An Improved Agrobacterium-Mediated Transformation Method for an Important Fresh Fruit: Kiwifruit (Actinidia deliciosa)
Abstract
1. Introduction
2. Results
2.1. Shoot Regeneration of Leaf Explants
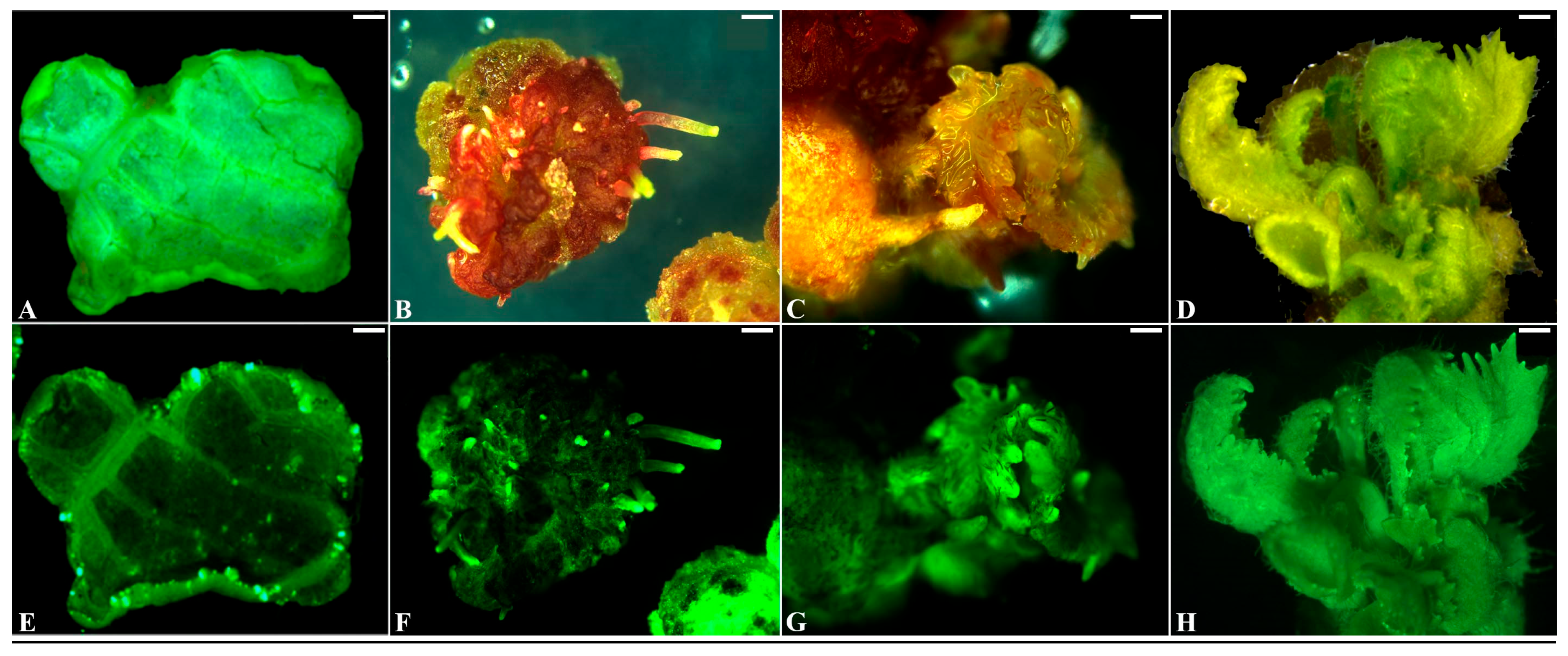
2.2. Transformation of Kiwifruit
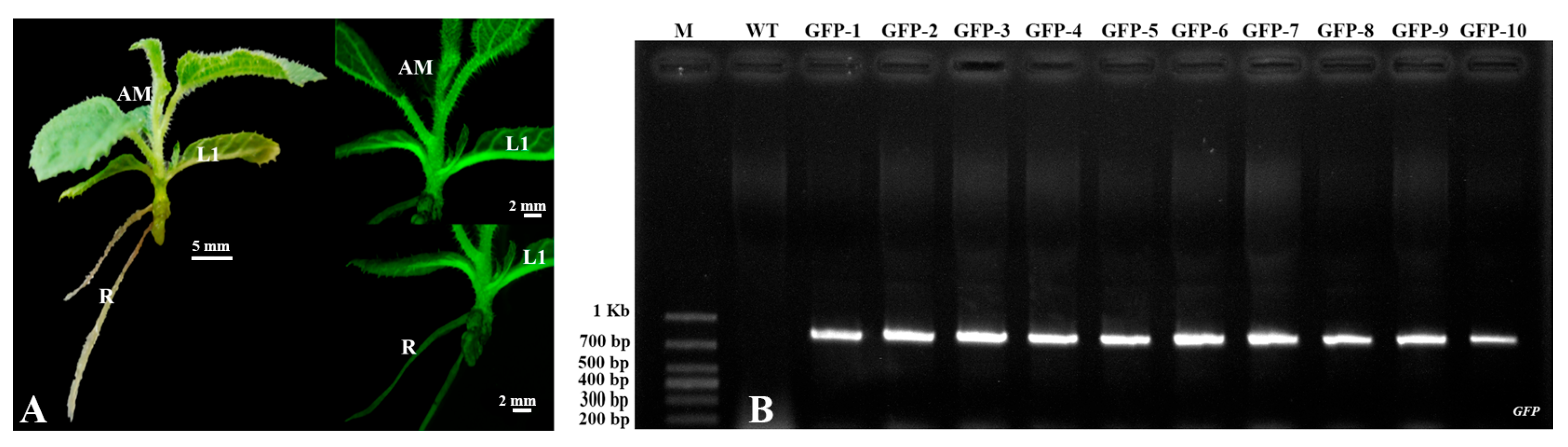
2.3. PCR Detection of Transgenic Kiwifruit
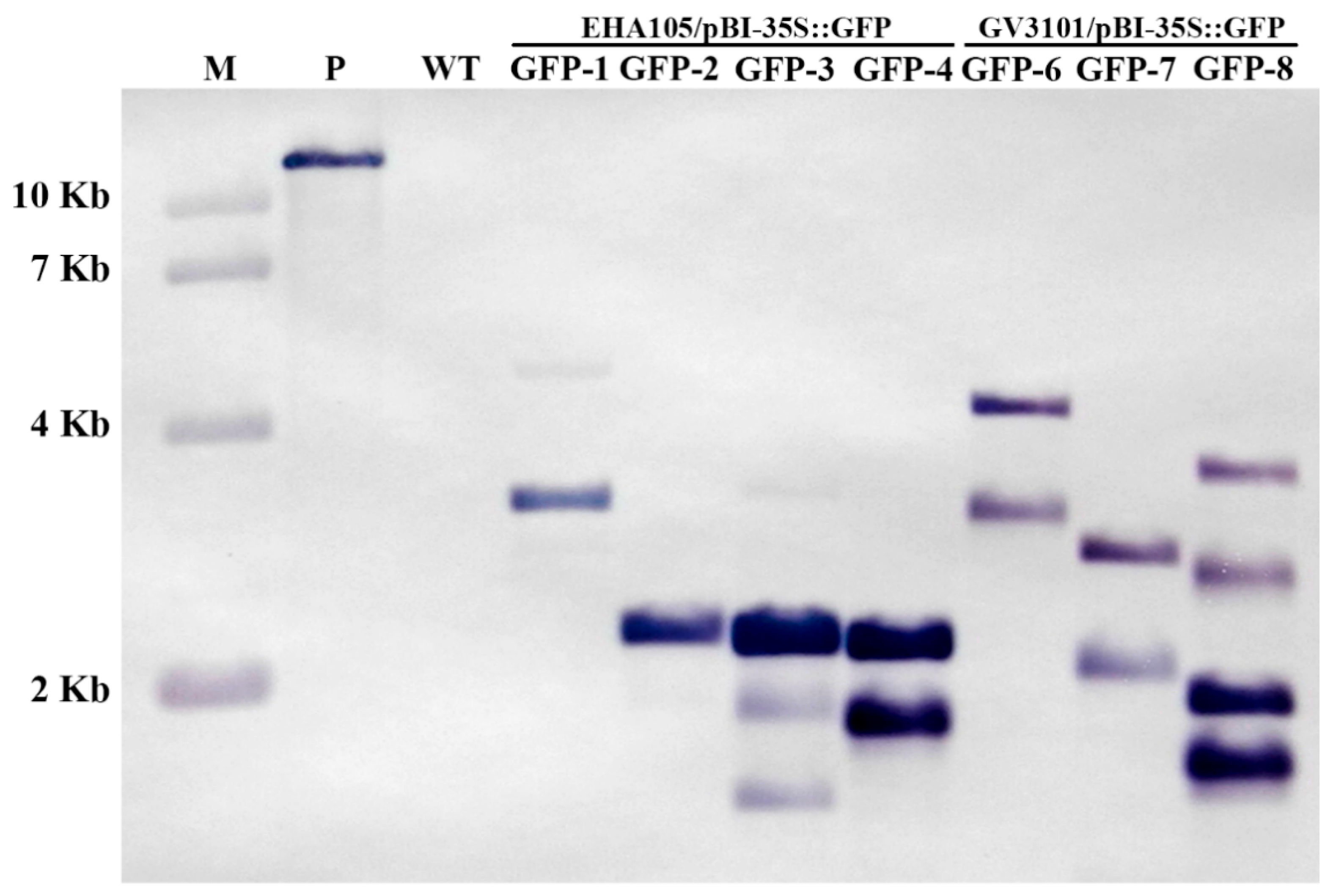
2.4. Southern Blot Analysis of Transgenic Kiwifruit
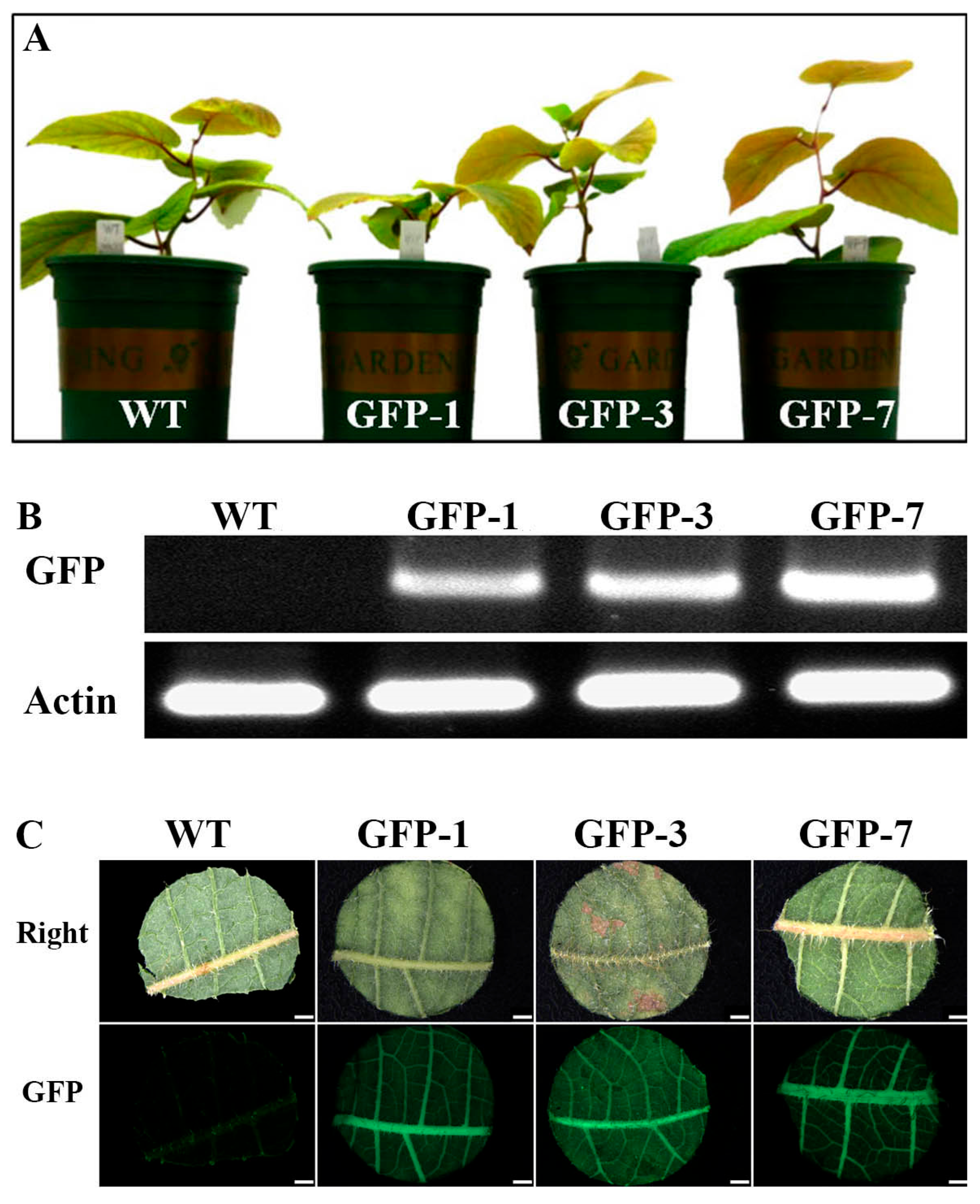
2.5. Observation of GFP Expression in Potted Kiwifruit
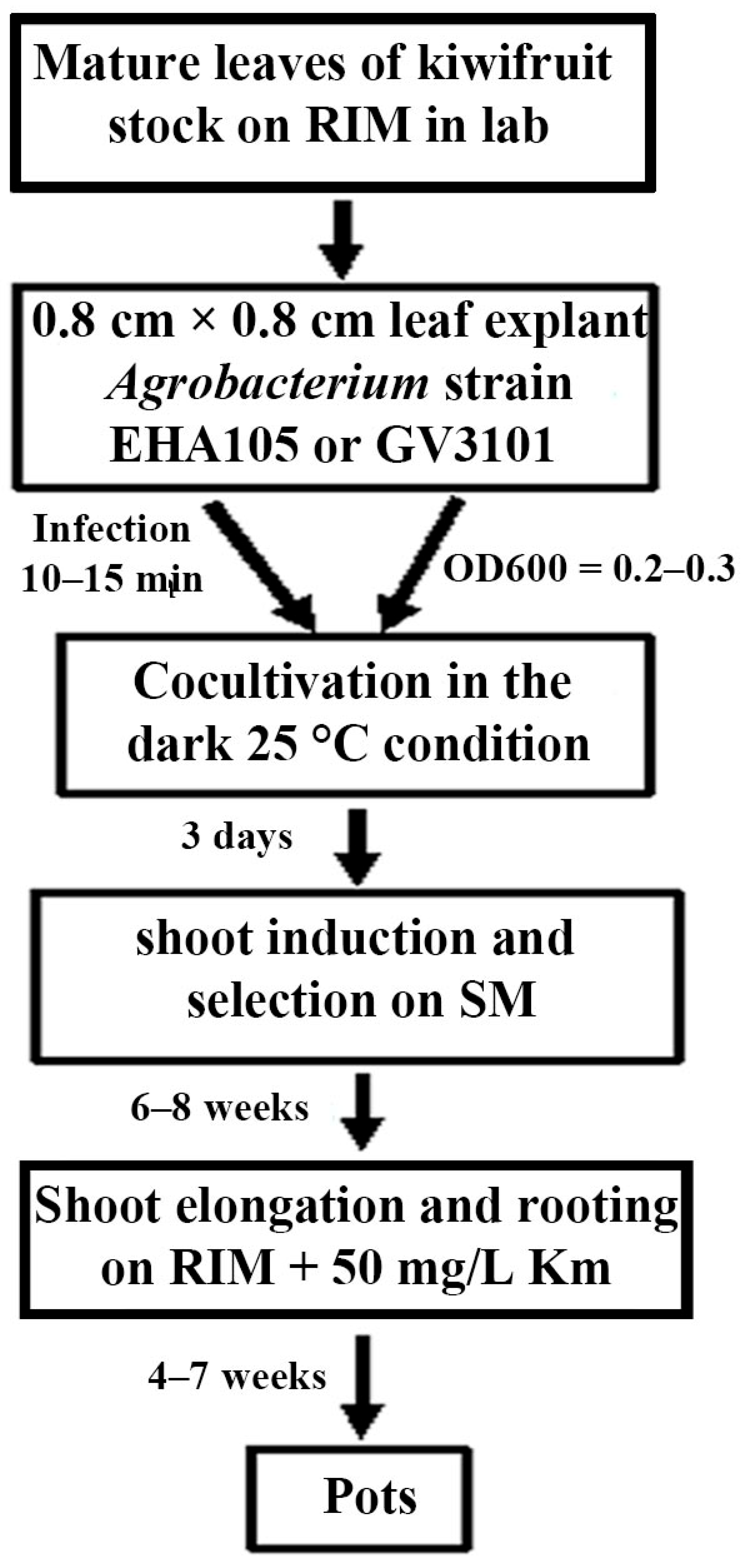
2.6. Procedure for Genetic Transformation of Kiwifruit
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Tissue Culture of Leaf Explants
4.3. Infection of Kiwifruit Leaf Explants
4.4. Observation of Visible GFP Expression
4.5. PCR Detection of Transformed Kiwifruit Plants
4.6. Southern Blot Analysis
4.7. Semiquantitative RT–PCR Analysis
4.8. Construction of pHEE401E-AcPDS Vector
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McGhie, T.K. Secondary metabolite components of kiwifruit. Adv. Food Nutr. Res. 2013, 68, 101–124. [Google Scholar]
- Vanneste, J.L. The scientific, economic, and social impacts of the New Zealand outbreak of bacterial canker of kiwifruit (Pseudomonas syringae pv. actinidiae). Annu. Rev. Phytopathol. 2017, 55, 377–399. [Google Scholar] [CrossRef]
