Photochemical Responses of Parmotrema tinctorum and Usnea barbata to Light Variations in Cerrado Landscapes
Abstract
1. Introduction
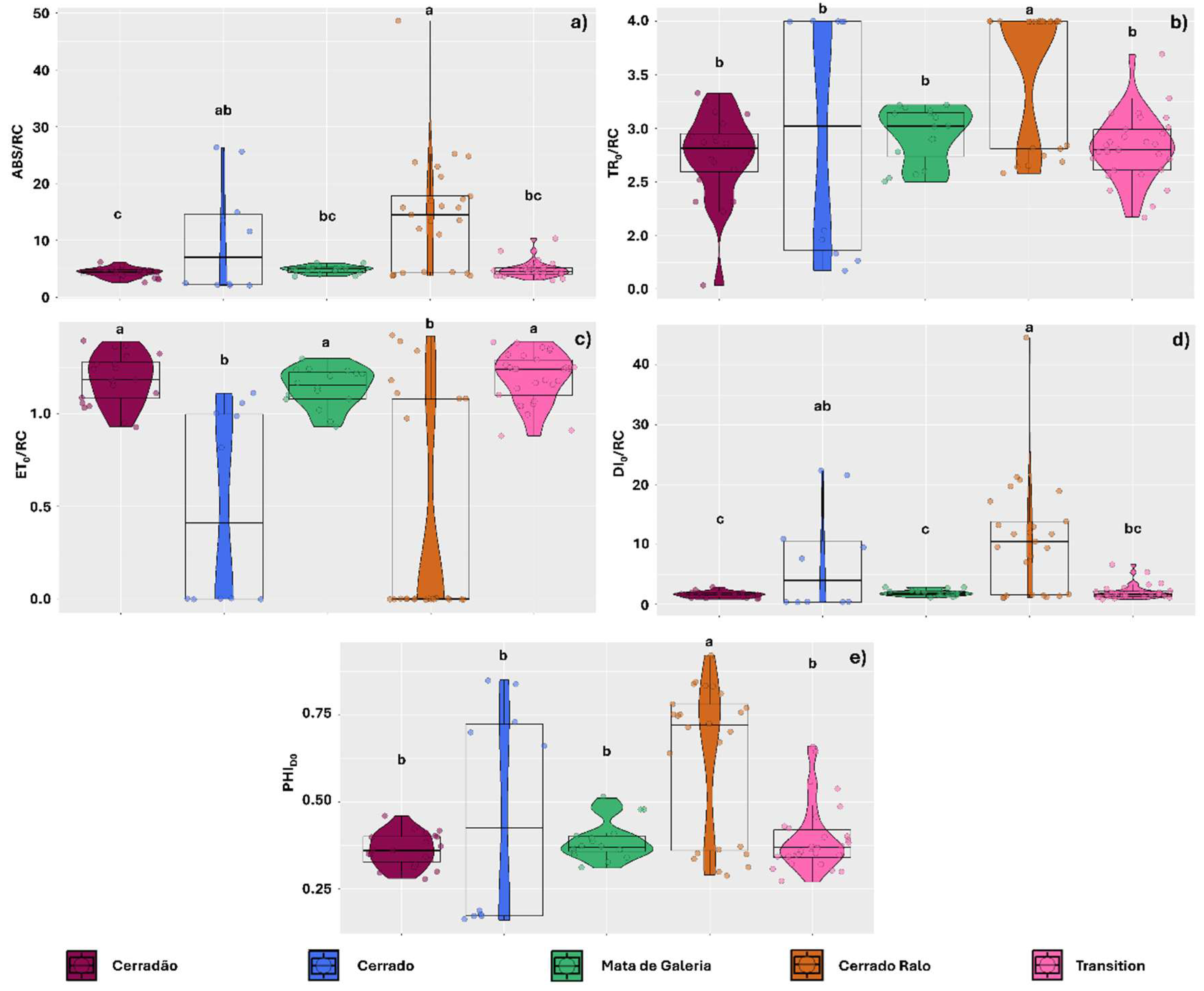
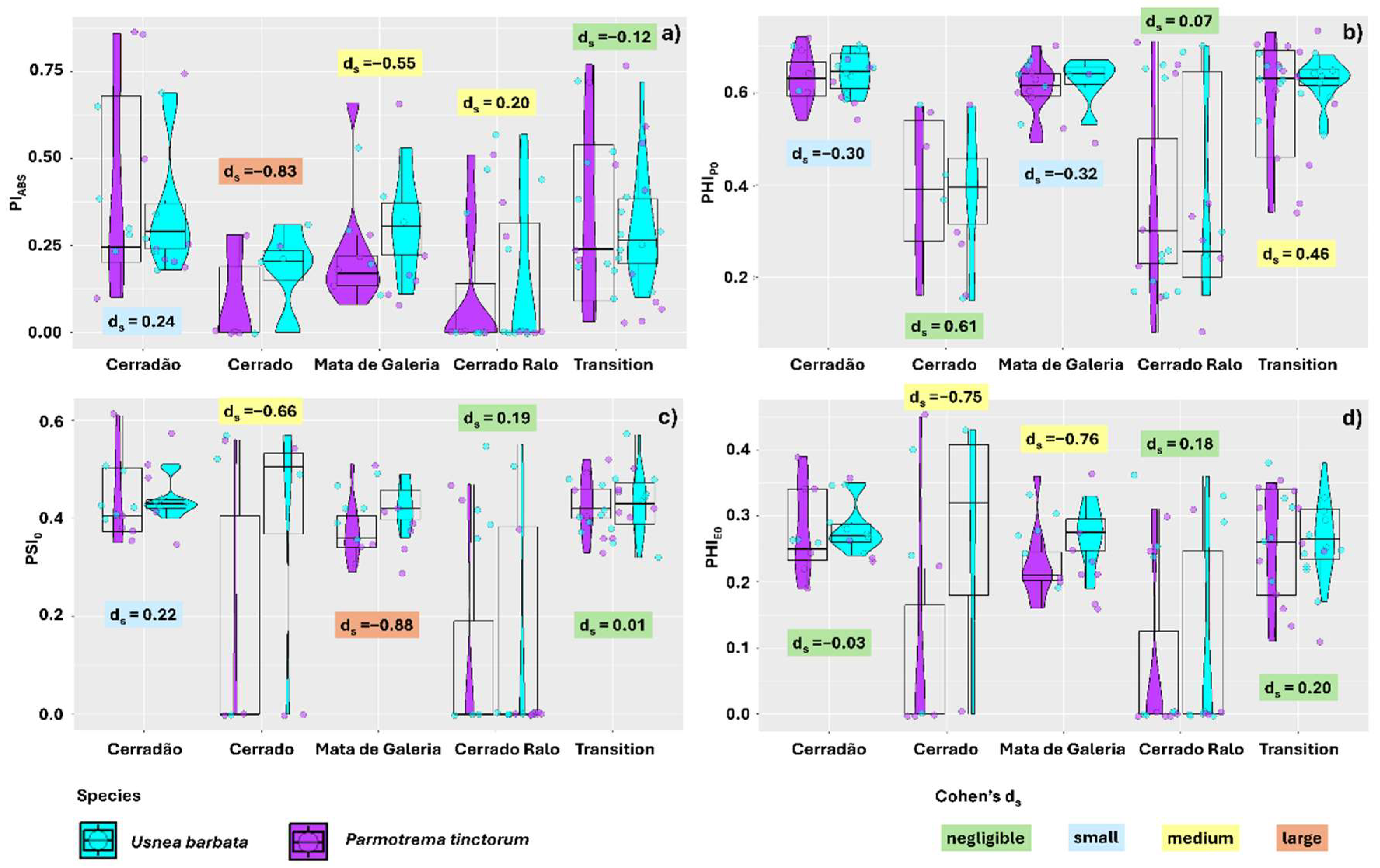
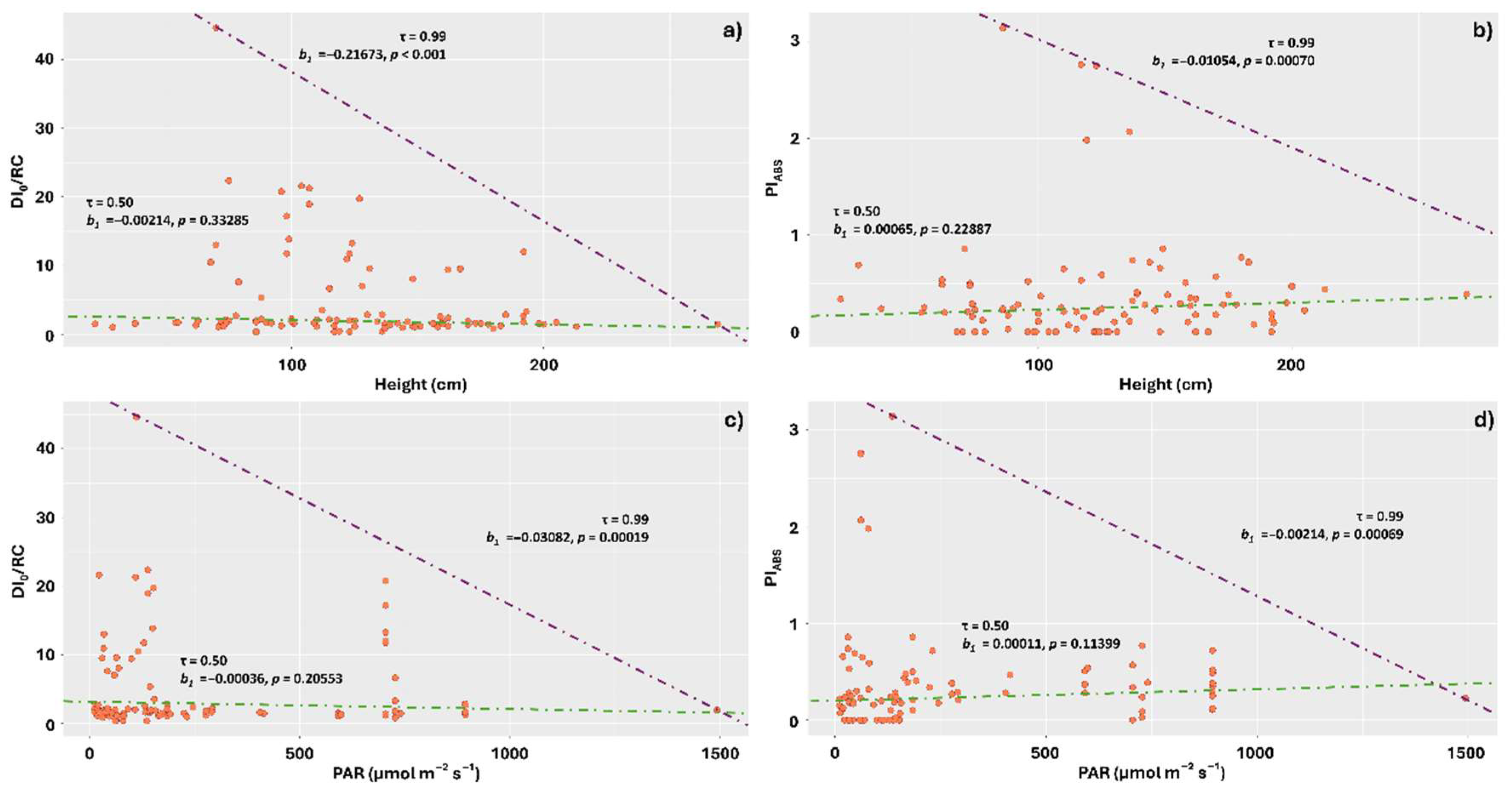
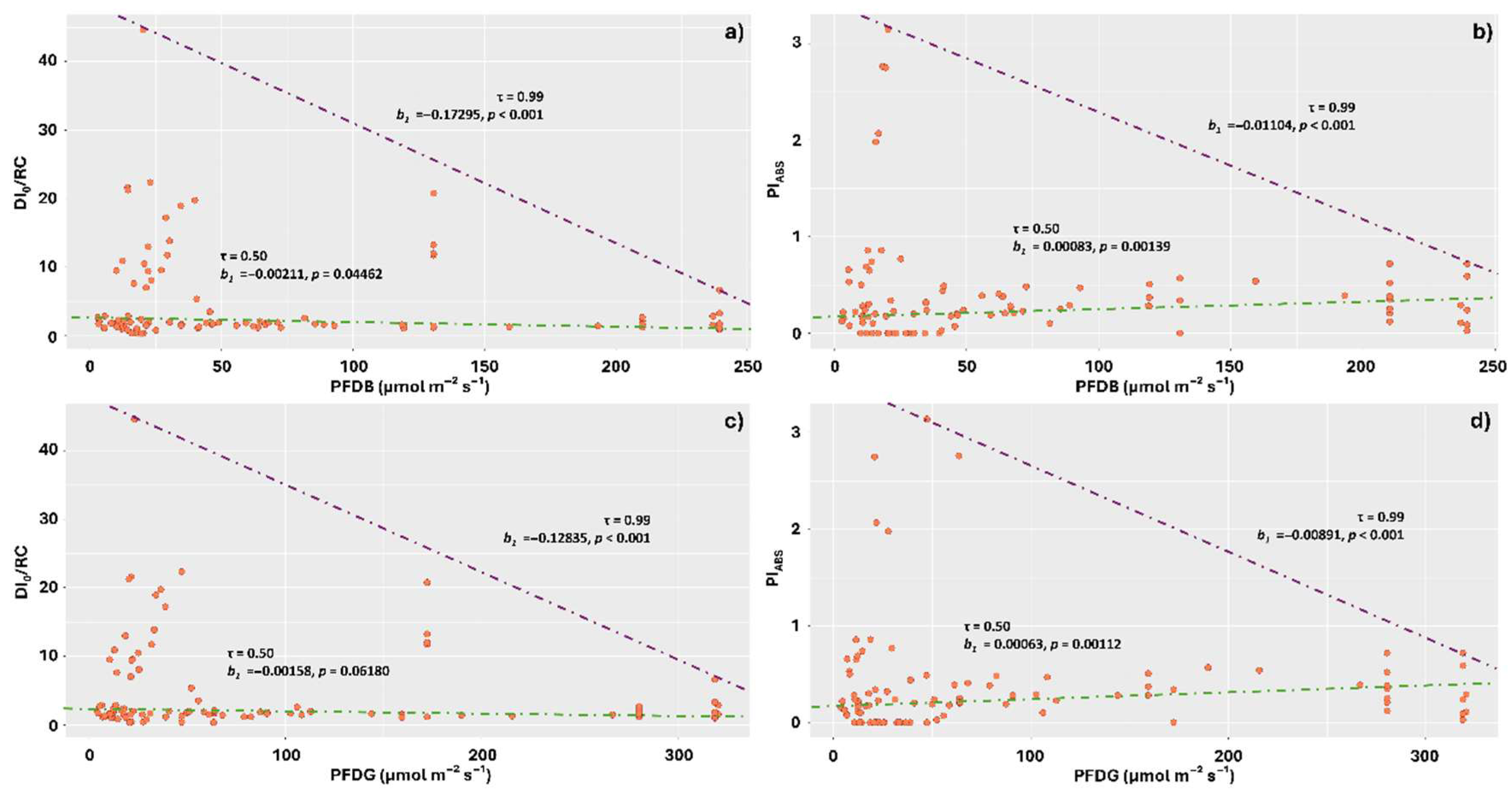
2. Results
3. Discussion
3.1. High Levels of Energy Dissipation Observed in Thalli Sampled from the Cerrado and Cerrado