Xylem Sap Bleeding as a Physiological Indicator in Grapevine: Genotype and Climate Influence
Abstract
1. Introduction
2. Results
2.1. Climatic Conditions During the Study (2022–2024)
2.2. Xylem Sap Exudation Main Parameters
2.2.1. The Sap Bleeding Onset
2.2.2. Bleeding Duration
2.2.3. Sap Bleeding Intensity
2.3. Sap Chemical Composition (2022–2024)
2.3.1. Total Soluble Solids (TSS)
2.3.2. Sap pH
2.3.3. Electrical Conductivity (EC)
2.3.4. Macronutrient Composition
2.3.5. Micronutrient Composition
2.3.6. Protein Content
2.3.7. Peroxidase Activity
2.3.8. Polyphenol Oxidase Activity
2.3.9. β-Glucosidase Activity
2.3.10. Organic Acid Content in Xylem Sap
2.3.11. Bleeding Sap Phenolic Compound Analysis (2022–2024)
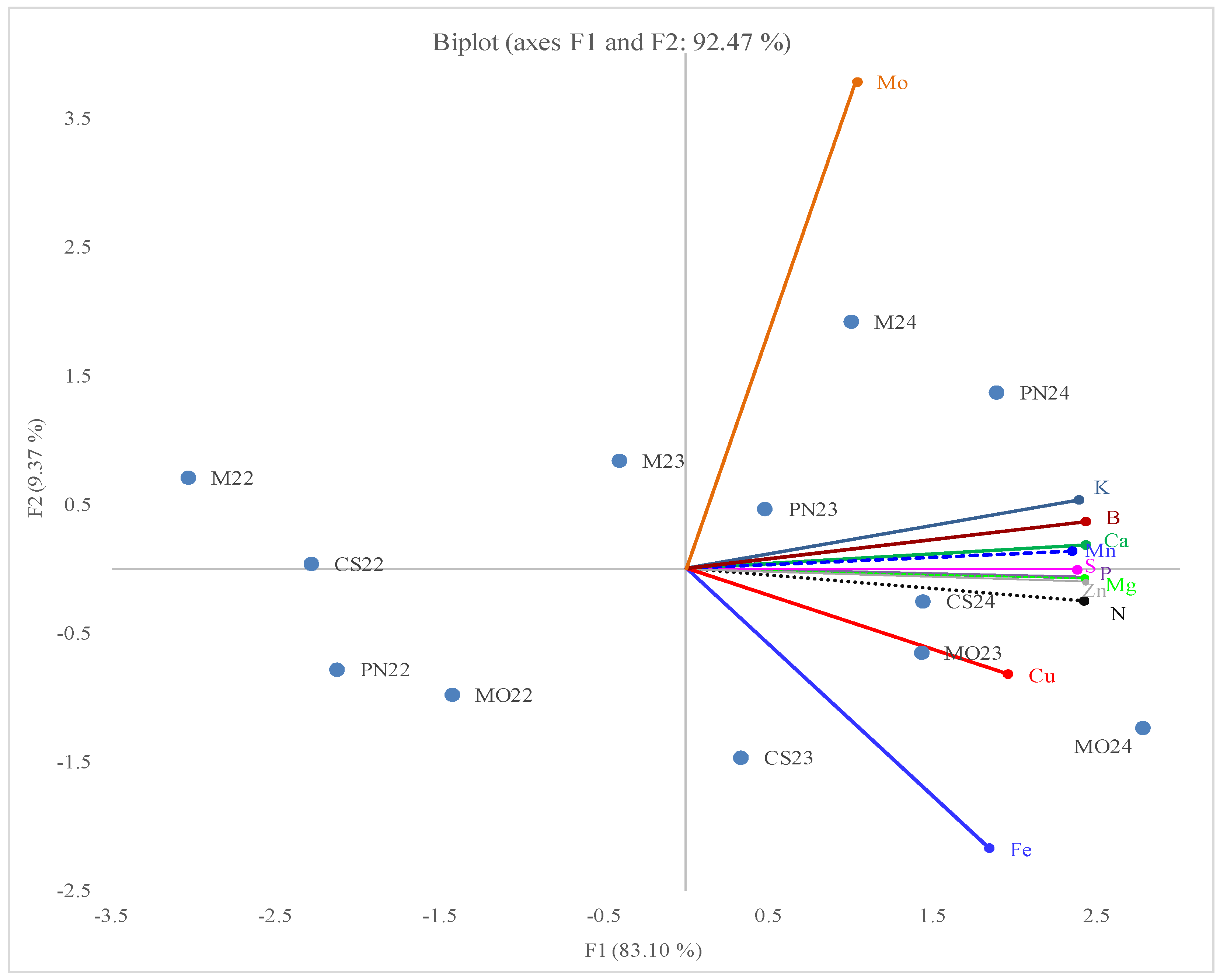
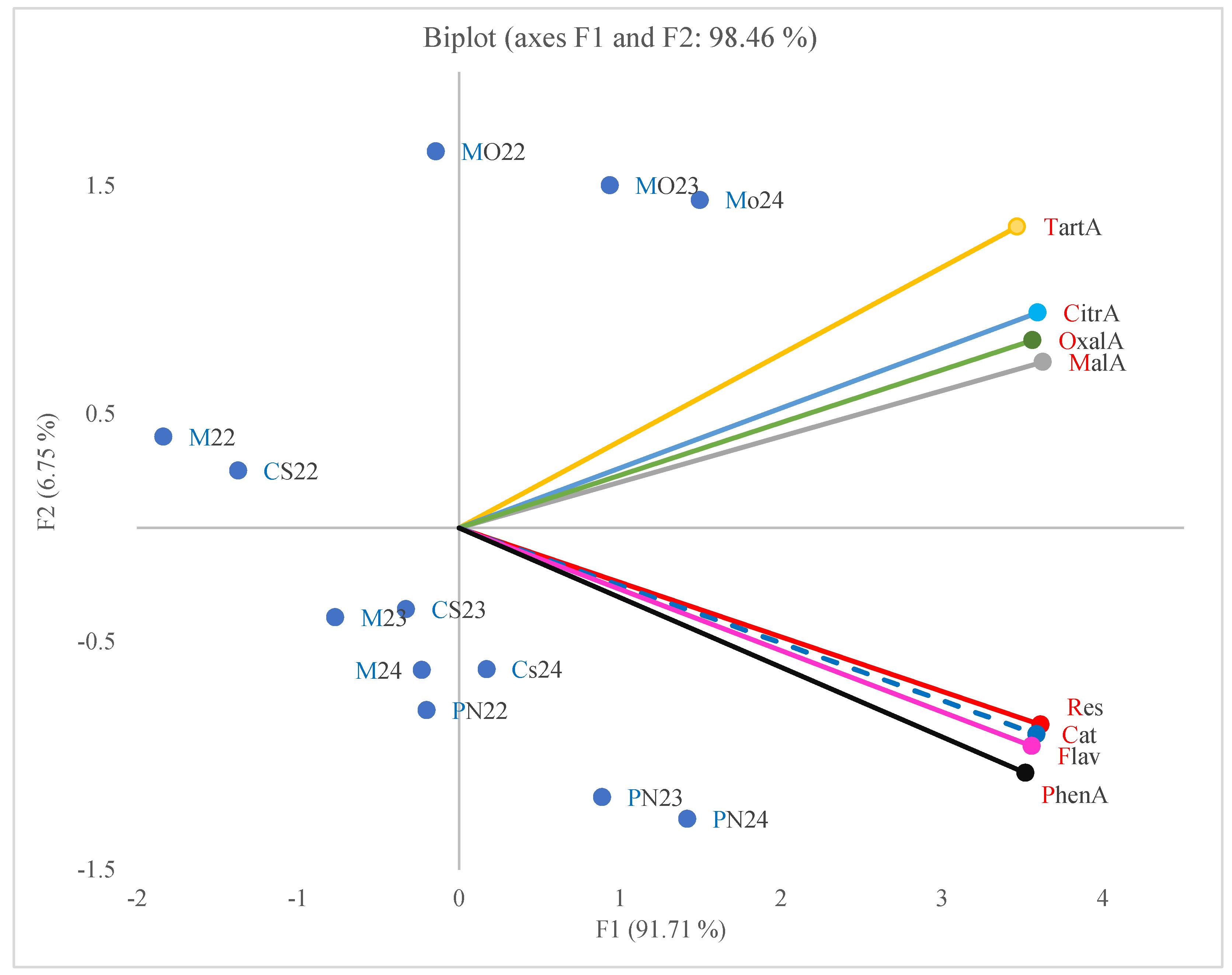
2.4. Principal Component Analysis
3. Discussion
3.1. Environmental Influence on Phenology and Bleeding Intensity
3.2. Sap Composition—Physiological Readiness and Environment Modulation
3.3. Macronutrient and Micronutrient Profile—Indicators of Cultivar-Specific Mobilization
3.4. Organic Acids and Phenolic Compounds—Indicators of Grapevine Vigor and Metabolism
3.5. Principal Component Analysis (PCA)
4. Materials and Methods
4.1. Study Area Description
4.2. Climate Data
4.3. Experimental Design
4.4. Plant Material
Sap Samples Collection
4.5. Measurement Parameters
Analytical Chemistry
4.6. Statistical Analysis
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TSS | Total soluble solids |
| PCA | The principal component analysis |
| N | Nitrogen |
| K | Potassium |
| Ca | Calcium |
| Mg | Magnesium |
| EC | Electrical conductivity |
| mS | millisiemens |
| S | Sulfur |
| Zn | Zinc |
| VSP | Guyot vertical trained |
| MTmin | average minimum temperature |
| MTmax | average maximum temperature |
| MalA | malic acid |
| TartA | tartaric acid |
| CitrA | citric acid |
| OxalA | oxalic acid |
| Res | resveratrol |
| Cat | catechins |
| Flav | flavonoids |
| PhenA | phenolic acids |
| CS | Cabernet Sauvignon |
| M | Merlot |
| MO | Muscat Ottonel |
| PN | Pinot Noir |
| TCA | tricarboxylic acid |
| IC | Ion Chromatography |
| HPLC | High-Performance Liquid Chromatography |
| HPLC-DAD | High-Performance Liquid Chromatography with Diode-Array Detection |
| UV-Vis | Ultraviolet–Visible Spectrophotometry |
| UV | Ultraviolet detection |
| DAD | Diode-Array Detector |
| KOH | Potassium hydroxide |
| MSA | Methanesulfonic acid |
| H2SO4 | Sulfuric acid |
| ACN | Acetonitrile |
| C18 column | Octadecylsilane-bonded silica reversed-phase column |
| AERS 500/CERS 500 | Anion/Cation Electrolytically Regenerated Suppressor (Thermo Dionex) |
| BSA | Bovine Serum Albumin (protein calibration standard) |
| r2 | Coefficient of determination (linearity of calibration curve) |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| QC | Quality Control |
| CCV | Continuing Calibration Verification |
| RSD | Relative Standard Deviation |
| N ≥ 5000 | Column efficiency expressed as theoretical plate number |
| Retention-time window ±2% | Accepted variation in peak retention time for compound identification |
| Spectral match ≥0.98 | Similarity threshold for confirming UV-DAD spectra |
| Matrix spikes (80–120% recovery) | QC test for accuracy in real sample matrix |
| NO3− | Nitrate |
| PO43− | Phosphate |
| SO42− | Sulfate |
| Cl− | Chloride |
| K+ | Potassium |
| Ca2+ | Calcium |
| Mg2+ | Magnesium |
| NH4+ | Ammonium |
| Na+ | Sodium |
References
