Partial Replacement of Peat: Effects on Substrate Physico-Hydrological Properties and Sage Growth
Abstract
1. Introduction
1.1. Wood Fiber
1.2. Coffee Silverskin
1.3. Brewer’s Spent Grain
1.4. Hydrophysical Recommended Ranges
1.5. Ornamental Sages
1.6. Aim of Research
2. Results
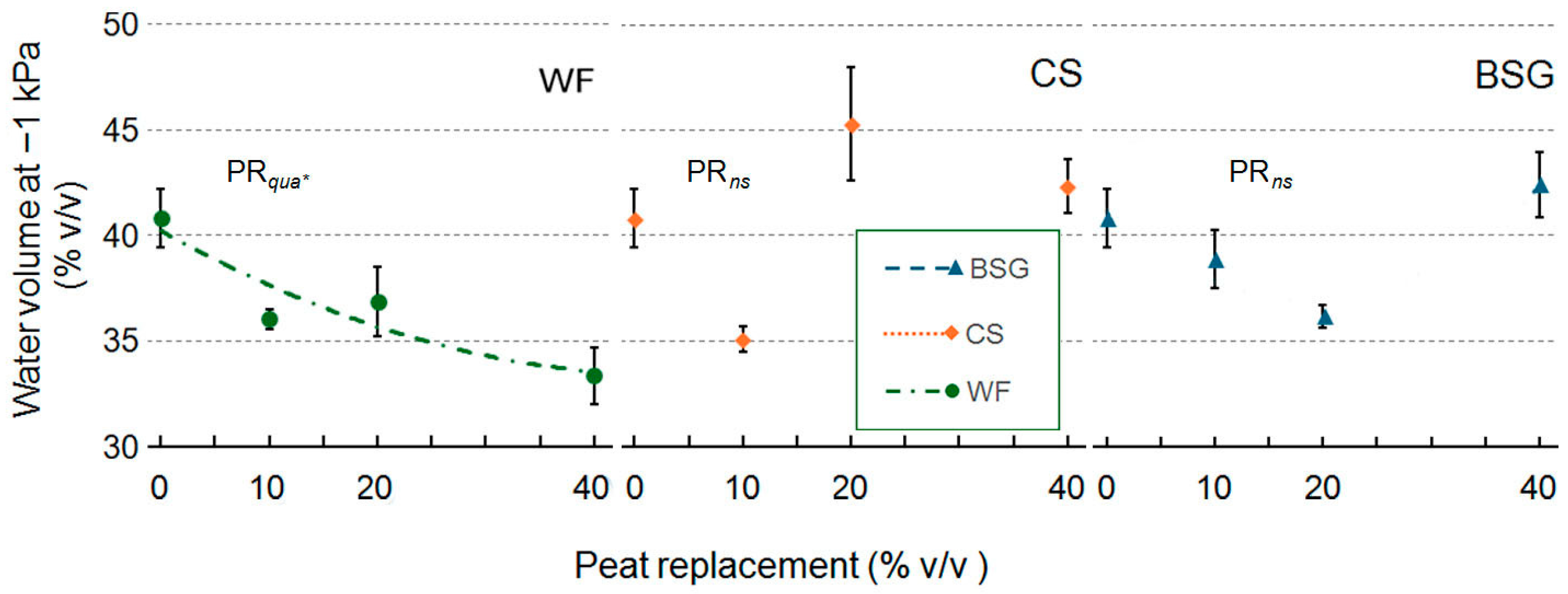
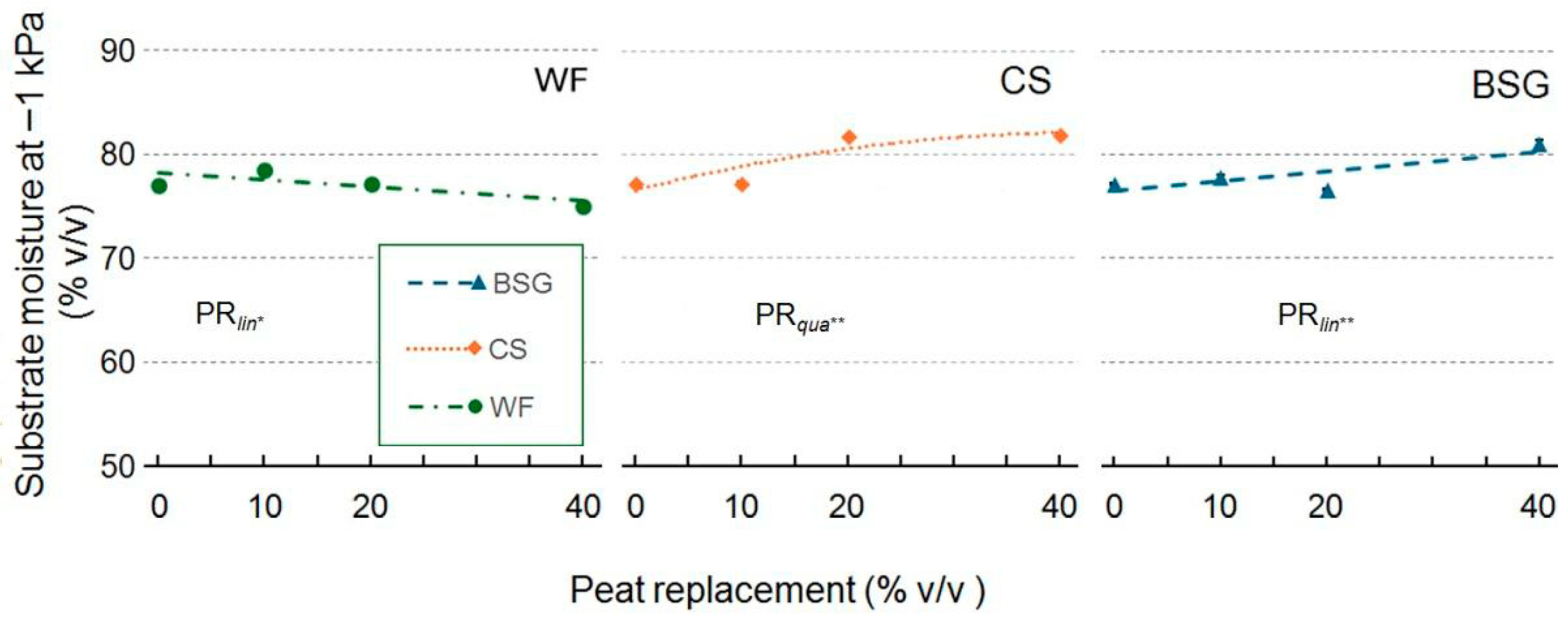
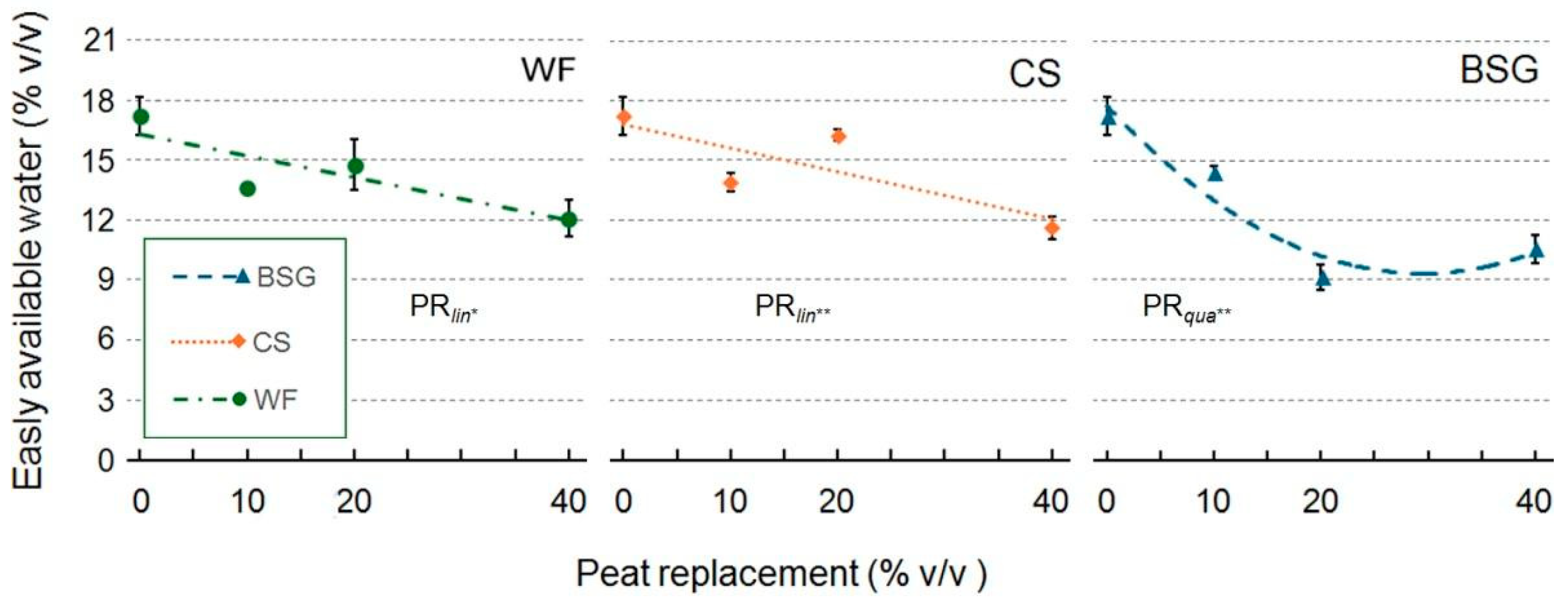
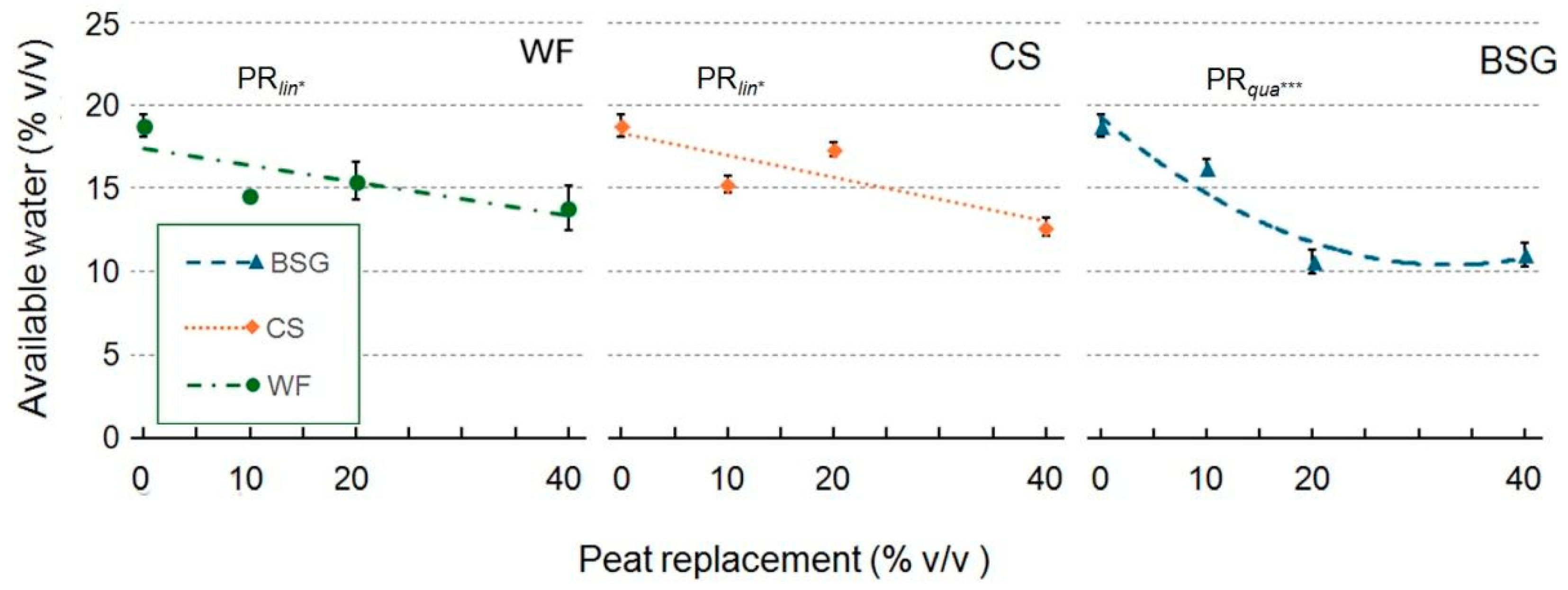
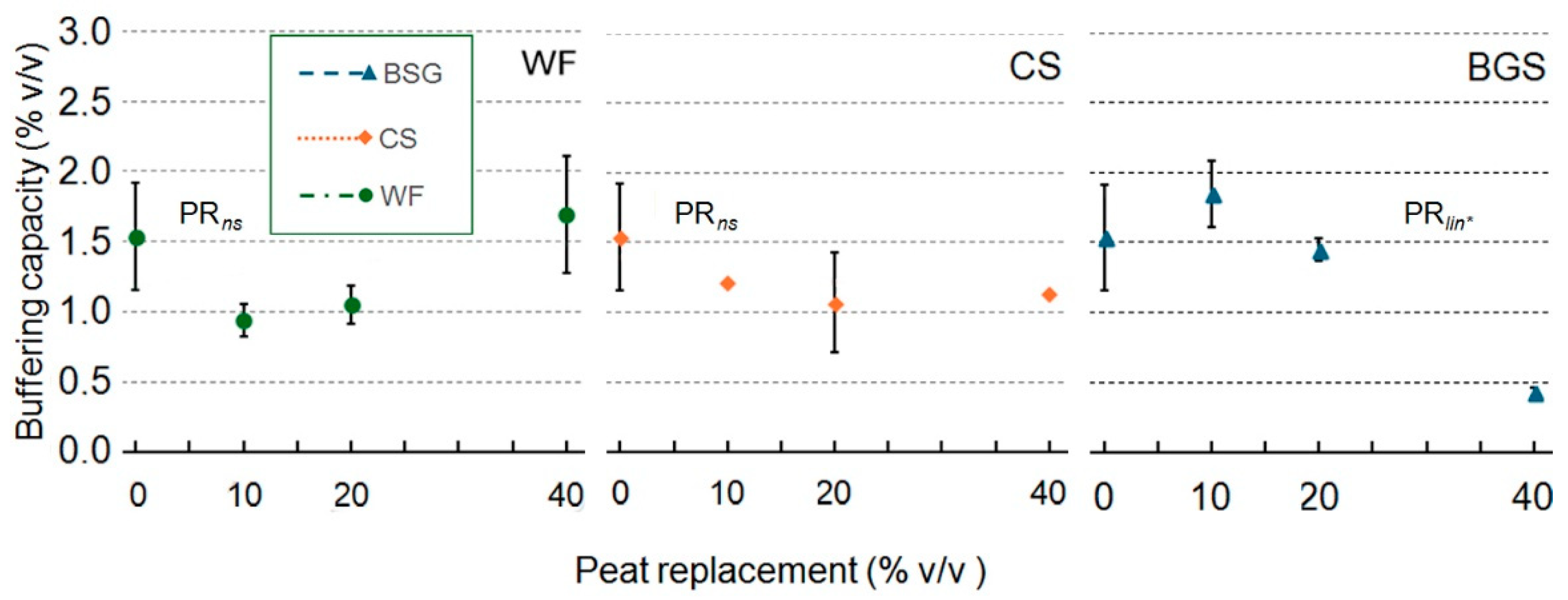
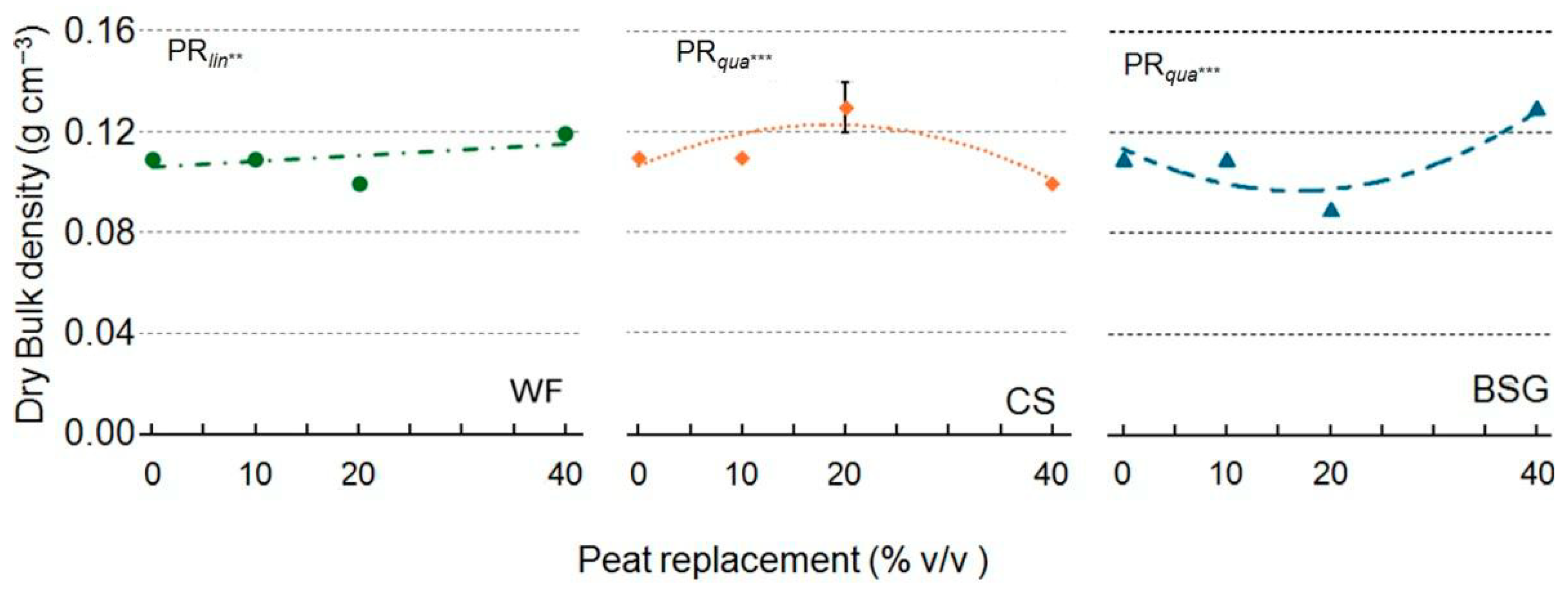
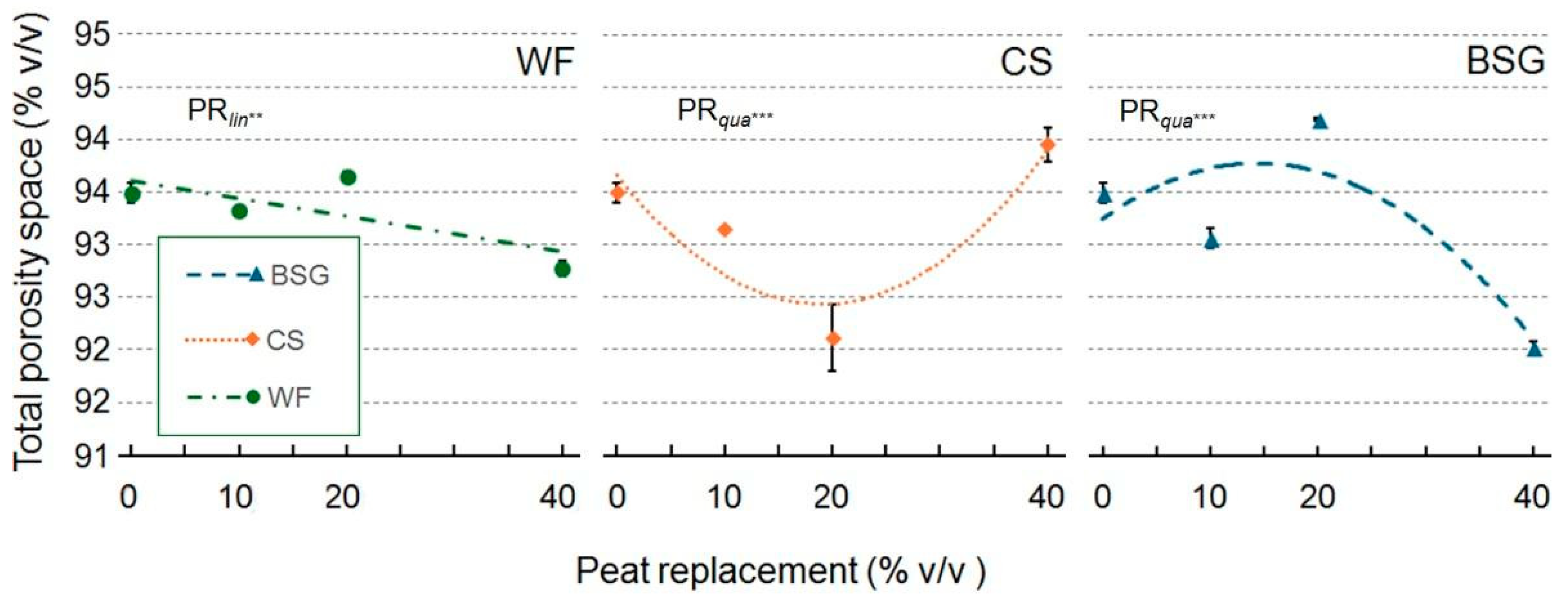
2.1. Physical and Hydrological Characterization
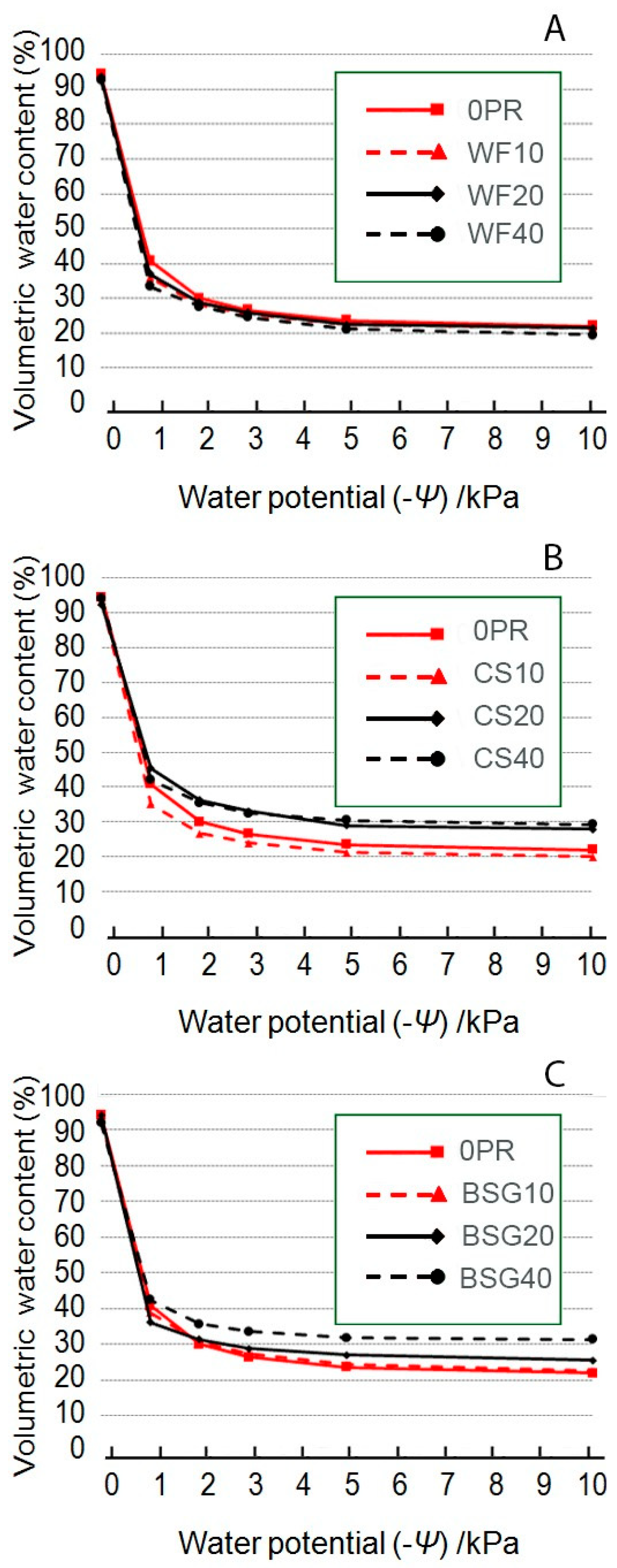
2.2. Volumetric Water Content
2.3. Chemical Characteristics of Substrates
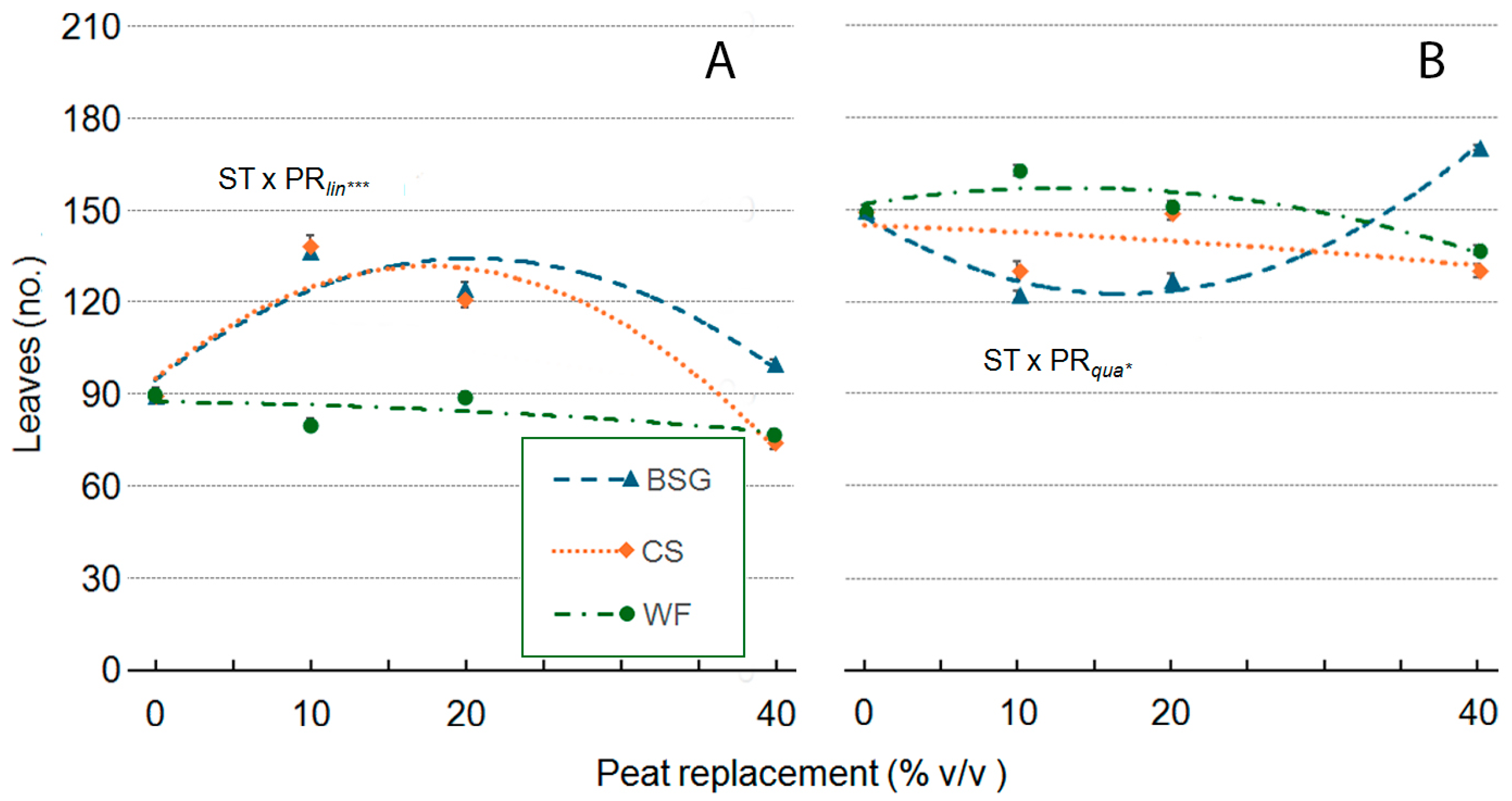
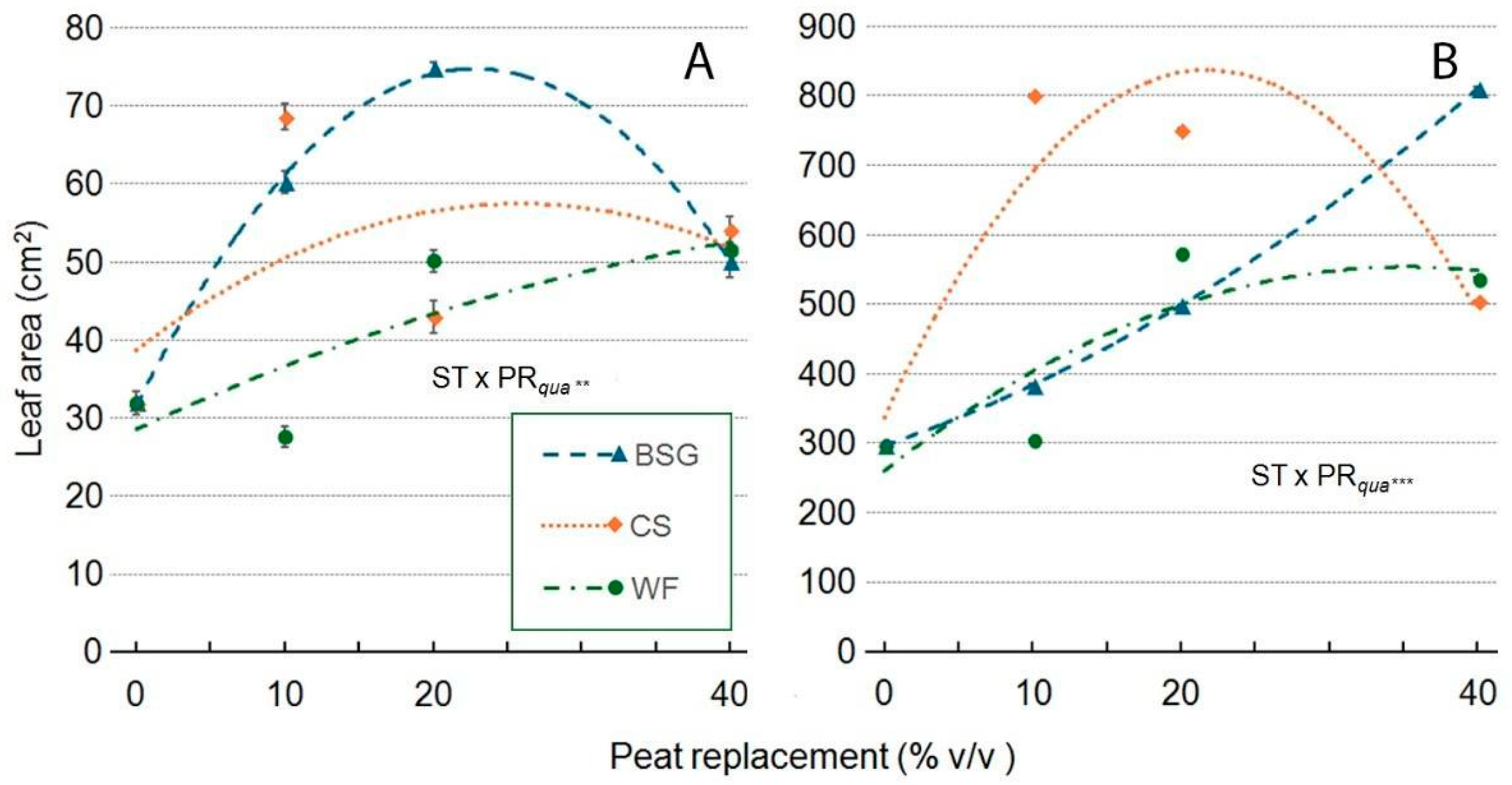
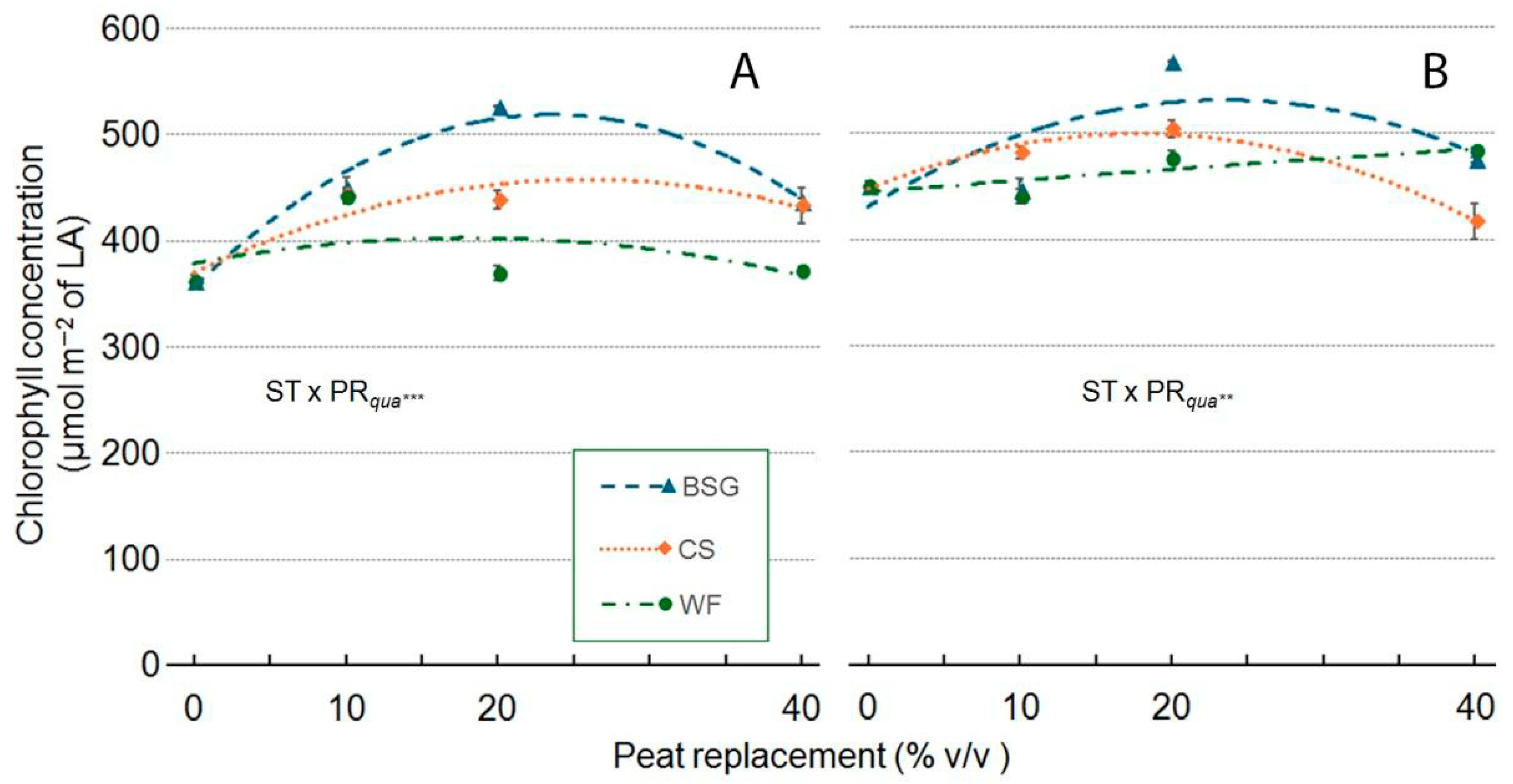
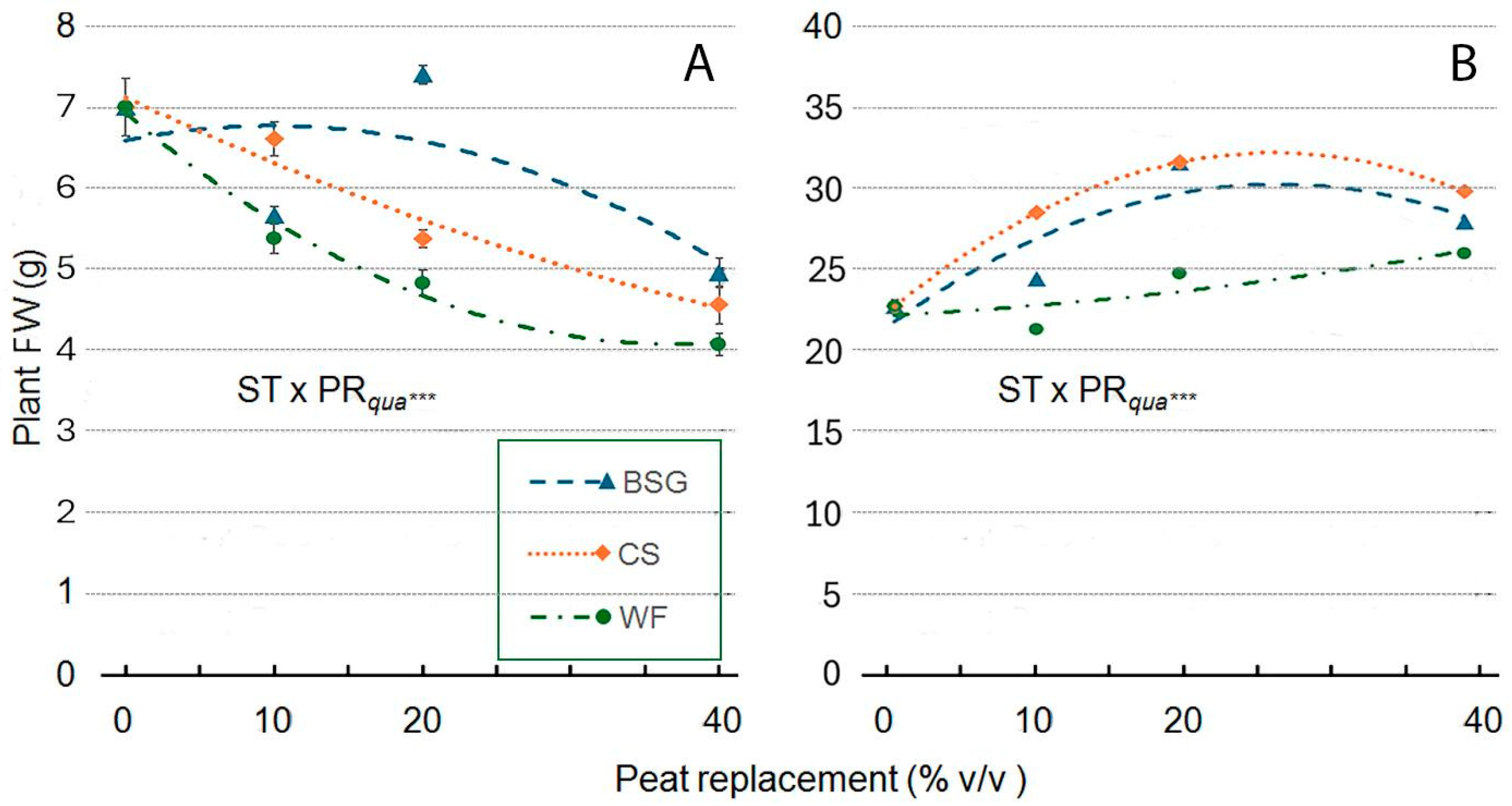
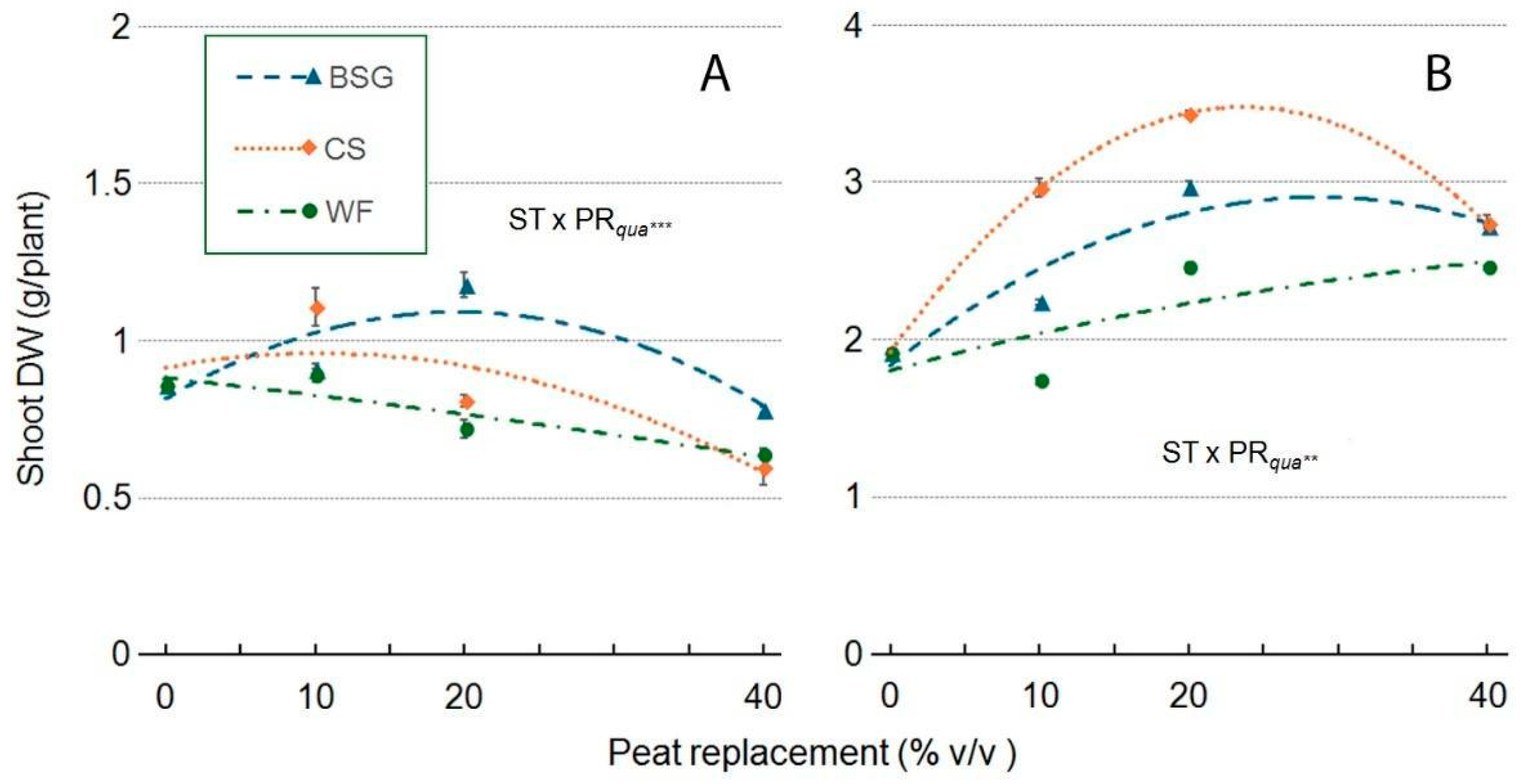
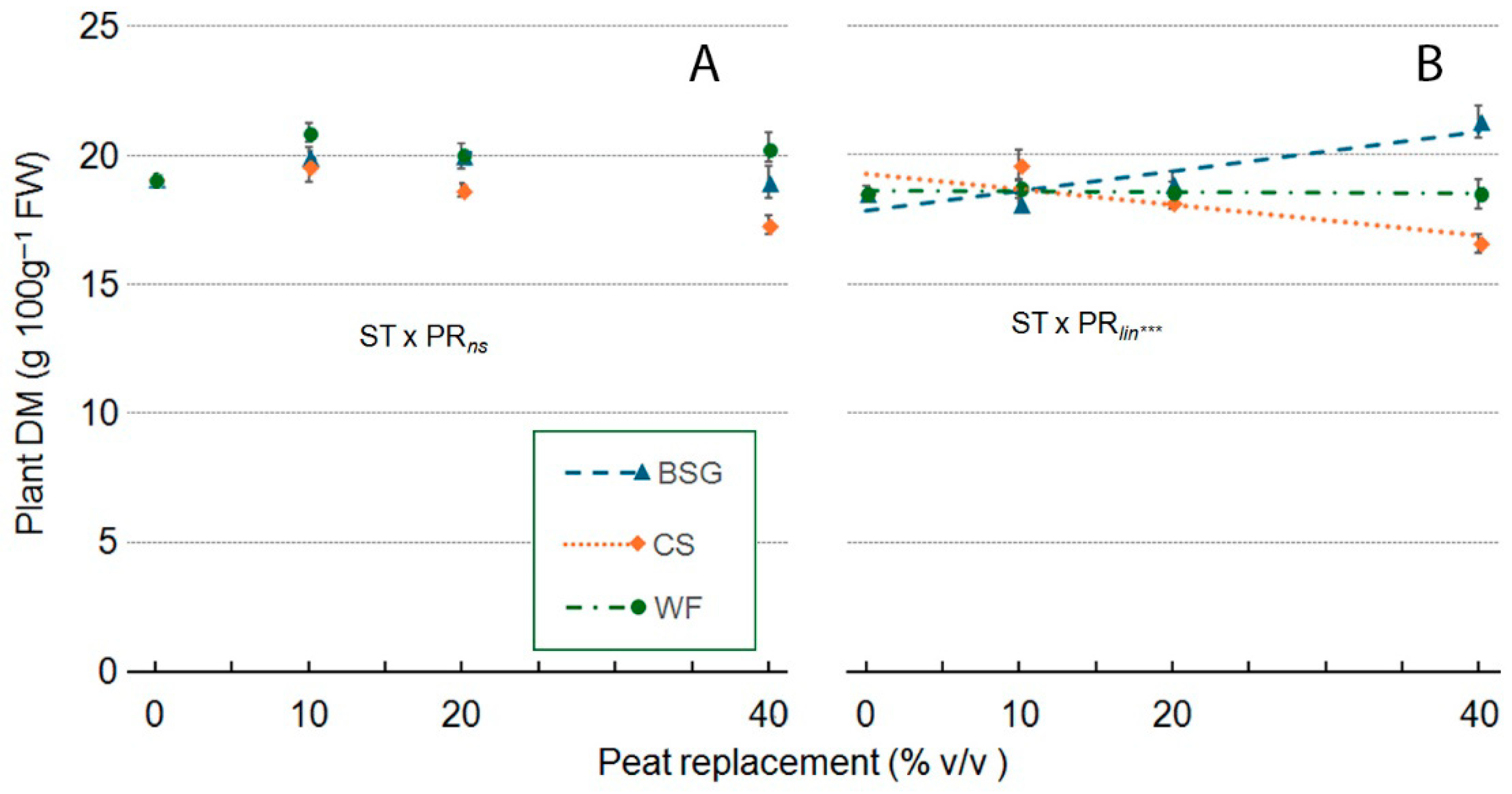
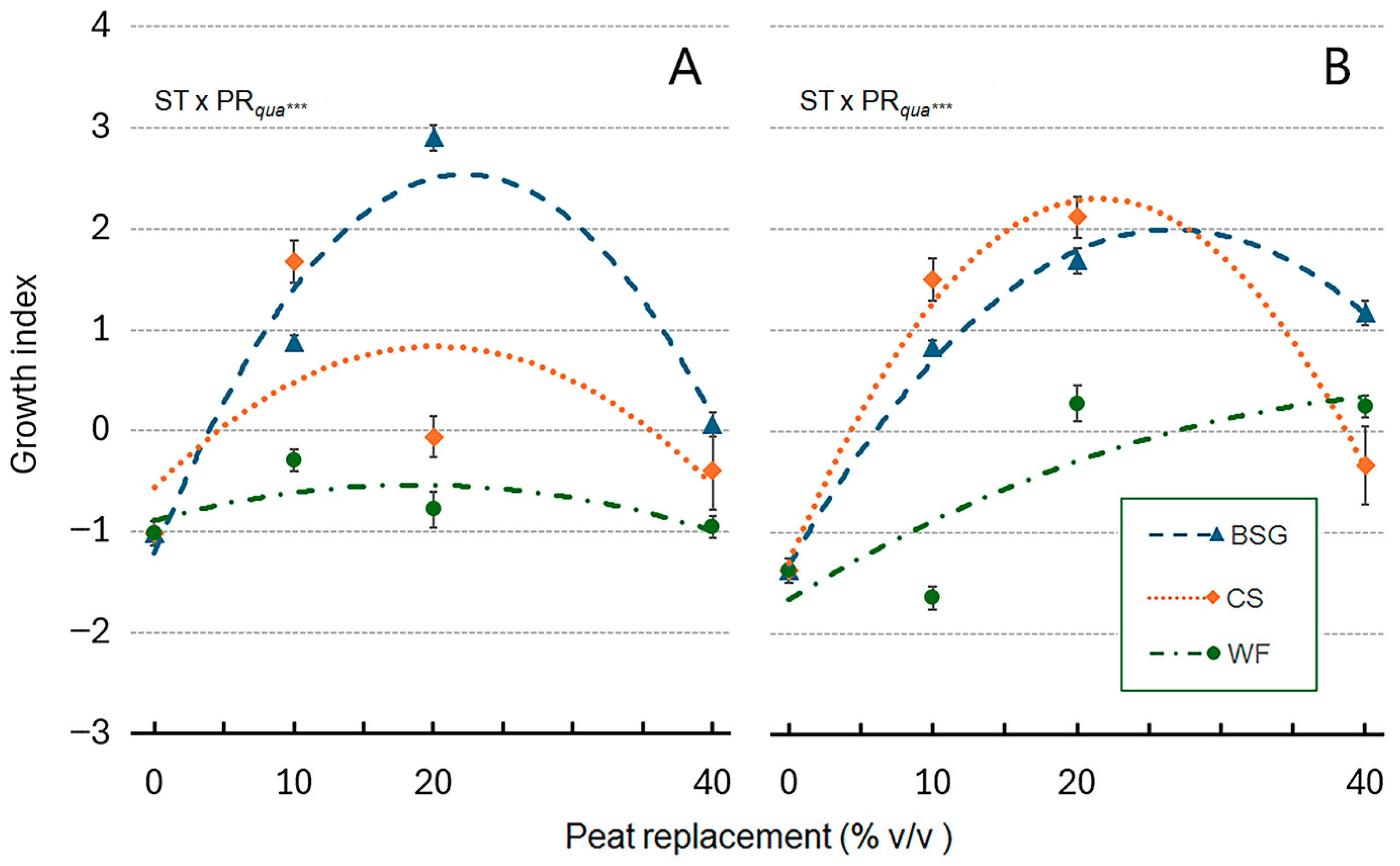
2.4. Sage Growth
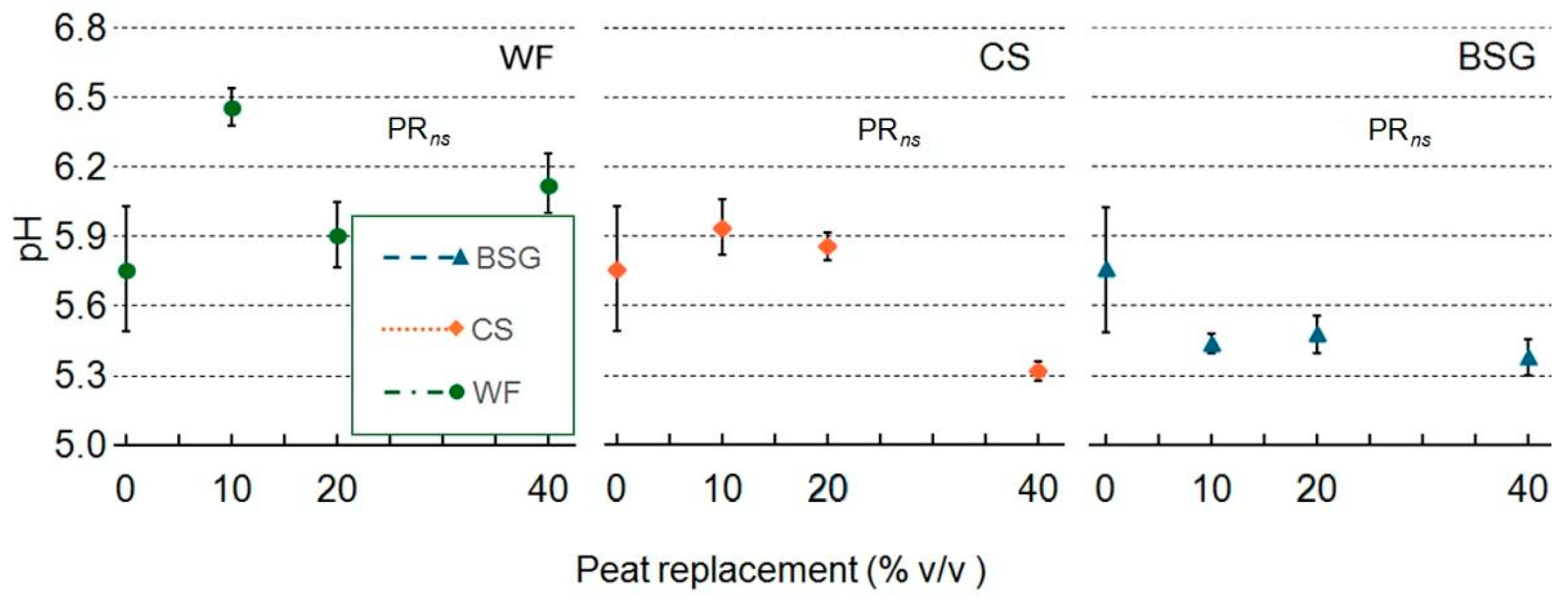
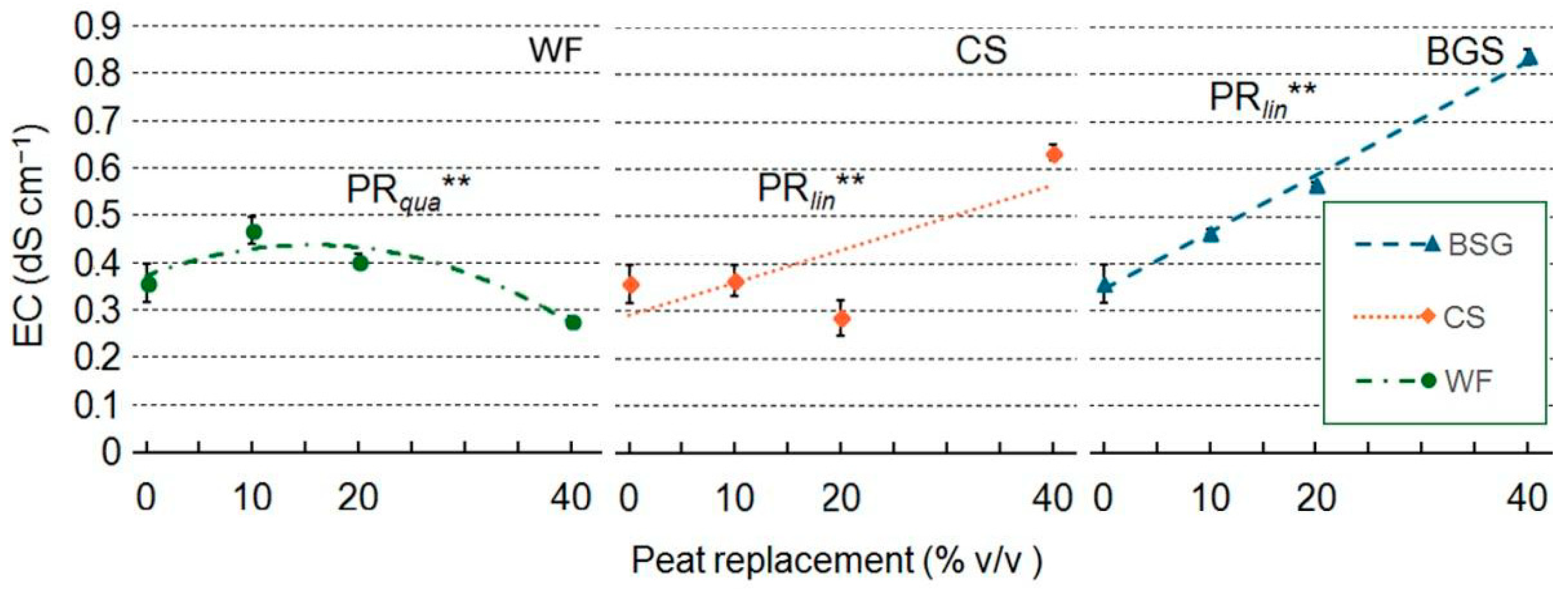
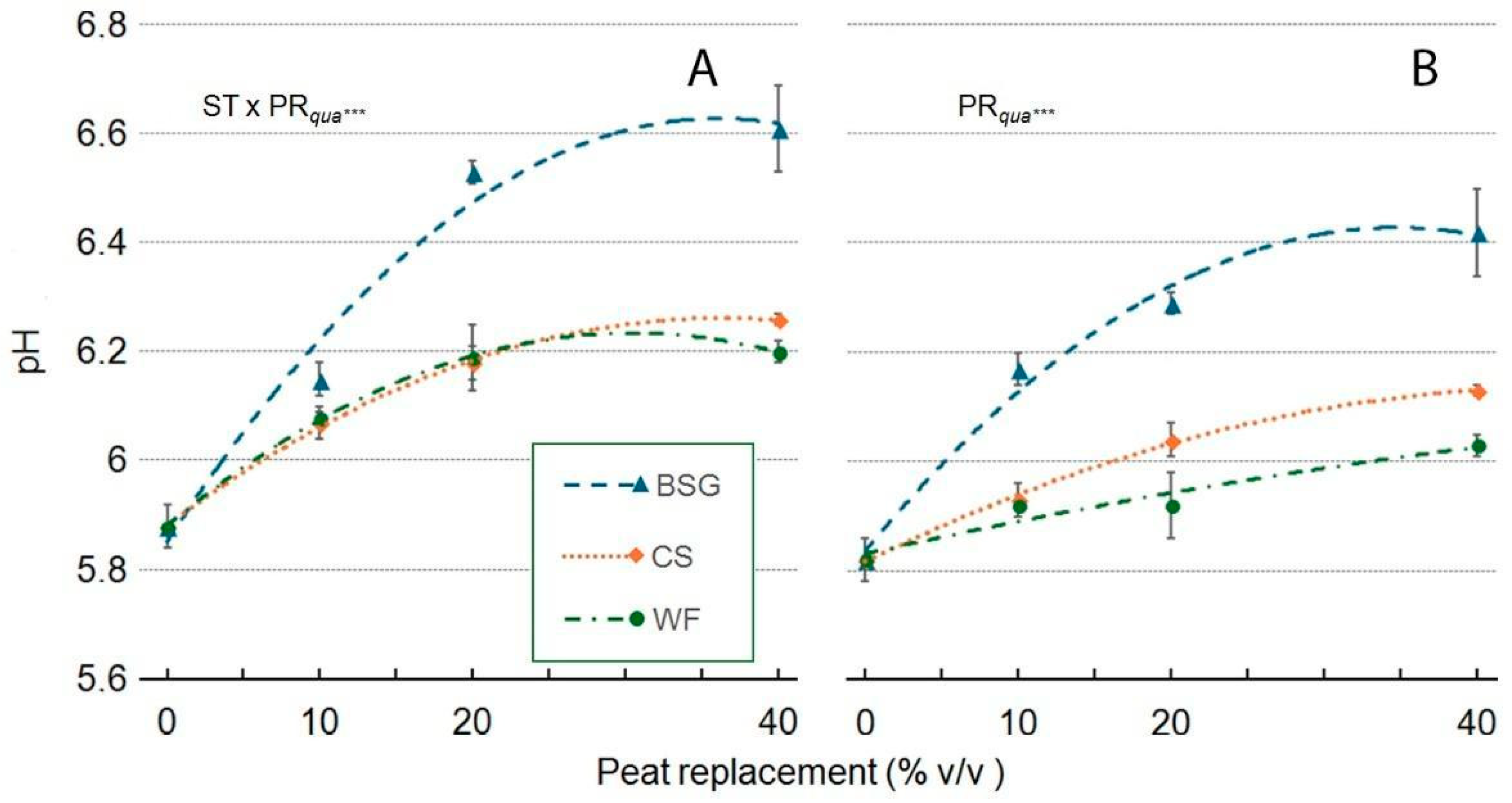
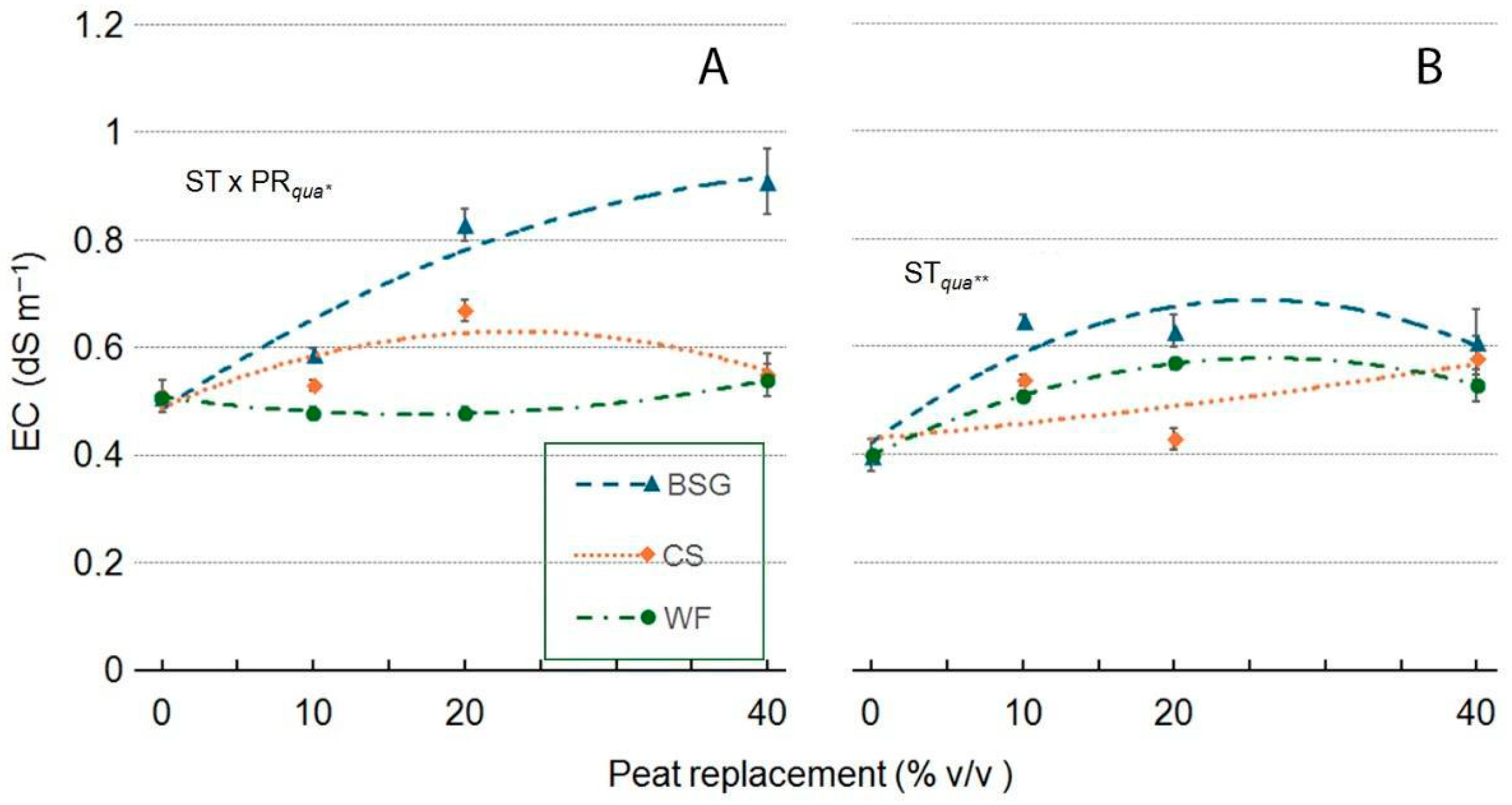
2.5. Leachate pH and EC
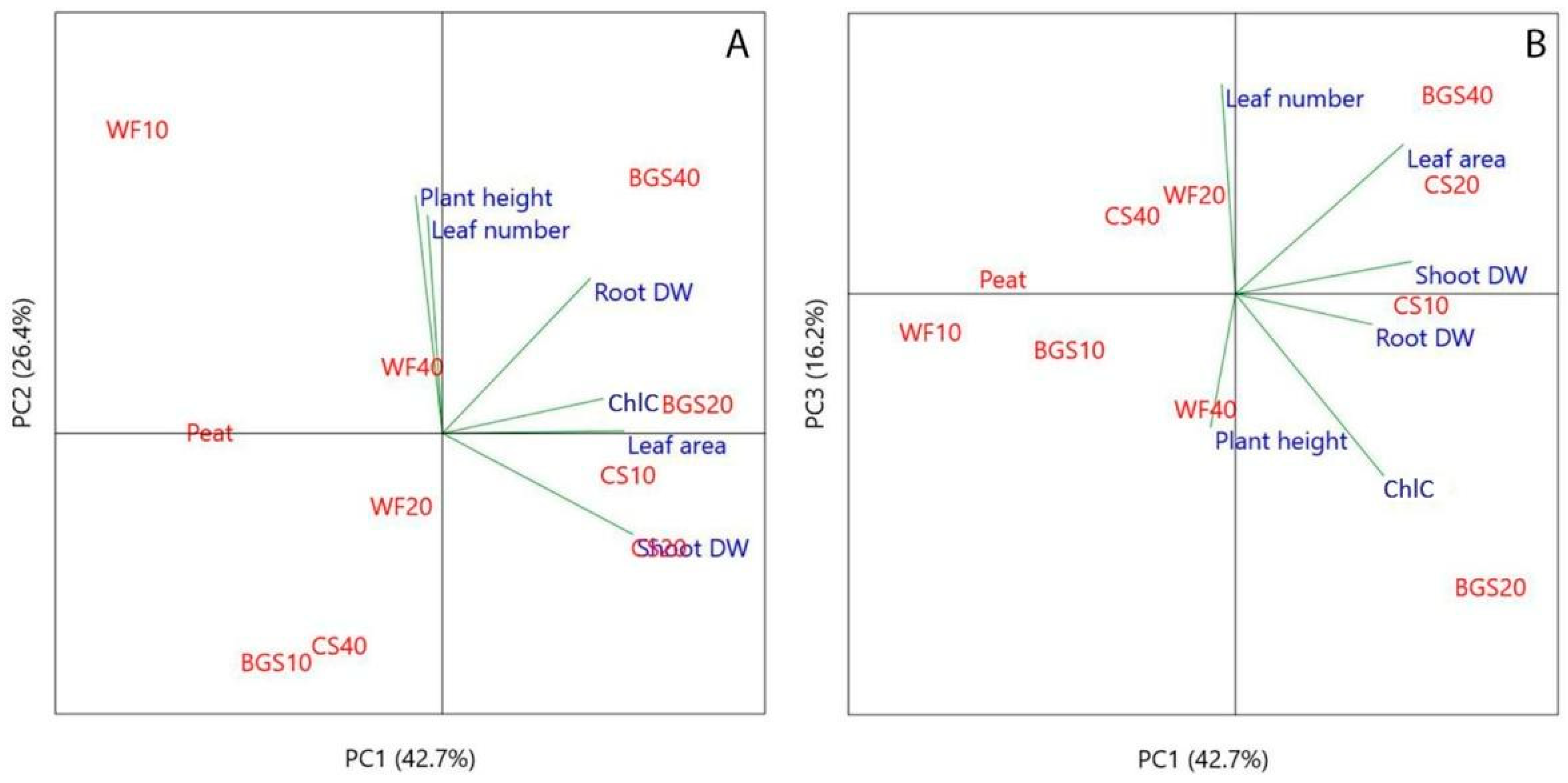
2.6. Principal Component Analysis (PCA) in Growth Parameters
3. Discussion
3.1. Physical and Hydrological Characterization
3.1.1. Wood Fiber as Peat Replacement
3.1.2. Coffee Silverskin as Peat Replacement
