Sustainable Production of Ginsenosides: Advances in Biosynthesis and Metabolic Engineering
Abstract
1. Introduction
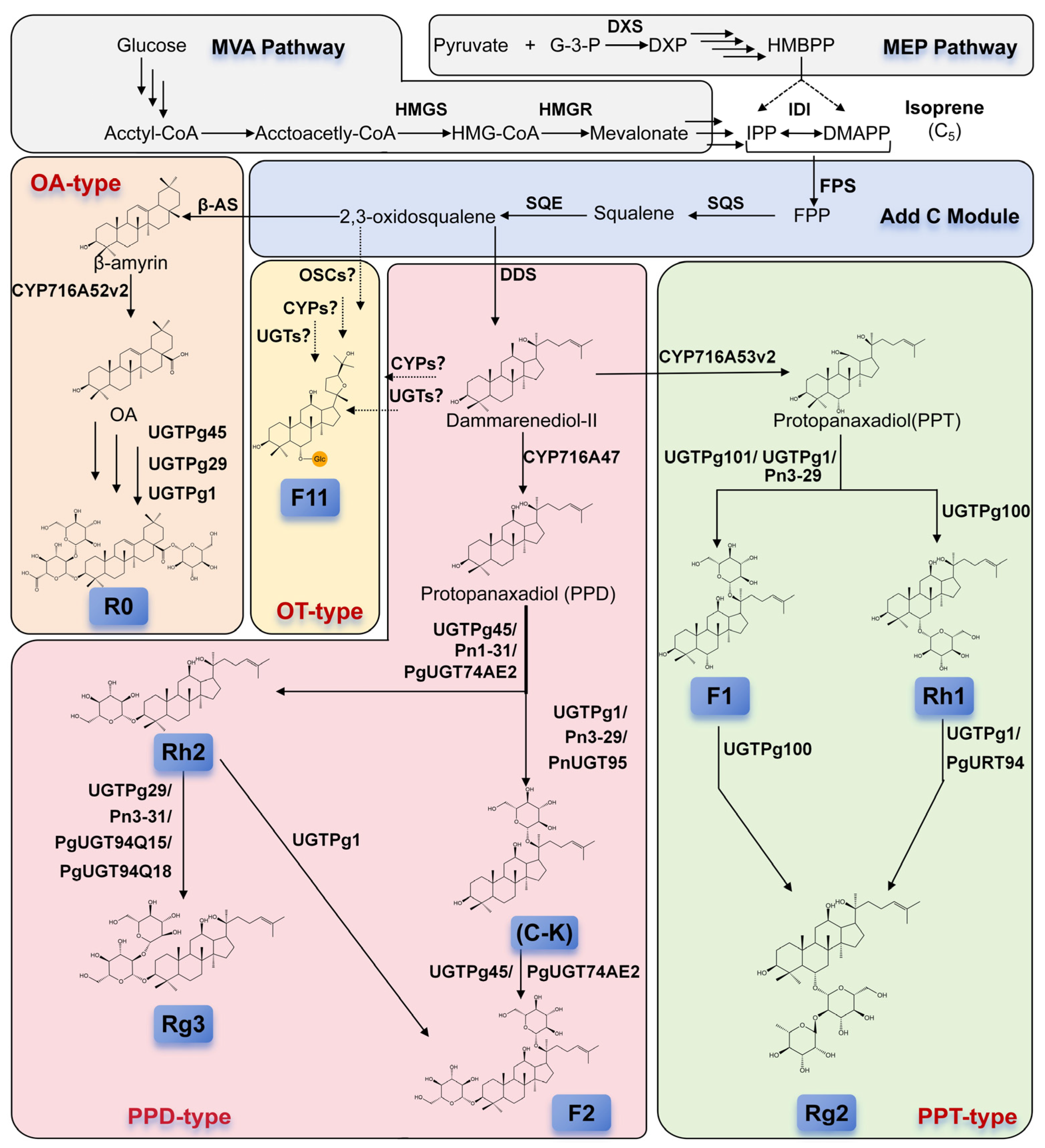
2. The Biosynthetic Pathway of Ginseno Sides
2.1. Classification of Ginsenosides
2.2. Biosynthetic Pathway of Ginsenosides
2.2.1. Upstream Biosynthesis Pathway of Isoprenoid Precursors
2.2.2. Formation of the Triterpenoid Saponin Skeleton: Squalene and Its Cyclization
2.2.3. Key Modifications Mediated by Cytochrome P450 (CYP450) Enzymes
2.2.4. Pivotal Role of UDP-Glycosyltransferases (UGTs) in Generating Structural Diversity
3. A Paradigm Shift for Sustainable Ginsenoside Production
3.1. Innovations in Agricultural Technology
3.2. Plant-Based Systems: Ginseng Cell, Tissue, and Hairy Root Cultures
3.3. Microbial Synthesis of Ginsenosides
3.4. Enzymatic Biocatalysis for Ginsenoside Production
4. Ginsenoside Bioprospecting: Advances and Applications
4.1. Pharmacological Significance and Multifaceted Bioactivities
4.2. Industrialization Prospects and Market Analysis
5. Challenges and Prospects
5.1. Current Technical Challenges
5.1.1. Insufficient Research on Enzyme Activity and Structural Optimization
5.1.2. Pathway Balancing and Metabolic Burden
5.1.3. Scaling-Up Challenges
5.2. Engineered Biosynthesis of Ginsenosides: A Sustainable Platform
5.3. Integration of New Technologies with Plant-Based Chassis for Ginsenoside Biosynthesis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ginseng | Panax ginseng |
| MVA | Mevalonate |
| MEP | Methylerythritol phosphate |
| IPP | Isopentenyl diphosphate |
| DMAPP | Isomer dimethylallyl diphosphate |
| HMGR | HMG-CoA reductase |
| DXP | 1-Deoxy-D-xylulose-5-phosphate |
| GPP | Geranyl diphosphate |
| DMAPP | 3,3-Dimethylallyl diphosphate |
| FPP | Farnesyl pyrophosphate |
| FPS | Farnesyl pyrophosphate synthase |
| SQS | Squalene synthase |
| DDS | Dammarenediol synthase |
| CYP716A47 | Cytochrome P450 oxidases enzymes |
| PPD | Protopanaxadiol |
| CYP716A53v2 | Cytochrome P450 oxidases enzymes |
| PPT | Protopanaxatriol |
| β-AS | β-Amyrin synthase |
| CYP716A52v2 | Cytochrome P450 oxidases enzymes |
| OA | Oleanolic acid |
| SQE | Squalene epoxidase |
| OSCs | Oxidosqualene cyclases |
| DD | Dammarenediol-II |
| DS | Dammarenediol-II synthase |
| CYP450 | Cytochrome P450 |
| UGTs | UDP-glycosyltransferases |
| notoginseng | Panax notoginseng |
| E. coli | Escherichia coli |
| B. subtilis | Bacillus subtilis |
| MeJA | Methyl jasmonic acid |
| JA | Jasmonic acid |
| CAGR | Compound annual growth rate |
References
- Lee, Y.-M.; Yoon, H.; Park, H.-M.; Song, B.C.; Yeum, K.-J. Implications of Red Panax ginseng in Oxidative Stress Associated Chronic Diseases. J. Ginseng Res. 2017, 41, 113–119. [Google Scholar] [CrossRef]
- Baeg, I.-H.; So, S.-H. The World Ginseng Market and the Ginseng (Korea). J. Ginseng Res. 2013, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Radad, K.; Gille, G.; Liu, L.L.; Rausch, W.D. Use of Ginseng in Medicine with Emphasis on Neurodegenerative Disorders. J. Pharmacol. Sci. 2006, 100, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Jeon, J.-N.; Jang, M.-G.; Oh, J.Y.; Kwon, W.-S.; Jung, S.-K.; Yang, D.-C. Ginsenoside Profiles and Related Gene Expression during Foliation in Panax ginseng Meyer. J Ginseng Res. 2014, 38, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P. Ginsenosides Chemistry, Biosynthesis, Analysis, and Potential Health Effects. Adv. Food Nutr. Res. 2009, 55, 1–99. [Google Scholar] [CrossRef]
- Zhang, C.X. Study on the Chemical Constituents from Ginseng and American Ginseng and Notoginseng. Ph.D. Thesis, Jilin Agricultural University, Changchun, China, 2004. Available online: https://www.dissertationtopic.net/doc/1579829 (accessed on 29 August 2025).