- Zheng, T.; Haider, M.S.; Zhang, K.; Jia, H.; Fang, J. Biological and functional properties of xylem sap extracted from grapevine (cv. Rosario Bianco). Sci. Hortic. 2020, 272, 109563. [Google Scholar] [CrossRef]
- Ollat, N.; Cookson, S.; Lauvergeat, V.; Marguerit, E.; Barrieu, F.; Gambetta, G.; Goutouly, J.-P.; Tandonnet, J.-P.; Vivin, P.; Delrot, S. Grapevine roots: The dark side. In X International Symposium on Grapevine Physiology and Biotechnology; International Society for Horticultural Science: Leuven, Belgium, 2016; Volume 1188, pp. 213–226. [Google Scholar] [CrossRef]
- Bouamama-Gzara, B.; Zemni, H.; Sleimi, N.; Ghorbel, A.; Gzara, L.; Mahfoudhi, N. Diversification of vascular occlusions and crystal deposits in the xylem sap flow of five Tunisian grapevines. Plants 2022, 11, 2177. [Google Scholar] [CrossRef]
- Wason, J.; Bouda, M.; Lee, E.F.; McElrone, A.J.; Phillips, R.J.; Shackel, K.A.; Matthews, M.A.; Brodersen, C. Xylem network connectivity and embolism spread in grapevine (Vitis vinifera L.). Plant Physiol. 2021, 186, 373–387. [Google Scholar] [CrossRef]
- McElrone, A.J.; Manuck, C.M.; Brodersen, C.R.; Patakas, A.; Pearsall, K.R.; Williams, L.E. Functional hydraulic sectoring in grapevines as evidenced by sap flow, dye infusion, leaf removal and micro-computed tomography. AoB Plants 2021, 13, plab003. [Google Scholar] [CrossRef]
- Esteves, E.; Locatelli, G.; Bou, N.A.; Ferrarezi, R.S. Sap analysis: A powerful tool for monitoring plant nutrition. Horticulturae 2021, 7, 426. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, L.; Li, H.; Li, C. Pruning effects on sap flow and sugar phloem unloading in current-year shoots of different aged grapevine shoots. Pak. J. Bot. 2021, 53, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Recent advances of polyphenol oxidases in plants. Molecules 2023, 28, 2158. [Google Scholar] [CrossRef] [PubMed]
- Benyahia, F.; Bastos Campos, F.; Ben Abdelkader, A.; Basile, B.; Tagliavini, M.; Andreotti, C.; Zanotelli, D. Assessing grapevine water status by integrating vine transpiration, leaf gas exchanges, chlorophyll fluorescence and sap flow measurements. Agronomy 2023, 13, 464. [Google Scholar] [CrossRef]
- Prinsi, B.; Simeoni, F.; Galbiati, M.; Meggio, F.; Tonelli, C.; Scienza, A.; Espen, L. Grapevine rootstocks differently affect physiological and molecular responses of the scion under water deficit condition. Agronomy 2021, 11, 289. [Google Scholar] [CrossRef]
- Beis, A.; Zotos, A.; Patakas, A. Influence of sampling time and sap extraction methodology on xylem pH values in two grapevine varieties grown under drought conditions. Environ. Exp. Bot. 2009, 67, 305–311. [Google Scholar] [CrossRef]
- Zhang, L.; Marguerit, E.; Rossdeutsch, L.; Ollat, N.; Gambetta, G.A. The influence of grapevine rootstocks on scion growth and drought resistance. Theor. Exp. Plant Physiol. 2016, 28, 143–157. [Google Scholar] [CrossRef]
- Zufferey, V.; Spring, J.L.; Verdenal, T.; Dienes, A.; Belcher, S.; Lorenzini, F.; Koestel, C.; Rösti, J.; Gindro, K.; Spangenberg, J.; et al. The influence of water stress on plant hydraulics, gas exchange, berry composition and quality of Pinot Noir wines in Switzerland. Oeno One 2017, 51, 37–57. [Google Scholar] [CrossRef]
- Santamaría, R.I.; Martínez-Carrasco, A.; Martín, J.; Tormo, J.R.; Pérez-Victoria, I.; González, I.; Genilloud, O.; Reyes, F.; Díaz, M. Grapevine Xylem Sap Is a Potent Elicitor of Antibiotic Production in Streptomyces spp. Antibiotics 2022, 11, 672. [Google Scholar] [CrossRef]
