Alleviation of Saline–Alkaline Stress in Alfalfa by a Consortium of Plant-Growth-Promoting Rhizobacteria
Abstract
1. Introduction
2. Results
2.1. Effects of Bacterial Consortium on Alfalfa Seed Germination Under Saline–Alkali Stress
2.2. Effects of Bacterial Consortium on Emergence Rate and Growth of Alfalfa Under Saline–Alkali Stress
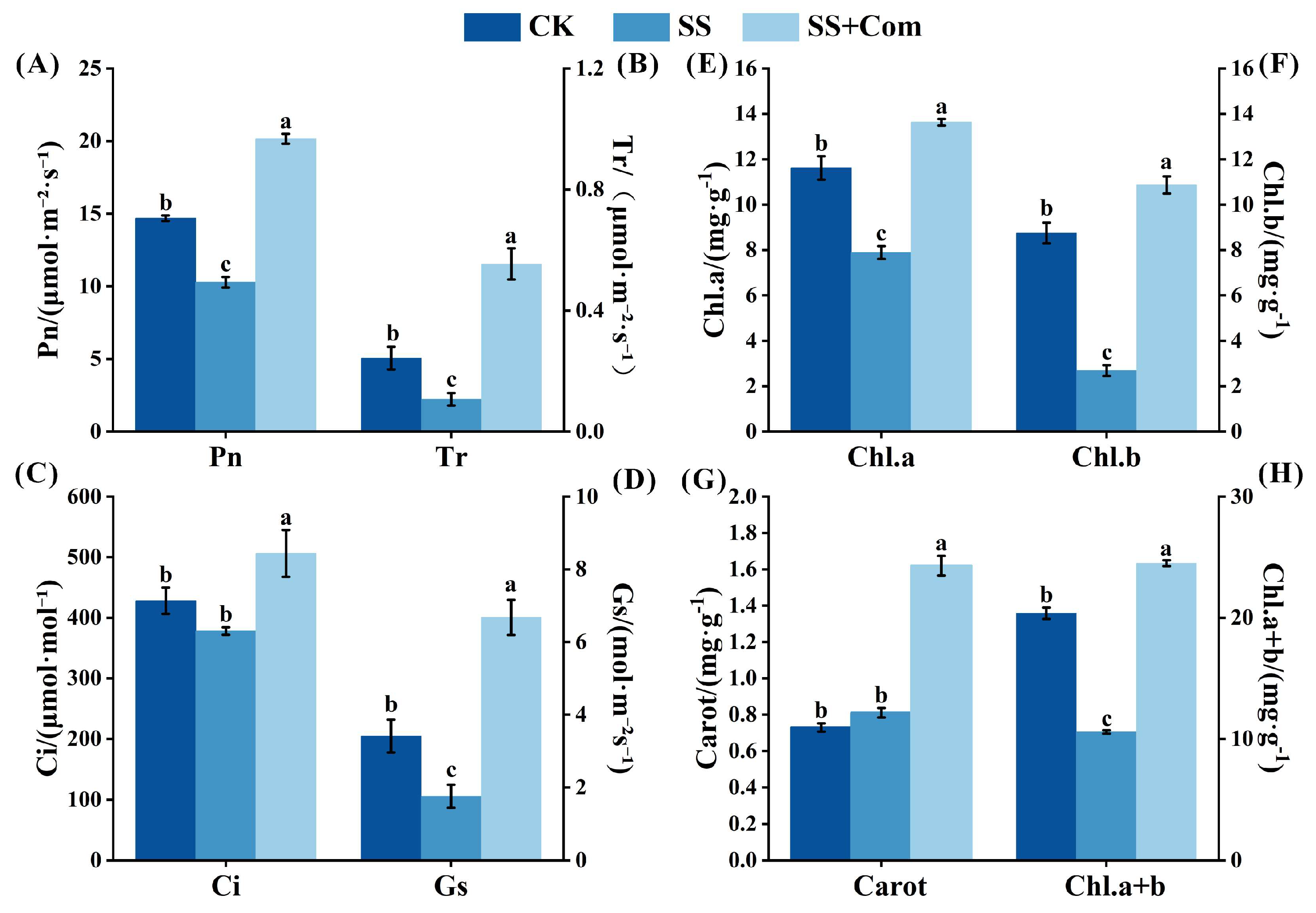
2.3. Effects of Bacterial Consortium on Photosynthetic Parameters and Photosynthetic Pigments of Alfalfa Under Saline–Alkali Stress
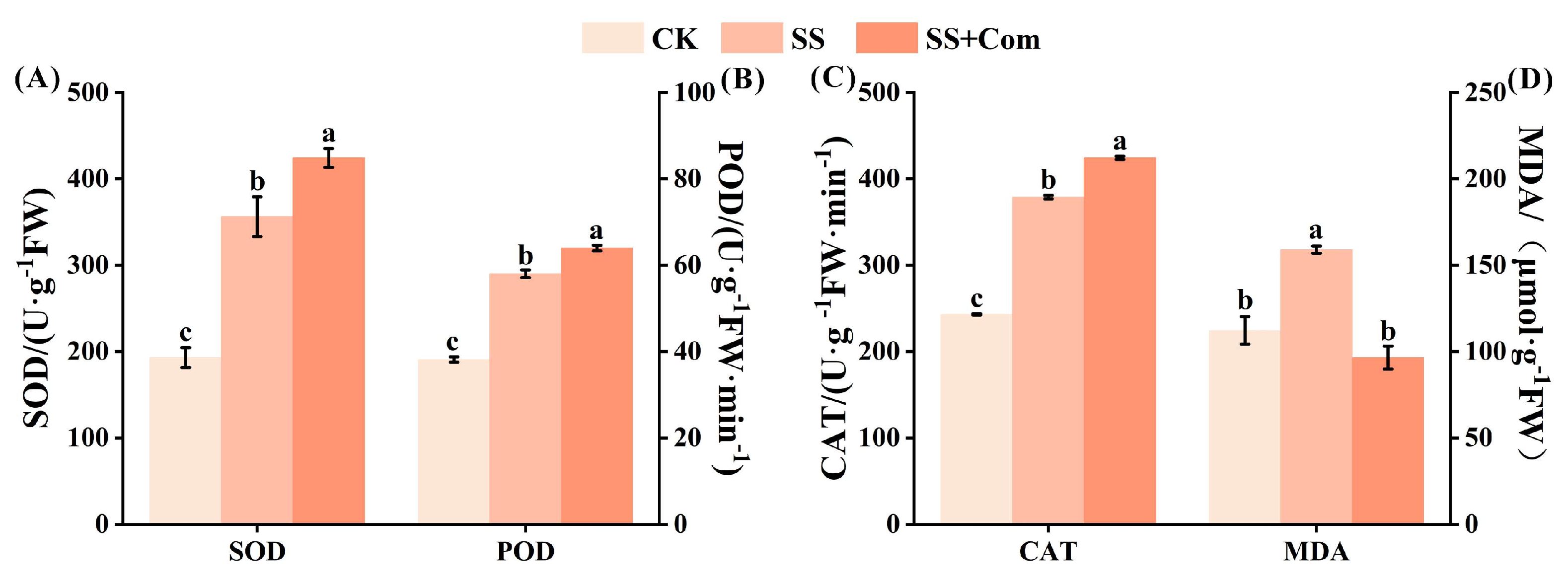
2.4. Effects of Bacterial Consortium on Antioxidant Enzyme Activities and MDA of Alfalfa Under Saline–Alkali Stress
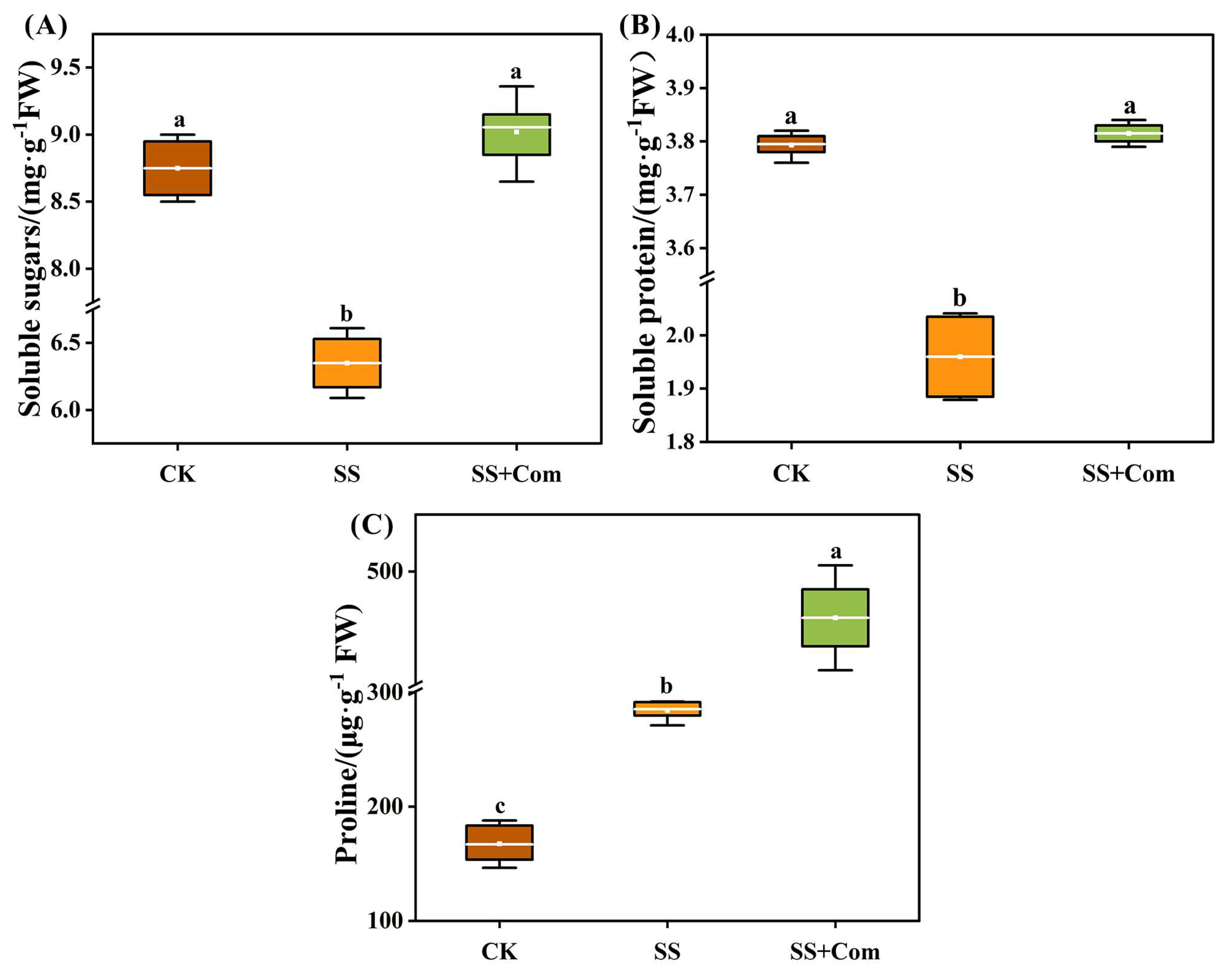
2.5. Effects of Bacterial Consortium on Osmoprotectant Levels of Alfalfa Under Saline–Alkali Stress
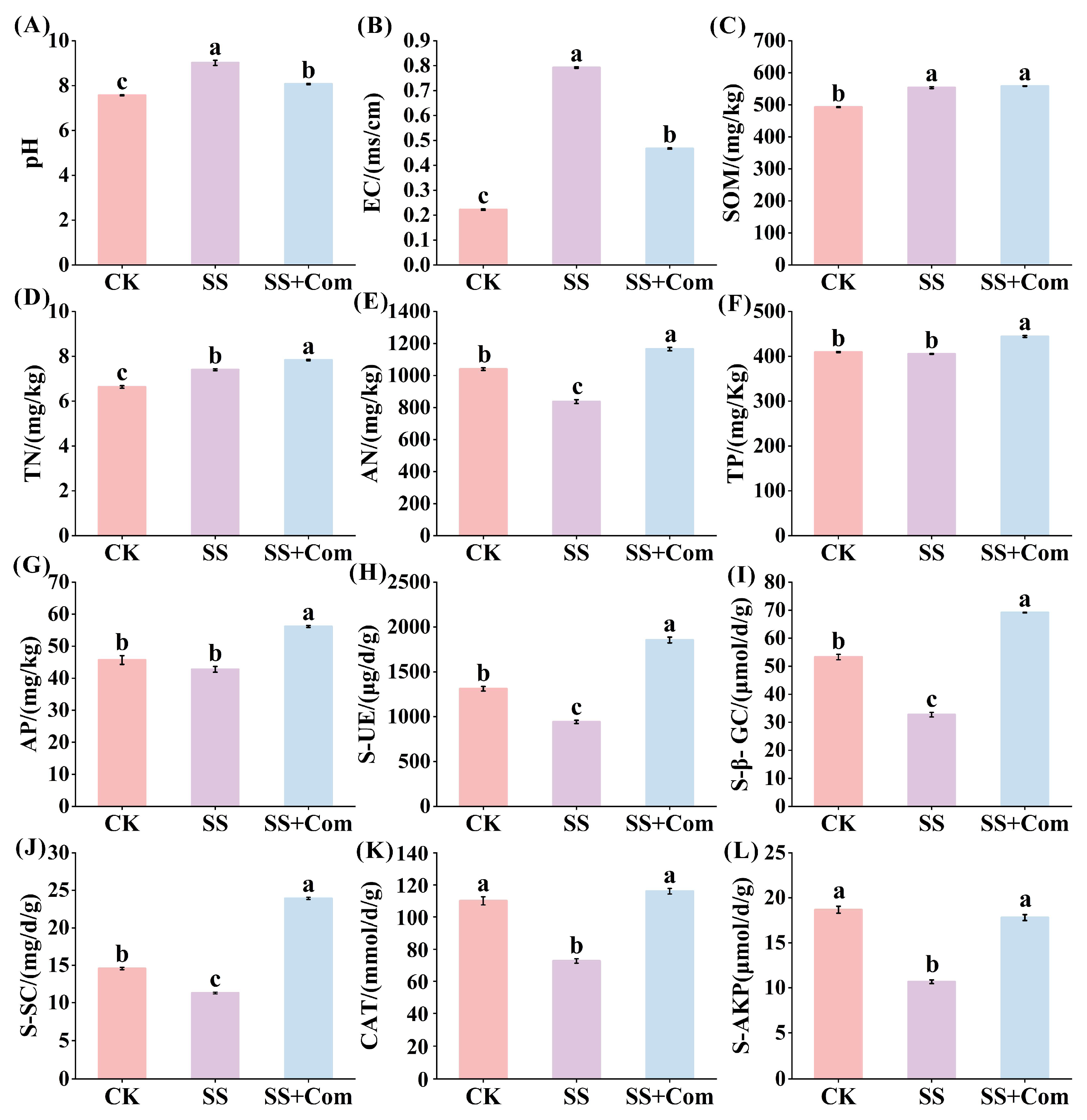
2.6. Effects of Bacterial Consortium on Rhizosphere Soil Physicochemical Properties
2.7. Correlation Analysis Between Growth Parameters and Physicochemical Properties
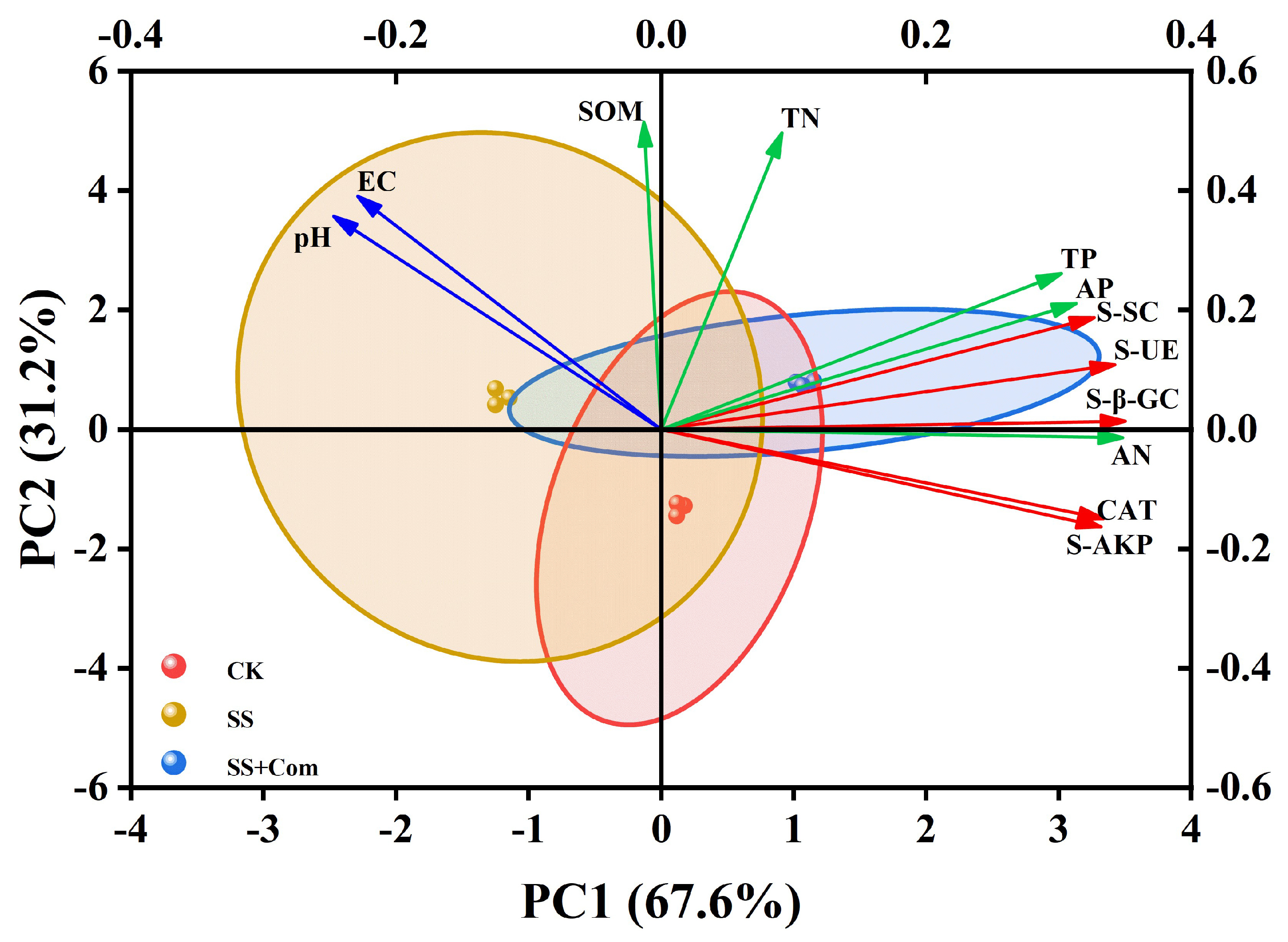
2.8. Principal Component Analysis (PCA) Between Soil Nutrients and Soil Enzyme Activities
3. Discussion
4. Materials and Methods
4.1. Preparation of Microbial Inoculant
4.2. Seed Inoculation
4.3. Seed Germination Experiment Design
4.4. Pot Experiment Design
4.5. Plant Growth Parameter Analysis
4.6. Determination of Photosynthetic Parameters and Photosynthetic Pigment
4.7. Determination of Antioxidant Enzyme Activities, Malondialdehyde (MDA), and Osmoprotectant Levels
4.8. Assessment of Soil Properties
4.9. Data Statistics and Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khoso, M.A.; Wang, M.; Zhou, Z.; Huang, Y.; Li, S.; Zhang, Y.; Qian, G.; Ko, S.N.; Pang, Q.; Liu, C. Bacillus altitudinis AD13-4 Enhances Saline-Alkali Stress Tolerance of Alfalfa and Affects Composition of Rhizosphere Soil Microbial Community. Int. J. Mol. Sci. 2024, 25, 5785. [Google Scholar] [CrossRef]
