Physiological Efficiency and Adaptability of Greek Indigenous Grapevine Cultivars Under Heat Stress and Elevated CO2: Insights into Photosynthetic Dynamics
Abstract
1. Introduction
2. Results
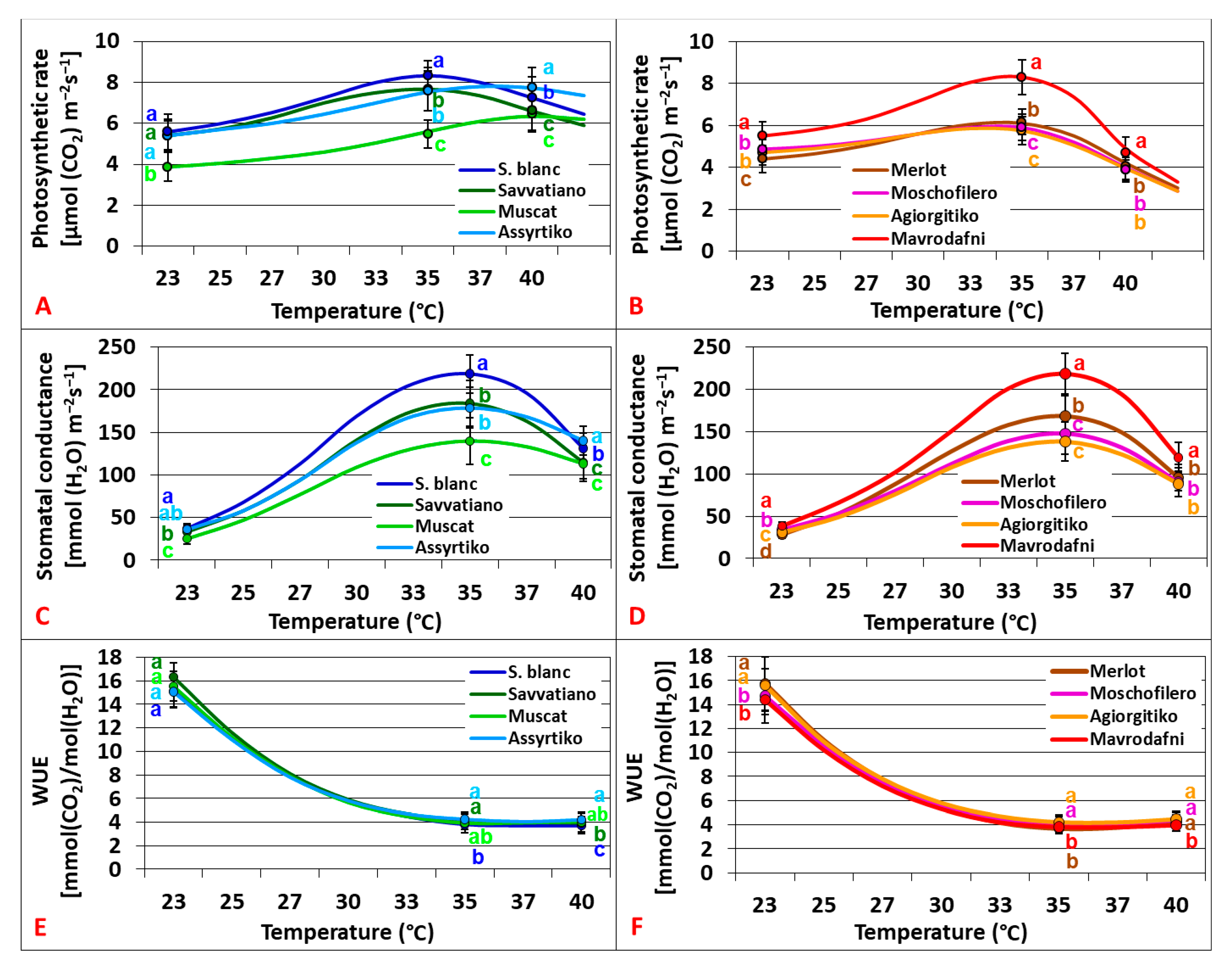
2.1. Physiological Responses Under Ambient CO2
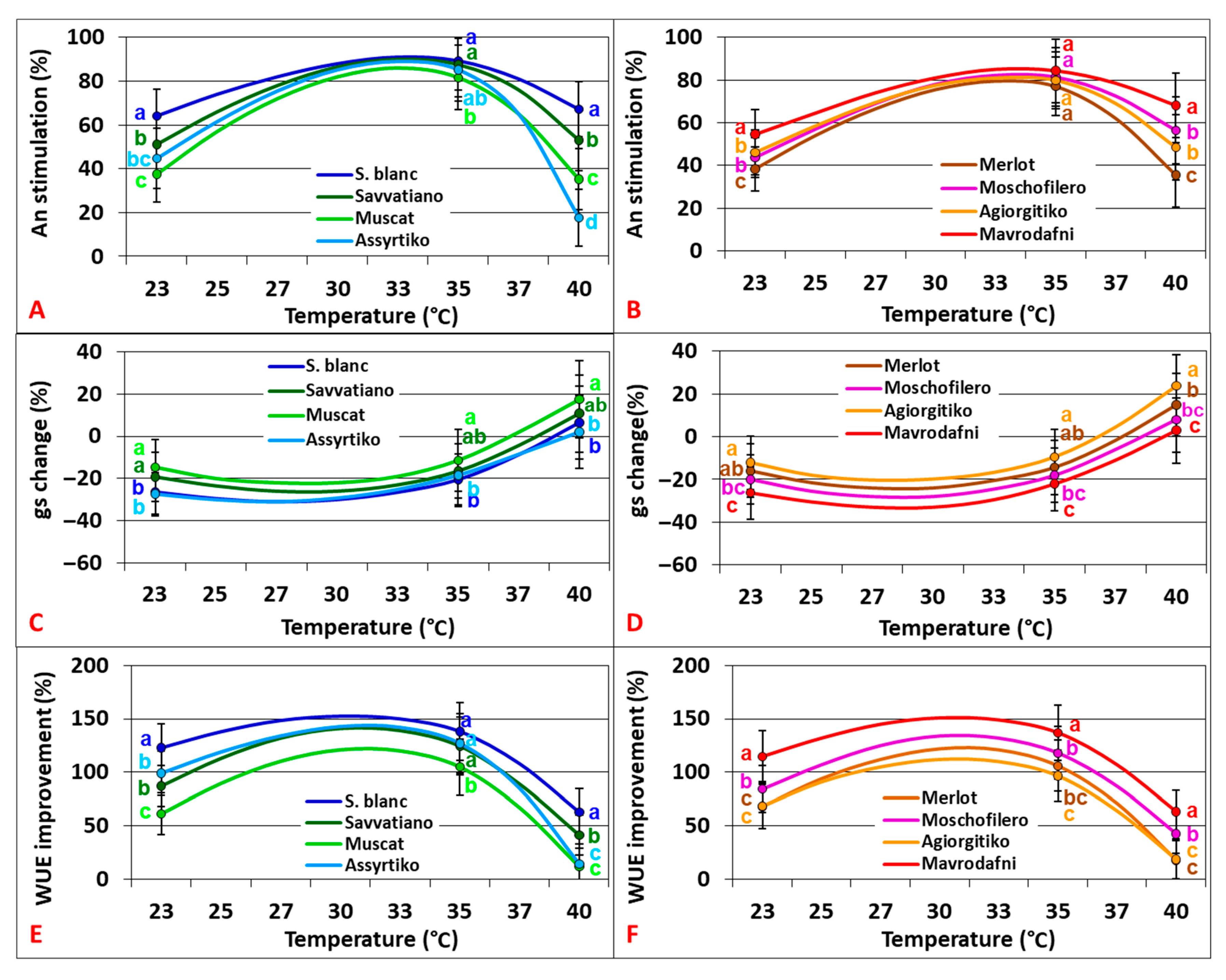
2.2. Physiological Responses Under Elevated CO2
2.3. Physiological Responses to CO2 Elevation
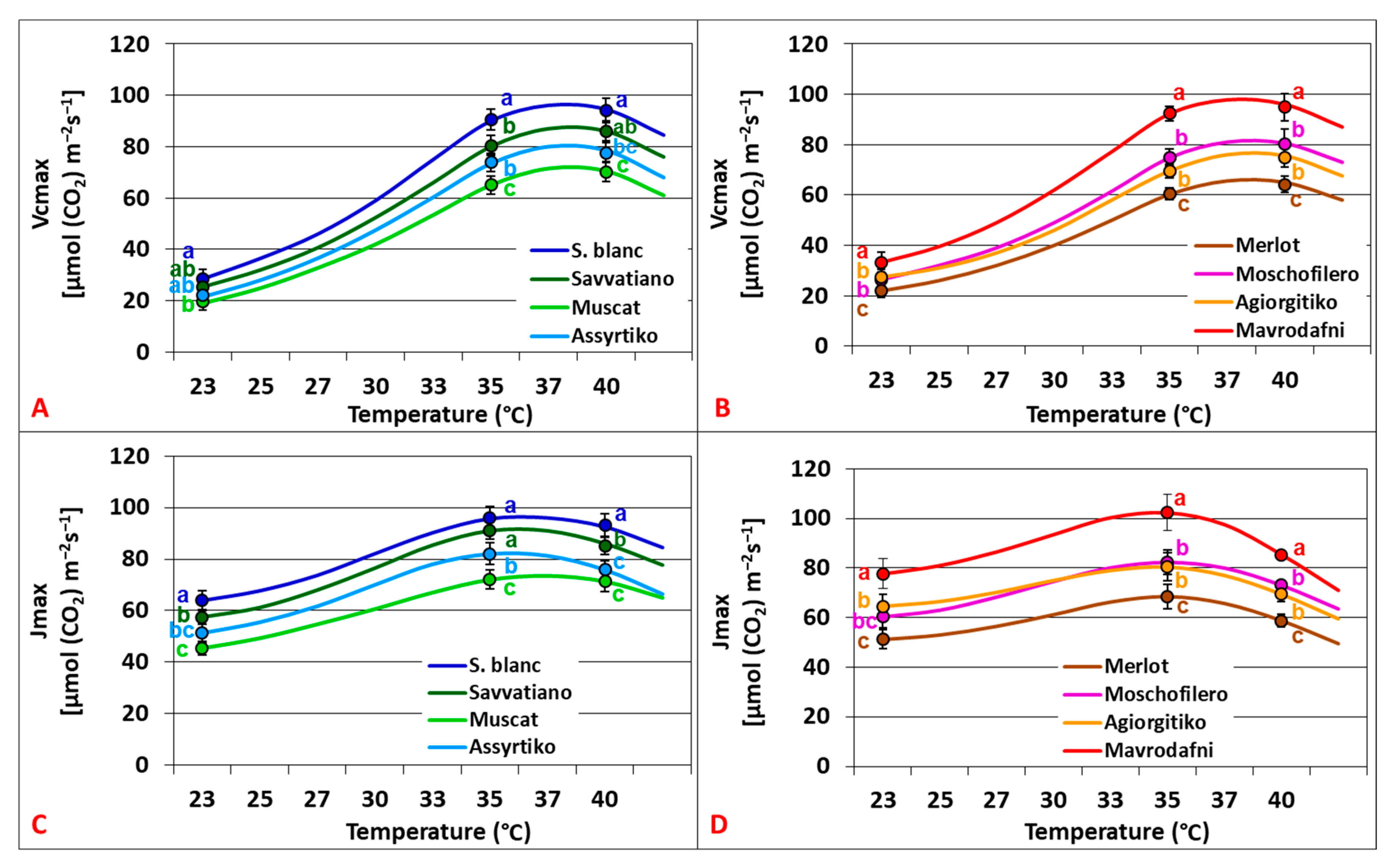
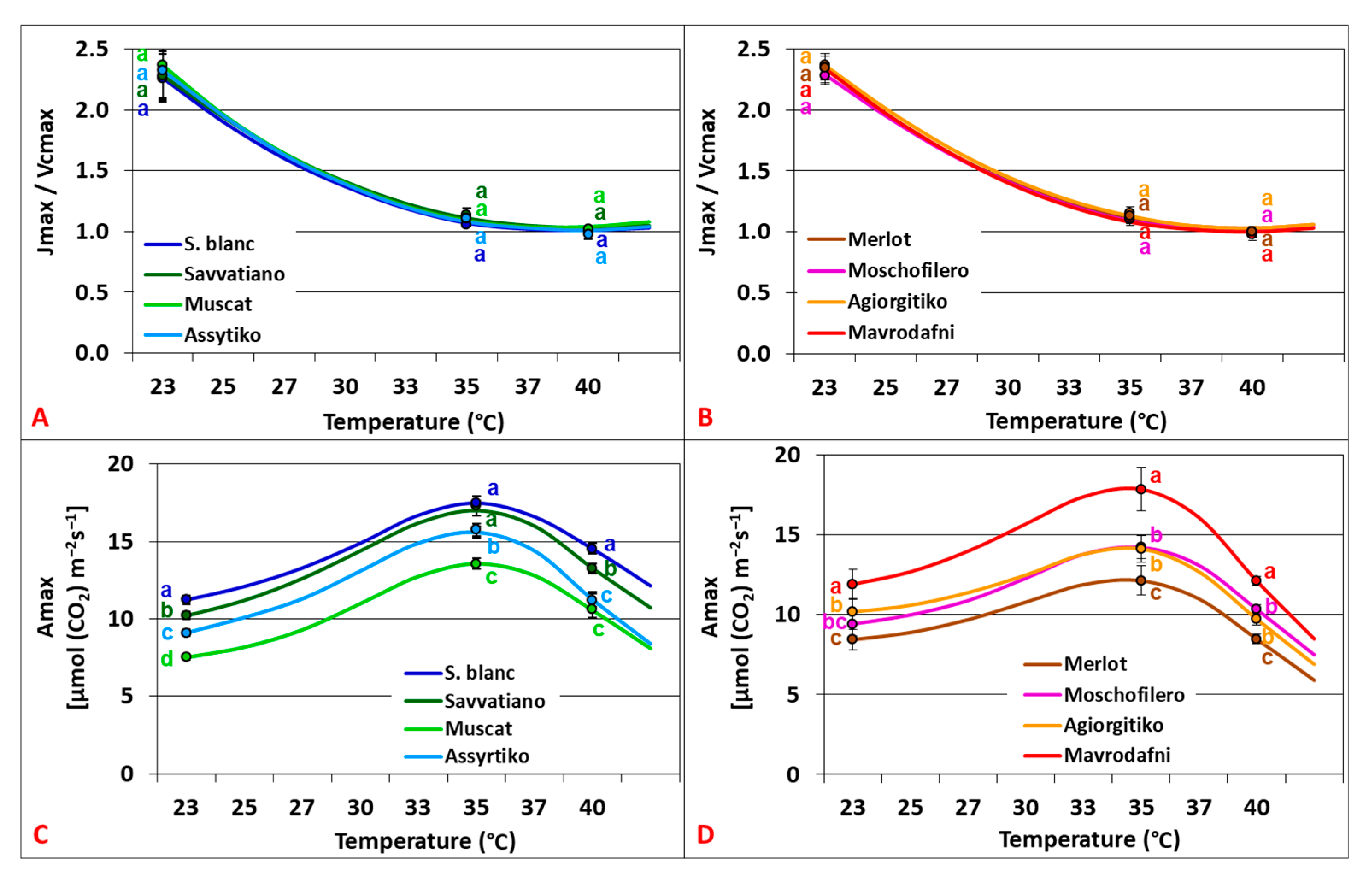
2.4. Temperature Dependence of Photosynthetic Capacity Parameters
2.5. Stomatal, Mesophyll, and Biochemical Limitations of Photosynthesis
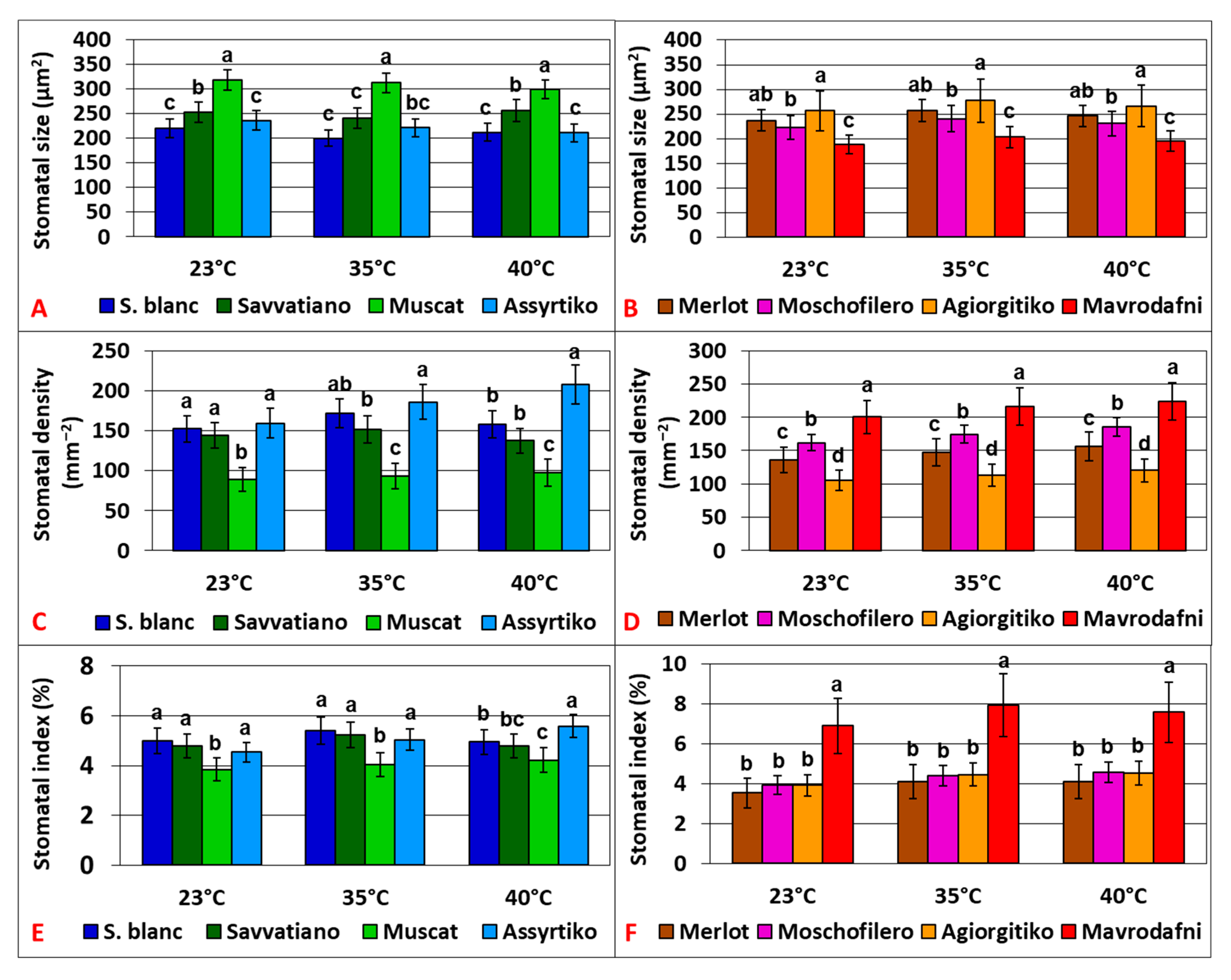
2.6. Morphological Leaf Traits Contributing to Physiological Responses
3. Discussion
3.1. Biochemical Contribution in Differential Photosynthetic Dynamics Under Elevated CO2
3.2. The Role of Stomata Traits in Modulating Photosynthesis Under Elevated CO2
3.3. WUE Benefits from CO2 Elevation
3.4. Biochemical Contribution in Photosynthetic Variations Under Ambient CO2
3.5. Stomatal Strategies and Cultivar Adaptation to Heat Stress Under Ambient CO2
3.6. The Role of Stomata Traits in Modulating Photosynthesis Under Ambient CO2
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Gas Exchange Measurements
4.3. Rapid A-Ci Response Curves (RACiR)
4.4. Biochemical Model Description
4.5. Mesophyll Conductance Estimation
4.6. Stomatal, Mesophyll, and Biochemical Limitations of Photosynthesis
4.7. Temperature Dependence Models for Curve Estimation
4.8. Stomatal Traits
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| An | Net rate of CO2 uptake |
| gs | Stomatal conductance |
| WUE | Water use efficiency |
| VPD | Vapor pressure deficit |
| a[CO2] | Ambient CO2 |
| e[CO2] | Elevated CO2 |
| Cc | Chloroplastic CO2 concentration |
| Ci | Intercellular CO2 concentration |
| Oi | Intercellular O2 concentration |
| Kc | Rubisco Michaelis constant for CO2 |
| Ko | Rubisco Michaelis constant for O2 |
| Rd | Mitochondrial respiration rate |
| Γ* | Chloroplast CO2 compensation point in the absence of mitochondrial respiration |
| gm | Mesophyll conductance |
| gi | Leaf internal conductance |
| J | Electron transport rate |
| PFD | Photosynthetic photon flux density |
| Jmax | Maximum electron transport rate |
| Vcmax | Maximum carboxylation rate |
| RuBP | Ribulose 1,5-bisphosphate |
| Ls | Stomatal limitations of photosynthesis |
| Lm | Mesophyll limitations of photosynthesis |
| Lb | Biochemical limitations of photosynthesis |
References
- Moriondo, M.; Giannakopoulos, C.; Bindi, M. Climate change impact assessment: The role of climate extremes in crop yield simulation. Clim. Change 2011, 104, 679–701. [Google Scholar] [CrossRef]
- Skinner, C.B.; Poulsen, C.J.; Mankin, J.S. Amplification of heat extremes by plant CO2 physiological forcing. Nat. Commun. 2018, 9, 1094. [Google Scholar] [CrossRef] [PubMed]
