Phytochemical Composition and Antioxidant Activity of a Viscum album Mother Tincture
Abstract
1. Introduction
2. Materials and Methods
2.1. Viscum Extracts and Products
2.2. Chemicals and Reagents
2.3. Cell Culture
2.4. MTT Assay
2.5. Antioxidant Activity
2.6. HPLC Profiling
2.7. Preparation of Samples by Solution Digestion
2.8. Untargeted LC-MS/MS
2.9. Mascot Identification
2.10. Targeted LC-MRM-MS
2.11. Targeted LC-MS/MS Analysis
Preparation of Standard Solutions and Samples
2.12. Untargeted GC-MS Analysis
Fatty Acids Analysis
2.13. Estimation of LD50 Based on In Vitro IC50 Values
2.14. Statistical Analyses
3. Results
3.1. Viscum album Mother Tincture Chemical Characterization
Viscum album Phenolic Compounds
3.2. Protein Analysis of Viscum album Extracts
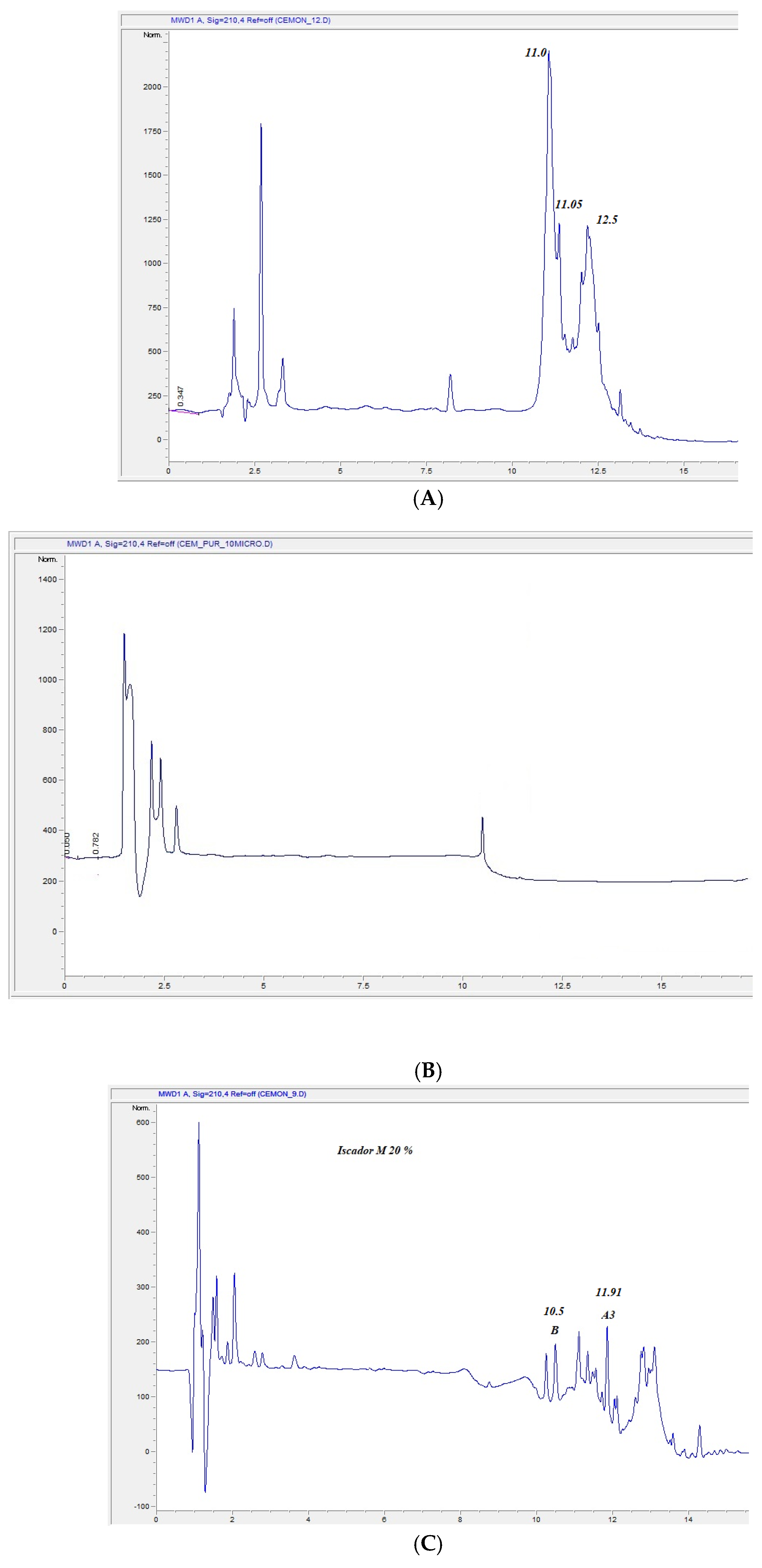
3.2.1. HPLC Analysis
3.2.2. Untargeted Proteomics
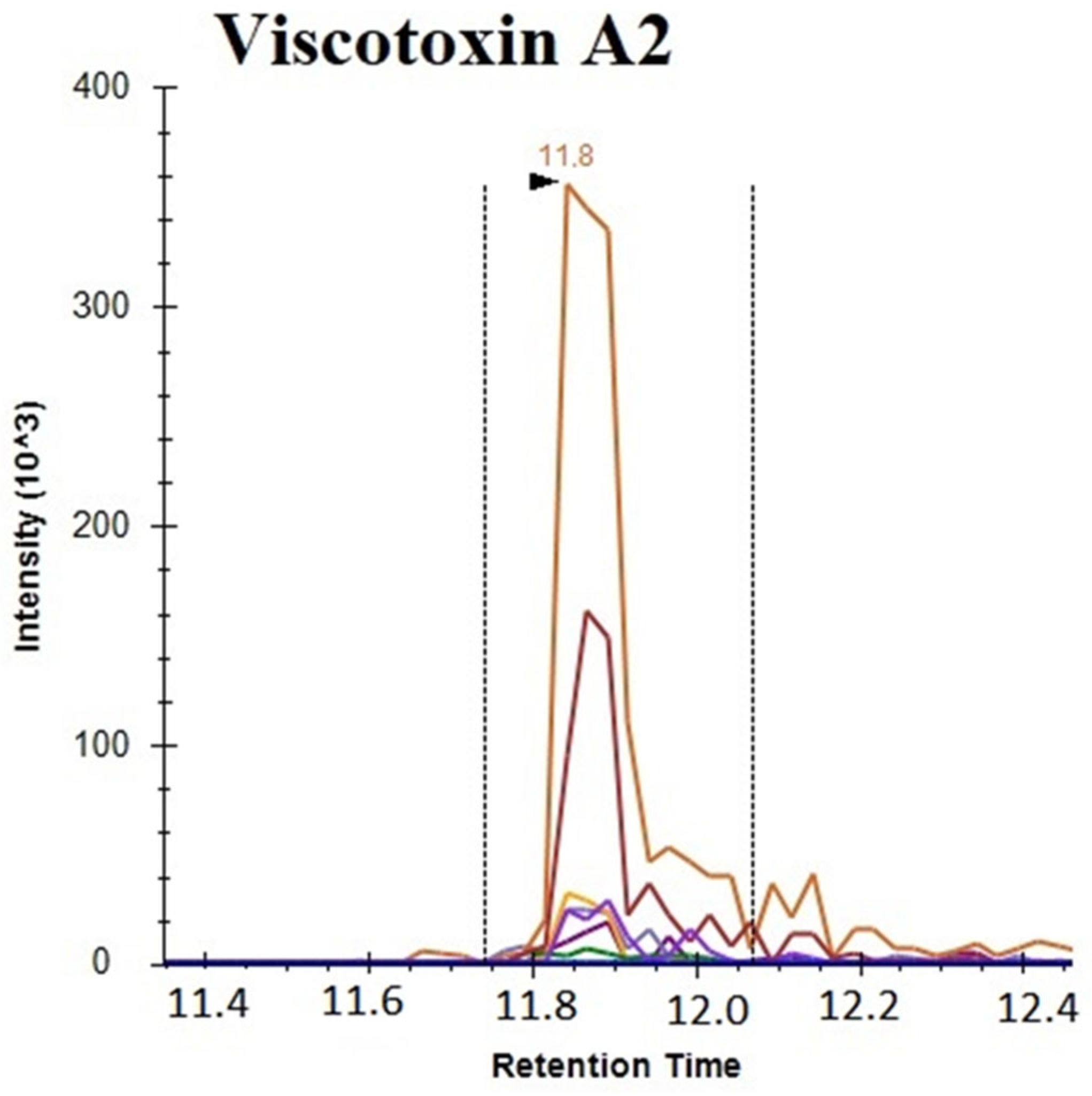
3.3. Multiple Reaction Monitoring Analysis of Viscotoxins
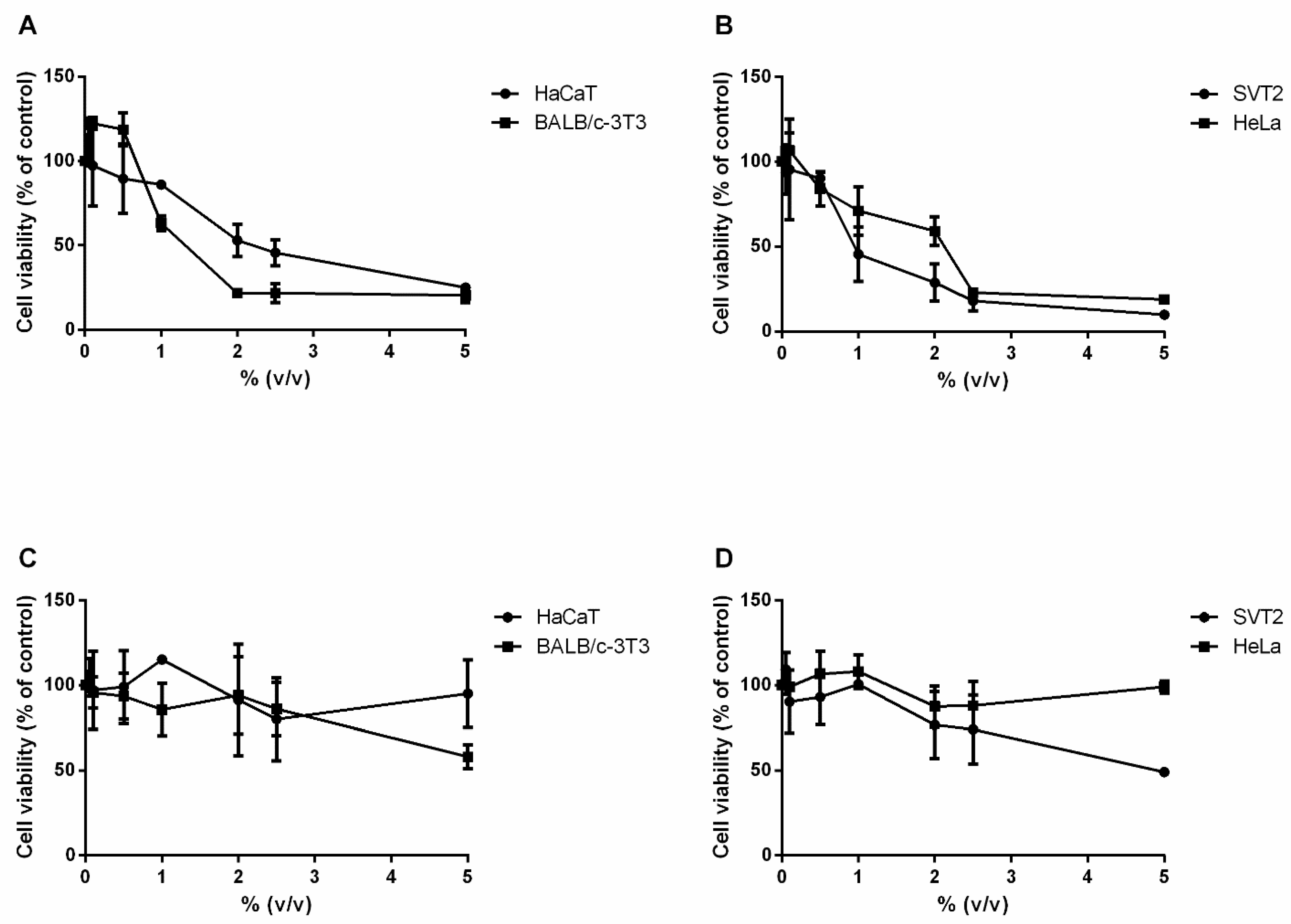
3.4. Effect of MT#39998 and vd-MT on Cell Viability
3.5. Estimation of LD50
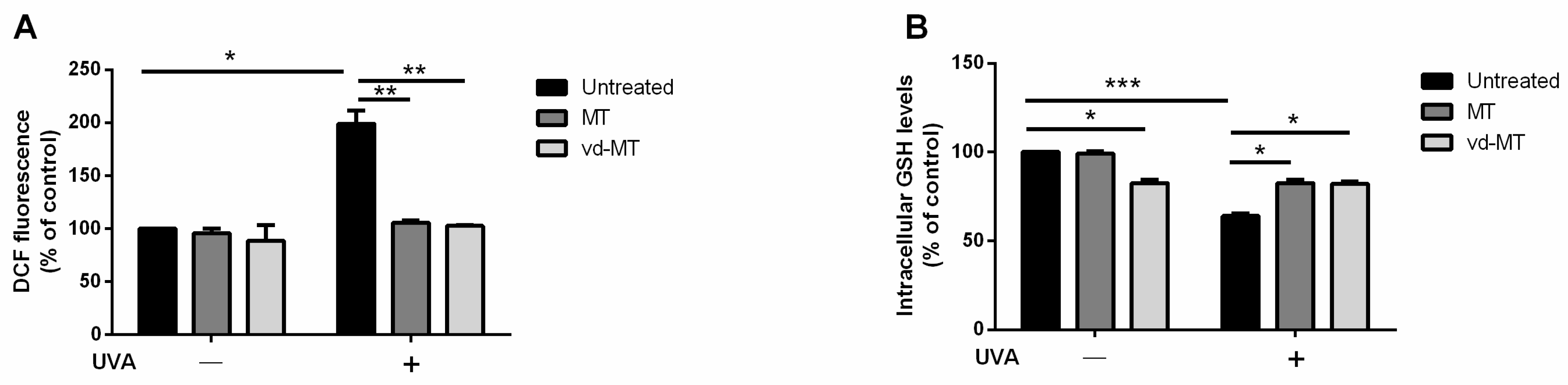
3.6. Antioxidant Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMBIC | Ammonium Bicarbonate |
| DCFDA | 2′,7′-dichlorofluorescin-diacetate |
| DTNB | 5,5′-Dithiobis(2-nitrobenzoic acid) |
| DTT | Dithiothreitol |
| GHS | Globally Harmonized System |
| HPLC | High-Performance Liquid Chromatography |
| IAM | IodoAcetaMide (IAM) |
| ICCVAM | Interagency Coordinating Committee on the Validation of Alternative Methods |
| LC-MRM | Liquid Chromatography-Multiple Reaction Monitoring |
| LC-MRM-MS | Liquid Chromatography-Multiple Reaction Monitoring-Mass Spectrometry |
| LC-MS/MS | Liquid Chromatography-Mass Chromatography/Mass Chromatography |
| LOQ | Limit of Quantification |
| MT | Mistletoe Tincture |
| MTT | Thiazolyl Blue Tetrazolium Bromide |
| ROS | Radical Oxygen Species |
| UVA | Ultraviolet Radiation of A-Type |
References
- Büssing, A. (Ed.) Mistletoe, the Genus Viscum (Medicinal and Aromatic Plants-Industrial Profiles); Harwood Academic Publishers; CRC: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Ernst, E.; Schmidt, K.; Steuer-Vogt, M.K. Mistletoe for cancer? A systematic review of randomised clinical trials. Int. J. Cancer 2003, 107, 262–267. [Google Scholar] [CrossRef]
- Bar-Sela, G. White-Berry Mistletoe (Viscum album L.) as complementary treatment in cancer: Does it help? Eur. J. Integr. Med. 2011, 3, e55–e62. [Google Scholar] [CrossRef]
- Cicchetti, J.T. Viscum album—Magical Plant of Complexity and Paradox. Homoepatic Links 2005, 28, 118–122. [Google Scholar]
- Miwa, Y.; Sakuma, R.A. Safety toxicology study of Equisetum arvense L. Pharmacometrics 2009, 76, 61–69. [Google Scholar]
- Sureshkumar, J.; Jenipher, C.; Sriramavaratharajan, V.; Gurav, S.S.; Rajiv Gandhi, G.; Ravichandran, K.; Ayyanar, M. Genus Equisetum L: Taxonomy, toxicology, phytochemistry and pharmacology. J. Ethnopharmacol. 2023, 314, 116630. [Google Scholar] [CrossRef]