- Richardson, D.P.; Ansell, J.; Drummond, L.N. The nutritional and health attributes of kiwifruit: A review. Eur. J. Nutr. 2018, 57, 2659–2676. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Abas, F.; Park, Y.S.; Park, Y.K.; Ham, K.S.; Kang, S.G.; Lubinska-Szczygeł, M.; Ezra, A.; Gorinstein, S. Bioactivities of phenolic compounds from kiwifruit and persimmon. Molecules 2021, 26, 4405. [Google Scholar] [CrossRef]
- Tavarini, S.; Degl’iNnocenti, E.; Remorini, D.; Massai, R.; Guidi, L. Antioxidant capacity, ascorbic acid, total phenols and carotenoids changes during harvest and after storage of Hayward kiwifruit. Food Chem. 2008, 107, 282–288. [Google Scholar] [CrossRef]
- Latocha, P.; Krupa, T.; Wołosiak, R.; Worobiej, E.; Wilczak, J. Antioxidant activity and chemical difference in fruit of different Actinidia sp. Int. J. Food Sci. Nutr. 2010, 61, 381–394. [Google Scholar] [CrossRef]
- Hunter, D.C.; Greenwood, J.; Zhang, J.; Skinner, M.A. Antioxidant and ‘natural protective’ properties of kiwifruit. Curr. Top Med. Chem. 2011, 11, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- Montefiori, M.; Espley, R.V.; Stevenso, D.; Cooney, J.; Datson, P.M.; Saiz, A.; Atkinson, R.G.; Hellens, R.P.; Allan, A.C. Identification and characterization of F3GT1 and F3GGT1, two glycosyltransferases responsible for anthocyanin biosynthesis in red-fleshed kiwifruit (Actinidia chinensis). Plant J. 2011, 65, 106–118. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Oliveras, M.J.; Quesada, J.; Rufián-Henares, J.A.; Pastoriza, S. Relationship between composition and bioactivity of persimmon and kiwifruit. Food Res. Int. 2018, 105, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xie, X.; Zhong, C.; Li, D. Comparative Transcriptome Analysis Revealed the Key Genes Regulating Ascorbic Acid Synthesis in Actinidia. Int. J. Mol. Sci. 2021, 22, 12894. [Google Scholar] [CrossRef]