3.1.3. Brewer’s Spent Grain as Peat Replacement
3.2. Chemical Characteristics of Growing Media
3.3. Principal Component Analysis (PCA) in Growth Parameters
4. Materials and Methods
4.1. Substrate Treatments
4.2. Experimental Setup
4.3. Physical and Hydrological Substrates Characterization
4.4. Chemical Substrates Characterization
4.5. Chemical Leachate Substrate Fraction Characterization
4.6. Plant Growth and Biomass Measurements
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trivellini, A.; Toscano, S.; Romano, D.; Ferrante, A. LED lighting to produce high-quality ornamental plants. Plants 2023, 12, 1667. [Google Scholar] [CrossRef] [PubMed]
- Wallach, R. Physical characteristics of soilless media. In Soilless Culture, 2nd ed.; Raviv, M., Lieth, J.H., Bar-Tal, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–112. [Google Scholar] [CrossRef]
- Schindler, U.; Müller, L.; Eulenstein, F. Measurement and evaluation of the hydraulic properties of horticultural substrates. Arch. Agron. Soil Sci. 2016, 62, 806–818. [Google Scholar] [CrossRef]
- Al Naddaf, O.; Livieratos, I.; Stamatakis, A.; Tsirogiannis, I.; Gizas, G.; Savvas, D. Hydraulic characteristics of composted pig manure, perlite, and mixtures of them, and their impact on cucumber grown on bags. Sci. Hortic. 2011, 129, 135–141. [Google Scholar] [CrossRef]
- Banitalebi, G.; Mosaddeghi, M.R.; Shariatmadari, H. Feasibility of agricultural residues and their biochars for plant growing media: Physical and hydraulic properties. Waste Manag. 2019, 87, 577–589. [Google Scholar] [CrossRef]