- Aldhouse-Green, M. The moon and the mistletoe in search of ancient druids: Story of an ancient priest-hood. In Caesar’s Druids; Yale University Press: New Haven, CT, USA, 2010; Chapter 1; p. 19. [Google Scholar]
- Lev, E.; Ephraim, M.; Ben-Arye, E. European and Oriental mistletoe: From mythology to contemporary integrative cancer care. Eur. J. Integr. Med. 2011, 3, e133–e137. [Google Scholar] [CrossRef]
- Zänker, K.S.; Kaveri, S.V. (Eds.) Mistletoe: From Mythology to Evidence-Based Medicine; Translational Research in Biomedicine; Karger: Basel, Switzerland, 2015; Volume 4, pp. 1–10. [Google Scholar]
- Jeong, S.Y.; Yu, H.-S.; Ra, M.-J.; Jung, S.-M.; Yu, J.-N.; Kim, J.-C.; Kim, K.H. Phytochemical Investigation of Equisetum arvense and Evaluation of Their Anti-Inflammatory Potential in TNFα/INFγ-Stimulated Keratinocytes. Pharmaceuticals 2023, 16, 1478. [Google Scholar] [CrossRef] [PubMed]
- Kienle, G.S.; Kiene, H. Complementary cancer therapy: A systematic review of prospective clinical trials on anthroposophic mistletoe extracts. Eur. J. Med. Res. 2007, 12, 103–119. [Google Scholar] [PubMed]
- Büssing, A. (Ed.) History of mistletoe uses. In Mistletoe. The Genus Viscum; Harwood Academic Publishers: Amsterdam, The Netherlands, 2000; pp. 1–6. [Google Scholar]
- Nicoletti, M. The Anti-Inflammatory Activity of Viscum album. Plants 2023, 12, 1460. [Google Scholar] [CrossRef]
- Gründemann, C.; Lengen, K.; Sauer, B.; Garcia-Käufer, M.; Zehl, M.; Huber, R. Equisetum arvense (common horsetail) modulates the function of inflammatory immunocompetent cells. BMC Complement Altern Med. 2014, 14, 283. [Google Scholar] [PubMed]
- Do Monte, F.H.; dos Santos, J.G., Jr.; Russi, M.; Lanziotti, V.M.; Leal, L.K.; Cunha, G.M. Antinociceptive and anti-inflammatory properties of the hydroalcoholic extract of stems from Equisetum arvense L. in mice. Pharmacol. Res. 2004, 49, 239–243. [Google Scholar] [CrossRef]
- Nagai, T.; Myoda, T.; Nagashima, T. Antioxidative activities of water extract and ethanol extract from field horsetail (tsukushi) Equisetum arvense L. Food Chem. 2005, 91, 389–394. [Google Scholar] [CrossRef]
- Huh, M.K.; Han, M.D. Inhibitory effect of hyaluronidase and DPPH radical scavenging activity using extraction of Equisetum arvense. J. Adv. Res. Biol. Life Sci. 2015, 3, 47–51. [Google Scholar]
- Valle, A.C.V.; Carvalho, A.C. Viscum album in Medicine Article Review. Int. J. Sci. Res. (IJSR) 2021, 10, 42–48. [Google Scholar] [CrossRef]
- Al-Snafi, P.D.A.E. The pharmacology of Equisetum arvense—A review. IOSR J. Pharm. (IOSRPHR) 2017, 7, 31–42. [Google Scholar] [CrossRef]
- Horneber, M.A.; Bueschel, G.; Huber, R.; Linde, K.; Rostok, M. Mistletoe therapy in oncology. Cochrane Database Syst. Rev. 2008, 2, CD003297. [Google Scholar] [CrossRef] [PubMed]
- Schnell-Inderst, P.; Steigenberger, C.; Mertz, M.; Otto, I.; Flatscher-Thöni, M.; Siebert, U. Additional treatment with mistletoe extracts for patients with breast cancer compared to conventional cancer therapy alone efficacy and safety, costs and cost-effectiveness, patients and social aspects, and ethical assessment. Ger. Med. Sci. 2002, 20, 10. [Google Scholar]