- Ferguson, A.R.; Huang, H. Genetic resources of kiwifruit: Domestication and breeding. Hortic. Rev. 2007, 33, 1–121. [Google Scholar]
- Ampomah-Dwamena, C.; McGhie, T.; Reginald, W.; Montefiori, M.; Hellens, R.P.; Allan, A.C. The kiwifruit lycopene beta-cyclase plays a significant role in carotenoid accumulation in fruit. J. Exp. Bot. 2009, 60, 3765–3779. [Google Scholar] [CrossRef] [PubMed]
- Nardozza, S.; Boldingh, H.L.; Osorio, S.; Höhne, M.; Wohlers, M.; Gleave, A.P.; MacRae, E.A.; Richardson, A.C.; Atkinson, R.G.; Sulpice, R.; et al. Metabolic analysis of kiwifruit (Actinidia deliciosa) berries from extreme genotypes reveals hallmarks for fruit starch metabolism. J. Exp. Bot. 2013, 64, 5049–5063. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, D.R.; Kwak, Y.S. Variations in kiwifruit microbiota across cultivars and tissues during developmental stage. Plant Pathol. J. 2023, 39, 245–254. [Google Scholar] [CrossRef]
- Noda, N.; Yoshioka, S.; Kishimoto, S.; Nakayama, M.; Douzono, M.; Tanaka, Y.; Aida, R. Generation of blue chrysanthemums by anthocyanin B-ring hydroxylation and glucosylation and its coloration mechanism. Sci. Adv. 2017, 3, e1602785. [Google Scholar] [CrossRef]
- Wu, L.; Lan, J.; Xiang, X.; Xiang, H.; Jin, Z.; Khan, S.; Liu, Y.; Han, Z. Transcriptome sequencing and endogenous phytohormone analysis reveal new insights in CPPU controlling fruit development in kiwifruit (Actinidia chinensis). PLoS ONE 2020, 15, e0240355. [Google Scholar] [CrossRef]