- Paradelo, R.; Basanta, R.; Barral, M.T. Water-holding capacity and plant growth in compost-based substrates modified with polyacrylamide, guar gum or bentonite. Sci. Hortic. 2019, 243, 344–349. [Google Scholar] [CrossRef]
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Van Gerrewey, T.; Vandecruys, M.; Ameloot, N.; Perneel, M.; Van Labeke, M.-C.; Boon, N.; Geelen, D. Microbe-plant growing media interactions modulate the effectiveness of bacterial amendments on lettuce performance inside a plant factory with artificial lighting. Agronomy 2020, 10, 1456. [Google Scholar] [CrossRef]
- Erdal, İ.; Aktaş, H. Comparison of the Perlite, Leonardite, Vermicompost and Peat Moss and Their Combinations with Cocopeat as Tomato Growing Media. J. Soil Sci. Plant Nutr. 2025, 25, 2726–2741. [Google Scholar] [CrossRef]
- Balawejder, M.; Matłok, N.; Szostek, M.; Kuboń, M. The Influence of a Biopolymer Coating on Selected Surface Properties of Soilless Substrates Made from Coconut Fibre. Appl. Sci. 2025, 15, 7039. [Google Scholar] [CrossRef]
- Barrett, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving environmentally sustainable growing media for soilless plant cultivation systems—A review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef]
- Fesendouz, S.O.; Ebrahimzadeh, A.; Rasouli, F. Using agricultural waste as an alter-native growing medium for cultivating Cucumis sativus L. greenhouse transplants. Sci. Rep. 2025, 15, 14899. [Google Scholar] [CrossRef]