- Guo, K.; Xu, Z.; Huo, Y.; Sun, Q.; Wang, Y.; Che, Y.; Wang, J.; Li, W.; Zhang, H. Effects of Salt Concentration, pH, and Their Interaction on Plant Growth, Nutrient Uptake, and Photochemistry of Alfalfa (Medicago sativa) Leaves. Plant Signal. Behav. 2020, 15, 1832373. [Google Scholar] [CrossRef]
- Fang, S.; Hou, X.; Liang, X. Response Mechanisms of Plants under Saline-Alkali Stress. Front. Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, L.; Cui, Y.; Yang, D.; Gao, H.; Chen, J.; Zheng, Z.; Guo, C. Inoculation of Plant Growth-Promoting Rhizobacteria and Rhizobia Changes the Protist Community of Alfalfa Rhizosphere Soil under Saline-Alkali Environment. Appl. Soil Ecol. 2025, 206, 105775. [Google Scholar] [CrossRef]
- De Andrade, L.A.; Santos, C.H.B.; Frezarin, E.T.; Sales, L.R.; Rigobelo, E.C. Plant Growth-Promoting Rhizobacteria for Sustainable Agricultural Production. Microorganisms 2023, 11, 1088. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Wang, X.; Feng, Z.; Yang, E.; Wu, M.; Jiang, Y.; Huang, J.; Gao, Z.; Du, Y. The Synergistic Effect of Extracellular Polysaccharide-Producing Salt-Tolerant Bacteria and Biochar Promotes Grape Growth under Saline-Alkaline Stress. Environ. Technol. Innov. 2025, 38, 104070. [Google Scholar] [CrossRef]
- De Oliveira, L.M.; Kavamura, V.N.; Clark, I.M.; Mauchline, T.H.; Desouza, J.T. Diversity and Multifunctional Potential for Plant Growth Promotion in Bacteria from Soil and the Rhizosphere. Soil Use Manag. 2024, 40, e13082. [Google Scholar] [CrossRef]
- Khalilpour, M.; Mozafari, V.; Abbaszadeh-Dahaji, P. Tolerance to Salinity and Drought Stresses in Pistachio (Pistacia vera L.) Seedlings Inoculated with Indigenous Stress-Tolerant PGPR Isolates. Sci. Hortic. 2021, 289, 110440. [Google Scholar] [CrossRef]
- Paul, S.; Parvez, S.S.; Goswami, A.; Banik, A. Exopolysaccharides from Agriculturally Important Microorganisms: Conferring Soil Nutrient Status and Plant Health. Int. J. Biol. Macromol. 2024, 262, 129954. [Google Scholar] [CrossRef]
- Han, L.; Zhang, M.; Du, L.; Zhang, L.; Li, B. Effects of Bacillus amyloliquefaciens QST713 on Photosynthesis and Antioxidant Characteristics of Alfalfa (Medicago sativa L.) under Drought Stress. Agronomy 2022, 12, 2177. [Google Scholar] [CrossRef]