- Lippi, P.; Mattii, G.B.; Cataldo, E. Biochar, Properties and Skills with a Focus on Implications for Vineyard Land and Grapevine Performance. Phyton-Int. J. Exp. Bot. 2025, 94, 33–64. [Google Scholar] [CrossRef]
- Cataldo, E.; Fucile, M.; Mattii, G.B. Composting from Organic Municipal Solid Waste: A Sustainable Tool for the Environment and to Improve Grape Quality. J. Agric. Sci. 2022, 160, 502–515. [Google Scholar] [CrossRef]
- Venios, X.; Gkizi, D.; Nisiotou, A.; Korkas, E.; Tjamos, S.E.; Zamioudis, C.; Banilas, G. Emerging Roles of Epigenetics in Grapevine and Winegrowing. Plants. 2024, 13, 515. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Coito, J.L.; Colaço, S.; Sangiogo, M.; Amancio, S. Heat stress in grapevine: The pros and cons of acclimation. Plant Cell Environ. 2015, 38, 777–789. [Google Scholar] [CrossRef]
- Kadir, S. Thermostability of photosynthesis of Vitis aestivalis and V. vinifera. J. Am. Soc. Hortic. Sci. 2006, 131, 476–483. [Google Scholar] [CrossRef]
- Faralli, M.; Bontempo, L.; Bianchedi, P.L.; Moser, C.; Bertamini, M.; Lawson, T.; Camin, F.; Stefanini, M.; Varotto, C. Natural variation in stomatal dynamics drives divergence in heat stress tolerance and contributes to seasonal intrinsic water-use efficiency in Vitis vinifera (subsp. sativa and sylvestris). J. Exp. Bot. 2022, 73, 3238–3250. [Google Scholar] [CrossRef]
- Sinclair, G.; Galarneau, E.R.; Hnizdor, J.F.; McElrone, A.J.; Walker, M.A.; Bartlett, M.K. Grape cultivars adapted to hotter, drier growing regions exhibit greater photosynthesis in hot conditions despite less drought-resistant leaves. Ann. Bot. 2024, 134, 205–218. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Silva, M.; Coito, J.L.; Rocheta, M.P.; Amâncio, S. Design of a custom RT-qPCR array for assignment of abiotic stress tolerance in traditional Portuguese grapevine varieties. Front. Plant Sci. 2017, 8, 1835. [Google Scholar] [CrossRef]
- Biasi, R.; Brunori, E.; Ferrara, C.; Salvati, L. Assessing impacts of climate change on phenology and quality traits of Vitis vinifera L.: The contribution of local knowledge. Plants 2019, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Venios, X.; Korkas, E.; Nisiotou, A.; Banilas, G. Grapevine responses to heat stress and global warming. Plants 2020, 9, 1754. [Google Scholar] [CrossRef] [PubMed]
- Banilas, G.; Korkas, E.; Kaldis, P.; Hatzopoulos, P. Olive and grapevine biodiversity in Greece and Cyprus—A review. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer: Dordrecht, The Netherlands, 2009; Volume 2, pp. 401–428. [Google Scholar]
- Koufos, G.C.; Mavromatis, T.; Koundouras, S.; Jones, G.V. Adaptive capacity of winegrape varieties cultivated in Greece to climate change: Current trends and future projections. Oeno One 2020, 54, 1201–1219. [Google Scholar]
- Tzortzakis, N.; Chrysargyris, A.; Aziz, A. Adaptive response of a native mediterranean grapevine cultivar upon short-term exposure to drought and heat stress in the context of climate change. Agronomy 2020, 10, 249. [Google Scholar] [CrossRef]