- Blok, C.; Eveleens, B.; van Winkel, A. Growing media for food and quality of life in the period 2020-2050. Acta Hortic. 2021, 1305, 341–356. [Google Scholar] [CrossRef]
- Hirschler, O.; Ostenburg, B. Peat extraction, trade and use in Europe: A material flow analysis. Mires Peat 2022, 28, 27. [Google Scholar] [CrossRef]
- Salimi, S.; Almuktar, S.A.; Scholz, M. Impact of climate change on wetland ecosystems: A critical review of experimental wetlands. J. Environ. Manag. 2021, 286, 112160. [Google Scholar] [CrossRef] [PubMed]
- Leifeld, J.; Menichetti, L. The underappreciated potential of peatlands in global climate change mitigation strategies. Nat. Commun. 2018, 9, 1071. [Google Scholar] [CrossRef] [PubMed]
- DEFRA. Ending the Retail Sale of Peat in Horticulture in England and Wales. Available online: https://consult.defra.gov.uk/soils-and-peatlands/endingtheretailsaleofpeatinhorticulture/ (accessed on 12 May 2025).
- Koseoglu, M.N.; Roberts, M. Supply Chain Dynamics of Moving from Peat-Based to Peat-Free Horticulture. Sustainability 2025, 17, 6159. [Google Scholar] [CrossRef]
- Sdao, A.E.; Gruda, N.S.; De Lucia, B. Beyond Peat: Wood Fiber and Two Novel Organic Byproducts as Growing Media—A Systematic Review. Plants 2025, 14, 1945. [Google Scholar] [CrossRef] [PubMed]
