Establishment of Iris laevigata Tissue Culture Using Hypocotyl and Root Explants
Abstract
1. Introduction
2. Results
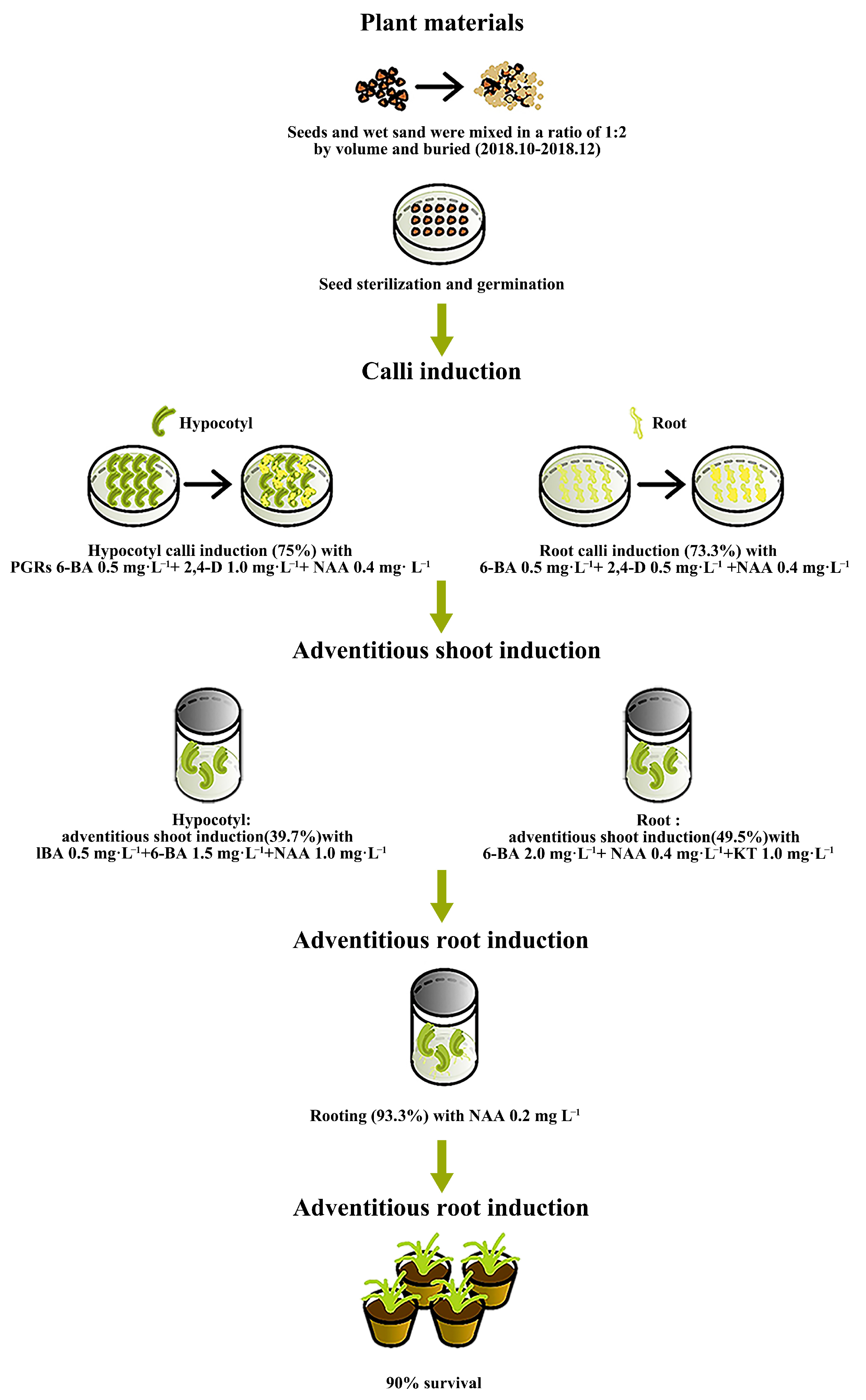
2.1. Effect of Different PGR Concentrations on I. laevigata Hypocotyl Callus Induction
2.2. Effects of Different PGR Concentrations on I. laevigata Root Callus Induction
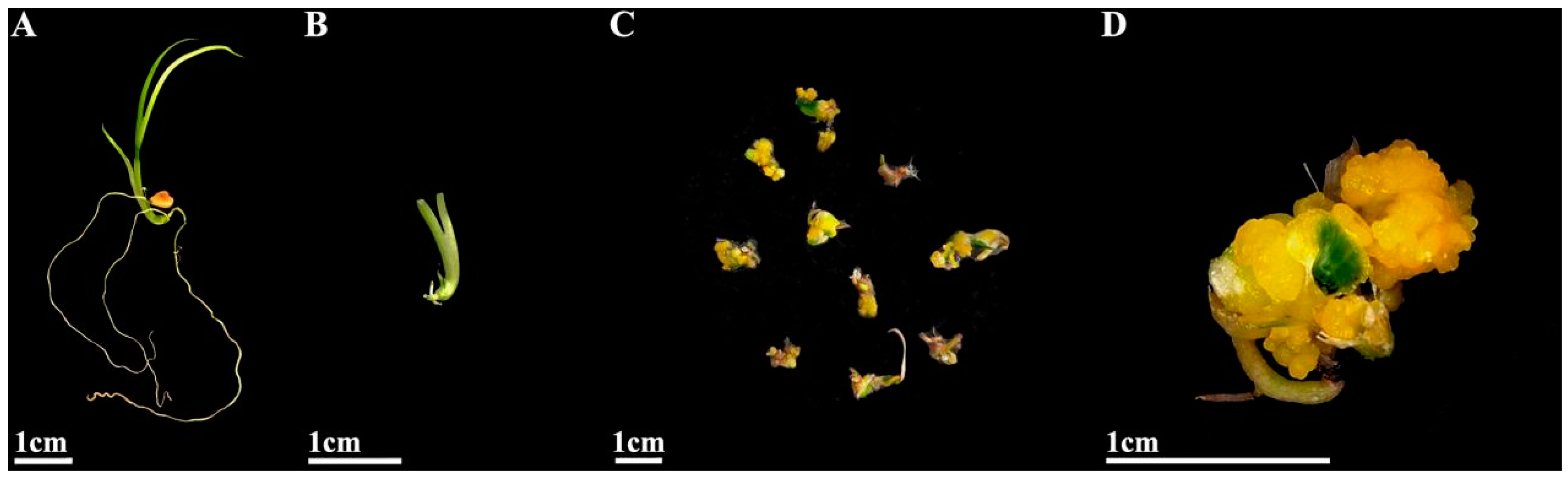
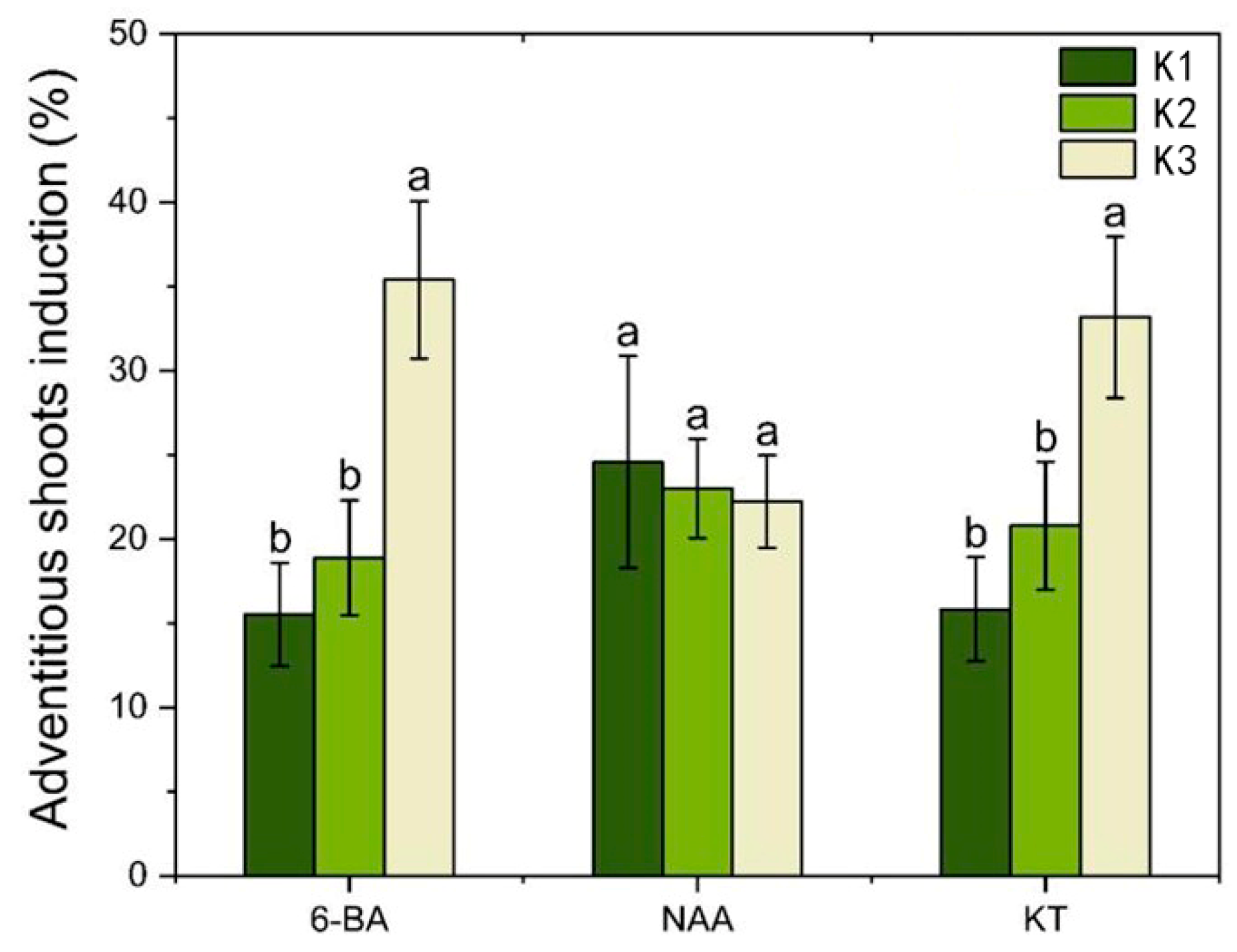
2.3. Effects of Different PGRs and Their Concentrations on Adventitious Shoot Induction of Hypocotyl and Root Calli
2.4. Effect of Different PGR Concentrations on Root Induction of Adventitious Shoots
2.5. Acclimatization and Transplanting of Plantlets
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Medium Preparation and Culture Conditions
4.3. Callus Induction from Explants
4.4. Adventitious Shoot Induction
4.5. Root Induction of Adventitious Shoots
4.6. Acclimatization of Plantlets
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, M.Z.; Li, M.R.; Shi, F.X.; Li, L.; Liu, Y.; Li, L.F.; Xiao, H.X. Genomic and EST-derived microsatellite markers for Iris laevigata (Iridaceae) and other congeneric species. Am. J. Bot. 2012, 99, e286–e288. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, H.; He, C.; Sheng, L.; Tang, Z. An irreversible division of labor through a sexually dependent system in the clonal plant Iris laevigata (Iridaceae). Ecosphere 2017, 8, e01757. [Google Scholar] [CrossRef]
- Inoue, K.; Tomita, T.; Yoshihara, N.; Yabuya, T. Interspecific hybrids between Iris setosa var. setosa and I. laevigata and their relationships to I. setosa var. hondoensis or I. setosa var. nasuensis. Cytologia 2008, 73, 401–410. [Google Scholar] [CrossRef][Green Version]