- Grillakis, M.G.; Doupis, G.; Kapetanakis, E.; Goumenaki, E. Future shifts in the phenology of table grapes on Crete under a warming climate. Agric. For. Meteorol. 2022, 318, 108915. [Google Scholar] [CrossRef]
- Karim, M.A.; Fracheboud, Y.; Stamp, P. Effect of high temperature on seedling growth and photosynthesis of tropical maize genotypes. J. Agric. Sci. 2000, 184, 217–223. [Google Scholar] [CrossRef]
- Camejo, D.; Rodríguez, P.; Morales, M.A.; Dell’Amico, J.M.; Torrecillas, A.; Alarcón, J.J. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 2005, 162, 281–289. [Google Scholar] [CrossRef]
- Greer, D.H.; Weedon, M.M. Modelling photosynthetic responses to temperature of grapevine (Vitis vinifera cv. Semillon) leaves on vines grown in a hot climate. Plant Cell Environ. 2012, 35, 1050–1064. [Google Scholar] [CrossRef]
- Luo, H.B.; Ma, L.; Xi, H.F.; Duan, W.; Li, S.H.; Loescher, W.; Wang, J.F.; Wang, L.J. Photosynthetic responses to heat treatments at different temperatures and following recovery in grapevine (Vitis amurensis L.) leaves. PLoS ONE 2011, 6, 23033. [Google Scholar] [CrossRef]
- Greer, D.H. Modelling seasonal changes in the temperature-dependency of CO2 photosynthetic responses in two Vitis vinifera cultivars. Funct. Plant Biol. 2017, 45, 315–327. [Google Scholar] [CrossRef]
- Gallo, A.E.; Peña, J.E.P.; Prieto, J.A. Mechanisms underlying photosynthetic acclimation to high temperature are different between Vitis vinifera cv. Syrah and Grenache. Funct. Plant Biol. 2020, 48, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Grossiord, C.; Buckley, T.N.; Cernusak, L.A.; Novick, K.A.; Poulter, B.; Siegwolf, R.T.; Sperry, J.S.; McDowell, N.G. Plant responses to rising vapor pressure deficit. New Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.K.; Takahashi, Y.; Merilo, E.; Costa, A.; Zhang, L.; Kernig, K.; Lee, K.H.; Schroeder, J.I. Raf-like kinases and receptor-like (pseudo) kinase GHR1 are required for stomatal vapor pressure difference response. Proc. Natl. Acad. Sci. USA 2021, 118, 2107280118. [Google Scholar] [CrossRef]
- Tomás, M.; Medrano, H.; Escalona, J.M.; Martorell, S.; Pou, A.; Ribas-Carbó, M.; Flexas, J. Variability of water use efficiency in grapevines. Environ. Exp. Bot. 2014, 103, 148–157. [Google Scholar] [CrossRef]
- Leakey, A.D.; Ferguson, J.N.; Pignon, C.P.; Wu, A.; Jin, Z.; Hammer, G.L.; Lobell, D.B. Water use efficiency as a constraint and target for improving the resilience and productivity of C3 and C4 crops. Annu. Rev. Plant Biol. 2019, 70, 781–808. [Google Scholar] [CrossRef]
- Soar, C.J.; Collins, M.J.; Sadras, V.O. Irrigated Shiraz vines (Vitis vinifera) upregulate gas exchange and maintain berry growth in response to short spells of high maximum temperature in the field. Funct. Plant Biol. 2009, 36, 801–814. [Google Scholar] [CrossRef]
- Makino, A.; Mae, T. Photosynthesis and Plant Growth at Elevated Levels of CO2. Plant Cell Physiol. 1999, 40, 999–1006. [Google Scholar] [CrossRef]