- Li, J.; Li, D.; Liu, B.; Wang, R.; Yan, Y.; Li, G.; Wang, L.; Ma, C.; Xu, W.; Zhao, L.; et al. Effects of root restriction on phytohormone levels in different growth stages and grapevine organs. Sci. Rep. 2022, 12, 1323. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Fu, S.; Chen, D.; Zheng, S.; Wang, T.; Bai, Y. Grapevine sap flow in response to physio-environmental factors under solar greenhouse conditions. Water 2020, 12, 3081. [Google Scholar] [CrossRef]
- Field, S.K.; Smith, J.P.; Morrison, E.N.; Emery, R.N.; Holzapfel, B.P. Soil temperature prior to veraison alters grapevine carbon partitioning, xylem sap hormones, and fruit set. Am. J. Enol. Vitic. 2020, 71, 52–61. [Google Scholar] [CrossRef]
- Deloire, A.; Dumont, C.; Giudici, M.; Rogiers, S.; Pellegrino, A. A few words on grapevine winter buds and pruning in consideration of sap flow. In IVES Technical Reviews Vine and Wine; International Viticulture and Enology Society: Villenave d’Ornon, France, 2022; pp. 1–2. [Google Scholar] [CrossRef]
- Poni, S.; Sabbatini, P.; Palliotti, A. Facing spring frost damage in grapevine: Recent developments and the role of delayed winter pruning—A review. Am. J. Enol. Vitic. 2022, 73, 211–226. [Google Scholar] [CrossRef]
- Sivilotti, P.; Bonetto, C.; Paladin, M.; Peterlunger, E. Effect of soil moisture availability on Merlot: From leaf water potential to grape composition. Am. J. Enol. Vitic. 2005, 56, 9–18. [Google Scholar] [CrossRef]
- Faúndez-López, P.; Delorenzo-Arancibia, J.; Gutiérrez-Gamboa, G.; Moreno-Simunovic, Y. Pruning cuts affect wood necrosis but not the percentage of budburst or shoot development on spur pruned vines for different grapevine varieties. Vitis 2021, 60, 137–141. [Google Scholar] [CrossRef]
- Mirás-Avalos, J.M.; Araujo, E.S. Optimization of vineyard water management: Challenges, strategies, and perspectives. Water 2021, 13, 746. [Google Scholar] [CrossRef]
- Cramer, G.R. Abiotic stress and plant responses from the whole vine to the genes. Aust. J. Grape Wine Res. 2010, 16, 86–93. [Google Scholar] [CrossRef]
- Barrios-Masias, F.H.; Knipfer, T.; McElrone, A.J. Differential responses of grapevine rootstocks to water stress are associated with adjustments in fine root hydraulic physiology and suberization. J. Exp. Bot. 2015, 66, 6069–6078. [Google Scholar] [CrossRef] [PubMed]
- Keller, M. Chapter 6—Developmental Physiology. In The Science of Grapevines: Anatomy and Physiology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 193–265. ISBN 9780124199873/9780124200081. [Google Scholar]
- Zufferey, V.; Verdenal, T.; Dienes, A.; Belcher, S.; Lorenzini, F.; Koestel, C.; Spring, J.L. The impact of plant water status on the gas exchange, berry composition and wine quality of Chasselas grapes in Switzerland: Impacts of water stress on grapevine physiology. Oeno One 2018, 52, 347–361. [Google Scholar] [CrossRef]
- Labarga, D.; Mairata, A.; Puelles, M.; Martín, I.; Albacete, A.; García-Escudero, E.; Pou, A. The rootstock genotypes determine drought tolerance by regulating aquaporin expression at the transcript level and phytohormone balance. Plants 2023, 12, 718. [Google Scholar] [CrossRef] [PubMed]
- Ouadi, L.; Bruez, E.; Bastien, S.; Yacoub, A.; Coppin, C.; Guérin-Dubrana, L.; Rey, P. Sap flow disruption in grapevine is the early signal predicting the structural, functional, and genetic responses to esca disease. Front. Plant Sci. 2021, 12, 695846. [Google Scholar] [CrossRef]
- Grall, S.; Roulland, C.; Guillaumès, J.; Manceau, C. Bleeding Sap and Old Wood Are the Two Main Sources of Contamination of Merging Organs of Vine Plants by Xylophilus ampelinus, the Causal Agent of Bacterial Necrosis. Appl. Environ. Microbiol. 2005, 71, 8292–8300. [Google Scholar] [CrossRef]