- Wang, J.-R.; Yau, L.-F.; Gao, W.-N.; Liu, Y.; Yick, P.-W.; Liu, L.; Jiang, Z.-H. Quantitative Comparison and Metabolite Profiling of Saponins in Different Parts of the Root of Panax notoginseng. J. Agric. Food Chem. 2014, 62, 9024–9034. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, M.; Wen, J.; Yang, Z.; Li, G.; Cao, Y.; Sun, L.; Ren, X. Panax japonicus CA Meyer: A Comprehensive Review on Botany, Phytochemistry, Pharmacology, Pharmacokinetics and Authentication. Chin. Med. 2023, 18, 148. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, S. Progress in Understanding of Ginsenoside Biosynthesis. Plant Biol. 2008, 10, 415–421. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biosynthesis and Biotechnological Production of Ginsenosides. Biotechnol. Adv. 2015, 33, 717–735. [Google Scholar] [CrossRef]
- Yang, J.-L.; Hu, Z.-F.; Zhang, T.-T.; Gu, A.-D.; Gong, T.; Zhu, P. Progress on the Studies of the Key Enzymes of Ginsenoside Biosynthesis. Molecules 2018, 23, 589. [Google Scholar] [CrossRef]
- Son, S.-H.; Kang, J.; Shin, Y.; Lee, C.; Sung, B.H.; Lee, J.Y.; Lee, W. Sustainable Production of Natural Products Using Synthetic Biology: Ginsenosides. J. Ginseng Res. 2024, 48, 140–148. [Google Scholar] [CrossRef]
- DesRochers, N.; Walsh, J.P.; Renaud, J.B.; Seifert, K.A.; Yeung, K.K.-C.; Sumarah, M.W. Metabolomic Profiling of Fungal Pathogens Responsible for Root Rot in American Ginseng. Metabolites 2020, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Lu, J.; Wang, J.; Gao, W.-Y. Advances in Biosynthesis of Triterpenoid Saponins in Medicinal Plants. Chin. J. Nat. Med. 2020, 18, 417–424. [Google Scholar] [CrossRef]
- Han, M.; Sha, X.Y.; Wu, Y.J.; Fang, X.L. Oral Absorption of Ginsenoside Rb1 Using in Vitro and in Vivo Models. Planta Med. 2006, 72, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.-N.; Luo, W.; Lv, C.-N.; Lu, J.-C. Research Progress on Naturally-Occurring and Semi-Synthetic Ocotillol-Type Ginsenosides in the genus Panax L. (Araliaceae). Chin. J. Nat. Med. 2021, 19, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Piao, X.; Zhang, H.; Kang, J.P.; Yang, D.U.; Li, Y.; Pang, S.; Jin, Y.; Yang, D.C.; Wang, Y. Advances in Saponin Diversity of Panax ginseng. Molecules 2020, 25, 3452. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, K.; Wang, J.; Cheng, H.; Ma, M.; Meng, Q.; Li, X.; Bi, Y. Design, Synthesis and Antibacterial Evaluation of Ocotillol Derivatives with Polycyclic Nitrogen-Containing Groups. Future Med. Chem. 2021, 13, 1025–1039. [Google Scholar] [CrossRef]
- Liu, J.; Gan, H.; Li, T.; Wang, J.; Du, G.; An, Y.; Yan, X.; Geng, C. The Metabolites and Biotransformation Pathwaysin Vivoafter Oral Administration of Ocotillol, RT5, and PF11. Biomed. Chromatogr. 2020, 34, e4856. [Google Scholar] [CrossRef]
- Zhang, D.; Cao, Y.; Wang, K.; Shi, Z.; Wang, R.; Meng, Q.; Bi, Y. Design, Synthesis, and Antibacterial Evaluation of Novel Ocotillol Derivatives and Their Synergistic Effects with Conventional Antibiotics. Molecules 2021, 26, 5969. [Google Scholar] [CrossRef]
- Kim, D.-H. Chemical Diversity of Panax ginseng, Panax quinquifolium, and Panax notoginseng. J. Ginseng Res. 2012, 36, 1–15. [Google Scholar] [CrossRef]
- Liu, J.-P.; Wang, F.; Li, P.-Y.; Lu, D. A New Ocotillol-Type Triterpenoid Saponin from Red American Ginseng. Nat. Prod. Res. 2012, 26, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Dou, W.; Wu, Z.; Huang, C.; Yang, X.; Ding, L.; Han, Z.; Yang, L.; Wang, Z.; Wang, R. Strategic Enrichment of Ocotillol-Type Ginsenosides F11, RT5 and Ocotillol from Panax quinquefolium. Ind. Crop. Prod. 2024, 218, 118953. [Google Scholar] [CrossRef]
- Kim, J.H.; Yi, Y.-S.; Kim, M.-Y.; Cho, J.Y. Role of Ginsenosides, the Main Active Components of Panax ginseng, in Inflammatory Responses and Diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Fang, X.-L. Difference in Oral Absorption of Ginsenoside Rg1 between in Vitro and in Vivo Models. Acta Pharmacol. Sin. 2006, 27, 499–505. [Google Scholar] [CrossRef]
- Han, J.Y.; Kwon, Y.S.; Yang, D.C.; Jung, Y.R.; Choi, Y.E. Expression and RNA Interference-Induced Silencing of the Dammarenediol Synthase Gene in Panax ginseng. Plant Cell Physiol. 2006, 47, 1653–1662. [Google Scholar] [CrossRef]
- Hasegawa, H.; Lee, K.S.; Nagaoka, T.; Tezuka, Y.; Uchiyama, M.; Kadota, S.; Saiki, I. Pharmacokinetics of Ginsenoside Deglycosylated by Intestinal Bacteria and Its Transformation to Biologically Active Fatty Acid Esters. Biol. Pharm. Bull. 2000, 23, 298–304. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, X.; Fan, D. Ginsenoside CK Inhibits Hypoxia-Induced Epithelial-Mesenchymal Transformation through the HIF-1α/NF-κB Feedback Pathway in Hepatocellular Carcinoma. Foods 2021, 10, 1195. [Google Scholar] [CrossRef]
- Jiang, G.-Z.; Yao, M.-D.; Wang, Y.; Zhou, L.; Song, T.-Q.; Liu, H.; Xiao, W.-H.; Yuan, Y.-J. Manipulation of GES and ERG20 for Geraniol Overproduction in Saccharomyces cerevisiae. Metab. Eng. 2017, 41, 57–66. [Google Scholar] [CrossRef]
- Yan, X.; Fan, Y.; Wei, W.; Wang, P.; Liu, Q.; Wei, Y.; Zhang, L.; Zhao, G.; Yue, J.; Zhou, Z. Production of Bioactive Ginsenoside Compound K in Metabolically Engineered Yeast. Cell Res. 2014, 24, 770–773. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Lee, O.R.; Oh, J.Y.; Jang, M.-G.; Yang, D.-C. Functional Analysis of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Encoding Genes in Triterpene Saponin-Producing Ginseng. Plant Physiol. 2014, 165, 373–387. [Google Scholar] [CrossRef]
- Hou, M.; Wang, R.; Zhao, S.; Wang, Z. Ginsenosides in Panax genus and Their Biosynthesis. Acta Pharm. Sin. B 2021, 11, 1813–1834. [Google Scholar] [CrossRef]
- Cordoba, E.; Porta, H.; Arroyo, A.; San Roman, C.; Medina, L.; Rodriguez-Concepcion, M.; Leon, P. Functional Characterization of the Three Genes Encoding 1-Deoxy-D-Xylulose 5-Phosphate Synthase in Maize. J. Exp. Bot. 2011, 62, 2023–2038. [Google Scholar] [CrossRef]
- Enfissi, E.M.A.; Fraser, P.D.; Lois, L.M.; Boronat, A.; Schuch, W.; Bramley, P.M. Metabolic Engineering of the Mevalonate and Non-Mevalonate Isopentenyl Diphosphate-Forming Pathways for the Production of Health-Promoting Isoprenoids in Tomato. Plant Biotechnol. J. 2005, 3, 17–27. [Google Scholar] [CrossRef]
- Lee, M.H.; Jeong, J.H.; Seo, J.W.; Shin, C.G.; Kim, Y.S.; In, J.G.; Yang, D.C.; Yi, J.S.; Choi, Y.E. Enhanced Triterpene and Phytosterol Biosynthesis in Panax ginseng Overexpressing Squalene Synthase Gene. Plant Cell Physiol. 2004, 45, 976–984. [Google Scholar] [CrossRef]