- van der Kooi, C.J.; Reich, M.; Löw, M.; de Kok, L.J.; Tausz, M. Growth and Yield Stimulation under Elevated CO2 and Drought: A Meta-Analysis on Crops. Environ. Exp. Bot. 2016, 122, 150–157. [Google Scholar] [CrossRef]
- Moutinho-Pereira, J.; Gonçalves, B.; Bacelar, E.; Cunha, J.B.; Countinho, J.; Correira, C.M. Effects of elevated CO2 on grapevine (Vitis vinifera L.): Physiological and yield attributes. VITIS-J. Grapevine Res. 2015, 48, 159. [Google Scholar]
- Edwards, E.J.; Unwin, D.; Kilmister, R.; Treeby, M. Multi-seasonal effects of warming and elevated CO2 on the physiology, growth and production of mature, field grown, Shiraz grapevines. Oeno One 2017, 51, 127–132. [Google Scholar] [CrossRef]
- Salazar-Parra, C.; Aguirreolea, J.; Sánchez-Díaz, M.; Irigoyen, J.J.; Morales, F. Photosynthetic response of Tempranillo grapevine to climate change scenarios. Ann. Appl. Biol. 2012, 161, 277–292. [Google Scholar] [CrossRef]
- Yang, J.; Feng, Y.; Chi, T.; Wen, Q.; Liang, P.; Wang, A.; Li, P. Mitigation of elevated CO2 concentration on warming-induced changes in wheat is limited under extreme temperature during the grain filling period. Agronomy 2023, 13, 1379. [Google Scholar] [CrossRef]
- Bindi, M.; Fibbi, L.; Miglietta, F. Free Air CO2 Enrichment (FACE) of grapevine (Vitis vinifera L.): II. Growth and quality of grape and wine in response to elevated CO2 concentrations. Eur. J. Agron. 2001, 14, 145–155. [Google Scholar] [CrossRef]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Rising atmospheric carbon dioxide: Plants FACE the future. Annu. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef]
- Nowak, R.S.; Ellsworth, D.S.; Smith, S.D. Functional responses of plants to elevated atmospheric CO2–do photosynthetic and productivity data from FACE experiments support early predictions? New Phytol. 2004, 162, 253–280. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Cen, Y.P.; Sage, R.F. The regulation of Rubisco activity in response to variation in temperature and atmospheric CO2 partial pressure in sweet potato. Plant Physiol. 2005, 139, 979–990. [Google Scholar] [CrossRef]
- Sage, R.F.; Kubien, D.S. The temperature response of C3 and C4 photosynthesis. Plant Cell Environ. 2007, 30, 1086–1106. [Google Scholar] [CrossRef]
- Bagley, J.; Rosenthal, D.M.; Ruiz-Vera, U.M.; Siebers, M.H.; Kumar, P.; Ort, D.R.; Bernacchi, C.J. The influence of photosynthetic acclimation to rising CO2 and warmer temperatures on leaf and canopy photosynthesis models. Glob. Biogeochem. Cycles 2015, 29, 194–206. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Rosenthal, D.M.; Ruiz-Vera, U.M.; Siebers, M.H.; Gray, S.B.; Bernacchi, C.J.; Ort, D.R. Biochemical acclimation, stomatal limitation and precipitation patterns underlie decreases in photosynthetic stimulation of soybean (Glycine max) at elevated [CO2] and temperatures under fully open air field conditions. Plant Sci. J. 2014, 226, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Bernacchi, C.J.; Morgan, P.B.; Ort, D.R.; Long, S.P. The Growth of Soybean under Free-Air CO2 Enrichment (FACE) Stimulates Photosynthesis while Decreasing In Vivo Rubisco Capacity. Planta 2005, 220, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Merilo, E.; Laanemets, K.; Waadt, R.; Pater, D.; Kollist, H.; Schroeder, J.I. Abscisic Acid-Independent Stomatal CO2 Signal Transduction Pathway and Convergence of CO2 and ABA Signaling Downstream of OST1 Kinase. Proc. Natl. Acad. Sci. USA 2018, 115, E9971–E9980. [Google Scholar] [CrossRef] [PubMed]
- Schultz, H.R.; Stoll, M. Some Critical Issues in Environmental Physiology of Grapevines: Future Challenges and Limitations. Aust. J. Grape Wine Res. 2010, 16, 4–24. [Google Scholar] [CrossRef]
- Leibar, U.; Aizpurua, A.; Unamunzaga, O.; Pascual, I.; Morales, F. How Will Climate Change Influence Grapevine cv. Tempranillo Photosynthesis under Different Soil Textures? Photosynth. Res. 2015, 124, 199–215. [Google Scholar] [CrossRef]
- Purcell, C.; Batke, S.P.; Yiotis, C.; Caballero, R.; Soh, W.K.; Murray, M.; McElwain, J.C. Increasing stomatal conductance in response to rising atmospheric CO2. Ann. Bot. 2018, 121, 1137–1149. [Google Scholar] [CrossRef]
- Wohlfahrt, Y.; Smith, J.P.; Tittmann, S.; Honermeier, B.; Stoll, M. Primary productivity and physiological responses of Vitis vinifera L. cvs. under Free Air Carbon dioxide Enrichment (FACE). Eur. J. Agron. 2018, 101, 149–162. [Google Scholar] [CrossRef]
- Lammertsma, E.I.; Boer, H.J.D.; Dekker, S.C.; Dilcher, D.L.; Lotter, A.F.; Wagner-Cremer, F. Global CO2 rise leads to reduced maximum stomatal conductance in Florida vegetation. Proc. Natl. Acad. Sci. USA 2011, 108, 4035–4040. [Google Scholar] [CrossRef]
- Monda, K.; Araki, H.; Kuhara, S.; Ishigaki, G.; Akashi, R.; Negi, J.; Kojima, M.; Sakakibara, H.; Takahashi, S.; Hashimoto-Sugimoto, M.; et al. Enhanced stomatal conductance by a spontaneous Arabidopsis tetraploid, Me-0, results from increased stomatal size and greater stomatal aperture. Plant Physiol. 2016, 170, 1435–1444. [Google Scholar] [CrossRef]
- Tang, X.; Zhao, J.; Zhou, J.; Zhu, Q.; Sheng, X.; Yue, C. Elevated CO2 Shifts Photosynthetic Constraint from Stomatal to Biochemical Limitations During Induction in Populus tomentosa and Eucalyptus robusta. Plants 2024, 14, 47. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Kozikova, D.; Goicoechea, N.; Pascual, I. Elevated CO2 alleviates the exacerbation of evapotranspiration rates of grapevine (Vitis vinifera) under elevated temperature. Agric. Water Manag. 2024, 302, 108971. [Google Scholar] [CrossRef]
- Zuniga, A.; Gaudin, A.C.; Gilbert, M.E.; Clemens, M.E.; Zona, D.; Oechel, W.C. Vitis vinifera L. varieties (cv. Cabernet Sauvignon and Chardonnay) vary in leaf water flux in response to elevated CO2 growing conditions and a gradual water deficit. AoB Plants 2025, 17, plaf011. [Google Scholar] [CrossRef]