Ralo Landscapes Resulted in Lower Photochemical Performance
3.2. U. barbata Thalli Exhibited Superior Photochemical Performance Compared to P. tinctorum Across All Sampled Landscapes
3.3. Lichens Sampled Higher on the Stem and Exposed to Higher PAR Levels Exhibited Lower Energy Dissipation as Heat and Reduced Photochemical Performance
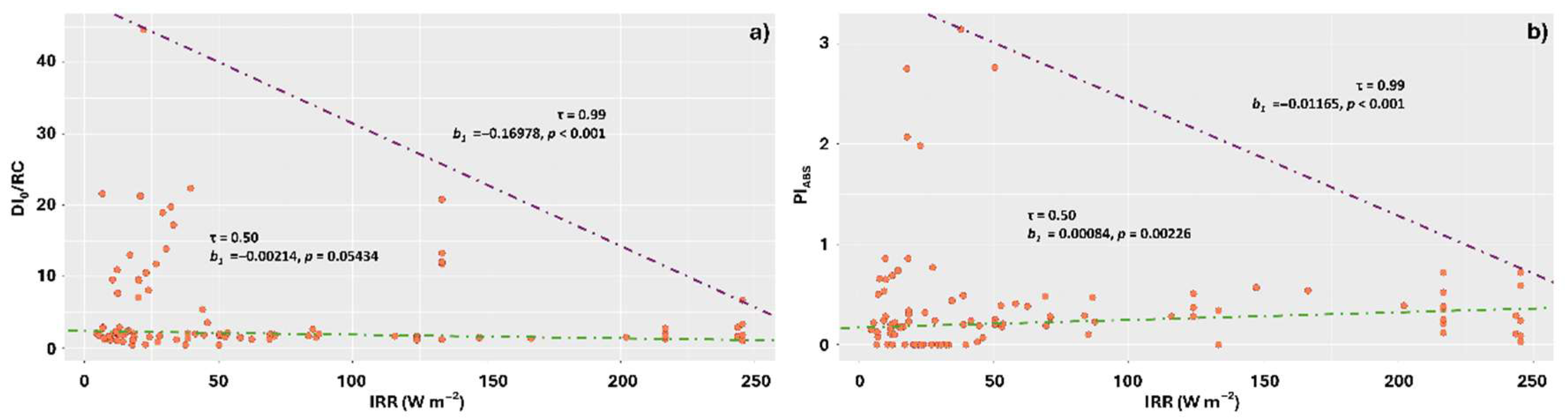