- Peuke, A.D. The chemical composition of xylem sap in Vitis vinifera L. cv. Riesling during vegetative growth on three different Franconian vineyard soils and as influenced by nitrogen fertilizer. Am. J. Enol. Vitic. 2000, 51, 329–339. [Google Scholar] [CrossRef]
- Gambetta, G.A.; Fei, J.; Rost, T.L.; Knipfer, T.; Matthews, M.A.; Shackel, K.A.; Walker, M.A.; McElrone, A.J. Water uptake along the length of grapevine fine roots: Developmental anatomy, tissue-specific aquaporin expression, and pathways of water transport. Plant Physiol. 2013, 163, 1254–1265. [Google Scholar] [CrossRef]
- Lo Gullo, M.A.; Castro Noval, L.; Salleo, S.; Nardini, A. Hydraulic architecture of plants of Helianthus annuus L. cv. Margot: Evidence for plant segmentation in herbs. J. Exp. Bot. 2004, 55, 1549–1556. [Google Scholar] [CrossRef]
- Villa-Llop, A.; Crespo-Martinez, S.; Ancín, M.; Marín, D.; Cookson, S.J.; Loupit, G.; Santesteban, L.G. Evaluation of the characteristics of rootstock hardwood cuttings on graft performance. In XI International Symposium on Grapevine Physiology and Biotechnology; International Society for Horticultural Science: Leuven, Belgium, 2021; Volume 1390, pp. 147–152. [Google Scholar] [CrossRef]
- Andersen, P.C.; Brodbeck, B.V. Temperature and temperature preconditioning on flux and chemical com-position of xylem exudate from Muscadine grapevines. J. Am. Soc. Hortic. Sci. 1989, 114, 440–444. [Google Scholar] [CrossRef]
- Clarke, S.J.; Lamont, K.J.; Pan, H.Y.; Barry, L.A.; Hall, A.; Rogiers, S.Y. Spring root-zone temperature regulates root growth, nutrient uptake and shoot growth dynamics in grapevines. Aust. J. Grape Wine Res. 2015, 21, 479–489. [Google Scholar] [CrossRef]
- Chitarra, W.; Balestrini, R.; Vitali, M.; Pagliarani, C.; Perrone, I.; Schubert, A.; Lovisolo, C. Gene expression in vessel-associated cells upon xylem embolism repair in Vitis vinifera L. petioles. Planta 2014, 239, 887–899. [Google Scholar] [CrossRef]
- Harris, Z.N.; Pratt, J.E.; Bhakta, N.; Frawley, E.; Klein, L.L.; Kwasniewski, M.T.; Migicovsky, Z.; Miller, A.J. Temporal and environmental factors interact with rootstock genotype to shape leaf elemental composition in grafted grapevines. Plant Direct 2022, 6, e440. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmed, N.; Zhang, B.; Chachar, Z.; Li, J.; Xiao, G.; Wang, Q.; Hayat, F.; Deng, L.; Narejo, M.-U.-N.; Bozdar, B.; et al. Micronutrients and their effects on Horticultural crop quality, productivity and sustainability. Sci. Hortic. 2024, 323, 112512. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Darriet, P. The impact of climate change on viticulture and wine quality. J. Wine Econ. 2016, 11, 150–167. [Google Scholar] [CrossRef]
- Alagna, F.; Cirilli, M.; Galla, G.; Carbone, F.; Daddiego, L.; Facella, P.; Lopez, L.; Colao, C.; Mariotti, R.; Cultrera, N.; et al. Correction: Transcript Analysis and Regulative Events during Flower Development in Olive (Olea europaea L.). PLoS ONE 2022, 17, e0263101. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.H.; Rafique, R.; Rafique, T.; Naseer, M.; Khalil, U.; Rafique, R. Chapter 13. Effect of climate change on polyphenols accumulation in grapevine. In Phenolic Compounds-Chemistry, Synthesis, Diversity, Non-Conventional Industrial, Pharmaceutical and Therapeutic Applications; IntechOpen: London, UK, 2021; pp. 221–240. ISBN 978-1-83969-347-2/978-1-83969-346-5/978-1-83969-348-9. ISSN 2632-0983. [Google Scholar] [CrossRef]
