Chemical Constituents from Euphorbia esula
Abstract
1. Introduction
2. Results
2.1. Separation and Structural Characterization for Compounds 1–11
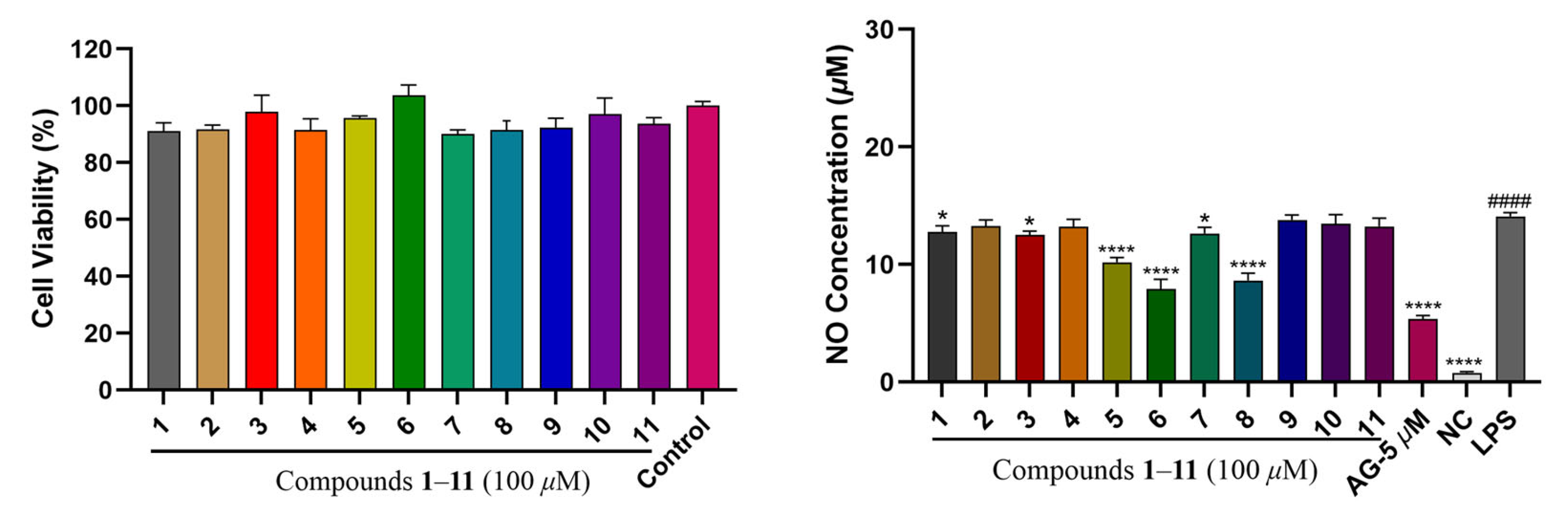
2.2. Anti-Inflammatory Activity Evaluation for Compounds 1–11
2.3. Cytotoxicity Evaluation for Compounds 1–11
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Separation
3.3.1. Methyl 4-[2-(2-Hydroxyacetyl)-1H-pyrrol-1-yl]butanoate (1)
3.3.2. (−)-Loliolide Ethyl Ether (2)
3.3.3. (−)-Loliolide (3)
3.3.4. Loliolide Acetate (4)
3.3.5. (3R)-3-Hydroxy-β-ionone (5)
3.3.6. (3S,5R,6S,7E)-5,6-Epoxy-3-hydroxy-7-megastigmen-9-one (6)
3.3.7. Blumenol A (Vomifoliol) (7)
3.3.8. (+)-Dehydrovomifoliol (8)
3.3.9. Inotopyrrole (9)
3.3.10. (5S)-5-Hydroxy-3,4-dimethyl-5-pentylfuran-2(5H)-one (10)
3.3.11. Chakyunglupulin A (11)
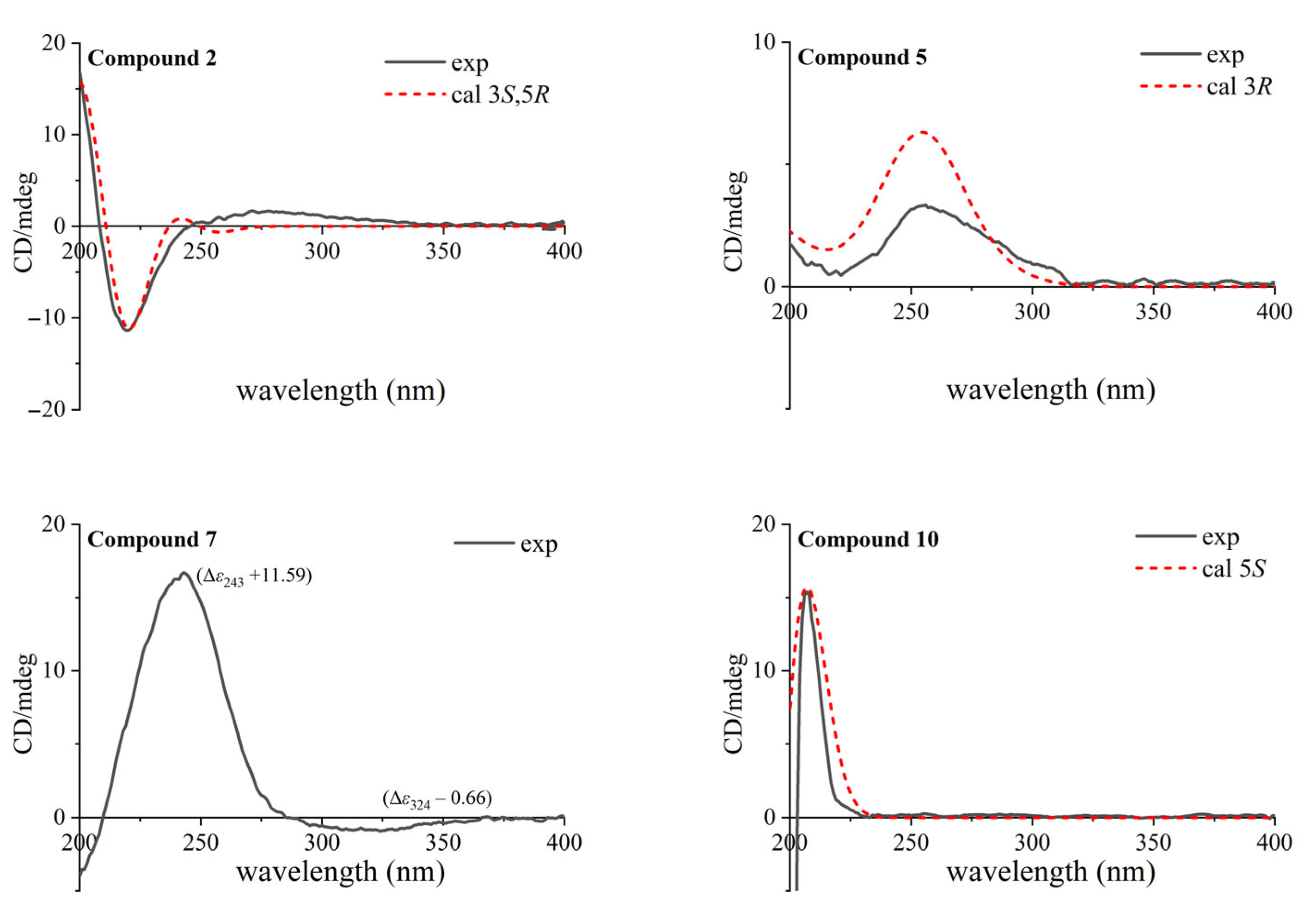
3.4. Calculations of ECD Spectra
3.5. Anti-Inflammatory Effect Assay
3.5.1. Cell Culture
3.5.2. Cell Viability Test Using the CCK-8 Method
3.5.3. NO Release Evaluation by the Griess Method
3.6. Cytotoxic Effect Assay
3.6.1. Cell Culture
3.6.2. Cell Growth Inhibition by MTT Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kgosiemang, I.K.R.; Lefojane, R.; Adegoke, A.M.; Ogunyemi, O.; Mashele, S.S.; Sekhoacha, M.P. Pharmacological significance, medicinal use, and toxicity of extracted and isolated compounds from Euphorbia species found in Southern Africa: A review. Plants 2025, 14, 469. [Google Scholar] [CrossRef]
- Webster, G.L. The saga of the spurges: A review of classification and relationships in the Euphorbiales. Bot. J. Linn. Soc. 1987, 94, 3–46. [Google Scholar] [CrossRef]