- Song, J.; Guan, X.; Chen, L.; Han, Z.; Cui, H.; Ma, S. Cooperative Interplay between PGPR and Trichoderma longibrachiatum Reprograms the Rhizosphere Microecology for Improved Saline Alkaline Stress Resilience in Rice Seedlings. Microorganisms 2025, 13, 1562. [Google Scholar] [CrossRef]
- Sarsan, S.; Pandiyan, A.; Rodhe, A.V.; Jagavati, S. Synergistic Interactions among Microbial Communities. In Microbes in Microbial Communities: Ecological and Applied Perspectives; Singh, R.P., Manchanda, G., Bhattacharjee, K., Panosyan, H., Eds.; Springer: Singapore, 2021; pp. 1–37. [Google Scholar] [CrossRef]
- Gao, H.; Yang, D.; Yang, L.; Han, S.; Liu, G.; Tang, L.; Chen, J.; Wang, D.; Guo, C. Co-Inoculation with Sinorhizobium meliloti and Enterobacter ludwigii Improves the Yield, Nodulation, and Quality of Alfalfa (Medicago sativa L.) under Saline-Alkali Environments. Ind. Crops Prod. 2023, 199, 116818. [Google Scholar] [CrossRef]
- Yuan, L.; Wu, Y.; Fan, Q.; Li, P.; Liang, J.; Liu, Y.; Ma, R.; Li, R.; Shi, L. Remediating Petroleum Hydrocarbons in Highly Saline-Alkali Soils Using Three Native Plant Species. J. Environ. Manag. 2023, 339, 117928. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, X.; Liu, Y.; Zhang, A.; Song, W.; Li, L.; Zhao, J.; Pang, Q. Salt-Alkali-Tolerant Growth-Promoting Streptomyces sp. Jrh8-9 Enhances Alfalfa Growth and Resilience under Saline-Alkali Stress through Integrated Modulation of Photosynthesis, Antioxidant Defense, and Hormone Signaling. Microbiol. Res. 2025, 296, 128158. [Google Scholar] [CrossRef]
- Yang, D.; Tang, L.; Chen, J.; Shi, Y.; Zhou, H.; Gao, H.; Jin, J.; Guo, C. Strategy of Endophytic Bacterial Communities in Alfalfa Roots for Enhancing Plant Resilience to Saline–Alkali Stress and Its Application. Biol. Fertil. Soils 2024, 60, 493–507. [Google Scholar] [CrossRef]
- Ling, L.; An, Y.; Wang, D.; Tang, L.; Du, B.; Shu, Y.; Bai, Y.; Guo, C. Proteomic Analysis Reveals Responsive Mechanisms for Saline-Alkali Stress in Alfalfa. Plant Physiol. Biochem. 2022, 170, 146–159. [Google Scholar] [CrossRef]