- Braedel-Ruoff, S. Immunomodulatory effects of Viscum album extracts on natural killer cells: Review of clinical trials. Complement. Med. Res. 2010, 17, 63–73. [Google Scholar]
- Freuding, M.; Keinki, C.; Kutschan, S.; Buentzel, J.; Huebner, J. Mistletoe in oncological treatment: A systematic review: Part 1 survival and safety and Part 2: Quality of life and toxicity of cancer treatment. J. Cancer Res. Clin. Oncol. 2019, 145, 695–707. [Google Scholar] [CrossRef]
- Ostermann, T.; Raak, C.; Büssing, A. Survival of cancer patients treated with mistletoe extract (Iscador): A systematic literature review. BMC Cancer 2009, 9, 451. [Google Scholar] [CrossRef]
- Kienle, G.S.; Kiene, H. Review article: Influence of Viscum album L. (European mistletoe) extracts on quality of life in cancer patients: A systematic review of controlled clinical studies. Integr. Cancer Ther. 2010, 9, 142–157. [Google Scholar] [CrossRef]
- Loef, M.; Walach, H. Quality of life in cancer patients treated with mistletoe: A systematic review and meta-analysis. BMC Complement. Med. Ther. 2020, 20, 227. [Google Scholar] [CrossRef]
- Brandenberger, M.; Simoes-Wüst, P.; Rist, L.; Saller, R. Impact of mistletoe therapy on the quality-of-life of cancer patients. Eur. J. Integr. Med. 2009, 1, 225–226. [Google Scholar] [CrossRef]
- Lu, H.; Ouyang, W.; Huang, C. Inflammation, a Key Event in Cancer Development. Mol. Cancer Res. 2006, 4, 221–233. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Philip, M.; Rowley, D.A.; Schreiber, H. Inflammation as a tumor promoter in cancer induction. Semin. Cancer Biol. 2004, 14, 433. [Google Scholar] [CrossRef] [PubMed]
- Okada, F. Inflammation and free radicals in tumor development and progression. Redox Rep. 2002, 7, 357. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2002, 357, 539. [Google Scholar] [CrossRef]
- Thronicke, A.; Schad, F.; Debus, M.; Graboski, J.; Soldner, G. Viscum album L. Therapy in Oncology: An Update of Current Evidence. Complement. Med. Res. 2022, 29, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Schad, F.; Thronicke, A. Safety of Combined Targeted and Helixor Viscum album L. Therapy in Breast and Gynecological Cancer Patients, a Real-World Data Study. Int. J. Environ. Res. Public Health 2023, 20, 2565. [Google Scholar] [CrossRef]
- Grossarth-Maticek, R.; Kiene, H.; Baumgartner, S.M.; Ziegler, R. Use of Iscador, an extract of European mistletoe (Viscum album), in cancer treatment: Prospective nonrandomized and randomized matched-pair studies nested within a cohort study. Altern. Ther. Health Med. 2001, 7, 57–66. [Google Scholar]
- Cazacu, M.; Oniu, T.; Lungoci, C.; Mihailov, A.; Cipak, A.; Klinger, R.; Weiss, T.; Zarkovic, N. The influence of isorel on the advanced colorectal cancer. Cancer Biother. Radiopharm. 2003, 18, 27–34. [Google Scholar] [CrossRef]
- Semiglazov, V.F.; Stepula, V.V.; Dudov, A.; Dudov, A.; Schnitker, J.; Mengs, U. Quality of life is improved in breast cancer patients by Standardised Mistletoe Extract PS76A2 during chemotherapy and follow-up: A randomised, placebo-controlled, double-blind, multicentre clinical trial. Anticancer Res. 2006, 26, 1519–1529. [Google Scholar]