- Yabuya, T.M.U.J. Chromosome associations and crossability with Iris ensata Thunb. in induced amphidiploids of I. laevigata Fisch. x I. ensata. Euphytica 1991, 55, 85–90. [Google Scholar] [CrossRef]
- Yabuya, T.; Nozaki, T.; Hanazaki, A.; Harada, S.; Tanaka, H.; Tomita, T.; Inoue, K. Origin of Japanese endemic species Iris setosa var. nasuensis and I. setosa var. hondoensis. Cytologia 2013, 78, 449–459. [Google Scholar] [CrossRef]
- Lee, E.H.; Lee, B.E.; Kim, J.G. Effects of water levels and soil nutrients on the growth of Iris laevigata seedlings. J. Ecol. Environ. 2018, 42, 5. [Google Scholar] [CrossRef]
- Bae, K.; Yoo, K.; Lee, H.; Yoon, E. Callus induction and plant regeneration of Iris dichotoma Pall. in endangered species. J. Plant Biotechnol. 2012, 39, 182–188. [Google Scholar] [CrossRef]
- Jehan, H.; Courtois, D.; Ehret, C.; Lerch, K.; Petiard, V. Plant regeneration of Iris pallida Lam. and Iris germanica L. via somatic embryogenesis from leaves, apices and young flowers. Plant Cell Rep. 1994, 13, 671–675. [Google Scholar] [CrossRef]
- González-Benito, M.E.; Martín, C. In vitro preservation of Spanish biodiversity. Vitr. Cell. Dev. Biol.-Plant 2011, 47, 46–54. [Google Scholar] [CrossRef]
- Wang, Y.; Jeknic, Z.; Ernst, R.C.; Chen, T.H. Improved plant regeneration from Suspension-cultured cells of Iris germanica L. ‘Skating Party’. Hortscience 1999, 34, 1271–1276. [Google Scholar] [CrossRef]
