Improving Sugarcane Biomass and Phosphorus Fertilization Through Phosphate-Solubilizing Bacteria: A Photosynthesis-Based Approach
Abstract
1. Introduction
2. Results
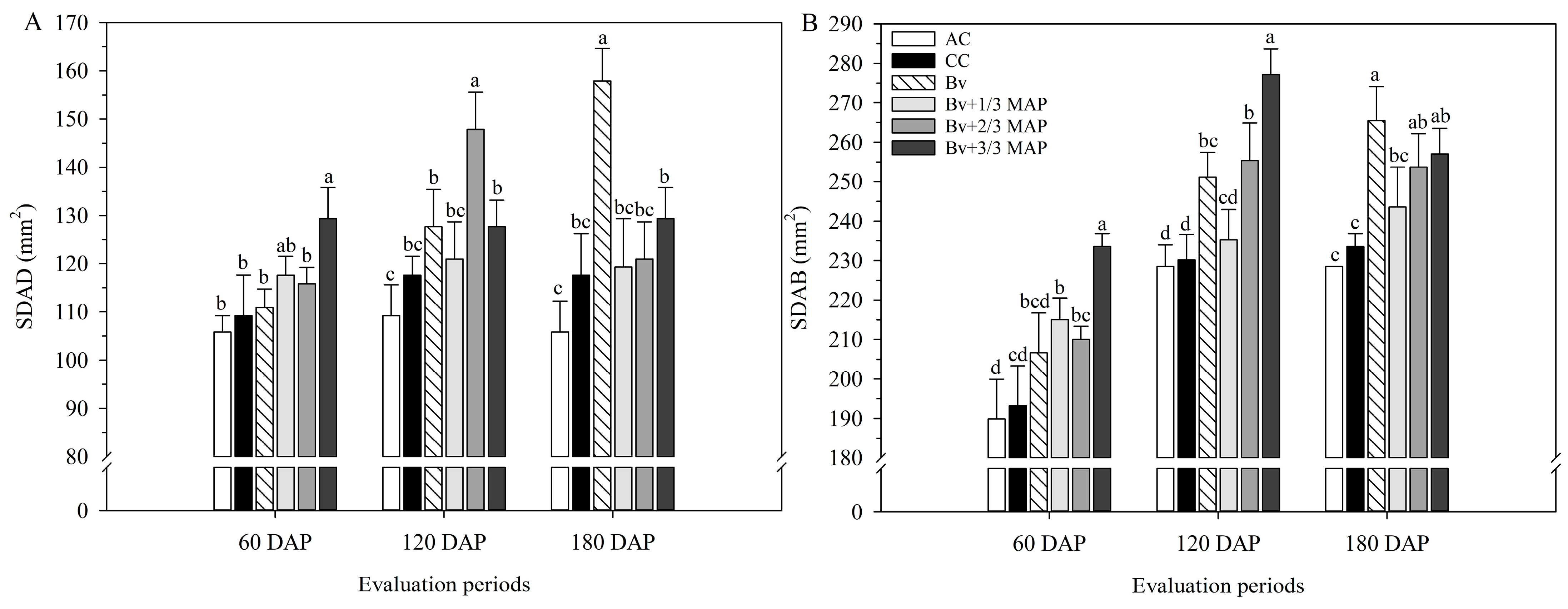
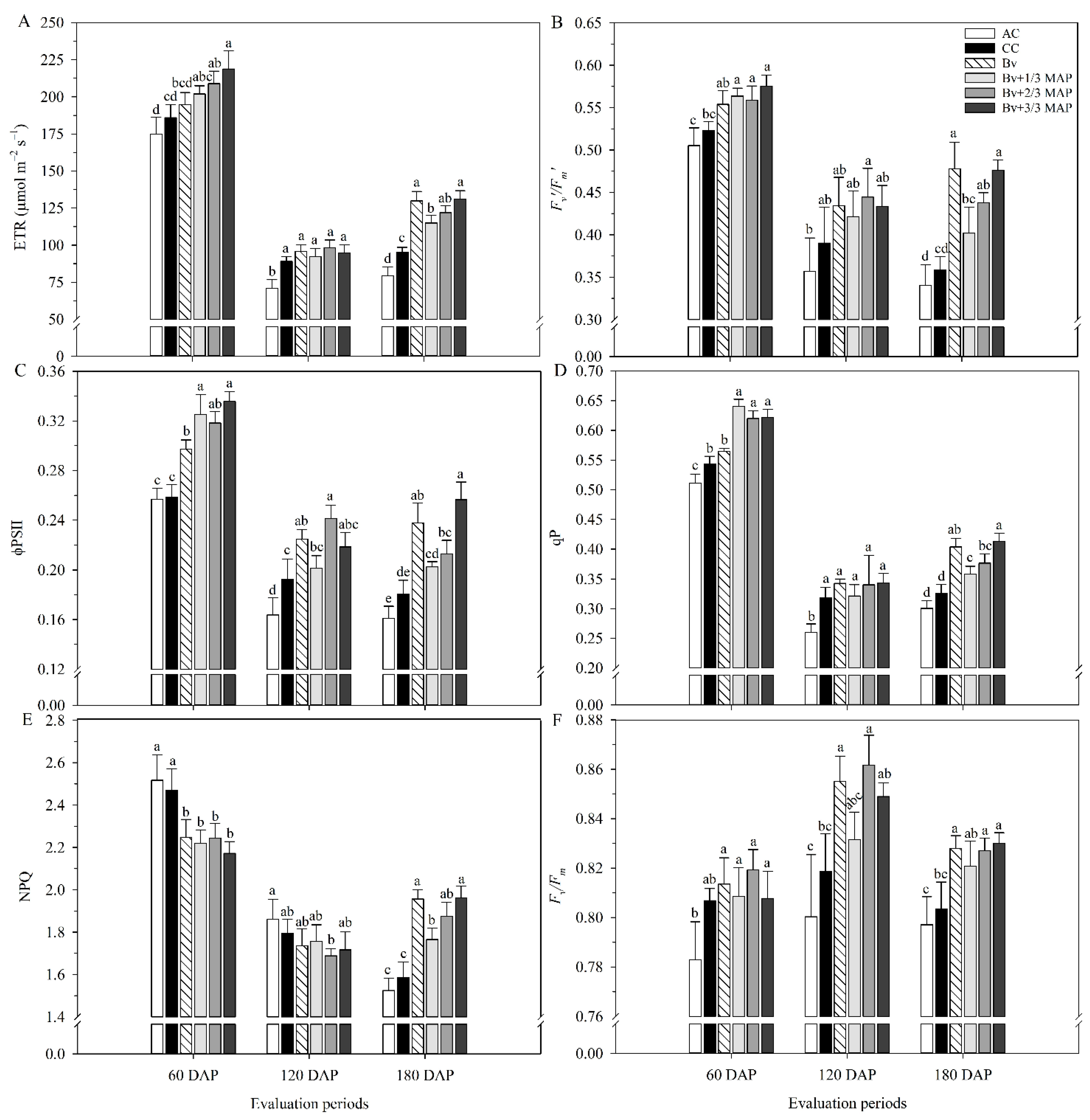