- Rostok, M. Mistletoe in the treatment of cancer. Bundesgesundheitsblatt Gesundheit. Gesund. 2020, 63, 535–540. [Google Scholar]
- Staupe, H.; Buentzel, J.; Keinki, C.; Buentzel, J.; Haiebner, J. Systematic analysis of mistletoe prescriptions in clinical studies. J. Cancer Res. Clin. Oncol. 2023, 149, 5559–5571. [Google Scholar] [CrossRef] [PubMed]
- Jaggy, C.; Musielski, H.; Urech, K.; Schaller, G. Quantitative determination of lectins in mistletoe preparations. Arzneim. Forsch./Drug Res. 1995, 45, 905–909. [Google Scholar]
- Urech, K.; Schaller, G.; Jäggy, C. Viscotoxins, mistletoe lectins and their isoforms in mistletoe (Viscum album L.) extracts Iscador. Arzneimittelforschung 2011, 56, 428–434. [Google Scholar] [CrossRef]
- Schaller, G.; Urech, K.; Giannattasio, M. Cytotoxicity of different viscotoxins and extracts from the European subspecies of Viscum album L. Phyther. Res. 1996, 10, 473–477. [Google Scholar] [CrossRef]
- Pietrzak, W.; Nowak, R. Impact of Harvest Conditions and Host Tree Species on Chemical Composition and Antioxidant Activity of Extracts from Viscum album L. Molecules 2021, 26, 3741. [Google Scholar] [CrossRef] [PubMed]
- Gallo, F.R.; Multari, G.; Federici, E.; Palazzino, G.; Giambenedetti, M.; Petitto, V.; Poli, F.; Nicoletti, M. Chemical fingerprinting of Equisetum arvense L. using HPTLC densitometry and HPLC. Nat. Prod. Res. 2011, 25, 1261–1270. [Google Scholar] [CrossRef]
- Tipke, I.; Bücker, L.; Middelstaedt, J.; Winterhalter, P.; Lubienski, M.; Beuerle, T. HILIC HPLC-ESI-MS/MS identification and quantification of the alkaloids from the genus Equisetum. Phytochem. Anal. 2019, 30, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Ibi, A.; Du, M.; Beuerle, T.; Melchert, D.; Solnier, J.; Chang, C. A Multi-Pronged Technique for Identifying Equisetum palustre and Equisetum arvense-Combining HPTLC, HPLC-ESI-MS/MS and Optimized DNA Barcoding Techniques. Plants 2022, 11, 2562. [Google Scholar] [CrossRef] [PubMed]
- Zia-ur, R.; Gurgul, A.; Youn, I.; Maldonado, A.; Wahid, F.; Che, C.-T.; Khan, T. UHPLC-MS/MS-GNPS based phytochemical investigation of Equisetum arvense L. and evaluation of cytotoxicity against human melanoma and ovarian cancer cells. Saudi J. Biol. Sci. 2022, 29, 103271. [Google Scholar] [CrossRef]
- Stan, R.L.; Hangan, A.C.; Dican, L.; Sevastre, B.; Hanganu, D.; Catoi, C.; Sarpataki, O.; Ionescu, C.M. Comparative study concerning mistletoe viscotoxins antitumor activity. Acta Biol. Hung. 2013, 64, 279–288. [Google Scholar] [CrossRef]
- Becker, H.; Exner, J. Comparative Studies of Flavonoids and Phenylcarboxylic Acids of Mistletoes from Different Host Trees. Z. Pflanzenphysiol. 2002, 97, 417–428. [Google Scholar] [CrossRef]
- Wójciak-Kosior, M.; Sowa, I.; Pucek, K.; Szymczak, G.; Kocjan, R.; Luchowsli, P. Evaluation of seasonal changes of triterpenic acid contents in Viscum album from different host trees. Pharm. Biol. 2017, 55, 1–4. [Google Scholar] [CrossRef]