- Fahim, S.; Ghanbari, A.; Naji, A.M.; Shokohian, A.A.; Lajayer, H.M.; Gohari, G.; Hano, C. Multivariate discrimination of some grapevine cultivars under drought stress in Iran. Horticulturae 2022, 8, 871. [Google Scholar] [CrossRef]
- Tangolar, S.; Cantürk, S.; Tangolar, S.; Turan, M.; Ada, M. Pruning Timing According to Lunar Phases: Impact on Yield, Grape Quality, and Biochemical Properties of Xylem Sap in ‘Black Magic’ Grapevines. Appl. Fruit Sci. 2025, 67, 243. [Google Scholar] [CrossRef]
- Teixeira, A.; Noronha, H.; Frusciante, S.; Diretto, G.; Gerós, H. The grapevine metabolite profile of phloem sap is modified by Flavescence dorée. Oeno One 2023, 57, 307–320. [Google Scholar] [CrossRef]

| Cultivar | n | 2022 Onset (±SD) | 2023 Onset (±SD) | 2024 Onset (±SD) |
|---|---|---|---|---|
| ‘Cabernet Sauvignon’ | 30 | March 20 ± 1.3 days | March 6 ± 1.2 days | March 4 ± 1.1 days |
| ‘Merlot’ | 30 | March 19 ± 1.5 days | March 5 ± 1.0 days | March 3 ± 0.9 days |
| ‘Muscat Ottonel’ | 30 | March 15 ± 1.4 days | March 3 ± 1.1 days | February 28 ± 1.1 days |
| ‘Pinot Noir’ | 30 | March 14 ± 1.2 days | March 2 ± 0.8 days | February 26 ± 0.9 days |
| Cultivar | n | 2022 Duration (±SD) | 2023 Duration (±SD) | 2024 Duration (±SD) |
|---|---|---|---|---|
| ‘Cabernet Sauvignon’ | 30 | 11 ± 2.0 days | 16 ± 2.1 days | 17 ± 1.6 days |
| ‘Merlot’ | 30 | 10 ± 1.5 days | 14 ± 2.0 days | 15 ± 1.7 days |
| ‘Muscat Ottonel’ | 30 | 13 ± 1.9 days | 18 ± 2.0 days | 20 ± 2.1 days |
| ‘Pinot Noir’ | 30 | 14 ± 2.1 days | 20 ± 1.8 days | 21 ± 1.6 days |
| Trait | Cultivar | n | 2022 (±SD) | 2023 (±SD) | 2024 (±SD) | ANOVA p-Value |
|---|---|---|---|---|---|---|
| Bleeding intensity (mL/vine/day) | ‘Cabernet Sauvignon’ | 30 | 4.9 ± 0.8 c | 6.1 ± 0.7 b | 6.8 ± 0.8 a | 0.027 |
| ‘Merlot’ | 30 | 4.3 ± 0.6 c | 5.7 ± 0.6 b | 6.4 ± 0.7 a | 0.015 | |
| ‘Muscat Ottonel’ | 30 | 7.1 ± 0.7 c | 8.5 ± 1.0 b | 9.1 ± 1.1 a | 0.031 | |
| ‘Pinot Noir’ | 30 | 8.2 ± 0.9 c | 9.4 ± 1.1 b | 10.8 ± 1.2 a | 0.012 | |
| Total Soluble Solids (TSS, °Brix) | ‘Cabernet Sauvignon’ | 30 | 2.1 ± 0.13 b | 2.3 ± 0.14 a | 2.3 ± 0.13 a | 0.032 |
| ‘Merlot’ | 30 | 1.3 ± 0.12 c | 1.9 ± 0.13 b | 2.2 ± 0.14 a | 0.018 | |
| ‘Muscat Ottonel’ | 30 | 3.3 ± 0.14 c | 3.8 ± 0.13 ab | 3.8 ± 0.12 ab | 0.009 | |
| ‘Pinot Noir’ | 30 | 2.3 ± 0.12 a | 2.4 ± 0.13 a | 2.4 ± 0.14 a | 0.051 (ns) | |
| Sap pH | ‘Cabernet Sauvignon’ | 30 | 6.3 ± 0.11 a | 6.6 ± 0.11 a | 6.6 ± 0.20 a | 0.513 (ns) |
| ‘Merlot’ | 30 | 6.1 ± 0.21 a | 6.3 ± 0.20 a | 6.4 ± 0.12 a | 0.436 (ns) | |
| ‘Muscat Ottonel’ | 30 | 6.5 ± 0.11 a | 6.8 ± 0.22 a | 6.9 ± 0.21 a | 0.388 (ns) | |
| ‘Pinot Noir’ | 30 | 6.2 ± 0.12 a | 6.5 ± 0.21 a | 6.5 ± 0.20 a | 0.482 (ns) | |
| Electrical Conductivity (EC, mS/cm) * | ‘Cabernet Sauvignon’ | 30 | 0.56 ± 0.06 b | 0.63 ± 0.05 a | 0.61 ± 0.04 a | 0.022 |
| ‘Merlot’ | 30 | 0.52 ± 0.05 c | 0.60 ± 0.04 ab | 0.58 ± 0.05 b | 0.028 | |
| ‘Muscat Ottonel’ | 30 | 0.43 ± 0.04 b | 0.46 ± 0.05 a | 0.44 ± 0.03 b | 0.039 | |
| ‘Pinot Noir’ | 30 | 0.49 ± 0.05 a | 0.52 ± 0.06 a | 0.51 ± 0.04 a | 0.061 (ns) |
| Cultivar | Year | n | N ± SD | P ± SD | K ± SD | Ca ± SD | Mg ± SD | S ± SD |
|---|---|---|---|---|---|---|---|---|
| ‘Cabernet Sauvignon’ | 2022 | 30 | 49.1 ± 3.2 a | 4.4 ± 0.3 a | 118.2 ± 6.0 a | 34.6 ± 2.2 a | 8.8 ± 0.5 a | 5.1 ± 0.4 a |
| 2023 | 30 | 56.7 ± 3.4 b | 5.2 ± 0.6 b | 134.8 ± 6.7 b | 38.1 ± 2.6 b | 9.6 ± 0.7 b | 5.4 ± 0.5 b | |
| 2024 | 30 | 58.5 ± 4.0 b | 5.5 ± 0.4 b | 139.2 ± 7.1 b | 39.4 ± 2.5 b | 10.2 ± 0.6 c | 5.6 ± 0.7 b | |