- Bal, H.B.; Nayak, L.; Das, S.; Adhya, T.K. Isolation of ACC Deaminase Producing PGPR from Rice Rhizosphere and Evaluating Their Plant Growth Promoting Activity under Salt Stress. Plant Soil 2013, 366, 93–105. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Huang, S.; Li, L.; Gao, Q.; Wang, Y.; Zhang, S.; Huang, S.; Yuan, L.; Wen, Y.; et al. A Highly Conserved Core Bacterial Microbiota with Nitrogen-Fixation Capacity Inhabits the Xylem Sap in Maize Plants. Nat. Commun. 2022, 13, 3361. [Google Scholar] [CrossRef]
- Damodaran, T.; Jha, S.K.; Kumari, S.; Gupta, G.; Mishra, V.K.; Sharma, P.C.; Gopal, R.; Singh, A.; Jat, H.S. Development of Halotolerant Microbial Consortia for Salt Stress Mitigation and Sustainable Tomato Production in Sodic Soils: An Enzyme Mechanism Approach. Sustainability 2023, 15, 5186. [Google Scholar] [CrossRef]
- Han, Z.; Chen, L.; Wang, W.; Guan, X.; Song, J.; Ma, S. Biochemical and Transcriptomic Analyses Reveal Key Salinity and Alkalinity Stress Response and Tolerance Pathways in Salix linearistipularis Inoculated with Trichoderma. Agronomy 2024, 14, 2358. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, P.; Wang, B.; Li, H.; Li, S.; Zhang, H.; Haider, F.U.; Li, X. Harnessing the Role of Rhizo-Bacteria to Mitigate Salinity Stress in Rice (Orzya sativa); Focus on Antioxidant Defense System, Photosynthesis Response, and Rhizosphere Microbial Diversity. Rhizosphere 2025, 33, 101043. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors Influencing Leaf Chlorophyll Content in Natural Forests at the Biome Scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Yu, M.; Wu, Q.; Zheng, D.; Feng, N.; Liang, X.; Liu, M.; Li, Y.; Mou, B. Plant Growth Regulators Enhance Saline-Alkali Tolerance by Upregulating the Levels of Antioxidants and Osmolytes in Soybean Seedlings. J. Plant Growth Regul. 2022, 41, 3218–3232. [Google Scholar] [CrossRef]