3.4. Increasing Intensities of the Different Spectrum Components Reduced DI0/RC and Increased PiABS
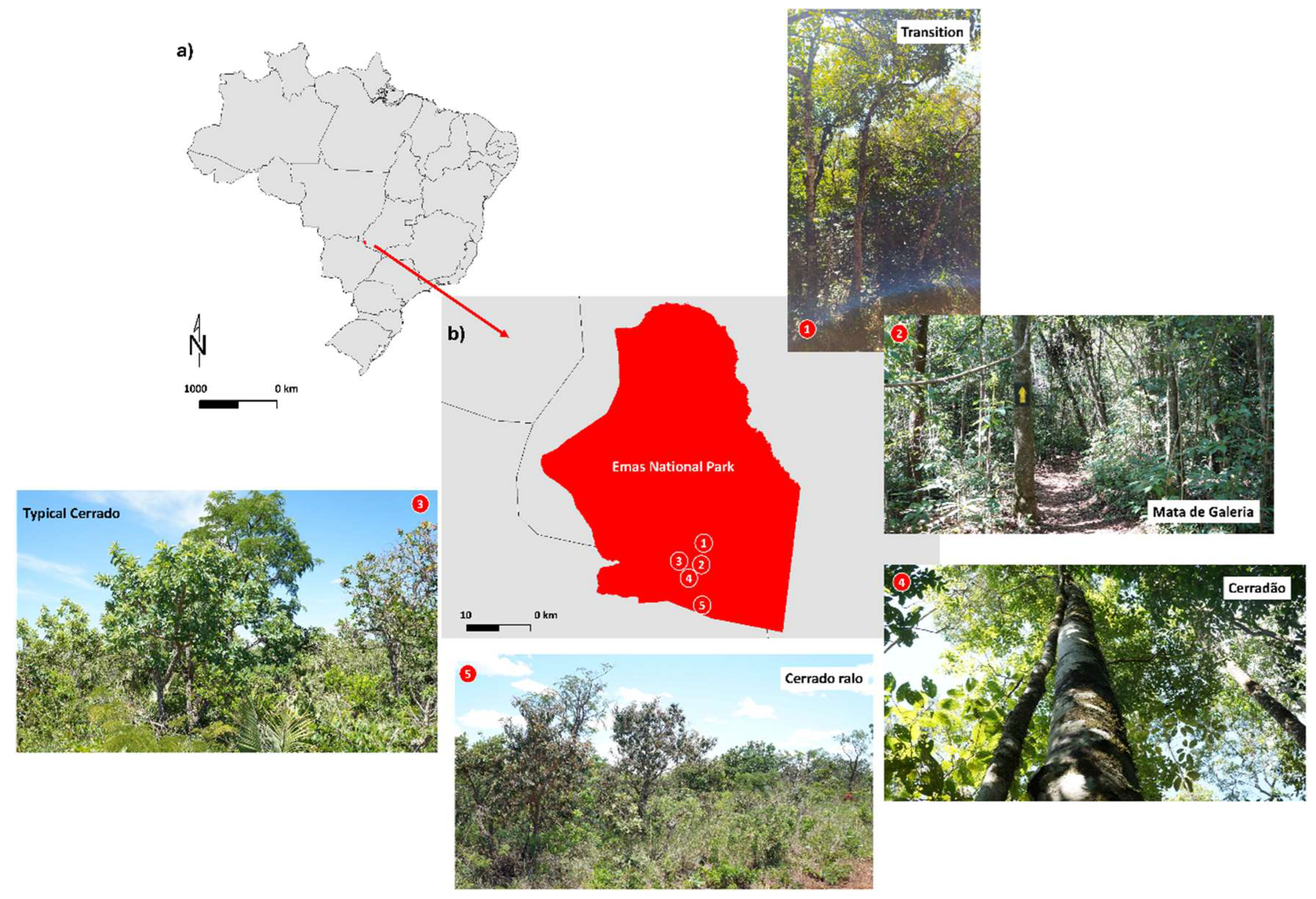
4. Materials and Methods
- Cerradão (18°15′05.2″ S; 52°53′12.2″ W)—Mean illuminance: 13,023 lm m−2. Characterized by dense forest vegetation with structural features intermediate between savanna and tropical forest. Dominated by a continuous tree stratum, emergent trees, and a relatively open understory.
