. A Chem. 2006, 178, 271–280. [Google Scholar] [CrossRef]
- Eijckelhoff, C.; Dekker, J.P.; Boekema, E.J. Characterization by electron microscopy of dimeric Photosystem II core complexes from spinach with and without CP43. Biochim. Biophys. Acta 1997, 1321, 10–20. [Google Scholar] [CrossRef]
- Wang, J.; Shan, J.; Xu, Q.; Ruan, X.; Gong, Y.; Kuang, T.; Zhao, N. Light- and heat-induced denaturation of Photosystem II core-antenna complexes CP43 and CP47. J. Photochem. Photobiol. B Biol. 1999, 50, 189–196. [Google Scholar] [CrossRef]
- Song, Q.; Wang, X.; Liu, Y.; Brestic, M.; Yang, X. StLTO1, a lumen thiol oxidoreductase in Solanum tuberosum L., enhances the cold resistance of potato plants. Plant Sci. 2022, 325, 111481. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Riaz, M.; Yan, L.; Zhang, Z.; Jiang, C. How the cells were injured and the secondary metabolites in the shikimate pathway were changed by boron deficiency in trifoliate orange root. Plant Physiol. Biochem. 2020, 151, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T. Phenylpropanoidd Biosynthesis. Mol. Plant. 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yu, W.; Yang, X.; Liang, J.; Sun, X.; Sun, M.; Xiao, Y.; Peng, F. Silicon enhances the drought resistance of peach seedlings by regulating hormone, amino acid, and sugar metabolism. BMC Plant Biol. 2022, 22, 422. [Google Scholar] [CrossRef]
- Shen, X.; Liu, Y.; Zeng, Y.; Zhao, Y.; Bao, Y.; Wu, Z.; Zheng, Y.; Jin, P. Hydrogen sulfide alleviates the chilling-induced lignification in loquat fruit by regulating shikimate, phenylpropanoid and cell wall metabolisms. Postharvest Biol. Technol. 2024, 113012. [Google Scholar] [CrossRef]
- Fabregas, N.; Fernie, A.R. The reliance of phytohormone biosynthesis on primary metabolite precursors. J. Plant Physiol. 2022, 268, 153589. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Ahmad, P. Role of Mineral Nutrients in Abiotic Stress Tolerance. In Plant Signaling Molecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 269–285. [Google Scholar]
- Wang, Y.; Ye, H.; Lin, W.; Wang, G.; Luo, T.; He, J. Cinnamic acid application inhibits the browning of cold-stored taro slicesby maintaining membrane function, reducing flavonoid biosynthesis and enhancing glutathione metabolism. Postharvest Biol. Technol. 2024, 218, 113180. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
| Day 0–6 | Day 7–12 | Day 13–14 | End of Day 14 | ||
|---|---|---|---|---|---|
| Pre-Chilling | Chilling | Recovery | Harvest | ||
| CK | 25 °C/18 °C (day/night) + 1/2 Hoagland solution | No additive | 25 °C/18 °C (day/night) + 1/2 Hoagland solution | 25 °C/18 °C (day/night) + 1/2 Hoagland solution | Harvest all |
| LL | 15 °C/5 °C (day/night) + 1/2 Hoagland solution | ||||
| LL-CGA | 0.05 g L−1 CGA | ||||
| Treatment | Shoot DM | Root DM | Root/Shoot DM | Shoot Moisture | Root Moisture |