- Kim, T.-D.; Han, J.-Y.; Huh, G.H.; Choi, Y.-E. Expression and Functional Characterization of Three Squalene Synthase Genes Associated with Saponin Biosynthesis in Panax ginseng. Plant Cell Physiol. 2011, 52, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Vincken, J.-P.; Heng, L.; de Groot, A.; Gruppen, H. Saponins, Classification and Occurrence in the Plant Kingdom. Phytochemistry 2007, 68, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-Y.; In, J.-G.; Kwon, Y.-S.; Choi, Y.-E. Regulation of Ginsenoside and Phytosterol Biosynthesis by RNA Interferences of Squalene Epoxidase Gene in Panax ginseng. Phytochemistry 2010, 71, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chu, Y.; Liao, B.; Xiao, S.; Yin, Q.; Bai, R.; Su, H.; Dong, L.; Li, X.; Qian, J.; et al. Panax ginseng Genome Examination for Ginsenoside Biosynthesis. GigaScience 2017, 6, gix093. [Google Scholar] [CrossRef]
- Lee, M.-H.; Han, J.-Y.; Kim, H.-J.; Kim, Y.-S.; Huh, G.H.; Choi, Y.-E. Dammarenediol-II Production Confers TMV Tolerance in Transgenic Tobacco Expressing Panax ginseng Dammarenediol-II Synthase. Plant Cell Physiol. 2012, 53, 173–182. [Google Scholar] [CrossRef]
- Ren, S.; Sun, Q.; Zhang, L.; Sun, W.; Li, Y.; Feng, X.; Li, C. Sustainable Production of Rare Oleanane-Type Ginsenoside Ro with an Artificial Glycosylation Pathway in Saccharomyces cerevisiae. Green Chem. 2022, 24, 8302–8313. [Google Scholar] [CrossRef]
- Han, J.-Y.; Kim, H.-J.; Kwon, Y.-S.; Choi, Y.-E. The Cyt P450 Enzyme CYP716A47 Catalyzes the Formation of Protopanaxadiol from Dammarenediol-II During Ginsenoside Biosynthesis in Panax ginseng. Plant Cell Physiol. 2011, 52, 2062–2073. [Google Scholar] [CrossRef]
- Han, J.-Y.; Hwang, H.-S.; Choi, S.-W.; Kim, H.-J.; Choi, Y.-E. Cytochrome P450 CYP716A53v2 Catalyzes the Formation of Protopanaxatriol from Protopanaxadiol During Ginsenoside Biosynthesis in Panax ginseng. Plant Cell Physiol. 2012, 53, 1535–1545. [Google Scholar] [CrossRef]
- Li, C.; Yan, X.; Xu, Z.; Wang, Y.; Shen, X.; Zhang, L.; Zhou, Z.; Wang, P. Pathway Elucidation of Bioactive Rhamnosylated Ginsenosides in Panax ginseng and Their de Novo High-Level Production by Engineered Saccharomyces cerevisiae. Commun. Biol. 2022, 5, 775. [Google Scholar] [CrossRef]
- Park, S.-B.; Chun, J.-H.; Ban, Y.-W.; Han, J.Y.; Choi, Y.E. Alteration of Panax ginseng Saponin Composition by Overexpression and RNA Interference of the Protopanaxadiol 6-Hydroxylase Gene (CYP716A53v2). J. Ginseng Res. 2016, 40, 47–54. [Google Scholar] [CrossRef]
- Han, J.-Y.; Kim, M.-J.; Ban, Y.-W.; Hwang, H.-S.; Choi, Y.-E. The Involvement of β-Amyrin 28-Oxidase (CYP716A52v2) in Oleanane-Type Ginsenoside Biosynthesis in Panax ginseng. Plant Cell Physiol. 2013, 54, 2034–2046. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.L.; Montecillo, J.A.V.; Bae, H. Recent Advances in the Metabolic Engineering of Yeasts for Ginsenoside Biosynthesis. Front. Bioeng. Biotechnol. 2020, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Malla, S.; Nguyen, T.T.; Dhakal, D.; Pokhrel, A.R.; Chu, L.L.; Sohng, J.K. Microbial Production of Astilbin, a Bioactive Rhamnosylated Flavanonol, from Taxifolin. World J. Microbiol. Biotechnol. 2017, 33, 36. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Song, X.; Zhang, Y.; Xu, Y.; Liu, Q. Insight on Structural Modification, Biological Activity, Structure-Activity Relationship of PPD-Type Ginsenoside Derivatives. Fitoterapia 2022, 158, 105135. [Google Scholar] [CrossRef]
- Guo, H.-Y.; Xing, Y.; Sun, Y.-Q.; Liu, C.; Xu, Q.; Shang, F.-F.; Zhang, R.-H.; Jin, X.-J.; Chen, F.; Lee, J.J.; et al. Ginsengenin Derivatives Synthesized from 20(R)-Panaxotriol: Synthesis, Characterization, and Antitumor Activity Targeting HIF-1 Pathway. J. Ginseng Res. 2022, 46, 738–749. [Google Scholar] [CrossRef]
- Seki, H.; Tamura, K.; Muranaka, T. P450s and UGTs: Key Players in the Structural Diversity of Triterpenoid Saponins. Plant Cell Physiol. 2015, 56, 1463–1471. [Google Scholar] [CrossRef]
- Zhao, J.-N.; Wang, R.-F.; Zhao, S.-J.; Wang, Z.-T. Advance in Glycosyltransferases, the Important Bioparts for Production of Diversified Ginsenosides. Chin. J. Nat. Med. 2020, 18, 643–658. [Google Scholar] [CrossRef]
- Lu, J.; Yao, L.; Li, J.-X.; Liu, S.-J.; Hu, Y.-Y.; Wang, S.-H.; Liang, W.-X.; Huang, L.-Q.; Dai, Y.-J.; Wang, J.; et al. Characterization of UDP-Glycosyltransferase Involved in Biosynthesis of Ginsenosides Rg1 and Rb1 and Identification of Critical Conserved Amino Acid Residues for Its Function. J. Agric. Food Chem. 2018, 66, 9446–9455. [Google Scholar] [CrossRef]
- Yang, C.; Li, C.; Wei, W.; Wei, Y.; Liu, Q.; Zhao, G.; Yue, J.; Yan, X.; Wang, P.; Zhou, Z. The Unprecedented Diversity of UGT94-Family UDP-Glycosyltransferases in Panax Plants and Their Contribution to Ginsenoside Biosynthesis. Sci. Rep. 2020, 10, 15394. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-C.; Kim, W.; Park, S.C.; Jeong, J.; Park, M.K.; Lim, S.; Lee, Y.; Im, W.-T.; Lee, J.H.; Choi, G.; et al. Two Ginseng UDP-Glycosyltransferases Synthesize Ginsenoside Rg3 and Rd. Plant Cell Physiol. 2014, 55, 2177–2188. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wei, Y.; Fan, Y.; Liu, Q.; Wei, W.; Yang, C.; Zhang, L.; Zhao, G.; Yue, J.; Yan, X.; et al. Production of Bioactive Ginsenosides Rh2 and Rg3 by Metabolically Engineered Yeasts. Metab. Eng. 2015, 29, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Diao, M.; Peng, L.; Huang, S.; Xie, N. Characterization of a Group of UDP-Glycosyltransferases Involved in the Biosynthesis of Triterpenoid Saponins of Panax notoginseng. ACS Synth. Biol. 2022, 11, 770–779. [Google Scholar] [CrossRef]
- Wei, W.; Wang, P.; Wei, Y.; Liu, Q.; Yang, C.; Zhao, G.; Yue, J.; Yan, X.; Zhou, Z. Characterization of Panax ginseng UDP-Glycosyltransferases Catalyzing Protopanaxatriol and Biosyntheses of Bioactive Ginsenosides F1 and Rh1 in Metabolically Engineered Yeasts. Mol. Plant 2015, 8, 1412–1424. [Google Scholar] [CrossRef]
- Zhang, H.; Hua, X.; Zheng, D.; Wu, H.; Li, C.; Rao, P.; Wen, M.; Choi, Y.-E.; Xue, Z.; Wang, Y.; et al. De Novo Biosynthesis of Oleanane-Type Ginsenosides in Saccharomyces cerevisiae Using Two Types of Glycosyltransferases from Panax ginseng. J. Agric. Food Chem. 2022, 70, 2231–2240. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J.; Yoon, H.I.; Son, J.E. Effect of Far-Red and UV-B Light on the Growth and Ginsenoside Content of Ginseng (Panax ginseng C. A. Meyer) Sprouts Aeroponically Grown in Plant Factories. Hortic. Environ. Biotechnol. 2022, 63, 77–87. [Google Scholar] [CrossRef]
- Gao, D.; Kim, J.H.; Vinh, L.B.; Seo, E.-Y.; Yang, S.Y.; Cho, C.W.; Kim, Y.H.; Kim, K.T.; Sim, J.; Kang, J.S. Effect of Citric Acid and Heat Treatment on the Content of Less-Polar Ginsenosides in Flower Buds of Panax ginseng. Prep. Biochem. Biotechnol. 2022, 52, 144–153. [Google Scholar] [CrossRef]
- Attele, A.S.; Wu, J.A.; Yuan, C.S. Ginseng Pharmacology: Multiple Constituents and Multiple Actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- Li, X.; Yan, Y.Z.; Jin, X.; Kim, Y.K.; Uddin, M.R.; Kim, Y.B.; Bae, H.; Kim, Y.C.; Lee, S.W.; Park, S.U. Ginsenoside Content in The Leaves and Roots of Panax ginseng at Different Ages. Life Sci. J. 2012, 9, 679–683. [Google Scholar]