- Li, G.; Shi, M.; Wan, W.; Wang, Z.; Ji, S.; Yang, F.; Jin, S.; Zhang, J. Maize Endophytic Plant Growth-Promoting Bacteria Peribacillus Simplex Can Alleviate Plant Saline and Alkaline Stress. Int. J. Mol. Sci. 2024, 25, 10870. [Google Scholar] [CrossRef]
- Xian, X.; Zhang, Z.; Wang, S.; Cheng, J.; Gao, Y.; Ma, N.; Li, C.; Wang, Y. Exogenous Melatonin Strengthens Saline-Alkali Stress Tolerance in Apple Rootstock M9-T337 Seedlings by Initiating a Variety of Physiological and Biochemical Pathways. Chem. Biol. Technol. Agric. 2024, 11, 58. [Google Scholar] [CrossRef]
- Bai, J.; Liu, J.; Zhang, N.; Yang, J.; Sa, R.; Wu, L. Effect of Alkali Stress on Soluble Sugar, Antioxidant Enzymes and Yield of Oat. J. Integr. Agric. 2013, 12, 1441–1449. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Einerich, D.W.; Sánchez-Díaz, M. Water Stress Induced Changes in Concentrations of Proline and Total Soluble Sugars in Nodulated Alfalfa (Medicago sativa) Plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Gandonou, C.B.; Bada, F.; Abrini, J.; Skali-Senhaji, N. Free Proline, Soluble Sugars and Soluble Proteins Concentration as Affected by Salt Stress in Two Sugarcane (Saccharum sp.) Cultivars Differing in Their Salt Tolerance. Int. J. Mol. Sci. 2011, 5, 2441–2453. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Kouna, T.; Antonopoulou, C.I.; Therios, I. Exogenous Proline Induces Soluble Sugar Accumulation and Alleviates Drought Stress Effects on Photosystem II Functioning of Arabidopsis Thaliana Leaves. Plant Growth Regul. 2011, 65, 315–325. [Google Scholar] [CrossRef]
- Bisht, N.; Singh, T.; Ansari, M.M.; Joshi, H.; Mishra, S.K.; Chauhan, P.S. Plant Growth-Promoting Bacillus amyloliquefaciens Orchestrate Homeostasis under Nutrient Deficiency Exacerbated Drought and Salinity Stress in Oryza sativa L. Seedlings. Planta 2024, 261, 8. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, F.; Mickan, B.; Wang, D.; Wang, W. Physiological, Proteomic, and Metabolomic Analysis Provide Insights into Bacillus Sp.