| ANOVA p-value | 0.019 | 0.023 | 0.016 | 0.025 | 0.031 | 0.033 | ||
| ‘Merlot’ | 2022 | 30 | 47.2 ± 2.8 a | 4.4 ± 0.5 a | 120.1 ± 5.7 a | 33.8 ± 2.0 a | 8.6 ± 0.4 a | 4.8 ± 0.3 a |
| 2023 | 30 | 54.5 ± 3.3 b | 4.8 ± 0.4 ab | 135.6 ± 7.2 b | 37.3 ± 2.3 b | 9.6 ± 0.5 b | 5.3 ± 0.4 b | |
| 2024 | 30 | 57.7 ± 3.6 c | 5.4 ± 0.5 b | 140.7 ± 6.6 b | 38.5 ± 2.4 b | 9.8 ± 0.7 b | 5.7 ± 0.4 b | |
| ANOVA p-value | 0.015 | 0.018 | 0.013 | 0.021 | 0.027 | 0.030 | ||
| ‘Muscat Ottonel’ | 2022 | 30 | 53.6 ± 3.2 a | 4.8 ± 0.6 a | 124.2 ± 6.1 a | 35.6 ± 2.5 a | 9.2 ± 0.7 a | 5.1 ± 0.5 a |
| 2023 | 30 | 60.2 ± 3.5 b | 5.4 ± 0.5 b | 141.7 ± 6.3 b | 39.2 ± 2.7 b | 10.2 ± 0.8 b | 5.7 ± 0.4 b | |
| 2024 | 30 | 62.8 ± 4.3 b | 5.8 ± 0.7 b | 145.1 ± 7.0 b | 40.2 ± 2.5 b | 10.5 ± 0.6 b | 6.1 ± 0.5 c | |
| ANOVA p-value | 0.011 | 0.016 | 0.015 | 0.017 | 0.026 | 0.028 | ||
| ‘Pinot Noir’ | 2022 | 30 | 51.7 ± 2.6 a | 4.6 ± 0.3 a | 122.6 ± 6.1 a | 34.2 ± 2.2 a | 8.7 ± 0.5 a | 5.2 ± 0.4 a |
| 2023 | 30 | 58.4 ± 3.1 b | 5.2 ± 0.5 b | 138.4 ± 7.2 b | 38.6 ± 2.6 b | 9.8 ± 0.7 b | 5.5 ± 0.6 b | |
| 2024 | 30 | 61.2 ± 3.8 b | 5.6 ± 0.4 b | 142.2 ± 6.7 b | 39.7 ± 2.5 b | 10.1 ± 0.8 b | 5.8 ± 0.5 b | |
| ANOVA p-value | 0.017 | 0.022 | 0.014 | 0.017 | 0.024 | 0.031 |
| Cultivar | Year | n | Fe ± SD | Mn ± SD | Zn ± SD | Cu ± SD | B ± SD | Mo ± SD |
|---|---|---|---|---|---|---|---|---|
| ‘Cabernet Sauvignon’ | 2022 | 30 | 1.2 ± 0.2 b | 0.31 ± 0.02 c | 0.51 ± 0.06 c | 0.18 ± 0.02 b | 0.80 ± 0.07 c | 0.04 ± 0.02 a |
| 2023 | 30 | 1.4 ± 0.2 a | 0.35 ± 0.03 b | 0.61 ± 0.05 b | 0.20 ± 0.01 a | 0.92 ± 0.06 b | 0.03 ± 0.01 a | |
| 2024 | 30 | 1.3 ± 0.1 ab | 0.38 ± 0.04 a | 0.64 ± 0.04 a | 0.21 ± 0.02 a | 1.01 ± 0.06 a | 0.04 ± 0.02 a | |
| ANOVA p-value | 0.021 | 0.027 | 0.026 | 0.035 | 0.018 | 0.030 | ||
| ‘Merlot’ | 2022 | 30 | 1.1 ± 0.1 b | 0.29 ± 0.02 c | 0.51 ± 0.03 c | 0.15 ± 0.01 b | 0.79 ± 0.06 c | 0.04 ± 0.02 b |
| 2023 | 30 | 1.3 ± 0.2 a | 0.33 ± 0.04 b | 0.57 ± 0.05 b | 0.17 ± 0.02 ab | 0.92 ± 0.05 b | 0.05 ± 0.01 ab | |
| 2024 | 30 | 1.2 ± 0.1 ab | 0.38 ± 0.03 a | 0.62 ± 0.06 a | 0.19 ± 0.02 a | 0.97 ± 0.05 a | 0.06 ± 0.02 a | |
| ANOVA p-value | 0.021 | 0.025 | 0.023 | 0.031 | 0.019 | 0.028 | ||
| ‘Muscat Ottonel’ | 2022 | 30 | 1.3 ± 0.2 b | 0.32 ± 0.04 c | 0.54 ± 0.06 c | 0.17 ± 0.02 b | 0.86 ± 0.06 c | 0.03 ± 0.02 a |
| 2023 | 30 | 1.4 ± 0.2 b | 0.36 ± 0.02 b | 0.62 ± 0.05 b | 0.21 ± 0.01 a | 0.98 ± 0.05 b | 0.04 ± 0.01 a | |
| 2024 | 30 | 1.6 ± 0.1 a | 0.41 ± 0.03 a | 0.68 ± 0.06 a | 0.20 ± 0.02 a | 1.03 ± 0.04 a | 0.04 ± 0.02 a | |
| ANOVA p-value | 0.016 | 0.024 | 0.022 | 0.027 | 0.021 | 0.031 | ||
| ‘Pinot Noir’ | 2022 | 30 | 1.2 ± 0.2 b | 0.28 ± 0.01 c | 0.54 ± 0.04 c | 0.18 ± 0.02 ab | 0.81 ± 0.05 c | 0.03 ± 0.02 b |
| 2023 | 30 | 1.4 ± 0.2 a | 0.34 ± 0.02 b | 0.60 ± 0.05 b | 0.17 ± 0.01 b | 0.93 ± 0.06 b | 0.05 ± 0.02 ab | |
| 2024 | 30 | 1.3 ± 0.1 ab | 0.37 ± 0.05 a | 0.65 ± 0.06 a | 0.21 ± 0.02 a | 1.02 ± 0.06 a | 0.06 ± 0.01 a | |
| ANOVA p-value | 0.018 | 0.023 | 0.021 | 0.028 | 0.021 | 0.026 |
| Trait | Cultivar | n | 2022 (±SD) | 2023 (±SD) | 2024 (±SD) | ANOVA p-Value |
|---|---|---|---|---|---|---|
| Total protein content (mg/mL) | ‘Cabernet Sauvignon’ | 30 | 0.62 ± 0.07 c | 0.75 ± 0.06 b | 0.79 ± 0.05 a | 0.018 |
| ‘Merlot’ | 30 | 0.59 ± 0.06 c | 0.72 ± 0.05 b | 0.77 ± 0.06 a | 0.015 | |
| ‘Muscat Ottonel’ | 30 | 0.84 ± 0.08 c | 0.93 ± 0.07 b | 0.95 ± 0.06 a | 0.032 | |
| ‘Pinot Noir’ | 30 | 0.89 ± 0.09 c | 0.97 ± 0.06 b | 1.05 ± 0.07 a | 0.022 | |