- Franks, P.J.; Beerling, D.J. CO2-forced evolution of plant gas exchange capacity and water-use efficiency over the Phanerozoic. Geobiology 2009, 7, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Drake, P.L.; Froend, R.H.; Franks, P.J. Smaller, faster stomata: Scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot. 2013, 64, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, K.; Ishikawa, K.; Borjigidai, A.; Muller, O.; Onoda, Y. Temperature acclimation of photosynthesis: Mechanisms involved in the changes in temperature dependence of photosynthetic rate. J. Exp. Bot. 2006, 57, 291–302. [Google Scholar] [CrossRef]
- Bernacchi, C.J.; Pimentel, C.; Long, S.P. In vivo temperature response functions of parameters required to model RuBP-limited photosynthesis. Plant Cell Environ. 2003, 26, 1419–1430. [Google Scholar] [CrossRef]
- Yamori, W.; Noguchi, K.O.; Terashima, I. Temperature acclimation of photosynthesis in spinach leaves: Analyses of photosynthetic components and temperature dependencies of photosynthetic partial reactions. Plant Cell Environ. 2005, 28, 536–547. [Google Scholar] [CrossRef]
- Kattge, J.; Knorr, W. Temperature acclimation in a biochemical model of photosynthesis: A reanalysis of data from 36 species. Plant Cell Environ. 2007, 30, 1176–1190. [Google Scholar] [CrossRef]
- Greer, D.H. Stomatal and non-stomatal limitations at different leaf temperatures to the photosynthetic process during the post-harvest period for Vitis vinifera cv. Chardonnay vines. N. Z. J. Crop Hortic. Sci. 2020, 48, 1–21. [Google Scholar] [CrossRef]
- Crous, K.Y.; Uddling, J.; De Kauwe, M.G. Temperature responses of photosynthesis and respiration in evergreen trees from boreal to tropical latitudes. New Phytol. 2022, 234, 353–374. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, E.; Fucile, M.; Manzi, D.; Peruzzi, E.; Mattii, G.B. Effects of Zeowine and compost on leaf functionality and berry composition in Sangiovese grapevines. J. Agric. Sci. 2023, 161, 412–427. [Google Scholar] [CrossRef]
- Alsina, M.M.; Smart, D.R.; Bauerle, T.; De Herralde, F.; Biel, C.; Stockert, C.; Negron, C.; Save, R. Seasonal changes of whole root system conductance by a drought-tolerant grape root system. J. Exp. Bot. 2011, 62, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, M.K.; Sinclair, G. Temperature and evaporative demand drive variation in stomatal and hydraulic traits across grape cultivars. J. Exp. Bot. 2021, 72, 1995–2009. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Greer, D.H.; Hatfield, J.M.; Hutton, R.J.; Clarke, S.J.; Hutchinson, P.A.; Somers, A. Stomatal response of an anisohydric grapevine cultivar to evaporative demand, available soil moisture and abscisic acid. Tree Physiol. 2012, 32, 249–261. [Google Scholar] [CrossRef]