- Kauczor, G.; Delebinski, C.; Jäger, S.; Seeger, K.; Seifert, G. Triterpene acid containing Viscum album L. extracts mediate apoptosis in pediatric solid cancer cells. BMC Complement. Altern. Med. 2012, 12, P18. [Google Scholar] [CrossRef][Green Version]
- Delebinski, C.I.; Jaeger, S.; Kemnitz-assanim, K.; Henze, G.; Lode, H.N.; Seifert, G.J. A new development of triterpene acid-containing extracts from Viscum album L. displays synergistic induction of apoptosis in acute lymphoblastic leukaemia. Cell Prolif. 2012, 45, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, W.; Nowak, R.; Olech, M. Effect of extraction method on phenolic content and antioxidant activity of mistletoe extracts from Viscum album subsp. abietis. Chem. Pap. 2014, 68, 976–982. [Google Scholar] [CrossRef]
- Jager, T.; Holandino, C.; Rehman, M.N.; Condori Penazola, E.M.; Oliveira, A.P.; Garrett, R.; Glauser, G.; Grazi, M.; Ramm, H.; Urech, K.; et al. Metabolomic by UHPLC-Q-TOF reveals host tree-dependent phytochemical variation in Viscum album L. Plants 2021, 10, 1726. [Google Scholar] [CrossRef]
- Peñaloza, E.; Holandino, C.; Scherr, C.; de Araujo, P.I.P.; Borges, R.M.; Urech, K.; Baumgartner, S.; Garrett, R. Comprehensive metabolome analysis of fermented aqueous extracts of Viscum album L. by liquid chromatography–high resolution tandem mass spectrometry. Molecules 2020, 25, 4006. [Google Scholar] [CrossRef]
- Pieme, C.A.; Ngogang, J.; Costache, M. In vitro antiproliferative and anti-oxidant activities of methanol extracts of Urena lobate and Viscum album against breast cancer cell lines. Toxicol. Environ. Chem. 2012, 94, 987–999. [Google Scholar] [CrossRef]
- Sárpataki, O.; Sevastre, B.; Stan, R.; Olah, N.K.; Hanganu, D.; Bedeceu, I.; Ionescu, C.; Marcus, I. Viscum album, L. Influence on the Antioxidant Enzymes Activity in Ehrlich Tumor Cells In Vivo. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca—Vet. Med. 2014, 71, 198–203. [Google Scholar]
- Mimica-Dukic, N.; Simin, N.; Cvejic, J.; Jovin, E.; Orcic, D.; Bozin, B. Phenolic compounds in field horsetail (Equisetum arvense L.) as natural antioxidants. Molecules 2008, 13, 1455–1464. [Google Scholar] [CrossRef]
- Papuc, C.; Crivineanu, M.; Goran, G.; Nicorescu, V.; Durdun, N. Free Radicals Scavenging and Antioxidant Activity of European Mistletoe (Viscum album) and European Birthwort (Aristolochia clematitis). Rev. Chim. 2010, 61, 619–622. [Google Scholar]
- Shahaboddin, M.E.; Pouramir, M.; Moghadamnia, A.A. Antihyperglycemic and antioxidant activity of Viscum album extract. Afr. J. Pharm. Pharm. 2011, 5, 432–436. [Google Scholar] [CrossRef]
- Vicaş, S.I.; Ruginǎ, D.; Socaciu, C. Comparative study about antioxidant activities of Viscum album from different host trees, harvested in different seasons. J. Med. Plants Res. 2011, 5, 2237–2244. [Google Scholar]
- Nicoletti, M. The Antioxidant Activity of Mistletoes (Viscum album and Other Species). Plants 2023, 12, 2707. [Google Scholar] [CrossRef] [PubMed]
- Homeopathic German Pharmacopoeia GHP 17th Supplement; English Edition; Medpharm Scientific Publishers: Pretoria, South Africa, 2020.