| Peroxidase activity (U/mL) * | ‘Cabernet Sauvignon’ | 30 | 5.4 ± 0.5 c | 6.8 ± 0.6 b | 7.1 ± 0.6 a | 0.021 |
| ‘Merlot’ | 30 | 4.9 ± 0.4 c | 6.1 ± 0.5 b | 6.6 ± 0.6 a | 0.016 | |
| ‘Muscat Ottonel’ | 30 | 6.2 ± 0.5 c | 7.0 ± 0.6 b | 7.4 ± 0.7 a | 0.039 | |
| ‘Pinot Noir’ | 30 | 6.9 ± 0.6 c | 7.6 ± 0.6 b | 8.1 ± 0.8 a | 0.025 | |
| Polyphenol oxidase activity (U/mL) ** | ‘Cabernet Sauvignon’ | 30 | 1.8 ± 0.2 c | 2.2 ± 0.2 b | 2.4 ± 0.2 a | 0.030 |
| ‘Merlot’ | 30 | 1.6 ± 0.2 c | 2.1 ± 0.3 b | 2.2 ± 0.2 a | 0.028 | |
| ‘Muscat Ottonel’ | 30 | 2.1 ± 0.3 c | 2.5 ± 0.2 b | 2.7 ± 0.2 a | 0.041 | |
| ‘Pinot Noir’ | 30 | 2.3 ± 0.2 c | 2.7 ± 0.3 b | 3.0 ± 0.3 a | 0.020 | |
| β-glucosidase activity (U/mL) * | ‘Cabernet Sauvignon’ | 30 | 0.94 ± 0.08 c | 1.18 ± 0.09 b | 1.25 ± 0.08 a | 0.019 |
| ‘Merlot’ | 30 | 0.87 ± 0.07 c | 1.10 ± 0.08 b | 1.15 ± 0.09 a | 0.015 | |
| ‘Muscat Ottonel’ | 30 | 1.05 ± 0.09 c | 1.24 ± 0.10 b | 1.30 ± 0.09 a | 0.026 | |
| ‘Pinot Noir’ | 30 | 1.10 ± 0.08 c | 1.31 ± 0.11 b | 1.38 ± 0.10 a | 0.018 |
| Cultivar | Year | n | Malic Acid ± SD | Tartaric Acid ± SD | Citric Acid ± SD | Oxalic Acid ± SD |
|---|---|---|---|---|---|---|
| ‘Cabernet Sauvignon’ | 2022 | 30 | 72.3 ± 4.0 c | 46.2 ± 2.6 c | 18.4 ± 1.3 c | 9.2 ± 0.5 c |
| 2023 | 30 | 79.1 ± 3.7 b | 49.6 ± 2.4 b | 21.0 ± 1.5 b | 9.7 ± 0.4 b | |
| 2024 | 30 | 81.5 ± 4.1 a | 50.8 ± 2.7 a | 22.2 ± 1.6 a | 10.2 ± 0.6 a | |
| ANOVA p-value | 0.017 | 0.041 | 0.021 | 0.035 | ||
| ‘Merlot’ | 2022 | 30 | 69.7 ± 3.8 c | 44.4 ± 2.2 c | 17.5 ± 1.2 c | 8.8 ± 0.3 c |
| 2023 | 30 | 75.5 ± 3.6 b | 47.7 ± 2.5 b | 19.7 ± 1.3 b | 9.5 ± 0.5 b | |
| 2024 | 30 | 78.0 ± 4.0 a | 49.1 ± 2.8 a | 20.6 ± 1.5 a | 10.2 ± 0.4 a | |
| ANOVA p-value | 0.021 | 0.037 | 0.024 | 0.030 | ||
| ‘Muscat Ottonel’ | 2022 | 30 | 84.6 ± 4.1 c | 52.5 ± 3.1 c | 23.2 ± 1.3 c | 10.1 ± 0.5 c |
| 2023 | 30 | 91.1 ± 3.9 b | 56.0 ± 3.2 b | 25.6 ± 1.5 b | 11.3 ± 0.7 b | |
| 2024 | 30 | 94.4 ± 4.5 a | 57.3 ± 3.0 a | 27.1 ± 1.6 a | 12.0 ± 0.8 a | |
| ANOVA p-value | 0.013 | 0.027 | 0.017 | 0.021 | ||
| ‘Pinot Noir’ | 2022 | 30 | 81.2 ± 4.0 c | 48.8 ± 2.7 c | 21.0 ± 1.5 c | 9.7 ± 0.5 c |
| 2023 | 30 | 87.5 ± 4.2 b | 52.2 ± 3.1 b | 23.8 ± 1.3 b | 10.6 ± 0.4 b | |
| 2024 | 30 | 91.0 ± 4.4 a | 53.4 ± 2.8 a | 25.2 ± 1.6 a | 11.2 ± 0.3 a | |
| ANOVA p-value | 0.018 | 0.031 | 0.020 | 0.024 |
| Cultivar | Year | n | Resveratrol ± SD | Catechins ± SD | Flavonoids ± SD | Phenolic Acids ± SD |
|---|---|---|---|---|---|---|
| ‘Cabernet Sauvignon’ | 2022 | 30 | 1.84 ± 0.11 c | 3.41 ± 0.17 c | 5.16 ± 0.25 c | 2.63 ± 0.13 c |
| 2023 | 30 | 2.13 ± 0.14 b | 3.67 ± 0.20 b | 5.45 ± 0.27 b | 2.91 ± 0.15 b | |
| 2024 | 30 | 2.24 ± 0.16 a | 3.88 ± 0.22 a | 5.57 ± 0.24 a | 3.00 ± 0.18 a | |
| ANOVA p-values | 0.018 | 0.021 | 0.026 | 0.031 | ||
| ‘Merlot’ | 2022 | 30 | 1.71 ± 0.10 c | 3.34 ± 0.16 c | 5.02 ± 0.23 c | 2.50 ± 0.12 c |
| 2023 | 30 | 2.00 ± 0.13 b | 3.58 ± 0.19 b | 5.36 ± 0.26 b | 2.82 ± 0.16 b | |
| 2024 | 30 | 2.11 ± 0.15 a | 3.80 ± 0.21 a | 5.47 ± 0.22 a | 2.93 ± 0.17 a | |
| ANOVA p-values | 0.015 | 0.020 | 0.023 | 0.025 | ||
| ‘Muscat Ottonel’ | 2022 | 30 | 2.04 ± 0.14 c | 3.67 ± 0.20 c | 5.43 ± 0.24 c | 2.72 ± 0.15 c |
| 2023 | 30 | 2.27 ± 0.16 b | 3.88 ± 0.21 b | 5.70 ± 0.28 b | 3.03 ± 0.18 b | |
| 2024 | 30 | 2.39 ± 0.17 a | 4.03 ± 0.24 a | 5.82 ± 0.23 a | 3.16 ± 0.20 a | |
| ANOVA p-values | 0.018 | 0.020 | 0.024 | 0.028 | ||
| ‘Pinot Noir’ | 2022 | 30 | 2.14 ± 0.13 c | 3.78 ± 0.20 c | 5.63 ± 0.26 c | 2.86 ± 0.14 c |