- Yu, D.J.; Kim, S.J.; Lee, H.J. Stomatal and non-stomatal limitations to photosynthesis in field-grown grapevine cultivars. Biol. Plant. 2009, 53, 133–137. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Greer, D.H.; Hutton, R.J.; Clarke, S.J. Transpiration efficiency of the grapevine cv. Semillon is tied to VPD in warm climates. Ann. Appl. Biol. 2011, 158, 106–114. [Google Scholar] [CrossRef]
- Collins, M.J.; Fuentes, S.; Barlow, E.W. Partial rootzone drying and deficit irrigation increase stomatal sensitivity to vapour pressure deficit in anisohydric grapevines. Funct. Plant Biol. 2010, 37, 128–138. [Google Scholar] [CrossRef]
- Soar, C.J.; Speirs, J.; Maffei, S.M.; Penrose, A.B.; McCarthy, M.G.; Loveys, B.R. Grape vine varieties Shiraz and Grenache differ in their stomatal response to VPD: Apparent links with ABA physiology and gene expression in leaf tissue. Aust. J. Grape Wine Res. 2006, 12, 2–12. [Google Scholar] [CrossRef]
- Hochberg, U.; Rockwell, F.E.; Holbrook, N.M.; Cochard, H. Iso/Anisohydry: A Plant–Environment Interaction Rather than a Simple Hydraulic Trait. Trends Plant Sci. 2018, 23, 112–120. [Google Scholar] [CrossRef]
- Keller, M. The Science of Grapevines, 3rd ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 179–294. [Google Scholar]
- Tanaka, Y.; Sugano, S.S.; Shimada, T.; Hara-Nishimura, I. Enhancement of leaf photosynthetic capacity through increased stomatal density in Arabidopsis. New Phytol. 2013, 198, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyama, Y.; Wakabayashi, Y.; Mercer, K.L.; Kawabata, S.; Kobayashi, T.; Tabuchi, T.; Yamori, W. Natural genetic variation in dynamic photosynthesis is correlated with stomatal anatomical traits in diverse tomato species across geographical habitats. J. Exp. Bot. 2024, 75, 6762–6777. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Dun, Z.; Wang, Y.; Yang, D.; Xiong, D.; Cui, K.; Peng, S.; Huang, J. Effect of stomatal morphology on leaf photosynthetic induction under fluctuating light in rice. Front. Plant Sci. 2022, 12, 754790. [Google Scholar] [CrossRef]
- Yin, Q.; Tian, T.; Kou, M.; Liu, P.; Wang, L.; Hao, Z.; Yue, M. The relationships between photosynthesis and stomatal traits on the Loess Plateau. Glob. Ecol. Conserv. 2020, 23, 01146. [Google Scholar] [CrossRef]
- Segev, R.; Nannapaneni, R.; Sindurakar, P.; Kim, H.; Read, H.; Lijek, S. The effect of stomatal index on the net rate of photosynthesis in the leaves of Spinacia oleracea, Vinca minor, Rhododendron spp, Epipremnum aureum and Hedera spp. J. Emerg. Investig 2015, 20, 2018. [Google Scholar] [CrossRef]
- Lawrence, E.H.; Stinziano, J.R.; Hanson, D.T. Using the rapid A-Ci response (RACiR) in the Li-Cor 6400 to measure developmental gradients of photosynthetic capacity in poplar. Plant Cell Environ. 2019, 42, 740–750. [Google Scholar] [CrossRef]
- Ethier, G.J.; Livingston, N.J. On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar–von Caemmerer–Berry leaf photosynthesis model. Plant Cell Environ. 2004, 27, 137–153. [Google Scholar] [CrossRef]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef]