- Typical Cerrado (18°19′42″ S; 52°52′51″ W)—Mean illuminance: 54,130 lm m−2. An open savanna formation with predominantly herbaceous and grassy vegetation, interspersed with a few shrubs and sparse trees.
- Mata de Galeria (Gallery Forest) (18°15′33.6″ S; 52°53′13.7″ W)—Mean illuminance: 26,967 lm m−2. Located along the banks of the Formoso River, this vegetation type is dense, with multiple layers of trees, shrubs, and epiphytes.
- Cerrado Ralo (18°15′33.4″ S; 52°53′21.6″ W)—Mean illuminance: 47,666 lm m−2. Slightly more open than the typical Cerrado, with a predominance of herbaceous vegetation, sparse shrubs, and widely spaced trees.
- Mata de Transição (Transition) (18°13′44.1″ S; 52°52′40.8″ W)—Mean illuminance: 17,010 lm m−2. Represents an intermediate stage between open areas (campo sujo) and forested formations (cerradão). The vegetation is mixed, consisting of trees, shrubs, and grasses. The canopy is discontinuous, with sparse trees and a well-developed herbaceous-shrub layer (Figure 10b).
4.1. Chlorophyll a Fluorescence in Thalli
4.2. Light Measurement
4.3. Position of Lichen on the Trunk
4.4. Experimental Design and Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osyczka, P.; Myśliwa-Kurdziel, B. The pattern of photosynthetic response and adaptation to changing light conditions in lichens is linked to their ecological range. Photosynth. Res. 2023, 157, 21–35. [Google Scholar] [CrossRef]
- Paoli, L.; Pisani, T.; Munzi, S.; Gaggi, C.; Loppi, S. Influence of sun irradiance and water availability on lichen photosynthetic pigments during a Mediterranean. Biologia 2010, 65, 776–783. [Google Scholar] [CrossRef]
- Piccotto, M.; Tretiach, M. Photosynthesis in chlorolichens: The influence of the habitat light regime. J. Plant Res. 2010, 123, 763–775. [Google Scholar] [CrossRef]
- Gasulla, F.; Del Campo, E.M.; Casano, L.M.; Guéra, A. Advances in understanding of desiccation tolerance of lichens and lichen-forming algae. Plants 2021, 10, 807. [Google Scholar] [CrossRef]
- Aubert, S.; Juge, C.; Boisson, A.M.; Gout, E.; Bligny, R. Metabolic processes sustaining the reviviscence of lichen Xanthoria elegans (Link) in high mountain environments. Planta 2007, 226, 1287–1297. [Google Scholar] [CrossRef]
- Lange, O.L.; Leisner, J.M.; Bilger, W. Chlorophyll fluorescence characteristics of the cyanobacterial lichen Peltigera rufescens under field conditions: II. Diel and annual distribution of metabolic activity and possible mechanisms to avoid photoinhibition. Flora 1999, 194, 413–430. [Google Scholar] [CrossRef]
- Boruah, T.; Dulal, K.; Das, P.N. Ecology of lichen. In Chemistry, Biology and Pharmacology of Lichen; Das, A.K., Sharma, A., Kathuria, D., Ansari, M.J., Bhardwaj, G., Eds.; Wiley: Hoboken, NJ, USA, 2024; pp. 49–69. [Google Scholar] [CrossRef]
- Morillas, L.; Roales, J.; Cruz, C.; Munzi, S. Resilience of epiphytic lichens to combined effects of increasing nitrogen and solar radiation. J. Fungi 2021, 7, 333. [Google Scholar] [CrossRef]
- Armstrong, R.A. Adaptation of lichens to extreme conditions. In Plant Adaptation Strategies in Changing Environment; Shukla, V., Kumar, S., Kumar, N., Eds.; Springer: Singapore, 2017; pp. 1–27. [Google Scholar] [CrossRef]
- Mikhaylov, A. Lichens as indicators of atmospheric pollution in urban ecosystems. Isr. J. Ecol. Evol. 2020, 67, 60–68. [Google Scholar] [CrossRef]
- Beckett, R.P.; Minibayeva, F.; Solhaug, K.A.; Roach, T. Photoprotection in lichens: Adaptations of photobionts to high light. Lichenologist 2021, 53, 21–33. [Google Scholar] [CrossRef]
- Veres, K.; Csintalan, Z.; Laufer, Z.; Engel, R.; Szabó, K.; Farkas, E. Photoprotection and high-light acclimation in semi-arid grassland lichens–a cooperation between algal and fungal partners. Symbiosis 2022, 86, 33–48. [Google Scholar] [CrossRef]
- Meeßen, J.; Sánchez, F.J.; Brandt, A.; Balzer, E.M.; de la Torre, R.; Sancho, L.G.; de Vera, J.P.; Ott, S. Extremotolerance and resistance of lichens: Comparative studies on five species used in astrobiological research I. Morphological and anatomical characteristics. Orig. Life Evol. Biosph. 2013, 43, 283–303. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Schneider, J.R.; Caverzan, A.; Chavarria, G. Water deficit stress, ROS involvement, and plant performance. Arch. Agron. Soil Sci. 2019, 65, 1160–1181. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Huiskes, A.H.L.; Gremmen, N.J.M.; Francke, J.W. Morphological effects on the water balance of Antarctic foliose and fruticose lichens. Antarct. Sci. 1997, 9, 36–42. [Google Scholar] [CrossRef]
- Spielmann, A.A.; Marcelli, M.P. Parmotrema sl (Parmeliaceae, lichenized Ascomycota) from Serra Geral slopes in central Rio Grande do Sul State, Brazil. Hoehnea 2009, 36, 551–595. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.; Gîrd, C.E.; Calcan, S.I.; Cucolea, E.I.; Costache, T.; Rambu, D.; Ungureanu-Iuga, M.; Oroian, M.; Mironeasa, S.; et al. Advances in the characterization of Usnea barbata (L.) Weber ex FH Wigg from Călimani Mountains, Romania. Appl. Sci. 2022, 12, 4234. [Google Scholar] [CrossRef]
- Souza, C.L.F.; de Oliveira, R.B.; Mustafé, D.N.; Nunes, K.A.C.; de Morais, E.M.B. O cerrado como o “berço das águas”: Potencialidades para a educação geográfica. Rev. Cerrados 2019, 17, 86–113. [Google Scholar] [CrossRef]
- Klink, C.A.; Machado, R.B. Conservation of the Brazilian cerrado. Conserv. Biol. 2005, 19, 707–713. [Google Scholar] [CrossRef]
- Colli, G.R.; Vieira, C.R.; Dianese, J.C. Biodiversity and conservation of the Cerrado: Recent advances and old challenges. Biodivers. Conserv. 2020, 29, 1465–1475. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Goward, T. Sunscreening pigments shape the horizontal distribution of pendent hair lichens in the lower canopy of unmanaged coniferous forests. Lichenologist 2023, 55, 81–89. [Google Scholar] [CrossRef]
- Sharma, N.; Nagar, S.; Thakur, M.; Suriyakumar, P.; Kataria, S.; Shanker, A.K.; Landi, M.; Anand, A. Photosystems under high light stress: Throwing light on mechanism and adaptation. Photosynthetica 2023, 61, 250. [Google Scholar] [CrossRef]
- Kataria, S.; Jajoo, A.; Guruprasad, K.N. Impact of increasing Ultraviolet-B (UV-B) radiation on photosynthetic processes. J. Photochem. Photobiol. B Biol. 2014, 137, 55–66. [Google Scholar] [CrossRef]
- Shao, N.; Krieger-Liszkay, A.; Schroda, M.; Beck, C.F. A reporter system for the individual detection of hydrogen peroxide and singlet oxygen: Its use for the assay of reactive oxygen species produced in vivo. Plant J. 2007, 50, 475–487. [Google Scholar] [CrossRef]