- Murthy, H.N.; Georgiev, M.I.; Kim, Y.-S.; Jeong, C.-S.; Kim, S.-J.; Park, S.-Y.; Paek, K.-Y. Ginsenosides: Prospective for Sustainable Biotechnological Production. Appl. Microbiol. Biotechnol. 2014, 98, 6243–6254. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhong, J.J. Production of Ginseng and Its Bioactive Components in Plant Cell Culture: Current Technological and Applied Aspects. J. Biotechnol. 1999, 68, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Wong, K.; Ho, K.P.; Zhou, L.G. Enhancement of Saponin Production in Panax ginseng Cell Culture by Osmotic Stress and Nutrient Feeding. Enzym. Microb. Technol. 2005, 36, 133–138. [Google Scholar] [CrossRef]
- Paek, K.-Y.; Murthy, H.N.; Hahn, E.-J.; Zhong, J.-J. Large Scale Culture of Ginseng Adventitious Roots for Production of Ginsenosides. In Biotechnology in China I: From Bioreaction to Bioseparation and Bioremediation; Zhong, J.J., Bai, F.W., Zhang, W., Eds.; Springer: Berlin, Germany, 2009; Volume 113, pp. 151–176. ISBN 978-3-540-88414-9. [Google Scholar]
- Baque, M.A.; Moh, S.-H.; Lee, E.-J.; Zhong, J.-J.; Paek, K.-Y. Production of Biomass and Useful Compounds from Adventitious Roots of High-Value Added Medicinal Plants Using Bioreactor. Biotechnol. Adv. 2012, 30, 1255–1267. [Google Scholar] [CrossRef]
- Gao, X.F.; Zhu, C.B.; Jia, W.; Gao, W.Y.; Qiu, M.F.; Zhang, Y.Y.; Xiao, P.G. Induction and Characterization of Adventitious Roots Directly from the Explants of Panax notoginseng. Biotechnol. Lett. 2005, 27, 1771–1775. [Google Scholar] [CrossRef]
- Mathur, A.; Gangwar, A.; Mathur, A.K.; Verma, P.; Uniyal, G.C.; Lal, R.K. Growth Kinetics and Ginsenosides Production in Transformed Hairy Roots of American Ginseng-Panax quinquefolium L. Biotechnol. Lett. 2010, 32, 457–461. [Google Scholar] [CrossRef]
- Gutierrez-Valdes, N.; Hakkinen, S.T.; Lemasson, C.; Guillet, M.; Oksman-Caldentey, K.-M.; Ritala, A.; Cardon, F. Hairy Root Cultures-A Versatile Tool with Multiple Applications. Front. Plant Sci. 2020, 11, 33. [Google Scholar] [CrossRef]
- Palazón, J.; Mallol, A.; Eibl, R.; Lettenbauer, C.; Cusidó, R.M.; Piñol, M.T. Growth and Ginsenoside Production in Hairy Root Cultures of Panax ginseng Using a Novel Bioreactor. Planta Med. 2003, 69, 344–349. [Google Scholar] [CrossRef]
- Zhao, B.; Agblevor, F.A.; Jelesko, J.G. Enhanced Production of Hairy Root Metabolites Using Microbubble Generator. Plant Cell Tissue Organ Cult. 2014, 117, 157–165. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Yeung, E.C.; Hahn, E.-J.; Paek, K.-Y. Combined Effects of Phytohormone, Indole-3-Butyric Acid, and Methyl Jasmonate on Root Growth and Ginsenoside Production in Adventitious Root Cultures of Panax ginseng CA Meyer. Biotechnol. Lett. 2007, 29, 1789–1792. [Google Scholar] [CrossRef]
- Wang, J.; Gao, W.; Zuo, B.; Zhang, L.; Huang, L. Effect of Methyl Jasmonate on the Ginsenoside Content of Panax ginseng Adventitious Root Cultures and on the Genes Involved in Triterpene Biosynthesis. Res. Chem. Intermed. 2013, 39, 1973–1980. [Google Scholar] [CrossRef]
- Kim, O.T.; Bang, K.H.; Kim, Y.C.; Hyun, D.Y.; Kim, M.Y.; Cha, S.W. Upregulation of Ginsenoside and Gene Expression Related to Triterpene Biosynthesis in Ginseng Hairy Root Cultures Elicited by Methyl Jasmonate. Plant Cell Tissue Organ Cult. 2009, 98, 25–33. [Google Scholar] [CrossRef]
- Wang, W.; Zhong, J.J. Manipulation of Ginsenoside Heterogeneity in Cell Cultures of Panax notoginseng by Addition of Jasmonates. J. Biosci. Bioeng. 2002, 93, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.-X.; Zhong, J.-J. Role of Jasmonic Acid in Alteration of Ginsenoside Heterogeneity in Elicited Cell Cultures of Panax notoginseng. J. Biosci. Bioeng. 2007, 104, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Thanh, N.T.; Murthy, H.N.; Yu, K.W.; Hahn, E.J.; Paek, K.Y. Methyl Jasmonate Elicitation Enhanced Synthesis of Ginsenoside by Cell Suspension Cultures of Panax ginseng in 5-l Balloon Type Bubble Bioreactors. Appl. Microbiol. Biotechnol. 2005, 67, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Zhong, X.; Hu, M.; Lu, L.; Deng, Z.; Liu, T. In Vitro Reconstitution of Mevalonate Pathway and Targeted Engineering of Farnesene Overproduction in Escherichia coli. Biotechnol. Bioeng. 2014, 111, 1396–1405. [Google Scholar] [CrossRef]
- Song, Y.; Guan, Z.; van Merkerk, R.; Pramastya, H.; Abdallah, I.I.; Setroikromo, R.; Quax, W.J. Production of Squalene in Bacillus Subtilis by Squalene Synthase Screening and Metabolic Engineering. J. Agric. Food Chem. 2020, 68, 4447–4455. [Google Scholar] [CrossRef]
- Li, D.; Wu, Y.; Zhang, C.; Sun, J.; Zhou, Z.; Lu, W. Production of Triterpene Ginsenoside Compound K in the Non-Conventional Yeast Yarrowia lipolytica. J. Agric. Food Chem. 2019, 67, 2581–2588. [Google Scholar] [CrossRef]
- Li, X.; Sun, L.; Zhao, D. Current Status and Problem-Solving Strategies for Ginseng Industry. Chin. J. Integr. Med. 2019, 25, 883–886. [Google Scholar] [CrossRef]
- Wang, D.; Wang, J.; Shi, Y.; Li, R.; Fan, F.; Huang, Y.; Li, W.; Chen, N.; Huang, L.; Dai, Z.; et al. Elucidation of the Complete Biosynthetic Pathway of the Main Triterpene Glycosylation Products of Panax notoginseng Using a Synthetic Biology Platform. Metab. Eng. 2020, 61, 131–140. [Google Scholar] [CrossRef]
- Nan, W.; Zhao, F.; Zhang, C.; Ju, H.; Lu, W. Promotion of Compound K Production in Saccharomyces cerevisiae by Glycerol. Microb. Cell. Fact. 2020, 19, 41. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, J.; Zhao, G.; Yan, X.; Zhou, Z. Systematic Optimization of the Yeast Cell Factory for Sustainable and High Efficiency Production of Bioactive Ginsenoside Compound K. Synth. Syst. Biotechnol. 2021, 6, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Pandey, R.P.; Jung, H.Y.; Chu, L.L.; Park, Y.I.; Sohng, J.K. In Vitro Single-Vessel Enzymatic Synthesis of Novel Resvera-A Glucosides. Carbohydr. Res. 2016, 424, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Gao, H.; Liu, R.; Xia, M.; Lu, Y.; Wang, J.; Chen, X.; Zhang, Y.; Li, D.; Tong, Y.; et al. Key Glycosyltransferase Genes of Panax notoginseng: Identification and Engineering Yeast Construction of Rare Ginsenosides. ACS Synth. Biol. 2022, 11, 2394–2404. [Google Scholar] [CrossRef]
- Jiang, F.; Zhou, C.; Li, Y.; Deng, H.; Gong, T.; Chen, J.; Chen, T.; Yang, J.; Zhu, P. Metabolic Engineering of Yeasts for Green and Sustainable Production of Bioactive Ginsenosides F2 and 3β,20S-Di-O-Glc-DM. Acta Pharm. Sin. B 2022, 12, 3167–3176. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yang, G.-Y.; Chen, X.; Liu, Q.; Zhang, X.; Deng, Z.; Feng, Y. Biosynthesis of Plant-Derived Ginsenoside Rh2 in Yeast via Repurposing a Key Promiscuous Microbial Enzyme. Metab. Eng. 2017, 42, 25–32. [Google Scholar] [CrossRef]
- Wang, P.; Wei, W.; Ye, W.; Li, X.; Zhao, W.; Yang, C.; Li, C.; Yan, X.; Zhou, Z. Synthesizing Ginsenoside Rh2 in Saccharomyces cerevisiae Cell Factory at High-Efficiency. Cell Discov. 2019, 5, 5. [Google Scholar] [CrossRef]