- Vasas, A.; Hohmann, J. Euphorbia diterpenes: Isolation, structure, biological activity, and synthesis (2008–2012). Chem. Rev. 2014, 114, 8579–8612. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wu, Y.; Dalal, S.; Cassera, M.B.; Yue, J.M. Euphorbesulins A-P, structurally diverse diterpenoids from Euphorbia esula. J. Nat. Prod. 2016, 79, 1952–1961. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.L.; Fan, R.Z.; Hu, R.; Luo, S.Y.; Tang, G.H.; Yin, S. Euphoresulanes A-M, structurally diverse jatrophane diterpenoids from Euphorbia esula. Bioorg. Chem. 2020, 98, 103763. [Google Scholar] [CrossRef]
- Vasas, A.; Sulyok, E.; Rédei, D.; Forgo, P.; Szabó, P.; Zupkó, I.; Berényi, A.; Molnár, J.; Hohmann, J. Jatrophane diterpenes from Euphorbia esula as antiproliferative agents and potent chemosensitizers to overcome multidrug resistance. J. Nat. Prod. 2011, 74, 1453–1461. [Google Scholar] [CrossRef]
- Hohmann, J.; Vasas, A.; Gunther, G.; Mathe, I.; Evanics, F.; Dombi, G.; Jerkovich, G. Macrocyclic diterpene polyesters of the jatrophane type from Euphorbia esula. J. Nat. Prod. 1997, 60, 331–335. [Google Scholar] [CrossRef]
- Yuan, S.H.; Zhang, Y.T.; Hua, P.; Zhou, H.H.; Xu, J.; Gu, Q. Discovery of ingenane and jatrophane diterpenoids from Euphorbia esula as inhibitors of RANKL-induced osteoclastogenesis. Fitoterapia 2020, 146, 104718. [Google Scholar] [CrossRef]
- Lu, Z.Q.; Yang, M.; Zhang, J.Q.; Chen, G.T.; Huang, H.L.; Guan, S.H.; Ma, C.; Liu, X.; Guo, D.A. Ingenane diterpenoids from Euphorbia esula. Phytochemistry 2008, 69, 812–819. [Google Scholar] [CrossRef]
- Onwukaeme, N.D.; Rowan, M.G. Jatrophane and lathyrane diterpenoid esters from north-american leafy spurge seed. Phytochemistry 1992, 31, 3479–3482. [Google Scholar] [CrossRef]
- Wagner, H.; Danninge, H.; Seligman, O.; Nogradi, M.; Farkas, L.; Farnswor, N. Isolierung von Kampferol-3-β-D-glucuronid aus Euphorbia esula L. und seine Synthese. Chem. Ber. 1970, 103, 3678–3683. [Google Scholar] [CrossRef]
- Farnsworth, N.R.; Wagner, H.; Hörhammer, L.; Hörhammer, H.P.; Fong, H.H.S. Euphorbia esula L. (Euphorbiaceae) I Preliminary phytochemical and biological evaluation. J. Pharm. Sci. 1968, 57, 933–939. [Google Scholar] [CrossRef]