- Laublin, G.; Saini, H.S.; Cappadocia, M. In vitro plant regeneration via somatic embryogenesis from root culture of some rhizomatous irises. Plant Cell Tissue Organ Cult. 1991, 27, 15–21. [Google Scholar] [CrossRef]
- Hussey, G. Totipotency in tissue explants and callus of some members of the Liliaceae, Iridaceae, and Amaryllidaceae. J. Exp. Bot. 1975, 26, 253–262. [Google Scholar] [CrossRef]
- Al-Gabbiesh, A.; Hassawi, D.S.; Afifi, F.U. In vitro propagation of endangered Iris species. J. Biol. Sci. 2006, 6, 1035–1040. [Google Scholar]
- Ascough, G.D.; Erwin, J.E.; van Staden, J. Micropropagation of iridaceae—A review. Plant Cell Tissue Organ Cult. (Pctoc) 2009, 97, 1–19. [Google Scholar] [CrossRef]
- Boltenkov, E.V.; Zarembo, E.V. In vitro regeneration and callogenesis in tissue culture of floral organs of the genus Iris (Iridaceae). Izv. Akad. Nauk Ser. Biol. 2005, 32, 174–179. [Google Scholar] [CrossRef]
- Stanišić, M.; Raspor, M.; Ninković, S.; Milošević, S.; Ćalić, D.; Bohanec, B.; Trifunović, M.; Petrić, M.; Subotić, A.; Jevremović, S. Clonal fidelity of Iris sibirica plants regenerated by somatic embryogenesis and organogenesis in leaf-base culture—RAPD and flow cytometer analyses. S. Afr. J. Bot. 2015, 96, 42–52. [Google Scholar] [CrossRef]
- Karakas, F.P.; Turker, A.U. Improvement of shoot proliferation and comparison of secondary metabolites in shoot and callus cultures of Phlomis armeniaca by LC-ESI-MS/MS analysis. Vitr. Cell. Dev. Biol.-Plant 2016, 52, 608–618. [Google Scholar] [CrossRef]