| 2023 | 30 | 2.40 ± 0.15 b | 4.04 ± 0.23 b | 5.92 ± 0.29 b | 3.15 ± 0.17 b | |
| 2024 | 30 | 2.51 ± 0.16 a | 4.17 ± 0.24 a | 6.05 ± 0.25 a | 3.28 ± 0.20 a | |
| ANOVA p-values | 0.017 | 0.021 | 0.027 | 0.031 |
| Analysis | Column and Conditions | Mobile Phase/Gradient | Detection | Standards and Calibration | LOD/LOQ | QC/Blanks |
|---|---|---|---|---|---|---|
| Ion Chromatography (IC)—anions (NO3−, PO43−, SO42−, Cl−) | Dionex IonPac AS11-HC (4 × 250 mm) + AG11-HC guard; 30 °C; 1.0 mL·min−1; injection 25 µL | KOH gradient: 10 mM (0–5 min) → 30 mM (5–15 min) → 60 mM (15–25 min) → 10 mM (25–30 min, re-eq.) | Suppressed conductivity (AERS 500, 78 mA; Thermo Fisher Scientific Inc. 168 Third Avenue Waltham, MA USA) | Certified standards: 0.05–50 mg L−1, 7 points, r2 ≥ 0.999 | 0.01–0.05/0.03–0.15 mg L−1 | Method blank and CCV every 10 samples; matrix spikes (80–120% recovery); duplicate injections (RSD ≤ 5%) |
| Ion Chromatography (IC)—cations (K+, Ca2+, Mg2+, NH4+, Na+) | Dionex IonPac CS12A (4 × 250 mm) + CG12A guard; 30 °C; 1.0 mL·min−1; injection 25 µL | 20 mM MSA, isocratic | Suppressed conductivity (CERS 500, 59 mA; Thermo Fisher Scientific Inc. 168 Third Avenue Waltham, MA USA) | Same as above; 0.02–50 mg L−1, 7 points | 0.02–0.05/0.06–0.15 mg L−1 | As above |
| HPLC (organic acids: malic, tartaric, citric, oxalic) | Bio-Rad Aminex HPX-87H (300 × 7.8 mm) + guard; 65 °C; 0.5 mL·min−1; injection 20 µL | 5 mM H2SO4, isocratic | UV 210 nm | Authentic standards: 0.05–20 mg L−1, r2 ≥ 0.999 | 0.01–0.03/0.03–0.10 mg L−1 | Blanks; CCV every 10 injections; duplicate injections (RSD ≤ 5%); system suitability N ≥ 5000 |
| HPLC-DAD (phenolics: phenolic acids, flavonoids, catechins, resveratrol) | C18 column (250 × 4.6 mm, 5 µm) + guard; 30 °C; 1.0 mL·min−1; injection 10 µL | A = H2O + 0.1% formic acid; B = ACN + 0.1% formic acid; gradient: 5% B (0 min) → 95% B (37 min), re-eq. 10 min | DAD: 280 nm (catechins), 306–310 nm (resveratrol), 320 nm (hydroxycinnamates), 360 nm (flavonols); spectra 200–400 nm | Authentic standards (gallic, caffeic, ferulic acids, catechin, quercetin, resveratrol); 0.05–25 mg L−1, r2 ≥ 0.998 | 0.005–0.02/0.02–0.06 mg L−1 | Mobile phase blanks; CCV every 10; matrix spikes (80–120%); duplicate injections; retention-time window ±2%; spectral match ≥0.98 |
| Bradford assay (protein) | — | — | UV-Vis at 595 nm (Shimadzu UV-1800) | BSA calibration curve 0–1.0 mg·mL−1, r2 ≥ 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nistor, E.; Dobrei, A.; Dragoescu-Petrica, A.; Cataldo, E.; Sala, F.; Ciorica, G.; Dobrei, A.G. Xylem Sap Bleeding as a Physiological Indicator in Grapevine: Genotype and Climate Influence. Plants 2025, 14, 2807. https://doi.org/10.3390/plants14172807
Nistor E, Dobrei A, Dragoescu-Petrica A, Cataldo E, Sala F, Ciorica G, Dobrei AG. Xylem Sap Bleeding as a Physiological Indicator in Grapevine: Genotype and Climate Influence. Plants. 2025; 14(17):2807. https://doi.org/10.3390/plants14172807
Chicago/Turabian StyleNistor, Eleonora, Alin Dobrei, Andreea Dragoescu-Petrica, Eleonora Cataldo, Florin Sala, Gabriel Ciorica, and Alina Georgeta Dobrei. 2025. "Xylem Sap Bleeding as a Physiological Indicator in Grapevine: Genotype and Climate Influence" Plants 14, no. 17: 2807. https://doi.org/10.3390/plants14172807
APA StyleNistor, E., Dobrei, A., Dragoescu-Petrica, A., Cataldo, E., Sala, F., Ciorica, G., & Dobrei, A. G. (2025). Xylem Sap Bleeding as a Physiological Indicator in Grapevine: Genotype and Climate Influence. Plants, 14(17), 2807. https://doi.org/10.3390/plants14172807