|---|---|---|---|---|---|
| g | g | % | % | ||
| CK | 0.22 ± 0.11 a | 0.03 ± 0.01 a | 0.13 ± 0.09 b | 94.1 ± 1.55 a | 94.2 ± 3.76 a |
| LL | 0.14 ± 0.06 b | 0.03 ± 0.01 a | 0.27 ± 0.21 a | 94.3 ± 3.17 a | 92.9 ± 1.96 a |
| LL-CGA | 0.18 ± 0.09 ab | 0.03 ± 0.02 a | 0.17 ± 0.08 b | 94.1 ± 2.25 a | 93.8 ± 3.20 a |
| Sig. | p = 0.06 | ns | p = 0.05 | ns | ns |
| Treatment * | MDA nmol g−1 | H2O2 μmol g−1 | O2 μmol min−1 g−1 | TOC % | T-AOC U mL−1 |
|---|---|---|---|---|---|
| CK | 77.7 ± 7.78 b | 1.142 ± 0.44 a | 107 ± 0.69 a | 84.6 ± 4.54 a | 26.8 ± 0.38 a |
| LL | 115.0 ± 14.90 a | 1.207 ± 0.19 a | 108 ± 0.47 a | 54.6 ± 8.54 b | 18.0 ± 3.18 b |
| LL-CGA | 72.6 ± 14.80 b | 1.119 ± 0.27 a | 107 ± 0.71 a | 73.8 ± 13.30 a | 25.2 ± 2.36 a |
| Sig. | *** | ns | ns | *** | *** |
| Treatment | N | P | K | Ca | Mg | Fe | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|
| g kg−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | mg kg−1 | mg kg−1 | |
| Shoot | |||||||||
| CK | 63.4 ± 1.85 a | 5.75 ± 0.53 a | 28.6 ± 1.77 a | 18.6 ± 0.88 a | 8.15 ± 0.48 a | 0.57 ± 0.06 a | 0.86 ± 0.25 ab | 40.1 ± 5.76 a | 10.1 ± 0.41 a |
| LL | 55.9 ± 1.12 b | 4.35 ± 0.25 b | 23.2 ± 1.85 b | 20.1 ± 0.70 b | 8.61 ± 0.32 a | 0.53 ± 0.05 a | 1.15 ± 0.06 a | 42.8 ± 8.81 a | 8.47 ± 0.13 a |
| LL-CGA | 56.7 ± 2.72 b | 4.36 ± 0.33 b | 25.3 ± 1.52 ab | 18.8 ± 1.08 b | 8.06 ± 0.23 a | 0.36 ± 0.01 b | 0.77 ± 0.10 b | 33.3 ± 3.76 a | 8.83 ± 1.35 a |
| Sig. | *** | *** | ** | * | p = 0.06 | *** | ** | p = 0.07 | * |
| Root | |||||||||
| CK | 41.9 ± 0.49 b | 4.47 ± 0.59 a | 25.3 ± 1.93 a | 5.85 ± 0.46 a | 6.48 ± 0.79 b | 5.19 ± 1.47 a | 0.92 ± 0.05 a | 156 ± 9.44 a | 10.4 ± 0.86 a |
| LL | 43.4 ± 0.62 b | 4.27 ± 0.43 a | 27.6 ± 1.39 a | 5.27 ± 0.25 b | 6.65 ± 0.06 b | 5.65 ± 0.46 a | 0.93 ± 0.17 a | 116 ± 11.7 b | 9.52 ± 0.59 a |
| LL-CGA | 77.0 ± 17.6 a | 4.16 ± 0.15 a | 28.3 ± 0.98 a | 5.31 ± 0.19 b | 7.44 ± 0.41 a | 6.42 ± 0.34 a | 0.89 ± 0.08 a | 117 ± 9.35 b | 9.74 ± 0.64 a |
| Sig. | *** | ns | * | * | * | ns | ns | *** | ns |
| Root to shoot translocation factor | |||||||||
| CK | 1.51 ± 0.03 a | 1.29 ± 0.08 a | 1.13 ± 0.04 a | 3.18 ± 0.10 b | 1.27 ± 0.22 a | 0.12 ± 0.03 a | 0.92 ± 0.17 b | 0.26 ± 0.04 b | 0.98 ± 0.06 a |
| LL | 1.29 ± 0.03 a | 1.03 ± 0.15 b | 0.85 ± 0.10 b | 3.81 ± 0.28 a | 1.29 ± 0.06 a | 0.09 ± 0.00 ab | 1.26 ± 0.22 a | 0.36 ± 0.04 a | 0.89 ± 0.05 a |
| LL-CGA | 0.77 ± 0.19 b | 1.05 ± 0.05 b | 0.89 ± 0.04 b | 3.54 ± 0.32 ab | 1.08 ± 0.08 a | 0.06 ± 0.00 b | 0.86 ± 0.11 b | 0.28 ± 0.03 b | 0.90 ± 0.09 a |
| Sig. | *** | ** | *** | ** | p = 0.06 | ** | * | * | ns |
| LL vs. CK | ||||
| Trend | KO name | Gene name | Encoding Protein | Protein Function |
| down | psbC | Solyc00g500057.1, Solyc00g500209.1 | Photosystem II CP43 Chlorophyll Apoprotein | Photosystem II (P680 chlorophyll a) |