- Han, X.; Zhang, Y.; Zhang, Q.; Ma, N.; Liu, X.; Tao, W.; Lou, Z.; Zhong, C.; Deng, X.W.; Li, D.; et al. Two haplotype-resolved, gap-free genome assemblies for Actinidia latifolia and Actinidia chinensis shed light on the regulatory mechanisms of vitamin C and sucrose metabolism in kiwifruit. Mol. Plant 2023, 16, 452–470. [Google Scholar] [CrossRef]
- Nazir, M.F.; Lou, J.; Wang, Y.; Zou, S.; Huang, H. Kiwifruit in the Omics Age: Advances in Genomics, Breeding, and Beyond. Plants 2024, 13, 2156. [Google Scholar] [CrossRef]
- Kusaba, M. RNA interference in crop plants. Curr. Opin. Biotechnol. 2004, 15, 139–143. [Google Scholar] [CrossRef]
- Cui, M.L.; Copsey, L.; Green, A.; Bangham, A.; Coen, E. Quantitative control of organ shape by combinatorial gene activity. PLoS Biol. 2010, 8, e1000538. [Google Scholar] [CrossRef][Green Version]
- Rössner, C.; Lotz, D.; Becker, A. VIGS goes viral: How VIGS transforms our understanding of plant science. Annu. Rev. Plant Biol. 2022, 73, 703–728. [Google Scholar] [CrossRef]
- Ding, M.; Piao, C.L.; Zhang, X.; Zhu, Y.; Cui, M.L. Establishment of a high-efficiency transformation and genome editing method for an essential vegetable and medicine Solanum nigrum. Physiol. Plantarum. 2023, 175, e14028. [Google Scholar] [CrossRef]