- Taparia, T.; Hendrix, E.; Nijhuis, E.; de Boer, W.; van der Wolf, J. Circular alternatives to peat in growing media: A microbial perspective. J. Clean. Prod. 2021, 327, 129375. [Google Scholar] [CrossRef]
- Poláková, K.; Bobková, A.; Demianová, A.; Bobko, M.; Lidiková, J.; Jurčaga, L.; Belej, Ľ.; Mesárošová, A.; Korčok, M.; Tóth, T. Quality attributes and sensory acceptance of different botanical coffee co-products. Foods 2023, 12, 2675. [Google Scholar] [CrossRef]
- Reineke, T.; Muhammed, H.H.A.; Anlauf, R.; Daum, D. Impact of thermo-hydrolytically treated wood fibers as a substrate component on the growth of petunias. Acta Hortic. 2024, 1389, 105–112. [Google Scholar] [CrossRef]
- Atzori, G.; Pane, C.; Zaccardelli, M.; Cacini, S.; Massa, D. The role of peat-free organic substrates in the sustainable management of soilless cultivations. Agronomy 2021, 11, 1236. [Google Scholar] [CrossRef]
- Zaccheo, P.; Cattivello, C.; Longo, C.; Crippa, L.; Notaristefano, P.; Orfeo, D. A comparative study on some locally available organic materials for their potential utilization in sustainable growing media blends. Acta Hortic. 2024, 1389, 123–130. [Google Scholar] [CrossRef]
- Gruda, N.S.; Hirschler, O.; Stuart, J. Peat reduction in horticulture: An overview of Europe. Acta Hortic. 2024, 1391, 545–560. [Google Scholar] [CrossRef]
- Miler, N.; Wojewódzki, P.; Woźny, A.; Rymarz, D.; Gołębiewska, A. Exploring Coffee Silverskin as a Sustainable Peat Additive in the Plant Nursery Production. Agronomy 2025, 15, 1633. [Google Scholar] [CrossRef]