- Dai, L.; Liu, C.; Li, J.; Dong, C.; Yang, J.; Dai, Z.; Zhang, X.; Sun, Y. One-Pot Synthesis of Ginsenoside Rh2 and Bioactive Unnatural Ginsenoside by Coupling Promiscuous Glycosyltransferase from Bacillus subtilis 168 to Sucrose Synthase. J. Agric. Food Chem. 2018, 66, 2830–2837. [Google Scholar] [CrossRef]
- Ma, W.; Zhao, L.; Ma, Y.; Li, Y.; Qin, S.; He, B. Oriented Efficient Biosynthesis of Rare Ginsenoside Rh2 from PPD by Compiling UGT-Yjic Mutant with Sucrose Synthase. Int. J. Biol. Macromol. 2020, 146, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y. Biotransformation of Ginsenoside Rb1 to Gyp-XVII and Minor Ginsenoside Rg3 by Endophytic Bacterium Flavobacterium Sp. GE 32 Isolated from Panax ginseng. Lett. Appl. Microbiol. 2019, 68, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.R.; Choi, K.J.; Suzuki, K.; Suzuki, Y. Enzymatic Preparation of Ginsenosides Rg2, Rh1, and F1. Chem. Pharm. Bull. 2003, 51, 404–408. [Google Scholar] [CrossRef]
- Ko, S.-R.; Suzuki, Y.; Suzuki, K.; Choi, K.-J.; Cho, B.-G. “Enzymatic Preparation of Minor Saponins and Intestinal Bacterial Saponin Metabolites of Ginseng.: Part III”.: Marked Production of Ginsenosides Rd, F2, Rg3, and Compound K by Enzymatic Method. Chem. Pharm. Bull. 2007, 55, 1522–1527. [Google Scholar] [CrossRef]
- Noh, K.-H.; Son, J.-W.; Kim, H.-J.; Oh, D.-K. Ginsenoside Compound K Production from Ginseng Root Extract by a Thermostable β-Glycosidase from Sulfolobus solfataricus. Biosci. Biotechnol. Biochem. 2009, 73, 316–321. [Google Scholar] [CrossRef]
- Yoo, M.-H.; Yeom, S.-J.; Park, C.-S.; Lee, K.-W.; Oh, D.-K. Production of Aglycon Protopanaxadiol via Compound K by a Thermostable β-Glycosidase from Pyrococcus furiosus. Appl. Microbiol. Biotechnol. 2011, 89, 1019–1028. [Google Scholar] [CrossRef]
- Wu, L.; Bai, L.; Dai, W.; Wu, Y.; Xi, P.; Zheng, L.; Zhang, J. Ginsenoside Rg3: A Review of Its Anticancer Mechanisms and Potential Therapeutic Applications. Curr. Top. Med. Chem. 2024, 24, 869–884. [Google Scholar] [CrossRef]
- Liang, L.-D.; He, T.; Du, T.-W.; Fan, Y.-G.; Chen, D.-S.; Wang, Y. Ginsenoside-Rg5 Induces Apoptosis and DNA Damage in Human Cervical Cancer Cells. Mol. Med. Rep. 2015, 11, 940–946. [Google Scholar] [CrossRef]
- Paik, S.; Song, G.Y.; Jo, E.-K. Ginsenosides for Therapeutically Targeting Inflammation through Modulation of Oxidative Stress. Int. Immunopharmacol. 2023, 121, 110461. [Google Scholar] [CrossRef]
- Feng, S.; Li, T.; Wei, X.; Zheng, Y.; Zhang, Y.; Li, G.; Zhao, Y. The Antioxidant and Anti-Fatigue Effects of Rare Ginsenosides and γ-Aminobutyric Acid in Fermented Ginseng and Germinated Brown Rice Puree. Int. J. Mol. Sci. 2024, 25, 10359. [Google Scholar] [CrossRef] [PubMed]
- Arring, N.M.; Millstine, D.; Marks, L.A.; Nail, L.M. Ginseng as a Treatment for Fatigue: A Systematic Review. J. Altern. Complement. Med. 2018, 24, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Balusamy, S.R.; Perumalsamy, H.; Huq, M.A.; Yoon, T.H.; Mijakovic, I.; Thangavelu, L.; Yang, D.C.; Rahimi, S. A Comprehensive and Systemic Review of Ginseng-Based Nanomaterials: Synthesis, Targeted Delivery, and Biomedical Applications. Med. Res. Rev. 2023, 43, 1374–1410. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-H.; Chung, K.-S.; Choi, J.-H.; Kim, D.-H.; Lee, K.-T. Compound K, a Metabolite of Ginseng Saponin, Induces Apoptosis via Caspase-8-Dependent Pathway in HL-60 Human Leukemia Cells. BMC Cancer 2009, 9, 449. [Google Scholar] [CrossRef] [PubMed]
- Han, G.C.; Ko, S.K.; Sung, J.H.; Chung, S.H. Compound K Enhances Insulin Secretion with Beneficial Metabolic Effects in Db/Db Mice. J. Agric. Food Chem. 2007, 55, 10641–10648. [Google Scholar] [CrossRef]
- Park, E.K.; Shin, Y.W.; Lee, H.U.; Kim, S.S.; Lee, Y.C.; Lee, B.Y.; Kim, D.H. Inhibitory Effect of Ginsenoside Rb1 and Compound K on NO and Prostaglandin E2 Biosyntheses of RAW264.7 Cells Induced by Lipopolysaccharide. Biol. Pharm. Bull. 2005, 28, 652–656. [Google Scholar] [CrossRef]
- Shin, Y.W.; Bae, E.A.; Kim, S.S.; Lee, Y.C.; Kim, D.H. Effect of Ginsenoside Rb1 and Compound K in Chronic Oxazolone-Induced Mouse Dermatitis. Int. Immunopharmacol. 2005, 5, 1183–1191. [Google Scholar] [CrossRef]
- Jeong, A.; Lee, H.-J.; Jeong, S.-J.; Lee, H.-J.; Lee, E.-O.; Bae, H.; Kim, S.-H. Compound K Inhibits Basic Fibroblast Growth Factor-Induced Angiogenesis via Regulation of P38 Mitogen Activated Protein Kinase and AKT in Human Umbilical Vein Endothelial Cells. Biol. Pharm. Bull. 2010, 33, 945–950. [Google Scholar] [CrossRef]
- Kim, S.; Kang, B.Y.; Cho, S.Y.; Sung, D.S.; Chang, H.K.; Yeom, M.H.; Kim, D.H.; Sim, Y.C.; Lee, Y.S. Compound K Induces Expression of Hyaluronan Synthase 2 Gene in Transformed Human Keratinocytes and Increases Hyaluronan in Hairless Mouse Skin. Biochem. Biophys. Res. Commun. 2004, 316, 348–355. [Google Scholar] [CrossRef]
- Park, J.-S.; Shin, J.A.; Jung, J.-S.; Hyun, J.-W.; Le, T.K.V.; Kim, D.-H.; Park, E.-M.; Kim, H.-S. Anti-Inflammatory Mechanism of Compound K in Activated Microglia and Its Neuroprotective Effect on Experimental Stroke in Mice. J. Pharmacol. Exp. Ther. 2012, 341, 59–67. [Google Scholar] [CrossRef]
- Shieh, P.-C.; Tsao, C.-W.; Li, J.-S.; Wu, H.-T.; Wen, Y.-J.; Kou, D.-H.; Cheng, J.-T. Role of Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) in the Action of Ginsenoside Rh2 against Beta-Amyloid-Induced Inhibition of Rat Brain Astrocytes. Neurosci. Lett. 2008, 434, 1–5. [Google Scholar] [CrossRef]
- Niu, C.-S.; Yeh, C.-H.; Yeh, M.-F.; Cheng, J.-T. Increase of Adipogenesis by Ginsenoside (Rh2) in 3T3-L1 Cell via an Activation of Glucocorticoid Receptor. Horm. Metab. Res. 2009, 41, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-T.; Kim, S.-H.; Lee, M.-S.; Kim, S.H.; Yang, H.-J.; Kim, M.-J.; Kim, H.-S.; Ha, J.; Kim, M.S.; Kwon, D.Y. Anti-Obesity Effects of Ginsenoside Rh2 Are Associated with the Activation of AMPK Signaling Pathway in 3T3-L1 Adipocyte. Biochem. Biophys. Res. Commun. 2007, 364, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Park, E.-M.; Kim, D.-H.; Jung, K.; Jung, J.-S.; Lee, E.-J.; Hyun, J.-W.; Kang, J.L.; Kim, H.-S. Anti-Inflammatory Mechanism of Ginseng Saponins in Activated Microglia. J. Neuroimmunol. 2009, 209, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, T.W.; Singh, S.V. Ginsenoside Rh2-Mediated G1 Phase Cell Cycle Arrest in Human Breast Cancer Cells Is Caused by P15 Ink4B and p27 Kip1-dependent inhibition of cyclin-dependent kinases. Pharm. Res. 2009, 26, 2280–2288. [Google Scholar] [CrossRef]
- Liu, J.; Shimizu, K.; Yu, H.; Zhang, C.; Jin, F.; Kondo, R. Stereospecificity of Hydroxyl Group at C-20 in Antiproliferative Action of Ginsenoside Rh2 on Prostate Cancer Cells. Fitoterapia 2010, 81, 902–905. [Google Scholar] [CrossRef]