- Uematsu, C.; Murase, M.; Ichikawa, H.; Imamura, J. Agrobacterium-mediated transformation and regeneration of kiwi fruit. Plant Cell Rep. 1991, 10, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Kohli, A.; Gahakwa, D.; Vain, P.; Laurie, D.A.; Christou, P. Transgene expression in rice engineered through particle bombardment: Molecular factors controlling stable expression and transgene silencing. Planta 1999, 208, 88–97. [Google Scholar] [CrossRef]
- Sailaja, M.; Tarakeswari, M.; Sujatha, M. Stable genetic transformation of castor (Ricinus communis L.) via particle gun-mediated gene transfer using embryo axes from mature seeds. Plant Cell Rep. 2008, 27, 1509–1519. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Z.; Piao, C.; Lu, K.; Wang, Z.; Cui, M.L. A stable and efficient Agrobacterium tumefaciens–mediated genetic transformation of the medicinal plant D. purpurea L. Appl. Biochem. Biotechnol. 2014, 172, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Legendre, M.; Demirer, G.S. Improving crop genetic transformation to feed the world. Trends Biotechnol. 2023, 41, 264–266. [Google Scholar] [CrossRef]
- Janssen, B.J.; Gardner, R. The use of transient GUS expression to develop an Agrobacterium-mediated gene transfer system for kiwifruit. Plant Cell Rep. 1993, 13, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ran, Y.; Atkinson, R.G.; Gleave, A.P.; Cohen, D. Transformation of Actinidia eriantha: A potential species for functional genomics studies in Actinidia. Plant Cell Rep. 2006, 25, 425–431. [Google Scholar] [CrossRef]