- Janeeshma, E.; Johnson, R.; Amritha, M.; Noble, L.; Aswathi, K.R.; Telesiński, A.; Kalaji, H.M.; Auriga, A.; Puthur, J.T. Modulations in chlorophyll a fluorescence based on intensity and spectral variations of light. Int. J. Mol. Sci. 2022, 23, 5599. [Google Scholar] [CrossRef]
- Bassi, R.; Dall’Osto, L. Dissipation of light energy absorbed in excess: The molecular mechanisms. Annu. Rev. Plant Biol. 2021, 72, 47–76. [Google Scholar] [CrossRef]
- Zavafer, A.; Mancilla, C. Concepts of photochemical damage of Photosystem II and the role of excessive excitation. J. Photochem. Photobiol. C Photochem. Rev. 2021, 47, 100421. [Google Scholar] [CrossRef]
- Phinney, N.H.; Solhaug, K.A.; Gauslaa, Y. Rapid resurrection of chlorolichens in humid air: Specific thallus mass drives rehydration and reactivation kinetics. Environ. Exp. Bot. 2018, 148, 184–191. [Google Scholar] [CrossRef]
- Muggia, L.; Vancurova, L.; Škaloud, P.; Peksa, O.; Wedin, M.; Grube, M. The symbiotic playground of lichen thalli–a highly flexible photobiont association in rock-inhabiting lichens. FEMS Microbiol. Ecol. 2013, 85, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, E.; Paoli, L.; Colzi, I.; Coppi, A.; Gonnelli, C.; Lazzaro, L.; Loppi, S.; Papini, A.; Vannini, A.; Benesperi, R. High-light stress in wet and dry thalli of the endangered Mediterranean lichen Seirophora villosa (Ach.) Frödén: Does size matter? Mycol. Prog. 2019, 18, 463–470. [Google Scholar] [CrossRef]
- Fatima, S.; APA; RKC; Sharma, B.; Gaikwad, S.; Mohan, A.; Sequeira, S. Deciphering the symbiosis of endemic Usnea ghattensis and their photobiont Trebouxia sp. through molecular tools from the northern Western Ghats, India. Microb. Biosyst. 2021, 6, 30–42. [Google Scholar] [CrossRef]
- Ohmura, Y.; Takeshita, S.; Kawachi, M. Photobiont diversity within populations of a vegetatively reproducing lichen, Parmotrema tinctorum, can be generated by photobiont switching. Symbiosis 2019, 77, 59–72. [Google Scholar] [CrossRef]
- Rafat, A.; Ridgway, H.J.; Cruickshank, R.H.; Buckley, H.L. Isolation and co-culturing of symbionts in the genus Usnea. Symbiosis 2015, 66, 123–132. [Google Scholar] [CrossRef]
- Ohmura, Y.; Kawachi, M.; Kasai, F.; Watanabe, M.M.; Takeshita, S. Genetic combinations of symbionts in a vegetatively reproducing lichen, Parmotrema tinctorum, based on ITS rDNA sequences. Bryologist 2006, 109, 43–59. [Google Scholar] [CrossRef]
- Muggia, L.; Candotto-Carniel, F.; Grube, M. The lichen photobiont Trebouxia: Towards an appreciation of species diversity and molecular studies. In Algal and Cyanobacteria Symbioses; Grube, M., Seckbach, J., Muggia, L., Eds.; World Scientific: Singapore, 2017; pp. 111–146. [Google Scholar] [CrossRef]
- Molins, A.; Chiva, S.; Calatayud, Á.; Marco, F.; García-Breijo, F.; Reig-Armiñana, J.; Carrasco, P.; Moya, P. Multidisciplinary approach to describe Trebouxia diversity within lichenized fungi Buellia zoharyi from the Canary Islands. Symbiosis 2020, 82, 19–34. [Google Scholar] [CrossRef]
- Casano, L.M.; del Campo, E.M.; García-Breijo, F.J.; Reig-Armiñana, J.; Gasulla, F.; Del Hoyo, A.; Guéra, A.; Barreno, E. Two Trebouxia algae with different physiological performances are ever-present in lichen thalli of Ramalina farinacea. Co-existence versus competition? Environ. Microbiol. 2011, 13, 806–818. [Google Scholar] [CrossRef]
- Muhetaer, G.; Jayasanka, S.M.; Fujino, T. Oxidative stress and antioxidant responses of Phormidium ambiguum and Microcystis aeruginosa under diurnally varying light conditions. Microorganisms 2020, 8, 890. [Google Scholar] [CrossRef] [PubMed]
- Han, L.J.; Fan, D.Y.; Wang, X.P.; Xu, C.Y.; Xia, X.L.; Chow, W.S. The protective role of non-photochemical quenching in PSII photosusceptibility: A case study in the field. Plant Cell Physiol. 2023, 64, 43–54. [Google Scholar] [CrossRef]
- Murchie, E.H.; Ruban, A.V. Dynamic non-photochemical quenching in plants: From molecular mechanism to productivity. Plant J. 2020, 101, 885–896. [Google Scholar] [CrossRef]
- Mlinarić, S.; Begović, L.; Katanić, Z.; Galić, V. Changes of chlorophyll a fluorescence parameters influenced by light and temperature Stress. In Chlorophyll a Fluorescence Measurements in Croatia—First Twenty Years; Hrvoje, L., Marija, V.V., Zvonimir, Z., Eds.; Instituto Agrícola: Osijek, Croatia, 2023; pp. 29–42. [Google Scholar]
- Kalaji, H.; Rastogi, A.; Živčák, M.; Brestic, M.; Daszkowska-Golec, A.; Sitko, K.; Alsharafa, K.; Lotfi, R.; Stypiński, P.; Samborska, I. Prompt chlorophyll fluorescence as a tool for crop phenotyping: An example of barley landraces exposed to various abiotic stress factors. Photosynthetica 2018, 56, 953–961. [Google Scholar] [CrossRef]
- Bayat, L.; Arab, M.; Aliniaeifard, S.; Seif, M.; Lastochkina, O.; Li, T. Effects of growth under different light spectra on the subsequent high light tolerance in rose plants. AoB Plants 2018, 10, ply052. [Google Scholar] [CrossRef]
- Markulj Kulundžić, A.; Viljevac Vuletić, M.; Matoša Kočar, M.; Antunović Dunić, J.; Varga, I.; Sudarić, A.; Cesar, V.; Zdunić, Z. Effect of elevated temperature and excess light on photosynthetic efficiency, pigments, and proteins in the field-grown sunflower during afternoon. Horticulturae 2022, 8, 392. [Google Scholar] [CrossRef]
- Beckett, R.P.; Roach, T.; Minibayeva, F.; Werth, S. Alternative electron transport pathways contribute to tolerance to high light stress in lichenized algae. Physiol. Plant. 2023, 175, e13904. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.; Popescu, A.; Schröder, V.; Costache, T.; Rambu, D.; Cucolea, I.E.; Gird, C.E.; Caraiane, A.; Gherghel, D.; et al. Antioxidant and cytotoxic activities of Usnea barbata (L.) FH Wigg. dry extracts in different solvents. Plants 2021, 10, 909. [Google Scholar] [CrossRef]
- White, P.A.S.; Oliveira, R.C.M.; Oliveira, A.P.; Serafini, M.R.; Araújo, A.A.S.; Gelain, D.P.; Moreira, J.C.F.; Almeida, J.R.G.S.; Quintans, J.S.S.; Quintans-Junior, L.J.; et al. Antioxidant activity and mechanisms of action of natural compounds isolated from lichens: A systematic review. Molecules 2014, 19, 14496–14527. [Google Scholar] [CrossRef]
- Eriksson, A.; Gauslaa, Y.; Palmqvist, K.; Ekström, M.; Esseen, P.-A. Morphology drives water storage traits in the globally widespread lichen genus Usnea. Fungal Ecol. 2018, 35, 51–61. [Google Scholar] [CrossRef]