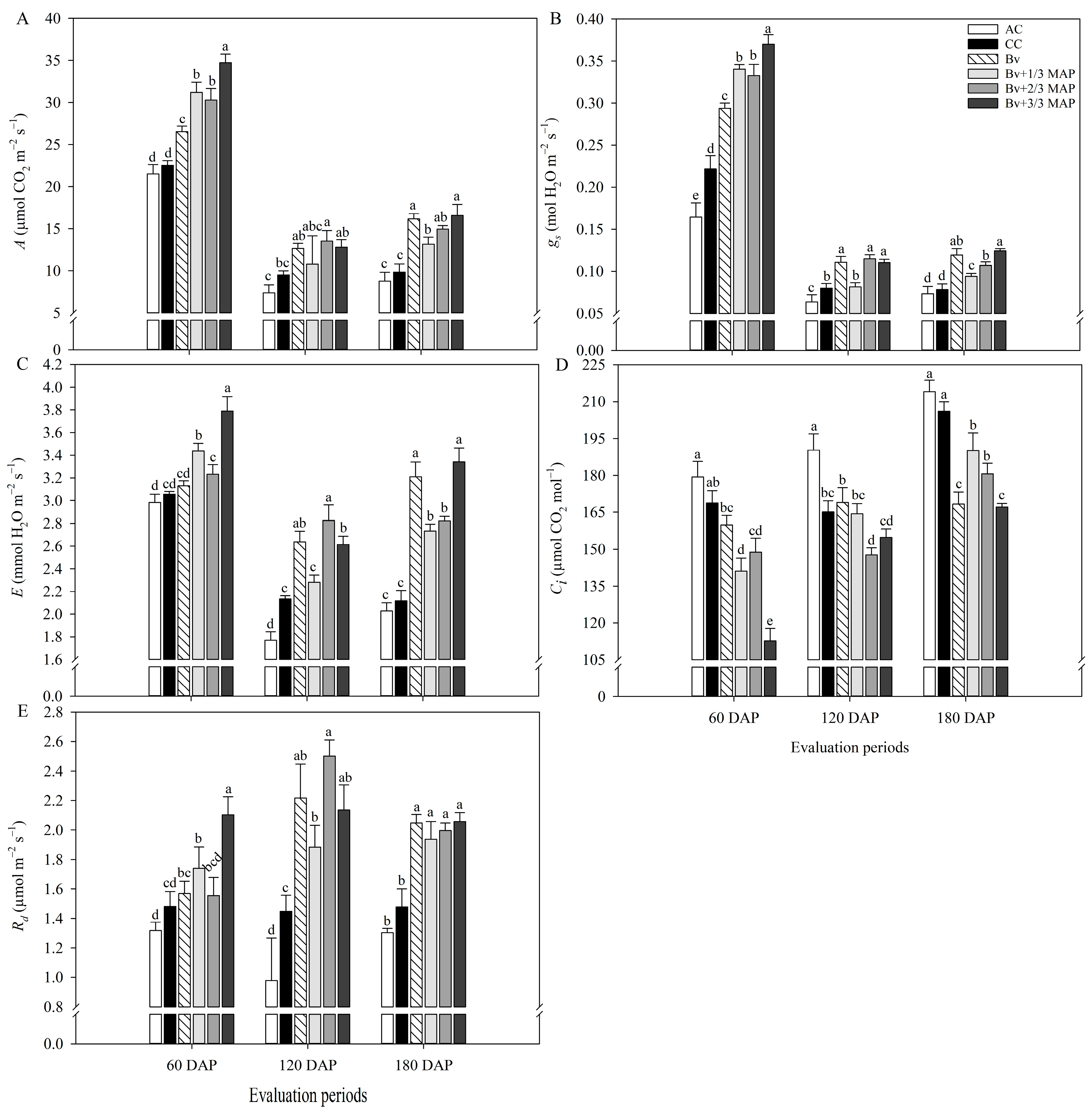
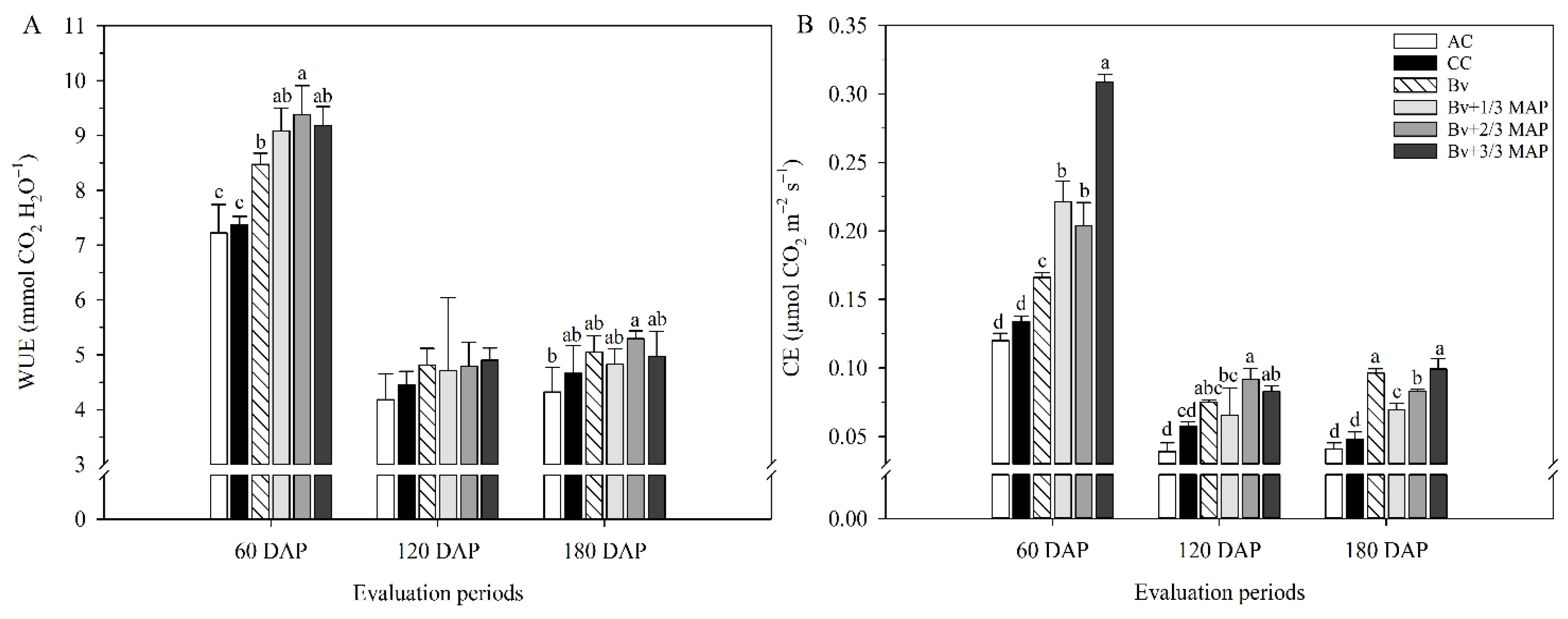
2.1. Physiological Assessments