-Mediated Salt Tolerance in Wheat. Plant Cell Rep. 2022, 41, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Wang, X.; Saleem, M.H.; Sumaira; Hafeez, A.; Afridi, M.S.; Khan, S.; Zaib-Un-Nisa; Ullah, I.; Amaral Júnior, A.T.D.; et al. PGPR-Mediated Salt Tolerance in Maize by Modulating Plant Physiology, Antioxidant Defense, Compatible Solutes Accumulation and Bio-Surfactant Producing Genes. Plants 2022, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; He, W.; Li, Z.; Ge, L.; Zhang, J.; Liu, T. Salt-Tolerant Endophytic Bacterium Enterobacter ludwigii B30 Enhance Bermudagrass Growth under Salt Stress by Modulating Plant Physiology and Changing Rhizosphere and Root Bacterial Community. Front. Plant Sci. 2022, 13, 959427. [Google Scholar] [CrossRef]
- Yan, N.; Wang, W.; Mi, T.; Zhang, X.; Li, X.; Du, G. Enhancing Tomato Growth and Soil Fertility under Salinity Stress Using Halotolerant Plant Growth-Promoting Rhizobacteria. Plant Stress 2024, 14, 100638. [Google Scholar] [CrossRef]
- Kumar, M.; Yusuf, M.A.; Chauhan, P.S.; Nigam, M. Pseudomonas putida and Bacillus amyloliquefaciens Alleviates the Adverse Effect of Pesticides and Poise Soil Enzymes Activities in Chickpea (Cicer arietinum L.) Rhizosphere. Trop. Plant Res. 2017, 4, 405–418. [Google Scholar] [CrossRef]
- Hidri, R.; Mahmoud, O.M.-B.; Zorrig, W.; Mahmoudi, H.; Smaoui, A.; Abdelly, C.; Azcon, R.; Debez, A. Plant Growth-Promoting Rhizobacteria Alleviate High Salinity Impact on the Halophyte Suaeda fruticosa by Modulating Antioxidant Defense and Soil Biological Activity. Front. Plant Sci. 2022, 13, 821475. [Google Scholar] [CrossRef]
- Han, L.; Su, D.; Lv, S.; Luo, Y.; Li, X.; Jiao, J.; Diao, Z.; Bu, H. Responses of Biogeochemical Characteristics and Enzyme Activities in Sediment to Climate Warming under a Simulation Experiment in Geographically Isolated Wetlands of the Hulunbuir Grassland, China. Int. J. Environ. Res. Public Health 2017, 14, 968. [Google Scholar] [CrossRef]