- Phinney, N.H.; Asplund, J.; Gauslaa, Y. The lichen cushion: A functional perspective of color and size of a dominant growth form on glacier forelands. Fungal Biol. 2022, 126, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Galanty, A.; Popiół, J.; Paczkowska-Walendowska, M.; Studzińska-Sroka, E.; Paśko, P.; Cielecka-Piontek, J.; Podolak, I. (+)-Usnic acid as a promising candidate for a safe and stable topical photoprotective agent. Molecules 2021, 26, 5224. [Google Scholar] [CrossRef]
- Solhaug, K.A.; Larsson, P.; Gauslaa, Y. Light screening in lichen cortices can be quantified by chlorophyll fluorescence techniques for both reflecting and absorbing pigments. Planta 2010, 231, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, K.A. Studies on lichen-dominated systems. XII. The ecological significance of thallus color. Can. J. Bot. 1975, 53, 660–667. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Deep, P.R.; Singh, S.; Nayak, B. Lichen secondary metabolites and its biological activity. Am. J. PharmTech Res. 2016, 6, 1–17. [Google Scholar] [CrossRef]
- Rojas, J.O.H.N.; Londoño, C.E.S.A.R.; Ciro, Y. The health benefits of natural skin UVA photoprotective compounds found in botanical sources. Int. J. Pharm. Pharm. Sci. 2016, 8, 13–23. [Google Scholar]
- Thakur, M.; Chander, H. Potential of lichens: A review of bioactive compounds with biological activities. Biol. Forum–Int. J. 2021, 13, 39–47. [Google Scholar]
- Engel, K.; Schmidt, U.; Reuter, J.; Weckesser, S.; Simon-Haarhaus, B.; Schempp, C.M. Usnea barbata extract prevents ultraviolet-B induced prostaglandin E2 synthesis and COX-2 expression in HaCaT keratinocytes. J. Photochem. Photobiol. B 2007, 89, 9–14. [Google Scholar] [CrossRef]
- Harikrishnan, A.; Veena, V.; Lakshmi, B.; Shanmugavalli, R.; Theres, S.; Prashantha, C.N.; Shah, T.; Oshin, K.; Togam, R.; Nandi, S. Atranorin, an antimicrobial metabolite from lichen Parmotrema rampoddense exhibited in vitro anti-breast cancer activity through interaction with Akt activity. J. Biomol. Struct. Dyn. 2021, 39, 1248–1258. [Google Scholar] [CrossRef]
- Passo, A.; Rodriguez, J.M.; Chiapella, J. New records of Antarctic lichens. N. Z. J. Bot. 2015, 53, 216–223. [Google Scholar] [CrossRef]
- Barták, M.; Hájek, J.; Halıcı, M.G.; Bednaříková, M.; Casanova-Katny, A.; Váczi, P.; Puhovkin, A.; Misha, K.B.; Giordano, D. Resistance of primary photosynthesis to photoinhibition in Antarctic lichen Xanthoria elegans: Photoprotective mechanisms activated during a short period of high light stress. Plants 2023, 12, 2259. [Google Scholar] [CrossRef] [PubMed]
- Rautenberger, R.; Hurd, C.L. Photoprotection by photoinhibitory and PSII-reaction centre quenching controls growth of Ulva rigida (Chlorophyta) and is a pre-requisite for green tide formation. Planta 2024, 259, 111. [Google Scholar] [CrossRef]
- Miyake, H.; Komura, M.; Itoh, S.; Kosugi, M.; Kashino, Y.; Satoh, K.; Shibata, Y. Multiple dissipation components of excess light energy in dry lichen revealed by ultrafast fluorescence study at 5 K. Photosynth. Res. 2011, 110, 39–48. [Google Scholar] [CrossRef]
- Adams, W.W.; Demmig-Adams, B.; Lange, O.L. Carotenoid composition and metabolism in green and blue-green algal lichens in the field. Oecologia 1993, 94, 576–584. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I. Photosynthetic apparatus: Major site of oxidative damage. In Reactive Oxygen Species in Plants: The Right Balance; Sachdev, S., Ansari, S.A., Ansari, M.I., Eds.; Springer Nature: Singapore, 2023; pp. 75–92. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Shimakawa, G. Regulation of the generation of reactive oxygen species during photosynthetic electron transport. Biochem. Soc. Trans. 2022, 50, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Mkhize, K.G.W.; Minibayeva, F.V.; Beckett, R.P. Adaptations of photosynthesis in sun and shade in populations of some Afromontane lichens. Lichenologist 2022, 54, 319–329. [Google Scholar] [CrossRef]
- Marečková, M.; Barták, M. Short-term responses of primary processes in PS II to low temperature are sensitively indicated by fast chlorophyll fluorescence kinetics in Antarctic lichen Dermatocarpon polyphyllizum. Czech Polar Rep. 2017, 7, 74–82. [Google Scholar] [CrossRef]
- Katanić, Z.; Atić, L.; Ferhatović, D.; Cesar, V.; Lepeduš, H. PSII photochemistry in vegetative buds and needles of Norway spruce (Picea abies L. Karst.) probed by OJIP chlorophyll a fluorescence measurement. Acta Biol. Hung. 2012, 63, 218–230. [Google Scholar] [CrossRef]
- Barták, M.; Hájek, J.; Orekhova, A.; Villagra, J.; Marín, C.; Palfner, G.; Casanova-Katny, A. Inhibition of primary photosynthesis in desiccating Antarctic lichens differing in their photobionts, thallus morphology, and spectral properties. Microorganisms 2021, 9, 818. [Google Scholar] [CrossRef]
- Dall’Osto, L.; Caffarri, S.; Bassi, R. A mechanism of nonphotochemical energy dissipation, independent from PsbS, revealed by a conformational change in the antenna protein CP26. Plant Cell 2005, 17, 1217–1232. [Google Scholar] [CrossRef]
- Boonpeng, C.; Pischom, M.; Butrid, P.; Noikrad, S.; Boonpragob, K. Laboratory and field measurements of water relations, photosynthetic parameters, and hydration traits in macrolichens in a tropical lower montane rainforest in Thailand. J. Plant Res. 2024, 137, 641–658. [Google Scholar] [CrossRef] [PubMed]
- Wieners, P.C.; Mudimu, O.; Bilger, W. Desiccation-induced non-radiative dissipation in isolated green lichen algae. Photosynth. Res. 2012, 113, 239–247. [Google Scholar] [CrossRef]
- Lopes-Assad, M.L. Produtos de Extrativismo do Cerrado: Preservando a caixa d’água do Brasil. Observatório de Conhecimento e Inovação em Bioeconomia; Fundação Getúlio Vargas—FGV-EESP: São Paulo, Brasil, 2023. [Google Scholar]
- Nobel, P.S.; Zaragoza, L.J.; Smith, W.K. Relation between mesophyll surface area, photosynthetic rate, and illumination level during development for leaves of Plectranthus parviflorus Henckel. Plant Physiol. 1975, 55, 1067–1070. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Chlorophyll fluorescence: A signature of photosynthesis. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S.; Price, B.; Adler, D.; Bates, D.; Baud-Bovy, G.; Bolker, B.; Ellison, S.; Firth, D.; Friendly, M.; et al. R-Core. Package Car. 2019. Available online: https://cran.r-project.org/web/packages/car/index.html (accessed on 1 January 2020).