- Hejna, A.; Aniśko-Michalak, J.; Skórczewska, K.; Barczewski, M.; Sulima, P.; Przyborowski, J.A.; Cieśliński, H.; Marć, M. Brewers’ Spent Grain from Different Types of Malt: A Comprehensive Evaluation of Appearance, Structure, Chemical Composition, Antimicrobial Activity, and Volatile Emissions. Molecules 2025, 30, 2809. [Google Scholar] [CrossRef]
- Paciulli, M.; Sogari, G.; Rodolfi, M.; Parenti, O.; Andreani, G.; Chiavaro, E. Fostering circular economy: Brewing by-products as innovative ingredients for cereal bar formulation. Foods 2024, 13, 2355. [Google Scholar] [CrossRef] [PubMed]
- Paksin, P.; Tangjaidee, P.; Klangpetch, W.; Unban, K.; Khumsap, T.; Klunklin, W.; Yawootti, A.; Jantanasakulwong, K.; Rachtanapun, P.; Phongthai, S. Quality Attributes, Structural Characteristics, and Functional Properties of Brewer’s Spent Grain Protein Concentrates as Affected by Alkaline and Pulsed Electric Field-Assisted Extraction. Foods 2025, 14, 1515. [Google Scholar] [CrossRef]
- Tumbure, A.; Pulver, C.; Black, L.; Walsh, L.; Prasad, M.; Leahy, J.J.; Corbett, E.; Gaffney, M.T. Bio-resource availability in Ireland: A practical review of potential replacement materials for use in horticultural growth media. Horticulturae 2025, 11, 378. [Google Scholar] [CrossRef]
- European Commission. Communication on the European Green Deal. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1588580774040&uri=CELEX%3A52019DC0640 (accessed on 11 June 2025).