| psbB | Solyc00g500206.1, Solyc00g500136.1, Solyc00g500054.1 | Photosystem II CP47 Chlorophyll Apoprotein | Photosystem II (P681 chlorophyll a) | |
| psaA | Solyc00g500024.1, Solyc00g500071.1 | Photosystem I P700 Chlorophyll A Apoprotein A1 | Photosystem I (P700 chlorophyll a) | |
| ndhA | Solyc00g500041.1 | NADH-Plastoquinone Oxidoreductase Subunit 1 | NADH: ubiquinone reductase (H + -translocating) | |
| ndhF | Solyc00g500195.1, Solyc00g160340.1 | NADH-Plastoquinone Oxidoreductase Subunit 5 | ||
| atpF | gene_Solyc00g500322.1 | ATP Synthase CF0 Subunit I | F-type ATPase | |
| atpB | Solyc00g500063.1, Solyc00g500064.1 | ATP Synthase CF1 Beta Subunit | ||
| atpA | Solyc00g500323.1 | ATP Synthase CF1 Alpha Subunit | ||
| up | atpA | Solyc00g500133.1 | ||
| LL-CGA vs. LL | ||||
| Trend | KO name | Gene name | Encoding Protein | Protein Function |
| up | psbK | Solyc00g277510.2, Solyc00g500329.1, Solyc00g500130.1, Solyc00g500296.1, Solyc00g500200.1 | Photosystem II Protein K | Photosystem II (P681 chlorophyll a) |
| psbC | Solyc00g500206.1, Solyc00g500136.1, Solyc00g500054.1 | Photosystem II CP43 Chlorophyll Apoprotein | Photosystem II (P680 chlorophyll a) | |
| psbA | Solyc00g500132.1 | Photosystem II P680 Reaction Center D1 Protein | ||
| petD | Solyc00g500028.1 | Cytochrome B6/F Complex Subunit IV | Cytochrome b6/f complex | |
| ndhB | Solyc00g500299.1 | NADH-Plastoquinone Oxidoreductase Subunit 2 | NADH: ubiquinone reductase (H + -translocating) | |
| ndhD | Solyc00g160280.1 | NAD(P)H-Quinone Oxidoreductase Subunit 4 | ||
| ndhF | Solyc00g160340.1 | NAD(P)H-Quinone Oxidoreductase Subunit 5 | ||
| atpB | Solyc00g500063.1 | ATP Synthase CF1 Beta Subunit | F-type ATPase | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Mur, L.A.J.; Guo, Q.; Xu, X. Pre-Chilling CGA Application Alleviates Chilling Injury in Tomato by Maintaining Photosynthetic Efficiency and Altering Phenylpropanoid Metabolism. Plants 2025, 14, 2026. https://doi.org/10.3390/plants14132026
Li Y, Mur LAJ, Guo Q, Xu X. Pre-Chilling CGA Application Alleviates Chilling Injury in Tomato by Maintaining Photosynthetic Efficiency and Altering Phenylpropanoid Metabolism. Plants. 2025; 14(13):2026. https://doi.org/10.3390/plants14132026
Chicago/Turabian StyleLi, Yanmei, Luis A. J. Mur, Qiang Guo, and Xiangnan Xu. 2025. "Pre-Chilling CGA Application Alleviates Chilling Injury in Tomato by Maintaining Photosynthetic Efficiency and Altering Phenylpropanoid Metabolism" Plants 14, no. 13: 2026. https://doi.org/10.3390/plants14132026
APA StyleLi, Y., Mur, L. A. J., Guo, Q., & Xu, X. (2025). Pre-Chilling CGA Application Alleviates Chilling Injury in Tomato by Maintaining Photosynthetic Efficiency and Altering Phenylpropanoid Metabolism. Plants, 14(13), 2026. https://doi.org/10.3390/plants14132026