- Hou, J.; Cui, C.; Kim, S.; Sung, C.; Choi, C. Ginsenoside F1 Suppresses Astrocytic Senescence-Associated Secretory Phenotype. Chem.-Biol. Interact. 2018, 283, 75–83. [Google Scholar] [CrossRef]
- Qin, M.; Luo, Y.; Lu, S.; Sun, J.; Yang, K.; Sun, G.; Sun, X. Ginsenoside F1 Ameliorates Endothelial Cell Inflammatory Injury and Prevents Atherosclerosis in Mice through A20-Mediated Suppression of NF-κB Signaling. Front. Pharmacol. 2017, 8, 953. [Google Scholar] [CrossRef]
- Li, B.; Qu, G. Inhibition of the Hypoxia-Induced Factor-1α and Vascular Endothelial Growth Factor Expression through Ginsenoside Rg3 in Human Gastric Cancer Cells. J. Cancer Res. Ther. 2019, 15, 1642–1646. [Google Scholar] [CrossRef]
- Sun, M.-Y.; Song, Y.-N.; Zhang, M.; Zhang, C.-Y.; Zhang, L.-J.; Zhang, H. Ginsenoside Rg3 Inhibits the Migration and Invasion of Liver Cancer Cells by Increasing the Protein Expression of ARHGAP9. Oncol. Lett. 2019, 17, 965–973. [Google Scholar] [CrossRef]
- Song, M.; Jia, F.; Cao, Z.; Zhang, H.; Liu, M.; Gao, L. Ginsenoside Rg3 Attenuates Aluminum-Induced Osteoporosis Through Regulation of Oxidative Stress and Bone Metabolism in Rats. Biol. Trace Elem. Res. 2020, 198, 557–566. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, D. The Preparation of Ginsenoside Rg5, Its Antitumor Activity against Breast Cancer Cells and Its Targeting of PI3K. Nutrients 2020, 12, 246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, A.; Feng, J.; Zhang, Q.; Liu, L.; Ren, H. Ginsenoside Rg5 Induces Apoptosis in Human Esophageal Cancer Cells through the Phosphoinositide-3 Kinase/Protein Kinase B Signaling Pathway. Mol. Med. Rep. 2019, 19, 4019–4026. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-F.; Zhang, J.-J.; Gong, X.-J.; Li, K.-K.; Zhang, L.-X.; Li, W. Ginsenoside Rg5: A Review of Anticancer and Neuroprotection with Network Pharmacology Approach. Am. J. Chin. Med. 2022, 50, 2033–2056. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Duan, Z.; Zhu, C.; Deng, J.; Fan, D. Anti-Anemia Effects of Ginsenoside Rk3 and Ginsenoside Rh4 on Mice with Ribavirin-Induced Anemia. Food Funct. 2018, 9, 2447–2455. [Google Scholar] [CrossRef]
- Duan, Z.; Wei, B.; Deng, J.; Mi, Y.; Dong, Y.; Zhu, C.; Fu, R.; Qu, L.; Fan, D. The Anti-Tumor Effect of Ginsenoside Rh4 in MCF-7 Breast Cancer Cells in Vitro and in Vivo. Biochem. Biophys. Res. Commun. 2018, 499, 482–487. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Kim, J.-E.; Song, G.-Y.; Bae, J.-S. Rgx365, a Rare Protopanaxatriol-Type Ginsenoside Fraction from Black Ginseng, Suppresses Inflammatory Gene iNOS via the Iinhibition of p-STAT-1 and NF-κB. Am. J. Chin. Med. 2020, 48, 1091–1102. [Google Scholar] [CrossRef]
- Yu, Q.; Zeng, K.-W.; Ma, X.-L.; Jiang, Y.; Tu, P.-F.; Wang, X.-M. Ginsenoside Rk1 Suppresses Pro-Inflammatory Responses in Lipopolysaccharide-Stimulated RAW264.7 Cells by Inhibiting the Jak2/Stat3 Pathway. Chin. J. Nat. Med. 2017, 15, 751–757. [Google Scholar] [CrossRef]
- Hu, J.-N.; Xu, X.-Y.; Li, W.; Wang, Y.-M.; Liu, Y.; Wang, Z.; Wang, Y.-P. Ginsenoside Rk1 Ameliorates Paracetamol-Induced Hepatotoxicity in Mice through Inhibition of Inflammation, Oxidative Stress, Nitrative Stress and Apoptosis. J. Ginseng Res. 2019, 43, 10–19. [Google Scholar] [CrossRef]
- Hu, M.; Yang, J.; Qu, L.; Deng, X.; Duan, Z.; Fu, R.; Liang, L.; Fan, D. Ginsenoside Rk1 Induces Apoptosis and Downregulates the Expression of PD-L1 by Targeting the NF-κB Pathway in Lung Adenocarcinoma. Food Funct. 2020, 11, 456–471. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, J.; Fu, R.; Zhu, C.; Fan, D. The Ginsenoside Rk3 Exerts Anti-Esophageal Cancer Activity in Vitro and in Vivo by Mediating Apoptosis and Autophagy through Regulation of the PI3K/Akt/mTOR Pathway. PLoS ONE 2019, 14, e0216759. [Google Scholar] [CrossRef]
- Tian, M.; Ma, P.; Zhang, Y.; Mi, Y.; Fan, D. Ginsenoside Rk3 Alleviated DSS-Induced Ulcerative Colitis by Protecting Colon Barrier and Inhibiting NLRP3 Inflammasome Pathway. Int. Immunopharmacol. 2020, 85, 106645. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, S.; Kim, J.; Mijakovic, I.; Jung, K.-H.; Choi, G.; Kim, S.-C.; Kim, Y.-J. Triterpenoid-Biosynthetic UDP-Glycosyltransferases from Plants. Biotechnol. Adv. 2019, 37, 107394. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wang, B.; Liu, Y.; Shi, M.; Wang, D.; Zhang, X.; Liu, T.; Huang, L.; Zhang, X. Producing Aglycons of Ginsenosides in Bakers’ Yeast. Sci. Rep. 2014, 4, 3698. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Valappil, A.K.; Mathiyalagan, R.; Tran, T.N.A.; Ramadhania, Z.M.; Awais, M.; Yang, D.C. In Vitro Cultivation and Ginsenosides Accumulation in Panax ginseng: A Review. Plants 2023, 12, 3165. [Google Scholar] [CrossRef]
- Chai, N.; Xu, J.; Zhang, R.; Li, G.; Wen, J.; Su, L.; Xue, Y.; Li, T.; Liu, J.; Zeng, D.; et al. Synthetic Metabolic Engineering of Functional Crops: Boosting Nutrition and Human Health. Crop J. 2025. [Google Scholar] [CrossRef]
- Zhu, Q.; Tan, J.; Liu, Y.-G. Molecular Farming Using Transgenic Rice Endosperm. Trends Biotechnol. 2022, 40, 1248–1260. [Google Scholar] [CrossRef]
- Zhu, Q.; Yu, S.; Zeng, D.; Liu, H.; Wang, H.; Yang, Z.; Xie, X.; Shen, R.; Tan, J.; Li, H.; et al. Development of “Purple Endosperm Rice” by Engineering Anthocyanin Biosynthesis in the Endosperm with a High-Efficiency Transgene Stacking System. Mol. Plant 2017, 10, 918–929. [Google Scholar] [CrossRef]
- Zhu, Q.; Zeng, D.; Yu, S.; Cui, C.; Li, J.; Li, H.; Chen, J.; Zhang, R.; Zhao, X.; Chen, L.; et al. From Golden Rice to aSTARice: Bioengineering Astaxanthin Biosynthesis in Rice Endosperm. Mol. Plant 2018, 11, 1440–1448. [Google Scholar] [CrossRef]
- Chen, K.; Sun, K.; Ye, C.; Liu, Y.; Hu, Y.; Jia, B.; Jiang, S.; Zou, Y.; Cao, R.; Guo, J.; et al. Metabolic Engineering of Theanine Biosynthesis in Rice Endosperm to Develop Biofortified theaRice for Benefiting Human Health. Plant Commun. 2025, 6, 101411. [Google Scholar] [CrossRef]
- Zhao, M.-L.; Li, X.-Y.; Lan, C.-X.; Yuan, Z.-L.; Zhao, J.-L.; Huang, Y.; Hu, Z.-L.; Jia, B. Promoting Photosynthetic Production of Dammarenediol-II in Chlamydomonas reinhardtii via Gene Loading and Culture Optimization. Int. J. Mol. Sci. 2023, 24, 11002. [Google Scholar] [CrossRef]
- Hu, Q.-R.; Hong, H.; Zhang, Z.-H.; Feng, H.; Luo, T.; Li, J.; Deng, Z.-Y.; Chen, F. Methods on Improvements of the Poor Oral Bioavailability of Ginsenosides: Pre-Processing, Structural Modification, Drug Combination, and Micro- or Nano- Delivery System. J. Ginseng Res. 2023, 47, 694–705. [Google Scholar] [CrossRef]