- Stein, G.M.; Pfüller, U.; Schietzel, M. Viscotoxins-free aqueous extracts from European mistletoe (Viscum album L.) stimulate activity of human granulocytes. Anticancer Res. 1999, 19, 2925–2928. [Google Scholar]
- Holandino, C.; Melo, M.N.O.; Oliveira, A.P.; Batista, J.V.D.C.; Capella, M.A.M.; Garrett, R.; Grazi, M.; Ramm, H.; Torre, C.D.; Schaller, G.; et al. Phytochemical analysis and in vitro anti-proliferative activity of Viscum album ethanolic extracts. BMC Complement. Med. Ther. 2020, 20, 215. [Google Scholar] [CrossRef]
- D’Elia, L.; Imbimbo, P.; Liberti, D.; Bolinesi, F.; Mangoni, O.; Pollio, A.; Olivieri, G.; Monti, D.M. Thermo Resistant Antioxidants from Photoautotrophic Microorganisms: Screening and Characterization. World J. Microbiol. Biotechnol. 2021, 37, 1–13. [Google Scholar] [CrossRef]
- Liberti, D.; Imbimbo, P.; Giustino, E.; D’Elia, L.; Silva, M.; Barreira, L.; Monti, D.M. Shedding Light on the Hidden Benefit of Porphyridium Cruentum Culture. Antioxidants 2023, 12, 1–12. [Google Scholar] [CrossRef]
- Petruk, G.; Di Lorenzo, F.; Imbimbo, P.; Silipo, A.; Bonina, A.; Rizza, L.; Piccoli, R.; Monti, D.M.; Lanzetta, R. Protective Effect of Opuntia Ficus-Indica L. Cladodes Against UVA-Induced Oxidative Stress in Normal Human Keratinocytes. Bioorganic Med. Chem. Lett. 2017, 27, 5485–5489. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantification of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- In Vitro Cytotoxicity Test Methods for Estimating Starting Doses For Acute Oral Systemic Toxicity Testing; NIH Publication No. 07-4519; National Institute for Environmental Health Sciences: Research Triangle Park, NC, USA, 2006.
- Schrage, A.; Hempel, K.; Schulz, M.; Kolle, S.N.; van Ravenzwaay, B.; Landsiedel, R. Refinement and reduction of acute oral toxicity testing: A critical review of the use of cytotoxicity data. Altern. Lab. Anim. 2011, 39, 273–295. [Google Scholar] [CrossRef] [PubMed]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B.; Yildiz, F. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar] [CrossRef]
- Shafi, S.; Ahmed, F.; Waheed, A.; Ahmad, S.S.; Khan, S.; Khan, M.A.; Pottoo, F.H.; Rabbani, S.A.; Singh, S.; Najmi, A.K. Phytochemicals and Nanotechnology: A Powerful Combination Against Breast Cancer. Mini Rev. Med. Chem. 2024, 25, 675–692. [Google Scholar] [CrossRef]
- Ozgen, S.; Kilins, O.K.; Selomoghu, Z. Antioxidant activity of Quercetin; A mechanicistic Review. Tyrkish J. Agric.—Food Sci. Technol. 2016, 4, 1134–1138. [Google Scholar]
- Brown, J.E.; Khodr, H.; Hider, R.C. Structural dependence of flavonoids interactions with Ca2+ ions: Implications for their antioxidant properties. Biochem. J. 1998, 330, v1173–v1178. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, S.H.; Doroudi, A. Review of the antioxidant potential of flavonoids as a subgroup of polyphenols and partial substitute for synthetic antioxidants. Avicenna J. Phytomed. 2023, 13, 354–376. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gosh, N.; Chakroborthy, T.; Mallick, S.; Mana, S.; Singha, D.; Gosh, B.; Roy, S. Synthesis, characterization and study of the antioxidant activity of quercetin-magnesium complex. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 151, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Botham, P.A. Acute systemic toxicity—Prospects for tiered testing strategies. Toxicol. In Vitro 2004, 18, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Schaller, G.; Urech, K.; Grazi, G.; Giannattasio, M. Viscotoxin composition of the three European subspecies of Viscum album. Planta Med. 1998, 64, 677. [Google Scholar] [CrossRef]
- Urech, K.; Schaller, G.; Ziska, P.; Giannattasio, M. Comparative study on the cytotoxic effect of viscotoxin and mistletoe lectin on tumor cells in culture. Phytother. Res. 1995, 9, 49–55. [Google Scholar] [CrossRef]
- MacLean, B.; Tomazela, D.M.; Shulman, N.; Chambers, M.; Finney, G.L.; Frewen, B.; Kern, R.; Tabb, D.L.; Liebler, D.C.; MacCoss, M.J. Skyline: An open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 2010, 26, 966–968. [Google Scholar] [CrossRef]
- Yang, H.O.; Park, S.Y.; Hong, K.H.; Kang, L.W.; Choe, K.H.; Kim, Y.K. Bleeding time prolongation effect of methanol extract of Viscum album var. coloratum. Nat. Prod. Sci. 2002, 8, 152–154. [Google Scholar]
- Yusuf, L.; Oladunmoye, M.K.; Ogundare, A.O.; Mohmo, A.O.; Daudu, O.A.Y. Comparative antifungal and toxicological effects of the extract of mistletoes growing on two different host plants in Akure North, Nigeria. Int. J. Biotechnol. Food Sci. 2014, 2, 31–34. [Google Scholar]