- Zhao, Z.Z.; Zhang, F.; Ji, B.Y.; Zhou, N.; Chen, H.; Sun, Y.J.; Feng, W.S.; Zheng, X.K. Pyrrole alkaloids from the fruiting bodies of edible mushroom Lentinula edodes. RSC Adv. 2023, 13, 18223–18228. [Google Scholar] [CrossRef]
- Yang, H.H.; Hwangbo, K.; Zheng, M.S.; Son, J.-K.; Baek, S.H.; Choi, H.C. Inhibitory effects of (—)-loliolide on cellular senescence in human dermal fibroblasts. Arch. Pharmacal Res. 2015, 38, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Jantaharn, P.; Mongkolthanaruk, W.; Senawon, T.; Jogloy, S.; McCloskey, S. Bioactive compounds from organic extracts of Helianthus tuberosus L. flowers. Ind. Crops Prod. 2018, 119, 57–63. [Google Scholar] [CrossRef]
- Perez, C.; Trujillo, J.; Almonacid, L.N.; Trujillo, J.; Navarro, E.; Alonso, S.J. Absolute structures of two new C13-norisoprenoids from Apollonias barbujana. J. Nat. Prod. 1996, 59, 69–72. [Google Scholar] [CrossRef]
- D’Abrosca, B.; DellaGreca, M.; Fiorentino, A.; Monaco, P.; Oriano, P.; Temussi, F. Structure elucidation and phytotoxicity of C13 nor-isoprenoids from Cestrum parqui. Phytochemistry 2004, 65, 497–505. [Google Scholar] [CrossRef]
- Gonzalez, A.G.; Guillermo, J.A.; Ravelo, A.G.; Jimenez, I.A.; Gupta, M.P. 4,5-Dihydroblumenol A, a new nor-isoprenoid from Perrottetia multiflora. J. Nat. Prod. 1994, 57, 400–402. [Google Scholar] [CrossRef]
- Kai, H.; Baba, M.; Okuyama, T. Two new megastigmanes from the leaves of Cucumis sativus. Chem. Pharm. Bull. 2007, 55, 133–136. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Bai, H.B.; Shan, W.G.; Zhan, Z.J. A new alkaloid from the mycelium of Inonotus obliquus. J. Chem. Res. 2014, 38, 245–246. [Google Scholar] [CrossRef]
- Li, T.; Wang, G.-C.; Huang, X.-J.; Ye, W.-C. Whitmanoside A, a new α-pyrone glycoside from the leech Whitmania pigra. Heterocycles 2013, 87, 1537–1543. [Google Scholar] [CrossRef]