- Hadfield, J.D. MCMC methods for multi-response generalized linear mixed models: The MCMCglmm R package. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- Zuur, A.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Pham, V.T.K.; Rediers, H.; Ghequire, M.G.K.; Nguyen, H.H.; De Mot, R.; Vanderleyden, J.; Spaepen, S. The plant growth-promoting effect of the nitrogen-fixing endophyte Pseudomonas stutzeri A15. Arch. Microbiol. 2017, 199, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.M.; Feinn, R. Using effect size—Or why the P value is not enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Cade, B.S.; Noon, B.R. A gentle introduction to quantile regression for ecologists. Front. Ecol. Environ. 2003, 1, 412–420. [Google Scholar] [CrossRef]
- Koenker, R.; Chernozhukov, V.; He, X.; Peng, L. Handbook of Quantile Regression; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 27 July 2024).




| ABS/RC | |||||
|---|---|---|---|---|---|
| Model | ∆AICc | wAIC | K | AIC | Pr (>F) |
| Landscape + Species | 0.0 | 0.6619 | 7 | 651.47 | - |
| Landscape + Species + Height | 1.4 | 0.3367 | 8 | 652.86 | 0.4332 |
| Landscape + Species + Height + PAR | 12.6 | 0.0012 | 9 | 653.47 | 0.3686 |
| Landscape + Species + Height + PFDUV + PFDG + PFDR + PFDFR + IRR + PAR | 20.1 | <0.001 | 14 | 648.87 | 0.0201 * |
| Landscape + Species + Height + PFDG + PFDR + PFDFR + IRR + PAR | 20.3 | <0.001 | 13 | 647.27 | 0.0127 * |
| Landscape + Species + Height + PFDFR + IRR + PAR | 21.0 | <0.001 | 11 | 651.28 | 0.0846 |
| Landscape + Species + Height + + IRR + PAR | 21.1 | <0.001 | 10 | 655.07 | 0.4936 |
| Landscape + Species + Height + PFDR + PFDFR + IRR + PAR | 21.1 | <0.001 | 12 | 648.67 | 0.0252 * |
| Landscape + Species + Height + PPFD + PFDUV + PFDG + PFDR + PFDFR + IRR + PAR | 26.3 | <0.001 | 15 | 648.98 | 0.0178 * |
| TR0/RC | |||||
| Model | ∆AICc | wAIC | K | AIC | Pr (>F) |
| Landscape + Species | 0.0 | 0.74 | 7 | 169.25 | - |
| Landscape + Species + Height | 2.0 | 0.27 | 8 | 171.25 | 0.9820 |
| Landscape + Species + Height + PAR | 18.1 | <0.001 | 9 | 171.92 | 0.4151 |
| Landscape + Species + Height + + IRR + PAR | 31.2 | <0.001 | 10 | 173.27 | 0.5754 |
| Landscape + Species + Height + PFDFR + IRR + PAR | 40.6 | <0.001 | 11 | 174.57 | 0.6126 |
| Landscape + Species + Height + PFDR + PFDFR + IRR + PAR | 47.3 | <0.001 | 12 | 174.03 | 0.3897 |
| Landscape + Species + Height + PFDG + PFDR + PFDFR + IRR + PAR | 52.6 | <0.001 | 15 | 174.11 | 0.3074 |
| Landscape + Species + Height + PFDUV + PFDG + PFDR + PFDFR + IRR + PAR | 56.0 | <0.001 | 13 | 174.29 | 0.2556 |
| Landscape + Species + Height + PPFD + PFDUV + PFDG + PFDR + PFDFR + IRR + PAR | 64.2 | <0.001 | 14 | 171.47 | 0.0876 |
| ET0/RC | |||||
| Model | ∆AICc | wAIC | K | AIC | Pr (>F) |
| Landscape + Species | 0.0 | 0.75 | 7 | 81.903 | - |
| Landscape + Species + Height | 2.0 | 0.29 | 8 | 83.851 | 0.8206 |
| Landscape + Species + Height + PAR | 16.3 | <0.001 | 9 | 81.206 | 0.0955 |
| Landscape + Species + Height + + IRR + PAR | 30.2 | <0.001 | 10 | 82.516 | 0.1456 |
| Landscape + Species + Height + PFDFR + IRR + PAR | 35.7 | <0.001 | 11 | 78.455 | 0.0221 * |
| Landscape + Species + Height + PFDR + PFDFR + IRR + PAR | 42 | <0.001 | 12 | 76.390 | 0.0083 ** |
| Landscape + Species + Height + PFDG + PFDR + PFDFR + IRR + PAR | 48.5 | <0.001 | 15 | 76.807 | 0.0089 ** |
| Landscape + Species + Height + PFDUV + PFDG + PFDR + PFDFR + IRR + PAR | 53.9 | <0.001 | 13 | 78.213 | 0.0134 * |
| Landscape + Species + Height + PPFD + PFDUV + PFDG + PFDR + PFDFR + IRR + PAR | 63.7 | <0.001 | 14 | 75.926 | 0.0049 ** |
| DI0/RC | |||||
| Model | ∆AICc | wAIC | K | AIC | Pr (>F) |
| Landscape + Species | 0.0 | 0.6619 | 7 | 639.23 | - |
| Landscape + Species + Height | 1.3 | 0.3367 | 8 | 640.56 | 0.4131 |
| Landscape + Species + Height + PAR | 12.6 | 0.0012 | 9 | 641.30 | 0.3802 |
| Landscape + Species + Height + PFDUV + PFDG + PFDR + PFDFR + IRR + PAR | 20.1 | <0.001 | 14 | 636.63 | 0.0201 * |
| Landscape + Species + Height + PFDG + PFDR + PFDFR + IRR + PAR | 20.3 | <0.001 | 13 | 634.92 | 0.0121 * |
| Landscape + Species + Height + PFDFR + IRR + PAR | 21 | <0.001 | 11 | 638.79 | 0.0765 |
| Landscape + Species + Height + + IRR + PAR | 21.1 | <0.001 | 10 | 642.94 | 0.5147 |
| Landscape + Species + Height + PFDR + PFDFR + IRR + PAR | 21.1 | <0.001 | 12 | 636.22 | 0.0232 * |
| Landscape + Species + Height + PPFD + PFDUV + PFDG + PFDR + PFDFR + IRR + PAR | 26.3 | <0.001 | 15 | 637.08 | 0.0200 * |
| PHIDO | |||||
| Model | ∆AICc | wAIC | K | AIC | Pr (>F) |
| Landscape + Species | 0.0 | 0.71 | 7 | 74.815 | - |
| Landscape + Species + Height | 1.8 | 0.29 | 8 | 75.516 | 0.6780 |
| Landscape + Species + Height + PAR | 20.5 | <0.001 | 9 | 74.815 | 0.4793 |
| Landscape + Species + Height + + IRR + PAR | 36.4 | <0.001 | 10 | 73.076 | 0.6297 |
| Landscape + Species + Height + PFDFR + IRR + PAR | 45.2 | <0.001 | 11 | 75.174 | 0.2122 |
| Landscape + Species + Height + PFDR + PFDFR + IRR + PAR | 52.6 | <0.001 | 12 | 77.874 | 0.0615 |