- Zhang, A.-D.; Wang, W.-Q.; Tong, Y.; Li, M.-J.; Grierson, D.; Ferguson, I.; Chen, K.S.; Yin, X.R. Transcriptome Analysis Identifies a Zinc Finger Protein Regulating Starch Degradation in Kiwifruit. Plant Physiol. 2018, 178, 850–863. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Y.; Liang, J.; Hu, X.; He, Y.; Miao, T.; Ouyang, Z.; Yang, Z.; Amin, A.K.; Ling, C.; et al. Agrobacterium rhizogenes-mediated marker-free transformation and gene editing system revealed that AeCBL3 mediates the formation of calcium oxalate crystal in kiwifruit. Mol. Hortic. 2024, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Handa, T. Genetic transformation of Antirrhinum majus L. and inheritance of altered phenotype induced by Ri T-DNA. Plant Sci. 1992, 81, 199–206. [Google Scholar] [CrossRef]
- Loyola-Vargas, V.M.; Ochoa-Alejo, N. An introduction to plant tissue culture: Advances and perspectives. Methods Mol. Biol. 2018, 1815, 3–13. [Google Scholar]
- Anjanappa, R.B.; Gruissem, W. Current progress and challenges in crop genetic transformation. J. Plant Physiol. 2021, 261, 153411. [Google Scholar] [CrossRef]
- Rugini, E. In vitro propagation of some olive (Olea europaea sativa L.) cultivars with different root ability, and medium development using analytical data from developing shoots and embryos. Sci. Hortic. 1984, 24, 123–134. [Google Scholar] [CrossRef]
- Abe, T.; Futsuhara, Y. Genotypic variability for callus formation and plant regeneration in rice (Oryza sativa L.). Theor. Appl. Genet. 1986, 72, 3–10. [Google Scholar] [CrossRef]