- Li, C.; Jia, Z.; Zhai, L.; Zhang, B.; Peng, X.; Liu, X.; Zhang, J. Effects of Mineral-Solubilizing Microorganisms on Root Growth, Soil Nutrient Content, and Enzyme Activities in the Rhizosphere Soil of Robinia pseudoacacia. Forests 2021, 12, 60. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, Z.; Sun, H.; Guo, H.; Song, Y.; Zhang, H.; Ruan, Y.; Xu, Q.; Huang, Q.; Shen, Q. Synthetic Community Derived from Grafted Watermelon Rhizosphere Provides Protection for Ungrafted Watermelon against Fusarium oxysporum Via Microbial Synergistic Effects. Microbiome 2024, 12, 101. [Google Scholar] [CrossRef]
- Kossalbayev, B.D.; Wei, M.; Wang, J.; Pang, Y.; Lv, M.; Sadvakasova, A.K.; Bauenova, M.O.; Zhang, X.; Zhao, W.; Xu, S.; et al. Growth Promotion of Synthetic Microbial Communities Influenced by the Function, Diversity and Interactions of Their Constituent Strains and Soil Types. World J. Microbiol. Biotechnol. 2025, 41, 181. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yuan, F.; Feng, Z.; Ding, T.; Song, J.; Wang, B. Comparative Study on Seed Germination Characteristics of Two Species of Australia Saltbush under Salt Stress. Acta Ecol. Sin. 2014, 34, 337–341. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Basit, F.; Chen, M.; Ahmed, T.; Shahid, M.; Noman, M.; Liu, J.; An, J.; Hashem, A.; Fahad Al-Arjani, A.B.; Alqarawi, A.A.; et al. Seed Priming with Brassinosteroids Alleviates Chromium Stress in Rice Cultivars Via Improving ROS Metabolism and Antioxidant Defense Response at Biochemical and Molecular Levels. Antioxidants 2021, 10, 1089. [Google Scholar] [CrossRef] [PubMed]
- Abdel Latef, A.; Sallam, M. Changes in Growth and Some Biochemical Parameters of Maize Plants Irrigated with Sewage Water. Austin J. Plant Biol. 2015, 1, 1004. [Google Scholar]
- Kirk, P.L. Kjeldahl Method for Total Nitrogen. Anal. Chem. 1950, 22, 354–358. [Google Scholar] [CrossRef]
- Fu, H.; Li, H.; Yin, P.; Mei, H.; Li, J.; Zhou, P.; Wang, Y.; Ma, Q.; Jeyaraj, A.; Thangaraj, K.; et al. Integrated Application of Rapeseed Cake and Green Manure Enhances Soil Nutrients and Microbial Communities in Tea Garden Soil. Sustainability 2021, 13, 2967. [Google Scholar] [CrossRef]
- Li, C.; Liu, L.; Guo, B.; Zhao, J.; Ren, Y. Potassium Dichromate Oxidation Methods’ Review and Application for Determination of Soil Organic Matter. Metall. Res. Technol. 2017, 4, 251–259. [Google Scholar] [CrossRef]