- Gwak, Y.S.; Han, J.Y.; Choi, Y.E. Production of Ginsenoside Aglycone (Protopanaxatriol) and Male Sterility of Transgenic Tobacco Co-Overexpressing Three Panax ginseng Genes: PgDDS, CYP716A47, and CYP716A53v2. J. Ginseng Res. 2019, 43, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Liu, T.; Chai, N.; Zeng, D.; Zhang, R.; Wu, Y.; Hang, J.; Liu, Y.; Deng, Q.; Tan, J.; et al. PhieDBEs: A DBD-Containing, PAM-Flexible, High-Efficiency Dual Base Editor Toolbox with Wide Targeting Scope for Use in Plants. Plant Biotechnol. J. 2024, 22, 3164–3174. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zeng, D.; Zheng, Z.; Lin, Z.; Xue, Y.; Li, T.; Xie, X.; Ma, G.; Liu, Y.-G.; Zhu, Q. The ScCas9++ Variant Expands the CRISPR Toolbox for Genome Editing in Plants. J. Integr. Plant Biol. 2021, 63, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, R.; Chai, N.; Su, L.; Zheng, Z.; Liu, T.; Guo, Z.; Ma, Y.; Xie, Y.; Xie, X.; et al. Programmable Genome Engineering and Gene Modifications for Plant Biodesign. Plant Commun. 2025, 6, 101427. [Google Scholar] [CrossRef]
- Sun, K.; Cheng, S.; Chai, N.; Mi, J.; Zhang, R.; Qian, Q.; Zheng, Z.; Chen, K.; Zeng, D.; Peng, X.; et al. Engineering Adenine Deaminase TadA for Precise and PAM-Flexible Point Mutagenesis and Gradient-Tuning Endogenous Protein Design. Adv. Sci. 2025, 6, e06644. [Google Scholar] [CrossRef]
- Tan, J.; Sun, K.; Qian, Q.; Chai, N.; Peng, X.; Shen, M.; Zhou, D.; Liu, Y.-G.; Zhu, Q.; Liu, Q. Development of PAM-Flexible Multiplex Genome Editors for Enhancing Cold-Tolerance in Indica Rice. Crop J. 2025, 13, 991–995. [Google Scholar] [CrossRef]
- Zhang, R.; Chai, N.; Liu, T.; Zheng, Z.; Lin, Q.; Xie, X.; Wen, J.; Yang, Z.; Liu, Y.-G.; Zhu, Q. The Type V Effectors for CRISPR/Cas-Mediated Genome Engineering in Plants. Biotechnol. Adv. 2024, 74, 108382. [Google Scholar] [CrossRef]
- Nielsen, J. Synthetic Biology for Engineering Acetyl Coenzyme A Metabolism in Yeast. mBio 2014, 5, e02153-14. [Google Scholar] [CrossRef]
- Sun, Z.; Meng, H.; Li, J.; Wang, J.; Li, Q.; Wang, Y.; Zhang, Y. Identification of Novel Knockout Targets for Improving Terpenoids Biosynthesis in Saccharomyces cerevisiae. PLoS ONE 2014, 9, e112615. [Google Scholar] [CrossRef]
- Gao, H.; Pei, X.; Song, X.; Wang, S.; Yang, Z.; Zhu, J.; Lin, Q.; Zhu, Q.; Yang, X. Application and Development of CRISPR Technology in the Secondary Metabolic Pathway of the Active Ingredients of Phytopharmaceuticals. Front Plant Sci. 2024, 15, 1477894. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Yang, W.; Wen, J.; Liu, W.; Zhi, S.; Li, G.; Chai, N.; Huang, J.; Xie, Y.; et al. PlantGPT: An Arabidopsis-Based Intelligent Agent That Answers Questions about Plant Functional Genomics. Adv. Sci. 2025, 12, e03926. [Google Scholar] [CrossRef]
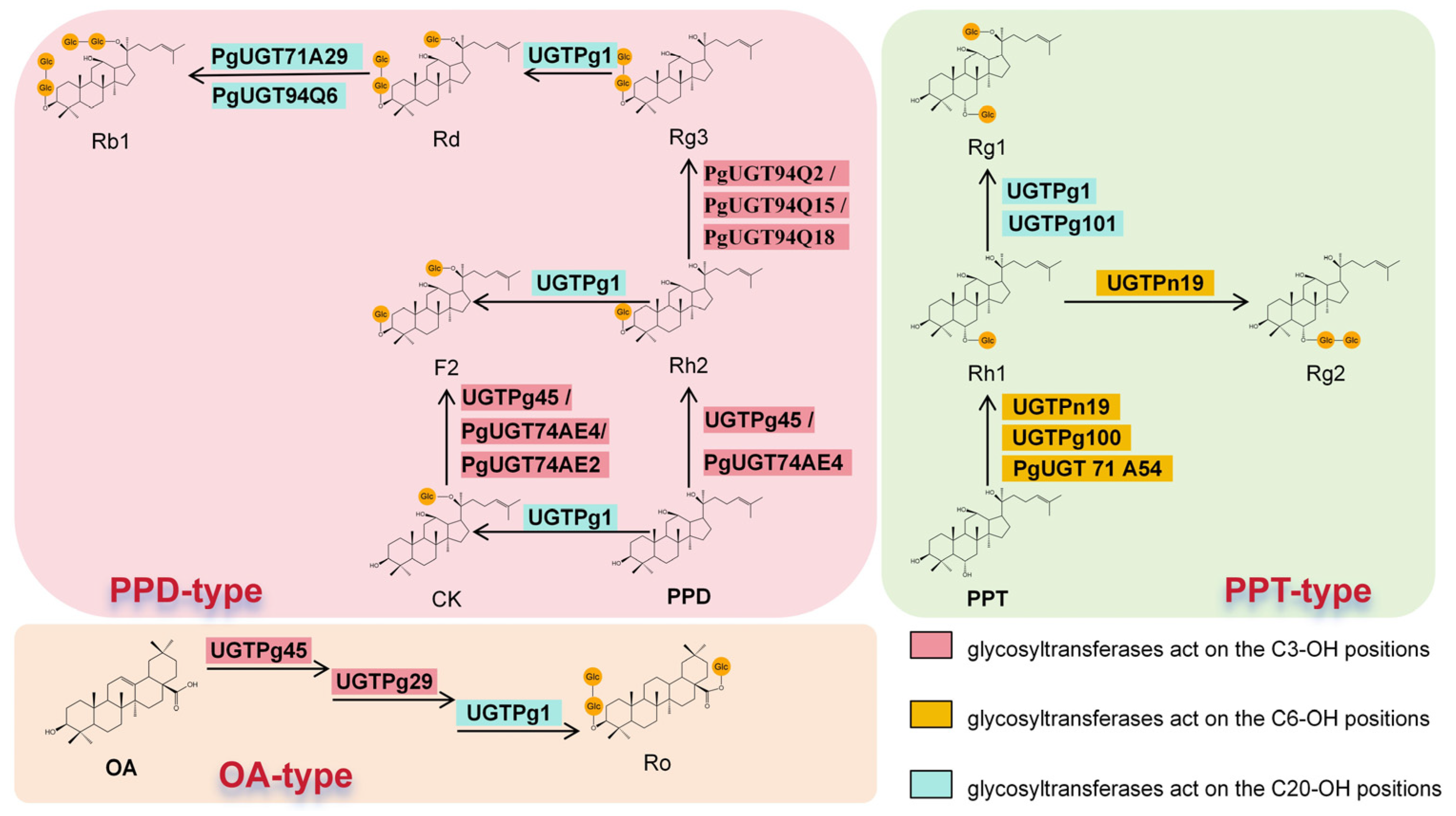

| Rare Ginsenoside | Synthetic Metabolic Engineering Strategies | Microbial Chassis | Yield | Reference |
|---|---|---|---|---|
| Ginsenoside CK | tHMGR, PPD, ATR2-1, UGTPg1 | S. cerevisiae | 1.4 mg/L | [30] |
| tHMG1, ERG9, ERG20, OpDDs, PPDDS, ATR1, UGT1 | Yarrowia lipolytica | 161.8 mg/L | [86] | |
| Synpggdds, SynPgPPDS, VvCPR, Pn3-29, PGM1, PGM2, UGP1 | S. cerevisiae | 1.17 g/L | [87] | |
| PGM2, UGP1, UGT1 | S. cerevisiae | 1.7 g/L | [88] | |
| tHMG1, PgCPR1, ERG1, ERG20, ERG9, PgDDS, PgPPDS, ERG12, ERG13, ERG8, ERG19, IDI, ERG10, PgUGT1, UGP1, PGM2, YNK1, ALG5 | S. cerevisiae | 5.74 g/L | [89] | |
| Ginsenoside Rg2 | CYP716A53v2, PgUGT71A54, PgURT94, RHM | S. cerevisiae | 1.3 g/L | [45] |
| Ginsenoside Rg3 | PgUGT74AE2, PgUGT94Q2 | S. cerevisiae | 1.3 mg/L | [56] |
| PgDDS, PgPPDS, ATR2.1, tHMG1, ERG20, PgERG1, ERG9, PgUGT45, PgUGT29 | S. cerevisiae | 3.49 μmol/g (DCW) | [90] | |
| PnUGT33 | S. cerevisiae | 51 mg/L | [91] | |
| Ginsenoside F1 | ERG20, ERG1, ERG9, tHMG1, CYP716A53v2, PgCPR1, UGTPg100 | S. cerevisiae | 42.1 mg/L | [60] |
| Ginsenoside F2 | PgDS, tHMG1, PgPPDS, IDI, AtATR2, PgUGT1, UGT74AE2, ERG20, ERG9, ERG1, ERG7, HAC1, PGM1, PGM2, UGP1 | S. cerevisiae | 21.0 mg/L | [92] |
| Ginsenoside Rh1 | ERG20, PgERG1, ERG9, tHMG1, CYP716A53v2, PgCPR1, UGTPg100 | S. cerevisiae | 98.2 mg/L | [60] |
| Ginsenoside Rh2 | ERG20, PgERG1, ERG9, tHMG1, M7-1, PGM.1, UGP1, PgPPDS | S. cerevisiae | 300 mg/L | [93] |
| PgDDS, synPgPPDS, ATR2.1, tHMG1, ERG20, ERG1, ERG9, UGT45 | S. cerevisiae | 1.45 μmol/g (DCW) | [90] | |
| ERG20, ERG9, ERG1, tHMG1, DS, CYP716A47-ATR1, RG12, ERG13, ERG19, ERG8, IDI, ERG1, tHMG1, UGTPn17, | S. cerevisiae | 354.69 mg/L | [61] | |
| tHMG1, synPgCPR1, ERG1, ERG20, ERG9, synDD, SynPPDS | S. cerevisiae | 2.25 g/L | [94] | |
| AtSuSy | E. coli | 0.20 g/L | [95] | |
| AtSuSy, M315F | E. coli | 3.7 g/L | [96] | |
| Ginsenoside Re | CYP716A53v2, PgUGT71A53, PgUGT71A54, PgURT94, RHM | S. cerevisiae | 3.6 g/L | [12] |
| Ginsenoside Ro | GgbAS, MtCPR, MtCYP716A12, tHMG1, ERG9, ERG1 | S. cerevisiae | 528.0 ± 18.0 mg/L | [42] |
| Rare Ginsenosides | Pharmacological Activity | Effects | Function | Reference |
|---|---|---|---|---|
| Ginsenoside CK | Anticancer | Caspase-8 plays a key role in Compound K-stimulated apoptosis via the activation of caspase-3 directly or indirectly through Bid cleavage | Inhibit the viability of HL-60 cells, andan IC50 values of 14 muM | [108] |
| Anti-diabetes | By elevating plasma adiponectin levels, hepatic glucose metabolism shifts from gluconeogenesis to glycogenesis, consequently improving insulin sensitivity and leading to upregulated expression of lipogenic genes and glucose transporters in adipose tissue | Oral glucose tolerance test (OGTT) using mice, revealed that CK improved glucose tolerance | [109] | |
| Anti-inflammatory | Inhibited prostaglandin E2, inducible NO synthase (iNOS) and COX-2 proteins expression in lipopolysaccharide (LPS)-induced RAW264.7 cell | Inhibited LPS-induced RAW 264.7 cells, IC50 values of 0.012 and 0.004 mM | [110] | |
| Anti-allergy | May ameliorate contact dermatitis or psoriasis by regulating COX-2 produced by macrophages cells and interferon-γ IL-5 and IL-4 induced by Th cells. | The level in interferon-γ IL-5 and IL-4 model group decreased 60–70%; 70–80%; 80–90% | [111] | |
| Anti-angiogenesis | It exerts anti-angiogenic activity by inhibiting p38 MAPK and AKT in HUVECs, with potential as a cancer chemopreventive agent. | Increased the phosphorylation level of p38(50%), blocked the AKT phosphorylation induced (10–20%). | [112] | |
| Antioxidant | Up-regulated the gene of hyaluronan synthase2 (HAS2), increased hyaluronan (HA) production in HaCaT cells. | The expression level in HaCaT cells was increased by 2.5-fold | [113] | |
| Anti-central neuroinflammatory disorders | Reduces the volume of ischemic cerebral infarction induced by middle cerebral artery occlusion and suppresses the activation of microglia in the ischemic cortex | The infarction volume was significantly reduced by 30–40%. | [114] | |
| Ginsenoside Rh2 | Anti-anxiety, anti-dementia | Can induce an increase in PACAP to activate PAC1, but not estrogen receptor, and thereby leads to attenuate Abeta-induced toxicity | The expression of PACAP and PCA1 mRNA increased by 200–250% and 180–220%. | [115] |