- European Commission. A Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52020DC0381 (accessed on 11 June 2025).
- European Commission. A New Circular Economy Action Plan for a Cleaner and More Competitive Europe. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM:2020:98:FIN (accessed on 25 June 2025).
- Caron, J.; Pepin, S.; Periard, Y. Physics of growing media in a green future. Acta Hortic. 2014, 1034, 309–317. [Google Scholar] [CrossRef]
- Schindler, U.; Lischeid, G.; Müller, L. Hydraulic performance of horticultural substrates—3. Impact of substrate composition and ingredients. Horticulturae 2017, 3, 7. [Google Scholar] [CrossRef]
- Muhammed, H.H.; Anlauf, R.; Daum, D. Estimation of Hydraulic Properties of Growing Media from Numerical Inversion of Mini Disk Infiltrometer Data. Hydrology 2025, 12, 100. [Google Scholar] [CrossRef]
- Noguera, P.; Abad, M.; Puchades, R.; Maquieira, A.; Noguera, V. Influence of particle size on physical and chemical properties of coconut coir dust as container medium. Commun. Soil Sci. Plant Anal. 2011, 34, 593–605. [Google Scholar] [CrossRef]
- Bilderback, T.E.; Warren, S.L.; Owen, J.S., Jr.; Albano, J.P. Healthy substrates need physicals too! HortTechnology 2005, 15, 747. [Google Scholar] [CrossRef]
- Plants of the World Online (POWO). Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:30000096-2 (accessed on 28 July 2025).
- Afonso, A.F.; Pereira, O.R.; Fernandes, Â.S.F.; Calhelha, R.C.; Silva, A.M.S.; Ferreira, I.C.F.R.; Cardoso, S.M. The health-benefits and phytochemical profile of Salvia apiana and Salvia farinacea var. Victoria Blue decoctions. Antioxidants 2019, 8, 241. [Google Scholar] [CrossRef] [PubMed]
- De Mastro, G.; El Mahdi, J.; Ruta, C. Bioherbicidal Potential of the Essential Oils from Mediterranean Lamiaceae for Weed Control in Organic Farming. Plants 2021, 10, 818. [Google Scholar] [CrossRef] [PubMed]
- Kamatou, G.P.; Makunga, N.P.; Ramogola, W.P.; Viljoen, A.M. South African Salvia species: A review of biological activities and phytochemistry. J. Ethnopharmacol. 2008, 119, 664–672. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Ozcelik, B.; Altın, G.; Daşkaya-Dikmen, C.; Martorell, M.; Ramirez-Alarcon, K.; Sharifi-Rad, J. Salvia spp. plants-from farm to food applications and phytopharmacotherapy. Trends Food Sci. Technol. 2018, 80, 242–263. [Google Scholar] [CrossRef]
- Lin, K.-H.; Lin, T.-Y.; Wu, C.-W.; Chang, Y.-S. Protective Effects of Salicylic Acid and Calcium Chloride on Sage Plants (Salvia officinalis L. and Salvia elegans Vahl) under High-Temperature Stress. Plants 2021, 10, 2110. [Google Scholar] [CrossRef]
- Papafotiou, M.; Martini, A.N.; Papanikolaou, E.; Stylias, E.G.; Kalantzis, A. Hybrids development between Greek Salvia species and their drought resistance evaluation along with Salvia fruticosa, under attapulgite-amended substrate. Agronomy 2021, 11, 2401. [Google Scholar] [CrossRef]
- Moughan, J.; McGinn, K.J.; Jones, L.; Rich, T.C.; Waters, E.; de Vere, N. Biological flora of the British Isles: Salvia pratensis. J. Ecol. 2021, 109, 4171–4190. [Google Scholar] [CrossRef]
- Mossi, A.; Cansian, R.; Paroul, N.; Toniazzo, G.; Oliveira, J.; Pierozan, M.; Serafini, L. Morphological characterization and agronomical parameters of different species of Salvia sp. (Lamiaceae). Braz. J. Biol. 2011, 71, 121–129. [Google Scholar] [CrossRef]
- Nanos, C.; Tsoulpha, P.; Kostas, S.; Hatzilazarou, S.; Michail, I.; Anastasiadi, V.; Pipinis, E.; Gklavakis, E.; Kanellis, A.K.; Nianiou-Obeidat, I. Asexual propagation of Greek Salvia officinalis L. populations selected for ornamental use. Horticulturae 2023, 9, 847. [Google Scholar] [CrossRef]
- Scaltrito, E.; Cristiano, G.; Sdao, A.E.; Gruda, N.S.; Loconsole, D.; De Lucia, B. Influence of water spraying intervals and indole-3-butyric acid concentrations on Salvia rooted cuttings quality in a closed aeroponics system. Sci. Hortic. 2024, 337, 113452. [Google Scholar] [CrossRef]
- Gokdogan, E.Y.; Burun, B. The studies on seed germination and in vitro cultures of Salvia L. species from Turkish flora. Nat. Prod. Biotechnol. 2022, 2, 60–73. [Google Scholar] [CrossRef]
- Greco, C.; Comparetti, A.; Fascella, G.; Febo, P.; La Placa, G.; Saiano, F.; Mammano, M.M.; Orlando, S.; Laudicina, V.A. Effects of vermicompost, compost and digestate as commercial alternative peat-based substrates on qualitative parameters of Salvia officinalis. Agronomy 2021, 11, 98. [Google Scholar] [CrossRef]
- Iacomino, G.; Cozzolino, A.; Idbella, M.; Amoroso, G.; Bertoli, T.; Bonanomi, G.; Motti, R. Potential of biochar as a peat substitute in growth media for Lavandula angustifolia, Salvia rosmarinus and Fragaria × ananassa. Plants 2023, 12, 3689. [Google Scholar] [CrossRef]
- Salvador, E.D.; Balas, J. Physical characteristics of soilless growing media: Basis for the development of methods to formulate substrates for ornamental plants in Brazil. In Floriculture, Ornamental and Plant Biotechnology: Advances and Topical Issues, 1st ed.; Teixeira da Silva, J.A., Ed.; Global Science Books: London, UK, 2006; pp. 161–171. [Google Scholar]
- Michel, J.-C.; Kerloch, E. Evolution of hydraulic properties and wettability of organic growing media during cultivation according to irrigation strategies. Sci. Hortic. 2017, 217, 28–35. [Google Scholar] [CrossRef][Green Version]
- Chrysargyris, A.; Antoniou, O.; Tzionis, A.; Prasad, M.; Tzortzakis, N. Alternative soilless media using olive-mill and paper waste for growing ornamental plants. Environ. Sci. Pollut. Res. 2018, 25, 35915–35927. [Google Scholar] [CrossRef]
- Grosbellet, C.; Beaudet, E.; Duclaud, E. Performances of new materials for peat substitution in horticulture. Acta Hortic. 2019, 1266, 315–322. [Google Scholar] [CrossRef]
- Thakur, T.; Garg, A. Growth and flowering of ornamental pot plants influenced by growing media—A review. J. Ornam. Hortic. 2025, 27, 95–106. Available online: https://epubs.icar.org.in/index.php/JOH/article/view/164891 (accessed on 29 July 2025).
- Awang, Y.; Shaharom, A.S.; Mohamad, R.B.; Selamat, A. Chemical and Physical Characteristics of Cocopeat-Based Media Mixtures and Their Effects on the Growth and Development of Celosia cristata. Am. J. Agric. Biol. Sci. 2009, 4, 63–71. [Google Scholar] [CrossRef]
- Di Lonardo, S.; Massa, D.; Orsenigo, S.; Zubani, L.; Rossi, G.; Fascella, G.; Cacini, S. Substitution of peat in the cultivation of two shrub species used for ecological restoration and recovery of degraded green areas. Acta Hortic. 2021, 1305, 97–102. [Google Scholar] [CrossRef]
- Di Lonardo, S.; Cacini, S.; Becucci, L.; Lenzi, A.; Orsenigo, S.; Zubani, L.; Rossi, G.; Zaccheo, P.; Massa, D. Testing new peat-free substrate mixtures for the cultivation of perennial herbaceous species: A case study on Leucanthemum vulgare Lam. Sci. Hortic. 2021, 288, 110472. [Google Scholar] [CrossRef]
- Caron, J.; Nkongolo, N.V. Assessing Gas Diffusion Coefficients in Growing Media from in situ Water Flow and Storage Measurements. Vadose Zone J. 2004, 3, 300–306. [Google Scholar] [CrossRef]
- Cannavo, P.; Michel, J.-C. Peat particle size effects on spatial root distribution, and changes on hydraulic and aeration properties. Sci. Hortic. 2013, 151, 148–154. [Google Scholar] [CrossRef]
- Mariotti, B.; Oliet, J.A.; Andivia, E.; Tsakaldimi, M.; Villar-Salvador, P.; Ivetić, V.; Cocozza, C. A global review on innovative, sustainable, and effective materials composing growing media for forest seedling production. Curr. For. Rep. 2023, 9, 413–428. [Google Scholar] [CrossRef]
- Carlile, B.; Coules, A. Towards sustainability in growing media. Acta Hortic. 2013, 1013, 341–349. [Google Scholar] [CrossRef]
- Schmilewski, G. Growing media constituents used in the EU in 2013. Acta Hortic. 2017, 1168, 85–92. [Google Scholar] [CrossRef]
- Jackson, B.E. The Role of Wood Substrates in Controlled Environment Vegetable and Fruit Production Systems. Available online: https://www.producegrower.com/article/the-role-of-wood-substrates-in-controlled-environment-vegetable-and-fruit-production-systems/ (accessed on 1 July 2025).