- Akama, K.; Shiraishi, S.; Nakamura, K.; Okada, K.; Shimura, Y. Efficient transformation of Arabidopsis thaliana: Comparison of the efficiencies with various organs, plant ecotypes and Agrobacterium strains. Plant Cell Rep. 1992, 12, 7–11. [Google Scholar] [CrossRef]
- Cui, M.L.; Handa, T.; Ezura, H. An improved protocol for Agrobacterium-mediated transformation of Antirrhinum majus L. Mol. Genet. Genomics 2003, 270, 296–302. [Google Scholar] [CrossRef]
- Yaroshko, O.; Pasternak, T.; Larriba, E.; Pérez-Pérez, J.M. Optimization of callus induction and shoot regeneration from tomato cotyledon explants. Plants 2023, 12, 2942. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, J.; Movafeghi, A.; Barzegari, A.; Barar, J. Efficient and stable transformation of Dunaliella pseudosalina by 3 strains of Agrobacterium tumefaciens. Bioimpacts 2017, 7, 247–254. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 1962, 15, 81–84. [Google Scholar] [CrossRef]
- Cui, M.L.; Liu, C.; Piao, C.L.; Liu, C.L. A stable Agrobacterium rhizogenes-mediated transformation of cotton (Gossypium hirsutum) and plant regeneration from transformed hairy root via embryogenesis. Front. Plant Sci. 2020, 11, 604255. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.C.; Flores-Vergara, M.A.; Krasynanski, S.; Kumar, S.; Thompson, W.F. A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat. Protoc. 2006, 1, 2320–2325. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.L.; Dong, L.; Wang, Z.P.; Zhang, H.Y.; Han, C.Y.; Liu, B.; Wang, X.C.; Chen, Q.J. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 2014, 14, 327. [Google Scholar] [CrossRef] [PubMed]