| Anti-obesity | Can promote preadipocytes differentiation through activating glucocorticoid receptor (GR). | The fat-producing capacity of 3T3-L1 cells was increased by 2.2 times, and the expression of adipogenic genes was upregulated by 2 to 8 times. | [116] | |
| Activation of the AMPK signaling pathway leads to the phosphorylation and inhibition of its downstream target ACC, ultimately downregulating the expression of key adipogenic transcription factors PPARγ and C/EBPα. | The level of p-AMPKα and p-ACC increased by 2.0–2.5 times and 2.0–3.0 times. | [117] | ||
| Anti-inflammatory | Significantly suppressed NF-kappaB and MAP kinase activities, which are upstream signaling molecules in inflammation. | Inhibit the degradation of IκB-α (by 70–80%) and the phosphorylation of ERK and p38 (by 60–80%) | [118] | |
| Anti-tumor | Rh2-mediated cell cycle arrest in human breast cancer cells is caused by p15 (Ink4B) and p27 (Kip1)-dependent inhibition of kinase activities of G(1)-S specific Cdks/cyclin complexes. | The expression of p27 (Kip1) protein and p15 (Ink4B) increased by 3 to 4 times and 2 to 3 times | [119] | |
| C20 may play an important role in antitumor activities. | IC50 values of 14 μM, induce 35% of cell apoptosis. | [120] | ||
| Ginsenoside F1 | Antioxidant | By inhibiting p38 MAPK-dependent NF-κB activity, it suppresses the senescence-associated secretory phenotype (SASP) in D-galactose-induced astrocytes. | The survival rate of neurons has recovered to approximately 85–90%. | [121] |
| Anti-atherosclerosis | Significantly increased A20 expression level and A20 siRNA markedly abolished the attenuation of GF1 on NF-κB nuclear translocation and inflammatory factors expression | The expression of A20 increased by 3.5 times, inhibiting approximately 70% of NF-κB pathway activations. | [122] | |
| Ginsenoside Rg3 | Anticancer | Can inhibit expression of HIF-1α and VEGF in human gastric cancer cells and may influence abdominal implantation metastasis of gastric cancer through inhibiting its expression. | Accumulation of HIF-1α protein and VEGF protein secretion lead to an inhibition rate of 60–70% | [123] |
| Effectively suppressed the migration and invasion of liver cancer cells by upregulating the protein expression of ARHGAP9. | Inhibits the migration and invasion abilities of liver cancer cells by 70–75%, and increases the expression of ARHGAP9 by 3 times. | [124] | ||
| Anti-osteoporosis | By activating the Nrf2/HO-1 signaling pathway, it enhances the body’s antioxidant capacity and alleviates oxidative stress-induced damage to bone cells. And, through activation of the Wnt/β-catenin signaling pathway, it upregulates osteogenesis- related factors (e.g., Runx2) and modulates the OPG/RANKL ratio, thereby promoting bone formation and inhibiting bone resorption. | The expression of β-catenin and Nrf2 protein in bone tissue increased by ~60–80% and 150–200%. | [125] | |
| Ginsenoside Rg5 | Anticancer | Decreased the phosphorylation levels of PI3K, Akt, mTOR, and Bad and suppressed the PI3K/Akt signaling pathway in breast cancer. | The IC50 values of 27–33 μM, induces approximately 40% cell apoptosis and inhibits approximately 50–60% of cell migration and invasion. | [126] |
| May have a tumor-suppressive effect on esophageal cancer by promoting apoptosis and may be associated with the downregulation of the PI3K/Akt signaling pathway. | The expression of p-PI3K p85 and p-Akt (Ser473) protein was inhibited by ~50–60% and 60–70%. | [127] | ||
| neuroprotective | Exerts its therapeutic effects mainly through PI3K/AKT, MAPK signaling pathways, and the regulation of apoptosis and cell cycle. | Protect neurons from damage (increase cell vitality by 30%). | [128] | |
| Ginsenoside Rh4 | Anti-anemia | Some positive regulators (EPO, erythroid transcription factor-1, and interleukin-3) related to hematopoiesis increased and some negative regulators (interferon-γ and tumor necrosis factor-α) decreased in vivo | The expression of Nrf2/HO-1 protein increased by 150–200%, inhibiting the apoptosis of bone marrow hematopoietic cells. | [129] |
| Anti-tumor | Decreasing Bcl-2, increasing Bax, and activating caspase-8, -3 and PARP. | Repressing 60% of tumor growth | [130] | |
| Ginsenoside Rs6 | Anti-inflammatory | By suppressing the activation of p-STAT-1 (phosphorylated signal transducer and activator of transcription 1) and NF-KB (nuclear factor kappa- light-chain-enhancer of activated B cells), it blocks the inflammatory signaling pathway. | Inhibit the NF-κB and STAT1 pathway by 50–60%. | [131] |
| Ginsenoside Rk1 | Anti-inflammatory | Inhibited the lipopolysaccharide-stimulated phosphorylation of NF-κB and janus kinase (Jak)2 and signal transducer and activator of transcription (Stat)3 at Ser727 and Tyr705. | The inhibition rates of p-Jak2 and p-Stat3 reached 80% and 90%. | [132] |
| Antioxidation | Due to its antioxidation, antiapoptosis, anti- inflammation, and antinitrative effects in APAP- induced hepatotoxicity. | The apoptotic index of liver tissue (TUNEL) decreased by 70–80% | [133] | |
| Anticancer | Reduced the high expression of PD-L1 in lung adenocarcinoma cells by inhibiting NF-κB signaling. | By inhibiting the NF-κB pathway (80% for p-p65), 50% of tumor growth can be suppressed. | [134] | |
| Ginsenoside Rk3 | Anticancer | Inhibit Eca109 and KYSE150 cell proliferation through activating apoptosis and autophagy by blocking the PI3K/Akt/mTOR pathway. | The volume and weight of the tumor in the body was reduced by 50–60%, and 55–65%. | [135] |
| Anti-inflammatory | Can improve DSS-induced ulcerative colitis by protecting intestinal barrier function and inhibiting NLRP3 inflammasome expression, | The length of the colon was improved by approximately 42%, tissue damage was reduced by about 60%, | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Zhang, R.; Li, T.; Deng, Q.; Luo, W.; Chang, R.; Zeng, D.; Tan, J.; Sun, T.; Liu, Y.-G.; et al. Sustainable Production of Ginsenosides: Advances in Biosynthesis and Metabolic Engineering. Plants 2025, 14, 2821. https://doi.org/10.3390/plants14182821
Xue Y, Zhang R, Li T, Deng Q, Luo W, Chang R, Zeng D, Tan J, Sun T, Liu Y-G, et al. Sustainable Production of Ginsenosides: Advances in Biosynthesis and Metabolic Engineering. Plants. 2025; 14(18):2821. https://doi.org/10.3390/plants14182821
Chicago/Turabian StyleXue, Yang, Ruixiang Zhang, Tie Li, Qindi Deng, Weidong Luo, Ruyue Chang, Dongchang Zeng, Jiantao Tan, Tianhu Sun, Yao-Guang Liu, and et al. 2025. "Sustainable Production of Ginsenosides: Advances in Biosynthesis and Metabolic Engineering" Plants 14, no. 18: 2821. https://doi.org/10.3390/plants14182821
APA StyleXue, Y., Zhang, R., Li, T., Deng, Q., Luo, W., Chang, R., Zeng, D., Tan, J., Sun, T., Liu, Y.-G., Xiang, Y., Zhu, Q., & Chai, N. (2025). Sustainable Production of Ginsenosides: Advances in Biosynthesis and Metabolic Engineering. Plants, 14(18), 2821. https://doi.org/10.3390/plants14182821