- Gupta, G.; Kazmi, I.; Afzal, M.; Rahman, M.; Saleem, S.; Ashraf, S.; Khusroo, M.J.; Nazeer, K.; Ahmed, S.; Mujeeb, M.; et al. Sedative, antiepileptic and antipsychotic effects of Viscum album L. (Loranthaceae) in mice and rats. J. Ethnopharmacol. 2012, 141, 810–816. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2022. [Google Scholar] [CrossRef]
- Olas, B. The cardioprotective potential of selected species of mistletoe. Front. Pharmacol. 2024, 11, 1395658. [Google Scholar] [CrossRef]
- Melo, M.N.O.; Ochioni, A.C.; Zancan, P.; Oliveira, A.P.; Grazi, M.; Garrett, R.; Holandino, C.; Baumgartner, S. Viscum album mother tinctures: Harvest conditions and host trees influence the plant metabolome and the glycolytic pathway of breast cancer cells. Front. Pharmacol. 2022, 31, 1027931. [Google Scholar] [CrossRef]
- Melo, M.N.O.; Batista, J.V.D.C.; Peñaloza, E.M.C.; Oliveira, A.P.; Garrett, R.; Baumgartner, S.; Holandino, C. A Scoping Review of Genus Viscum: Biological and Chemical Aspects of Alcoholic Extracts. Plants 2023, 28, 1811. [Google Scholar] [CrossRef]
- Hegde, P.; Maddur, M.S.; Friboulet, A.; Bayry, J.; Kaveri, S.V. Viscum album exerts anti-inflammatory effect by selectively inhibiting cytokine-induced expression of cyclooxygenase-2. PLoS ONE 2011, 6, e26312. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, J.H.; Kwon, D.Y.; Yang, H.J.; Kim, D.S.; Kang, S.; Shin, B.K.; Moon, N.R.; Song, B.S.; Kim, J.H.; et al. The supplementation of Korean mistletoe water extracts reduces hot flushes, dyslipidemia, hepatic steatosis, and muscle loss in ovariectomized rats. Exp. Biol. Med. 2015, 240, 477–487. [Google Scholar] [CrossRef]
- Mirmiran, P.; Hosseini-Esfahani, F.; Esfandiar, Z.; Hosseinpour-Niazi, S.; Azizi, F. Associations between dietary antioxidant intakes and cardiovascular disease. Sci. Rep. 2022, 12, 1504. [Google Scholar] [CrossRef] [PubMed]
- Mangge, H.; Becker, K.; Fuchs, D.; Gostner, J.M. Antioxidants, inflammation and cardiovascular disease. World J. Cardiol. 2014, 26, 462–477. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A. Antioxidants and coronary artery disease: From pathophysiology to preventive therapy. Coron. Artery Dis. 2015, 26, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.; Newport, E.; Morten, K.J. The Warburg effect: 80 years on. Biochem. Soc. Trans. 2016, 44, 1499–1505. [Google Scholar] [CrossRef]
- Halle, W.; Spielmann, H. Two Procedures for the Prediction of Acute Toxicity (LD50) from Cytotoxicity Data. Altern. Lab. Anim. 1992, 20, 40–49. [Google Scholar] [CrossRef]
| Product | Name/Batch | Host Plant | Harvesting Time | Marketed as |
|---|---|---|---|---|
| Viscum MT#39998 | #39998 | Malus domestica Borkh. | November | Homeopathic starting material |
| Viscum vd-MT | #39998 | M. domestica | November | N.A. |
| Viscum MT#40286 | #40286 | M. domestica | February | Homeopathic starting material |
| Viscum MT#40293 | #40293 | M. domestica | March | Homeopathic starting material |
| Viscum–MT leaves | #00293421 | M. domestica | September | Starting material |
| Iscador M#442 | M#442 | M. domestica | Unknown | Anthroposophic medicine (Swiss) |
| Vischio Centofiori | VC#011473 | Unknown | Unknown | Food supplement (Italy) |
| Coragil | Co#82102 | Unknown | Unknown | Homeopathic medicine (Germany) |
| Rubaxx Arthro | RA#210071 | Unknown | Unknown | Homeopathic medicine (Germany) |
| Vischio Biokyma | VB#217 | Unknown | Unknown | Food supplement (Italy) |
| Component | MT#39998 | MT#40293 | AV |
|---|---|---|---|
| Phenolic compounds | 66.87 | 96.63 | 81.75 ± 14.88 |
| Phytols | 20 | 30 | 25 ± 5 |
| Sugars | 14 | 15 | 14.5 ± 0.5 |
| Fatty acid esters | 16.8 | 21.09 | 18.95 ± 2.5 |
| Fatty acids | 1 | 1.5 | 1.25 ± 0.25 |
| Amino acids | 63.011 | 87.253 | 75.132 ± 12.12 |
| Vitamins | 0.56 | 0.68 | 0.62 ± 0.06 |
| Phenolic Compound | MT#39998 | MT#40293 |
|---|---|---|
| Vitexin | 0.07 | 0.07 |
| 5-Caffeoylquinic acid | 0.17 | 0.2 |
| Neochlorogenic acid | 20.04 | 34.11 |
| Catechin | 0.03 | 0.05 |
| Chlorogenic acid | 32.88 | 48.95 |
| cis-Resveratrol-3-O-glucoside | 0.15 | 0.12 |
| Coumaric Acid | 0.92 | 0.65 |
| Diosmetin | 2.68 | 3.08 |
| Epigallocatechin-3,3′-digallate | 0.01 | 0.01 |
| Epigallocatechin-3-gallate | 0.01 | 0.01 |
| Eriodictyol | 0.08 | 0.14 |
| Ethyl gallate | 0.03 | 0.04 |
| Ferulic acid | 0.79 | 1.15 |
| Gallic acid | 0.03 | 0.03 |
| Genistein | 0.01 | 0.01 |
| Genistin | 0.07 | 0.04 |
| Hesperetin | 0.04 | 0.07 |
| Isorhamentin-3-O-neohesperidoside | 0.69 | 0.69 |
| Isorhamnetin | 0.05 | 0.06 |
| Kaempferol | 0.16 | 0.18 |
| Linarin | 0.03 | 0.04 |
| Luteolin | 0.34 | 0.02 |
| Luteolin-7-O-glucoside | 0.13 | 0.17 |
| Myricetin | 0.01 | 0.02 |
| Myricetin-3-O-glucoside | 0.18 | 0.19 |