- Kim, K.H.; Clardy, J.; Senger, D.; Cao, S.G. Chakyunglupulins A and B, two novel 4,8,8-trimethylcyclooct-2-enone derivatives from Barleria lupulina. Tetrahedron Lett. 2015, 56, 2732–2734. [Google Scholar] [CrossRef]
- Takasugi, M.; Anetai, M.; Katsui, N.; Masamune, T. The occurrence of vomifoliol, dehydrovomifoliol and dihydrophaseic acid in the roots of “kidney bean” (Phaseolus vulgaris L.). Chem. Lett. 1973, 2, 245–248. [Google Scholar] [CrossRef]
- Weiss, G.; Koreeda, M.; Nakanishi, K. Stereochemistry of theaspirone and the blurnenols. J. Chem. Soc. Chem. Commun. 1973, 565–566. [Google Scholar] [CrossRef]
- Çalis, I.; Kuruüzüm-Uz, A.; Lorenzetto, P.A.; Rüedi, P. (6S)-Hydroxy-3-oxo-α-ionol glucosides from Capparis spinosa fruits. Phytochemistry 2002, 59, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Shimada, H.; Saka, M.; Yoshizumi, S.; Yamahara, J.; Matsuda, H. Medicinal foodstuffs V. Moroheiya.(1): Absolute stereostructures of corchoionosides A, B, and C, histamine release inhibitors from the leaves of Vietnamese Corchorus olitorius L. (Tiliaceae). Chem. Pharm. Bull. 1997, 45, 464–469. [Google Scholar] [CrossRef]
- Steffen, C.; Thomas, K.; Huniar, U.; Hellweg, A.; Rubner, O.; Schroer, A. TmoleX-A graphical user interface for TURBOMOLE. J. Comput. Chem. 2010, 31, 2967–2970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Nueraihemaiti, M.; Kamoldinov, K.; Yan, D.F.; Song, Y.Q.; Xu, N.N.; Zou, G.A.; Aisa, H.A. Sesquiterpenoids from Ferula samarkandica. Phytochemistry 2025, 237, 114529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Kamoldinov, K.; Nueraihemaiti, M.; Turdu, G.; Zou, G.A.; Aisa, H.A. Sesquiterpene coumarins with anti-vitiligo and cytotoxic activities from Ferula samarkandica. Phytochem. Lett. 2024, 61, 21–28. [Google Scholar] [CrossRef]

 ), HMBC (
), HMBC ( ) and NOESY (
) and NOESY ( ) correlations of compounds 1–2.