- Yu, H.; Si, P.; Shao, W.; Qiao, X.; Yang, X.; Gao, D.; Wang, Z. Response of Enzyme Activities and Microbial Communities to Soil Amendment with Sugar Alcohols. Microbiologyopen 2016, 5, 604–615. [Google Scholar] [CrossRef]
- Xu, R.; Li, J.; Li, X.; Zhang, J.; Song, W. Effect of Coal Mining Subsidence on Soil Enzyme Activity in Mining Areas with High Underground Water Levels. Water 2024, 16, 1704. [Google Scholar] [CrossRef]


| Treatment | Germination Rate (%) | Germination Potential (%) | Germination Index | Vigor Index |
|---|---|---|---|---|
| SS | 52.75 ± 1.14 c | 37.40 ± 3.03 d | 13.01 ± 2.35 c | 286.50 ± 1.71 c |
| SS + B1 | 68.25 ± 2.48 b | 46.40 ± 2.21 c | 16.60 ± 1.62 b | 359.49 ± 3.32 b |
| SS + B2 | 71.50 ± 3.49 b | 45.20 ± 1.57 c | 16.33 ± 1.75 b | 364.87 ± 4.26 b |
| SS + B3 | 73.75 ± 1.85 b | 53.60 ± 1.86 b | 17.74 ± 3.30 b | 360.14 ± 2.22 b |
| SS + Com | 79.75 ± 1.72 a | 68.60 ± 3.13 a | 27.78 ± 1.68 a | 417.49 ± 1.32 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.; Li, Y.; Ma, Z.; Li, B.; Liang, Y.; Gao, P.; Zhao, X. Alleviation of Saline–Alkaline Stress in Alfalfa by a Consortium of Plant-Growth-Promoting Rhizobacteria. Plants 2025, 14, 2744. https://doi.org/10.3390/plants14172744
Han L, Li Y, Ma Z, Li B, Liang Y, Gao P, Zhao X. Alleviation of Saline–Alkaline Stress in Alfalfa by a Consortium of Plant-Growth-Promoting Rhizobacteria. Plants. 2025; 14(17):2744. https://doi.org/10.3390/plants14172744
Chicago/Turabian StyleHan, Lingjuan, Yixuan Li, Zheng Ma, Bin Li, Yinping Liang, Peng Gao, and Xiang Zhao. 2025. "Alleviation of Saline–Alkaline Stress in Alfalfa by a Consortium of Plant-Growth-Promoting Rhizobacteria" Plants 14, no. 17: 2744. https://doi.org/10.3390/plants14172744
APA StyleHan, L., Li, Y., Ma, Z., Li, B., Liang, Y., Gao, P., & Zhao, X. (2025). Alleviation of Saline–Alkaline Stress in Alfalfa by a Consortium of Plant-Growth-Promoting Rhizobacteria. Plants, 14(17), 2744. https://doi.org/10.3390/plants14172744