| Naringenin | 4.16 | 2.46 |
| Naringenin-7-O-neohesperidoside | 0.02 | 0.03 |
| Naringin | 0.78 | 1.38 |
| Nicotinflorin | 0.04 | 0.05 |
| Orientin | 0.17 | 0.19 |
| Piceatannol | 0.01 | 0.01 |
| Procynadin C1 | 0.02 | 0.02 |
| Protocatechuic acid | 1.35 | 1.48 |
| Quercetin | 0.1 | 0.14 |
| Quercetin-3-O-rutinoside | 0.02 | 0.02 |
| Rutin | 0.53 | 0.64 |
| Theaflavin-3,3-digallate | 0.01 | 0.01 |
| Theaflavin-3-gallate | 0.01 | 0.01 |
| Vanillic acid | 0.05 | 0.09 |
| Viscotoxin | µg/mL |
|---|---|
| VTA1 | 75.629 |
| VTA2 | 165.185 |
| VTA3 | 221.442 |
| VTA B | 0 |
| VT Tot | 462.256 |
| Protein | Specie |
|---|---|
| ABC transporter G family member 30 | Arabidopsis thaliana |
| Allene oxide synthase 2, chloroplastic | Solanum lycopersicum |
| Beta-galactoside-specific lectin 1 | Viscum album |
| Beta-galactoside-specific lectin 2 | Viscum album |
| Beta-galactoside-specific lectin 3 | Viscum album |
| Calmodulin | Bryonia dioica; Triticum aestivum |
| Calmodulin-1 | Solanum tuberosum |
| DEAD-box ATP-dependent RNA helicase 40 | Arabidopsis thaliana |
| FBD-associated F-box protein At4g10400 | Arabidopsis thaliana |
| F-box protein At3g19890 | Arabidopsis thaliana |
| NAD(P)H-quinone oxidoreductase subunit 5, chloroplastic | Morus indica |
| Oxygen-evolving enhancer protein 1, chloroplastic | Chlamydomonas reinhardtii |
| Pentatricopeptide repeat-containing protein At1g18485 | Arabidopsis thaliana |
| Peroxidase 23 | Arabidopsis thaliana |
| Peroxidase A2 | Armoracia rusticana |
| Putative heat shock protein 2 (Fragment) OS= | Pseudotsuga menziesii |
| Putative UPF0496 protein 5 O | Oryza sativa subsp. indica |
| Remorin 4.1 OS | Arabidopsis thaliana |
| Ribulose bisphosphate carboxylase small chain 1A, chloroplastic | Arabidopsis thaliana |
| Ribulose bisphosphate carboxylase small chain SSU1, chloroplastic | Lemna gibba |
| Spermidine hydroxycinnamoyltransferase 1 | Oryza sativa subsp. japonica |
| Superoxide dismutase [Cu-Zn] OS= | Ananas comosus |
| Superoxide dismutase [Cu-Zn], chloroplastic | Petunia hybrida |
| Terpenoid synthase 9 | Arabidopsis thaliana |
| Thionin OS | Pyrularia pubera. |
| Viscotoxin-1-PS | OS = Viscum album |
| Viscotoxin-A3 OS = Viscum album; | OS = Viscum album |
| Viscotoxin-B (Fragment) OS = Viscum album | OS = Viscum album |
| VT | MT #39998 | pv #39998 | MT #40286 | MT#40293 | L-MT | VC | IM | Co | RA | VB | vd-MT #39998 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 2.4 | 5.4 | 2.31 | 1.69 | 0.63 | 1.23 | 6.3 | 0.83 | 0.75 | 0.23 | - |
| A3 | 31.6 | 38.8 | 31.20 | 33.50 | 43.69 | 19.02 | 32.2 | 50.31 | 57.04 | 26.35 | - |
| C | 1.5 | 1.1 | 0.42 | 0.59 | 0.13 | 0.58 | 14.3 | - | - | - | - |
| A1 | 11.5 | 10.5 | 28.60 | 21.52 | 17.09 | 12.30 | 10.2 | 0.56 | 0.52 | 0.21 | - |
| A1PS | 1.6 | 3.2 | 1.88 | 3.44 | 3.07 | 2.40 | 1.0 | 1.78 | 0.66 | 0.13 | - |
| A2 | 24.2 | 21.1 | 19.50 | 27.30 | 17.60 | 21.60 | 26.2 | 35.20 | 27.60 | 10.52 | - |
| B | 4.2 | 3.8 | 4.70 | 4.10 | 3.70 | 3.60 | 9.5 | 1.69 | 1.00 | 0.25 | - |
| ToT | 77 | 83.9 | 88.61 | 92.14 | 85.91 | 60.73 | 99.7 | 90.37 | 87.57 | 37.69 | - |
| Cell Line | MT#39998 | vd-MT |
|---|---|---|
| HaCaT | 2.6 ± 0.2 | >5 |
| BALB/c-3T3 | 1.6 ± 0.2 | >5 |
| SVT2 | 1.3 ± 0.8 | 5.0 ± 0.1 |
| HeLa | 2.1 ± 0.1 | >5 |
| Cells | IC50 vol% | LD50 g/kg Bw |
|---|---|---|
| HaCaT | 2.60 | 4.57 |
| Hela | 2.10 | 4.15 |
| BALB/c-3T3 | 1.60 | 3.82 |
| SVT2 | 1.30 | 3.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imbimbo, P.; Fontanarosa, C.; Amoresano, A.; Monti, D.M.; Battaglia, G.; Nicoletti, M.; Spinelli, M.; Schaller, G.; Rocco, V. Phytochemical Composition and Antioxidant Activity of a Viscum album Mother Tincture. Plants 2025, 14, 2762. https://doi.org/10.3390/plants14172762
Imbimbo P, Fontanarosa C, Amoresano A, Monti DM, Battaglia G, Nicoletti M, Spinelli M, Schaller G, Rocco V. Phytochemical Composition and Antioxidant Activity of a Viscum album Mother Tincture. Plants. 2025; 14(17):2762. https://doi.org/10.3390/plants14172762
Chicago/Turabian StyleImbimbo, Paola, Carolina Fontanarosa, Angela Amoresano, Daria Maria Monti, Gennaro Battaglia, Marcello Nicoletti, Michele Spinelli, Gerhard Schaller, and Vincenzo Rocco. 2025. "Phytochemical Composition and Antioxidant Activity of a Viscum album Mother Tincture" Plants 14, no. 17: 2762. https://doi.org/10.3390/plants14172762
APA StyleImbimbo, P., Fontanarosa, C., Amoresano, A., Monti, D. M., Battaglia, G., Nicoletti, M., Spinelli, M., Schaller, G., & Rocco, V. (2025). Phytochemical Composition and Antioxidant Activity of a Viscum album Mother Tincture. Plants, 14(17), 2762. https://doi.org/10.3390/plants14172762