) correlations of compounds 1–2.
| No. | 1 | 2 | ||
|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | - | - | - | 37.2, C |
| 2 | - | 128.1, C | 1.99, ddd (14.4, 3.1, 2.3), β 1.53, dd (14.4, 3.7), α | 48.0, CH2 |
| 3 | 7.07, overlapped | 120.5, CH | 4.22, m | 67.3, CH |
| 4 | 6.17, dd (3.9, 2.7) | 109.7, CH | 2.42, dt (13.4, 2.3), β 1.75, overlapped, α | 46.4, CH2 |
| 5 | 7.08, overlapped | 132.5, CH | - | 89.0, C |
| 6 | - | 189.9, C | - | 185.7, C |
| 7 | 4.63, s | 65.4, CH2 | 5.75, s | 113.3, CH |
| 8 | - | - | - | 174.4, C |
| 9 | - | - | 1.47, s | 27.0, CH3 |
| 10 | - | - | 1.28, s | 31.0, CH3 |
| 11 | - | - | 1.76, s | 27.4, CH3 |
| 1′ | 4.41, t (6.9) | 49.5, CH2 | 3.61, q (7.1) | 58.3, CH2 |
| 2′ | 2.03, m | 27.7, CH2 | 1.18, t (7.1) | 18.4, CH3 |
| 3′ | 2.28, t (7.4) | 31.5, CH2 | - | - |
| 4′ | - | 175.1, C | - | - |
| 4′-OCH3 | 3.64, s | 52.1, CH3 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, D.; Zhang, M.; Song, Y.; Liu, L.; Begmatov, N.; Turginov, O.T.; Zhao, B.; Yang, H.; Zou, G. Chemical Constituents from Euphorbia esula. Plants 2025, 14, 2822. https://doi.org/10.3390/plants14182822
Yan D, Zhang M, Song Y, Liu L, Begmatov N, Turginov OT, Zhao B, Yang H, Zou G. Chemical Constituents from Euphorbia esula. Plants. 2025; 14(18):2822. https://doi.org/10.3390/plants14182822
Chicago/Turabian StyleYan, Defeng, Miaomiao Zhang, Yuqing Song, Liu Liu, Nurmirza Begmatov, Orzimat Turdimatovich Turginov, Bo Zhao, Hequn Yang, and Guoan Zou. 2025. "Chemical Constituents from Euphorbia esula" Plants 14, no. 18: 2822. https://doi.org/10.3390/plants14182822
APA StyleYan, D., Zhang, M., Song, Y., Liu, L., Begmatov, N., Turginov, O. T., Zhao, B., Yang, H., & Zou, G. (2025). Chemical Constituents from Euphorbia esula. Plants, 14(18), 2822. https://doi.org/10.3390/plants14182822