- Wang, L.; Du, Y.; Rahman, M.M.; Tang, B.; Fan, L.; Kilaru, A. Establishment of an efficient in vitro propagation system for Iris sanguinea. Sci. Rep. 2018, 8, 17100. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Sugimoto, K.; Iwase, A. Plant callus: Mechanisms of induction and repression. Plant Cell 2013, 25, 3159–3173. [Google Scholar] [CrossRef]
- Boltenkov, E.V.; Mironova, L.N.; Zarembo, E.V. Effect of phytohormones on plant regeneration in callus culture of Iris ensata Thunb. Izv. Akad. Nauk Ser. Biol. 2007, 34, 539–544. [Google Scholar] [CrossRef]
- Da Costa, C.T.; Gaeta, M.L.; de Araujo Mariath, J.E.; Offringa, R.; Fett-Neto, A.G. Comparative adventitious root development in pre-etiolated and flooded Arabidopsis hypocotyls exposed to different auxins. Plant Physiol. Biochem. 2018, 127, 161–168. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, N.; Fu, H.; Liu, Y.; Kilaru, A.; Behera, J.R.; Wang, L. Establishment of Iris laevigata Tissue Culture Using Hypocotyl and Root Explants. Plants 2025, 14, 2733. https://doi.org/10.3390/plants14172733
Xu N, Fu H, Liu Y, Kilaru A, Behera JR, Wang L. Establishment of Iris laevigata Tissue Culture Using Hypocotyl and Root Explants. Plants. 2025; 14(17):2733. https://doi.org/10.3390/plants14172733
Chicago/Turabian StyleXu, Nuo, Haijing Fu, Yujia Liu, Aruna Kilaru, Jyoti R. Behera, and Ling Wang. 2025. "Establishment of Iris laevigata Tissue Culture Using Hypocotyl and Root Explants" Plants 14, no. 17: 2733. https://doi.org/10.3390/plants14172733
APA StyleXu, N., Fu, H., Liu, Y., Kilaru, A., Behera, J. R., & Wang, L. (2025). Establishment of Iris laevigata Tissue Culture Using Hypocotyl and Root Explants. Plants, 14(17), 2733. https://doi.org/10.3390/plants14172733