- Bernacchi, C.J.; Portis, A.R.; Nakano, H.; Von Caemmerer, S.; Long, S.P. Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. J. Plant Physiol. 2002, 130, 1992–1998. [Google Scholar] [CrossRef]
- Pinelli, P.; Loreto, F. 12CO2 emission from different metabolic pathways measured in illuminated and darkened C3 and C4 leaves at low, atmospheric and elevated CO2 concentration. J. Exp. Bot. 2003, 54, 1761–1769. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal Conductance and Photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Johnson, F.H.; Eyring, H.; Williams, R.W. The nature of enzyme inhibitors in bacterial luminescence: Sulfanilamide, urethane, temperature and pressure. J. Cell. Physiol. 1942, 20, 247–268. [Google Scholar] [CrossRef]
- Medlyn, B.E.; Dreyer, E.; Ellsworth, D.; Forstreuter, M.; Harley, P.C.; Kirschbaum, M.U.F.; Le Roux, X.; Montpied, P.; Strassemeyer, J.; Walcroft, A.; et al. Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant Cell Environ. 2002, 25, 1167–1179. [Google Scholar] [CrossRef]
- June, T.; Evans, J.R.; Farquhar, G.D. A simple new equation for the reversible temperature dependence of photosynthetic electron transport: A study on soybean leaf. Funct. Plant Biol. 2004, 31, 275–283. [Google Scholar] [CrossRef]
- Taylor, S.H.; Franks, P.J.; Hulme, S.P.; Spriggs, E.; Christin, P.A.; Edwards, E.J.; Woodward, F.I.; Osborne, C.P. Photosynthetic pathway and ecological adaptation explain stomatal trait diversity amongst grasses. New Phytol. 2012, 193, 387–396. [Google Scholar] [CrossRef]
- Zhu, C.; Hu, Y.; Mao, H.; Li, S.; Li, F.; Zhao, C.; Luo, L.; Liu, W.; Yuan, X. A deep learning-based method for automatic assessment of stomatal index in wheat microscopic images of leaf epidermis. Front. Plant Sci. 2021, 12, 716784. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venios, X.; Banilas, G.; Beris, E.; Biniari, K.; Korkas, E. Physiological Efficiency and Adaptability of Greek Indigenous Grapevine Cultivars Under Heat Stress and Elevated CO2: Insights into Photosynthetic Dynamics. Plants 2025, 14, 2518. https://doi.org/10.3390/plants14162518
Venios X, Banilas G, Beris E, Biniari K, Korkas E. Physiological Efficiency and Adaptability of Greek Indigenous Grapevine Cultivars Under Heat Stress and Elevated CO2: Insights into Photosynthetic Dynamics. Plants. 2025; 14(16):2518. https://doi.org/10.3390/plants14162518
Chicago/Turabian StyleVenios, Xenophon, Georgios Banilas, Evangelos Beris, Katerina Biniari, and Elias Korkas. 2025. "Physiological Efficiency and Adaptability of Greek Indigenous Grapevine Cultivars Under Heat Stress and Elevated CO2: Insights into Photosynthetic Dynamics" Plants 14, no. 16: 2518. https://doi.org/10.3390/plants14162518
APA StyleVenios, X., Banilas, G., Beris, E., Biniari, K., & Korkas, E. (2025). Physiological Efficiency and Adaptability of Greek Indigenous Grapevine Cultivars Under Heat Stress and Elevated CO2: Insights into Photosynthetic Dynamics. Plants, 14(16), 2518. https://doi.org/10.3390/plants14162518