- Lemaire, F.; Dartigues, A.; Riviere, L.M.; Charpentier, S. Cultures en pots et Conteneurs: Principes Agronomiques et Applications, 1st ed.; INRA: Paris, France, 1989; ISBN 2-7380-0157-2. [Google Scholar]
- Gruda, N.; Schnitzler, W.H. Suitability of wood fiber substrate for production of vegetable transplants: I. Physical properties of wood fiber substrates. Sci. Hortic. 2004, 100, 309–322. [Google Scholar] [CrossRef]
- Domeño, I.; Irigoyen, I.; Muro, J. New Wood Fibre Substrates Characterization and Evaluation in Hydroponic Tomato Culture. Eur. J. Hortic. Sci. 2010, 75, 89. [Google Scholar] [CrossRef]
- Anlauf, R.; Muhammed, H.H.A.; Reineke, T.; Daum, D. Water retention properties of wood fiber based growing media and their impact on irrigation strategy. Acta Hortic. 2024, 1389, 227–236. [Google Scholar] [CrossRef]
- Durand, S.; Jackson, B.E.; Fonteno, W.C.; Michel, J.C. Advances in substrate particle characterization using dynamic image analysis compared to sieving procedure for predicting water retention properties. Acta Hortic. 2023, 1377, 537–544. [Google Scholar] [CrossRef]
- Caron, J.; Parent, L.E.; Elrick, D.E.; Naasz, R.; Carter, M.R.; Gregorich, E.G. Physical properties of organic soils and growing media: Water and air storage and flow dynamics. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 885–912. [Google Scholar]
- Fields, J.S.; Fonteno, W.C.; Jackson, B.E.; Heitman, J.L.; Owen, J.S. Hydrophysical properties, moisture retention, and drainage profiles of wood and traditional components for greenhouse substrates. HortScience 2014, 49, 827–832. [Google Scholar] [CrossRef]
- Durand, S.; Jackson, B.E.; Fonteno, W.C.; Michel, J.-C. The Use of Wood Fiber for Reducing Risks of Hydrophobicity in Peat-Based Substrates. Agronomy 2021, 11, 907. [Google Scholar] [CrossRef]
- Michiels, P.; Hartmann, R.; Coussens, C. Physical properties of peat substrates in an ebb/flood irrigation system. Acta Hortic. 1993, 342, 205–220. [Google Scholar] [CrossRef]
- Raviv, M.; Lieth, J.H.; Bar-Tal, A. Soilless Culture: Theory and Practice; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Eveleens, B.; van Winkel, A.; Blok, C. Wood fiber in pot plant culture; peat replacement up to 50% in volume? Acta Hortic. 2021, 1317, 165–174. [Google Scholar] [CrossRef]
- Arancon, N.Q.; Pant, A.; Radovich, T.; Hue, N.V.; Potter, J.K.; Converse, C.E. Seed germination and seedling growth of tomato and lettuce as affected by vermicompost water extracts (teas). HortScience 2012, 47, 1722–1728. [Google Scholar] [CrossRef]
- Woznicki, T.L.; Sønsteby, A.; Aurdal, S.M.; Kusnierek, K.; Haraldsen, T.K. Optimizing Peat and Wood Fiber Blends: Impacts of Liming and Fertilization on Growth of Petunia (Petunia x hybrida Vilm.) and Basil (Ocimum basilicum L.). Horticulturae 2024, 10, 895. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioproc. Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Pourfarzad, A.; Mahdavian-Mehr, H.; Sedaghat, N. Coffee silverskin as a source of dietary fiber in bread-making: Optimization of chemical treatment using response surface methodology. LWT-Food Sci. Technol. 2013, 50, 599–606. [Google Scholar] [CrossRef]
- Martuscelli, M.; Esposito, L.; Di Mattia, C.D.; Ricci, A.; Mastrocola, D. Characterizationof coffee silver skin as potential food-safe ingredient. Foods 2021, 10, 1367. [Google Scholar] [CrossRef]
- De Boodt, M.; Verdonck, O.; Cappaert, I. Method for measuring the water release curve of organic substrates. Acta Hortic. 1974, 37, 2054–2063. [Google Scholar] [CrossRef]
- De Boodt, M.A.V.O.; Verdonck, O. The physical properties of the substrates in horticulture. In Proceedings of the III Symposium on Peat in Horticulture 26, Dublin, Ireland, 28 June–3 July 1971; pp. 37–44. [Google Scholar]
- Abad, M.; Noguera, P.; Burés, S. National inventory of organic wastes for use as growing media for ornamental potted plant production: Case study in Spain. Bioresour. Technol. 2001, 77, 197–200. [Google Scholar] [CrossRef]
- Naibaho, J.; Korzeniowska, M. The Variability of Physico-Chemical Properties of Brewery Spent Grain from 8 Different Breweries. Heliyon 2021, 7, e06329. [Google Scholar] [CrossRef]
- Castro, L.E.N.; Colpini, L.M.S. All-Around Characterization of Brewers’ Spent Grain. Eur. Food Res. Technol. 2021, 247, 3013–3021. Available online: https://link.springer.com/article/10.1007%2Fs00217-021-03860-5 (accessed on 4 June 2025). [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Xiros, C.; Christakopoulos, P. Biotechnological potential of brewers’ spent grain and its recent applications. Waste Biomass Valorization 2012, 3, 213–232. [Google Scholar] [CrossRef]
- Beldman, G.; Hennekam, J.; Voragen, A.G. Enzymatic hydrolysis of beer brewers’ spent grain and the influence of pretreatments. Biotechnol. Bioeng. 1987, 30, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Hauck, D.; Lohr, D.; Meinken, E.; Schmidhalter, U. Plant availability of secondary phosphates depending on pH in a peat-based growing medium. Acta Hortic. 2021, 1305, 437–442. [Google Scholar] [CrossRef]
- Cacace, C.; Cocozza, C.; Traversa, A.; Coda, R.; Rizzello, C.G.; Pontonio, E.; De Mastro, F.; Brunetti, G.; Verni, M. Potential of Native and Bioprocessed Brewers’ Spent Grains as Organic Soil Amendments. Front. Sustain. Food Syst. 2022, 6, 1010890. [Google Scholar] [CrossRef]
- Kyriacou, M.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.; Giordano, M.; Palladino, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Phenolic Constitution, Phytochemical and Macronutrient Content in Three Species of Microgreens as Modulated by Natural Fiber and Synthetic Substrates. Antioxidants 2020, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Zawadzińska, A.; Salachna, P.; Nowak, J.S.; Kowalczyk, W. Response of Interspecific Geraniums to Waste Wood Fiber Substrates and Additional Fertilization. Agriculture 2021, 11, 119. [Google Scholar] [CrossRef]
- Dickson, R.W.; Helms, K.M.; Jackson, B.E.; Machesney, L.M.; Lee, J.A. Evaluation of Peat Blended with Pine Wood Components for Effects on Substrate Physical Properties, Nitrogen Immobilization, and Growth of Petunia (Petunia × hybrida Vilm.-Andr.). HortScience 2022, 57, 304–311. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Uranga, J.A.; del Castillo, M.D.; Abalo, R. Effects of Coffee and Its Components on the Gastrointestinal Tract and the Brain–Gut Axis. Nutrients 2021, 13, 88. [Google Scholar] [CrossRef]
- Sdao, A.E.; Mondelli, D.; Piscitelli, L.; Scaltrito, E.; Leoni, B.; Cristiano, G.; De Lucia, B. Morpho-physiological response of bedding plants quality to unconventional agro-industrial organic matrices to peat replacing: Preliminary outcomes. Acta Hortic. 2025, 1417, 125–132. [Google Scholar] [CrossRef]
- Yang, H.; Yang, J.; Lv, Y.; He, J. SPAD Values and Nitrogen Nutrition Index for the Evaluation of Rice Nitrogen Status. Plant Prod. Sci. 2014, 17, 81–92. [Google Scholar] [CrossRef]
- Cangussu, L.B.; Melo, J.C.; Franca, A.S.; Oliveira, L.S. Chemical Characterization of Coffee Husks, a By-Product of Coffea arabica Production. Foods 2021, 10, 3125. [Google Scholar] [CrossRef]
- European Standard 13038; Determination of Electrical Conductivity, in Soil Improvers and Growing Media. European Committee for Standardization: Brussels, Belgium, 1999.
- European Standard 13037; Determination of pH, in Soil Improvers and Growing Media. European Committee for Standardization: Brussels, Belgium, 1999.
- Cavins, T.; Whipker, B.; Fonteno, W. PourThru: A Method for Monitoring Nutrition in the Greenhouse. Acta Hortic. 2008, 779, 289–298. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT® User’s Guide, Version 8; SAS Institute Inc.: Cary, NC, USA, 1999. [Google Scholar]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]



| WV | AC | SM | EAW | BC | TAW | TPS | BD | |
|---|---|---|---|---|---|---|---|---|
| Wood fiber | ||||||||
| Peat replacement (PR) | * a | ns | * | * | ns | * | *** | *** |
| PRlin | ** | ns | * | * | ns | * | *** | *** |
| PRqua | * | ns | ns | ns | ns | * | ns | ns |
| Coffee silverskin | ||||||||
| PR | * | * | *** | ** | ns | ** | *** | ** |
| PRlin | ns | ns | *** | *** | ns | *** | ns | ns |
| PRqua | ns | ns | ** | ns | ns | * | ** | ** |
| Brewer’s spent grain | ||||||||
| PR | * | * | * | ** | * | *** | *** | *** |
| PRlin | ns | ns | ** | *** | * | *** | *** | *** |
| PRqua | ns | ns | ns | *** | ns | *** | *** | *** |
| Treatment | pH | EC |
|---|---|---|
| Wood fiber | ||
| PR | ns | *** |
| PRlin | ns | ** |
| PRqua | ns | ** |
| Coffee silverskin | ||
| PR | ns | ** |
| PRlin | ns | ** |
| PRqua | ns | ns |
| Brewer’s spent grain | ||
| PR | ns | *** |
| PRlin | ns | *** |
| PRqua | ns | ns |
| Treatment | Plant Height (cm) | Leaves (no./Plant) | Leaf Area (cm2/Plan) | ChlC (µmol m−2 LA) | Plant FW (g/Plant) | Shoot DW (g/Plant) | Root DW (g/Plant) | Plant DM (g 100 g−1 FW) |
|---|---|---|---|---|---|---|---|---|
| Substrate Type (ST) | *** a | *** | *** | *** | *** | *** | ns | *** |
| Peat replacement (PR) | *** | *** | *** | *** | *** | *** | *** | ** |
| PRlin | ns | *** | *** | *** | *** | *** | *** | ns |
| PRqua | ns | *** | *** | *** | ns | * | *** | ns |
| ST × PR | *** | *** | *** | *** | *** | *** | ns | ns |
| ST × PRlin | ns | *** | *** | *** | * | *** | ns | ns |
| ST × PRqua | *** | ns | *** | *** | *** | *** | ns | ns |
| Treatment | Plant Height (cm) | Leaves (no./Plant) | Leaf Area (cm2/Plant) | ChlC (µmol m−2 LA) | Plant FW (g/Plant) | Shoot DW (g/Plant) | Root DW (g/Plant) | Plant DM (g 100 g−1 FW) |
|---|---|---|---|---|---|---|---|---|
| Substrate type (ST) | ns a | * | *** | *** | *** | *** | *** | * |
| Peat Replacement (PR) | ns | ns | *** | *** | *** | *** | * | ns |
| PRlin | ns | ns | *** | * | *** | *** | ** | ns |
| PRqua | ns | * | *** | *** | *** | *** | ns | ns |
| ST × PR | * | *** | *** | *** | *** | *** | *** | *** |
| ST × PRlin | ns | *** | *** | *** | ** | ns | *** | *** |
| ST × PRqua | ns | * | *** | ** | *** | ** | * | ns |
| ‘Victoria’ | pH | EC (dS m−1) |
|---|---|---|
| Substrate type (ST) | *** a | *** |
| Peat replacement (PR) | *** | *** |
| PRlin | *** | *** |
| PRqua | *** | *** |
| ST × PR | *** | *** |
| ST × PRlin | *** | *** |
| ST × PRqua | *** | * |
| ‘Amistad’ | pH | EC (dS m−1) |
|---|---|---|
| Substrate type (ST) | *** a | ns |
| Peat replacement (PR) | *** | ** |
| PRlin | *** | ** |
| PRqua | *** | ** |
| ST × PR | ns | ns |
| ST × PRlin | ns | ns |
| ST × PRqua | ns | ns |
| Substrate Treatment Code | Peat | Peat Replacement | Matrices Used as Peat Replacement |
|---|---|---|---|
| (% v/v of the Organic Fraction of the Substrate) | |||
| 0PR | 100 | 0 | None |
| WF10 | 90 | 10 | Wood fiber |
| WF20 | 80 | 20 | |
| WF40 | 60 | 40 | |
| CS10 | 90 | 10 | Coffee silverskin |
| CS20 | 80 | 20 | |
| CS40 | 60 | 40 | |
| BSG10 | 90 | 10 | Brewer’s spent grain |
| BSG20 | 80 | 20 | |
| BSG40 | 60 | 40 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sdao, A.E.; Cacini, S.; Loconsole, D.; Conversa, G.; Cristiano, G.; Elia, A.; De Lucia, B. Partial Replacement of Peat: Effects on Substrate Physico-Hydrological Properties and Sage Growth. Plants 2025, 14, 2801. https://doi.org/10.3390/plants14172801
Sdao AE, Cacini S, Loconsole D, Conversa G, Cristiano G, Elia A, De Lucia B. Partial Replacement of Peat: Effects on Substrate Physico-Hydrological Properties and Sage Growth. Plants. 2025; 14(17):2801. https://doi.org/10.3390/plants14172801
Chicago/Turabian StyleSdao, Anna Elisa, Sonia Cacini, Danilo Loconsole, Giulia Conversa, Giuseppe Cristiano, Antonio Elia, and Barbara De Lucia. 2025. "Partial Replacement of Peat: Effects on Substrate Physico-Hydrological Properties and Sage Growth" Plants 14, no. 17: 2801. https://doi.org/10.3390/plants14172801
APA StyleSdao, A. E., Cacini, S., Loconsole, D., Conversa, G., Cristiano, G., Elia, A., & De Lucia, B. (2025). Partial Replacement of Peat: Effects on Substrate Physico-Hydrological Properties and Sage Growth. Plants, 14(17), 2801. https://doi.org/10.3390/plants14172801