| A. tumefaciens Strains (Plasmid) | Replicate | No. of Leaf Explants a | Km+ Shoot Numbers b | GFP-Positive Shoot | Transformation Frequency (%) d | |
|---|---|---|---|---|---|---|
| Numbers | Frequency (%) c | |||||
| EHA105 (pBI-35S::GFP) | 1 | 30 | 24 | 17 | 70.8 | 80 |
| 2 | 30 | 21 | 15 | 71.4 | 70 | |
| Total | 60 | 45 | 32 | 71.1 | 75 | |
| GV3101 (pBI-35S::GFP) | 1 | 30 | 21 | 15 | 71.4 | 70 |
| 2 | 30 | 19 | 14 | 73.7 | 63.3 | |
| Total | 60 | 40 | 29 | 72.5 | 66.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piao, C.-L.; Ding, M.; Gao, Y.; Song, T.; Zhu, Y.; Cui, M.-L. An Improved Agrobacterium-Mediated Transformation Method for an Important Fresh Fruit: Kiwifruit (Actinidia deliciosa). Plants 2025, 14, 2353. https://doi.org/10.3390/plants14152353
Piao C-L, Ding M, Gao Y, Song T, Zhu Y, Cui M-L. An Improved Agrobacterium-Mediated Transformation Method for an Important Fresh Fruit: Kiwifruit (Actinidia deliciosa). Plants. 2025; 14(15):2353. https://doi.org/10.3390/plants14152353
Chicago/Turabian StylePiao, Chun-Lan, Mengdou Ding, Yongbin Gao, Tao Song, Ying Zhu, and Min-Long Cui. 2025. "An Improved Agrobacterium-Mediated Transformation Method for an Important Fresh Fruit: Kiwifruit (Actinidia deliciosa)" Plants 14, no. 15: 2353. https://doi.org/10.3390/plants14152353
APA StylePiao, C.-L., Ding, M., Gao, Y., Song, T., Zhu, Y., & Cui, M.-L. (2025). An Improved Agrobacterium-Mediated Transformation Method for an Important Fresh Fruit: Kiwifruit (Actinidia deliciosa). Plants, 14(15), 2353. https://doi.org/10.3390/plants14152353