| Landscape + Species + Height + PFDG + PFDR + PFDFR + IRR + PAR | 57.9 | <0.001 | 13 | 80.515 | 0.0189 * |
| Landscape + Species + Height + PFDUV + PFDG + PFDR + PFDFR + IRR + PAR | 65.3 | <0.001 | 14 | 78.653 | 0.0322 * |
| Landscape + Species + Height + PPFD + PFDUV + PFDG + PFDR + PFDFR + IRR + PAR | 77.4 | <0.001 | 15 | 80.007 | 0.0167 * |
| PIABS | |||||
| Model | ∆AICc | wAIC | K | AIC | Pr (>F) |
| Landscape + Species | 0.0 | 0.76 | 7 | 140.86 | - |
| Landscape + Species + Height | 1.8 | 0.28 | 8 | 142.87 | 0.9989 |
| Landscape + Species + Height + PAR | 19.2 | <0.001 | 9 | 144.38 | 0.7888 |
| Landscape + Species + Height + + IRR + PAR | 32.9 | <0.001 | 10 | 146.12 | 0.8639 |
| Landscape + Species + Height + PFDFR + IRR + PAR | 41.2 | <0.001 | 11 | 145.94 | 0.5712 |
| Landscape + Species + Height + PFDR + PFDFR + IRR + PAR | 49.8 | <0.001 | 12 | 147.12 | 0.5872 |
| Landscape + Species + Height + PFDG + PFDR + PFDFR + IRR + PAR | 52.1 | <0.001 | 15 | 143.56 | 0.1576 |
| Landscape + Species + Height + PFDUV + PFDG + PFDR + PFDFR + IRR + PAR | 56.4 | <0.001 | 13 | 144.51 | 0.1698 |
| Landscape + Species + Height + PPFD + PFDUV + PFDG + PFDR + PFDFR + IRR + PAR | 64.1 | <0.001 | 14 | 140.69 | 0.0491 * |
| PHIPO | |||||
| Model | ∆AICc | wAIC | K | AIC | Pr (>F) |
| Landscape + Species | 0.0 | 0.71 | 7 | 76.859 | - |
| Landscape + Species + Height | 1.8 | 0.29 | 8 | 75.026 | 0.6831 |
| Landscape + Species + Height + PAR | 20.6 | <0.001 | 9 | 74.305 | 0.4852 |
| Landscape + Species + Height + + IRR + PAR | 36.5 | <0.001 | 10 | 72.591 | 0.6298 |
| Landscape + Species + Height + PFDFR + IRR + PAR | 45.2 | <0.001 | 11 | 74.700 | 0.2113 |
| Landscape + Species + Height + PFDR + PFDFR + IRR + PAR | 52.5 | <0.001 | 12 | 77.460 | 0.0598 |
| Landscape + Species + Height + PFDG + PFDR + PFDFR + IRR + PAR | 57.7 | <0.001 | 13 | 80.246 | 0.0174 * |
| Landscape + Species + Height + PFDUV + PFDG + PFDR + PFDFR + IRR + PAR | 65.1 | <0.001 | 14 | 78.392 | 0.0297 * |
| Landscape + Species + Height + PPFD + PFDUV + PFDG + PFDR + PFDFR + IRR + PAR | 77.1 | <0.001 | 15 | 79.842 | 0.0149 * |
| PSI0 | |||||
| Model | ∆AICc | wAIC | K | AIC | Pr (>F) |
| Landscape + Species | 0.0 | 0.73 | 7 | 90.765 | - |
| Landscape + Species + Height | 2.0 | 0.27 | 8 | 88.765 | 0.9987 |
| Landscape + Species + Height + PAR | 18.8 | <0.001 | 9 | 90.286 | 0.1720 |
| Landscape + Species + Height + + IRR + PAR | 34.1 | <0.001 | 10 | 89.339 | 0.2057 |
| Landscape + Species + Height + PFDFR + IRR + PAR | 43.2 | <0.001 | 11 | 91.304 | 0.0737 |
| Landscape + Species + Height + PFDR + PFDFR + IRR + PAR | 51.0 | <0.001 | 12 | 93.565 | 0.0253 * |
| Landscape + Species + Height + PFDG + PFDR + PFDFR + IRR + PAR | 58.8 | <0.001 | 13 | 93.652 | 0.0211 * |
| Landscape + Species + Height + PFDUV + PFDG + PFDR + PFDFR + IRR + PAR | 65.2 | <0.001 | 14 | 93.020 | 0.0228 * |
| Landscape + Species + Height + PPFD + PFDUV + PFDG + PFDR + PFDFR + IRR + PAR | 75.0 | <0.001 | 15 | 97.146 | 0.0042 ** |
| PHIE0 | |||||
| Model | ∆AICc | wAIC | K | AIC | Pr (>F) |
| Landscape + Species | 0.0 | 0.74 | 7 | 143.35 | - |
| Landscape + Species + Height | 1.6 | 0.27 | 8 | 141.35 | 0.9899 |
| Landscape + Species + Height + PAR | 20.3 | <0.001 | 9 | 142.0 | 0.2610 |
| Landscape + Species + Height + + IRR + PAR | 36.6 | <0.001 | 10 | 140.59 | 0.3541 |
| Landscape + Species + Height + PFDFR + IRR + PAR | 46.3 | <0.001 | 11 | 142.54 | 0.1257 |
| Landscape + Species + Height + PFDR + PFDFR + IRR + PAR | 55.1 | <0.001 | 12 | 144.26 | 0.0530 |
| Landscape + Species + Height + PFDG + PFDR + PFDFR + IRR + PAR | 62.7 | <0.001 | 13 | 145.13 | 0.0321 * |
| Landscape + Species + Height + PFDUV + PFDG + PFDR + PFDFR + IRR + PAR | 70.1 | <0.001 | 14 | 143.97 | 0.0411 * |
| Landscape + Species + Height + PPFD + PFDUV + PFDG + PFDR + PFDFR + IRR + PAR | 80.8 | <0.001 | 15 | 147.75 | 0.0089 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitorino, L.C.; Rosa, M.; Cruvinel, B.G.; Marques, M.M.d.S.; Santos, A.M.D.; Bessa, L.A. Photochemical Responses of Parmotrema tinctorum and Usnea barbata to Light Variations in Cerrado Landscapes. Plants 2025, 14, 2802. https://doi.org/10.3390/plants14172802
Vitorino LC, Rosa M, Cruvinel BG, Marques MMdS, Santos AMD, Bessa LA. Photochemical Responses of Parmotrema tinctorum and Usnea barbata to Light Variations in Cerrado Landscapes. Plants. 2025; 14(17):2802. https://doi.org/10.3390/plants14172802
Chicago/Turabian StyleVitorino, Luciana Cristina, Márcio Rosa, Bárbara Gonçalves Cruvinel, Matheus Mendonça de Souza Marques, Alex Marcelino Dos Santos, and Layara Alexandre Bessa. 2025. "Photochemical Responses of Parmotrema tinctorum and Usnea barbata to Light Variations in Cerrado Landscapes" Plants 14, no. 17: 2802. https://doi.org/10.3390/plants14172802
APA StyleVitorino, L. C., Rosa, M., Cruvinel, B. G., Marques, M. M. d. S., Santos, A. M. D., & Bessa, L. A. (2025). Photochemical Responses of Parmotrema tinctorum and Usnea barbata to Light Variations in Cerrado Landscapes. Plants, 14(17), 2802. https://doi.org/10.3390/plants14172802