2.2. Biochemical Assessments
2.3. Phosphorus Content in the Soil and Shoot
2.4. Shoot Biomass
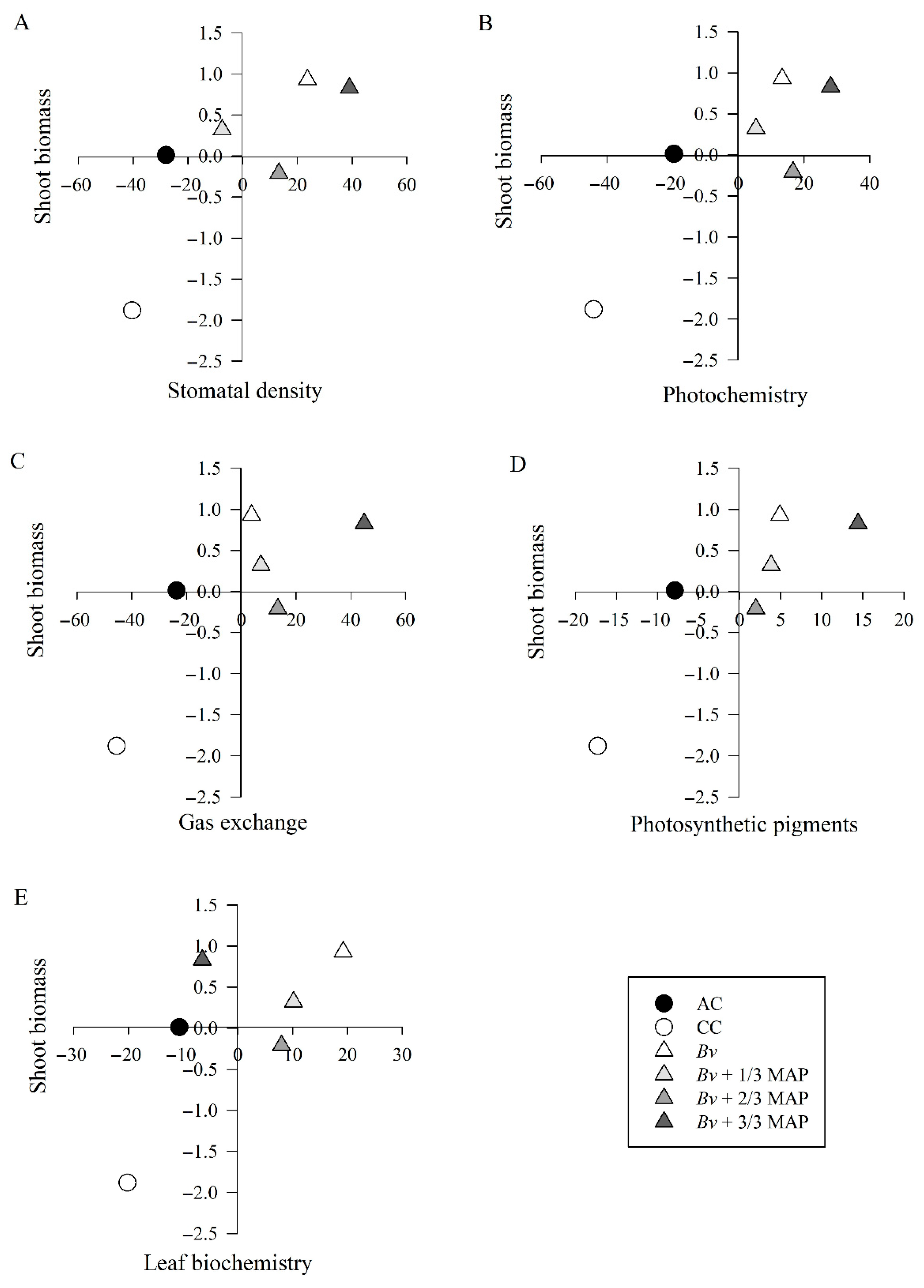
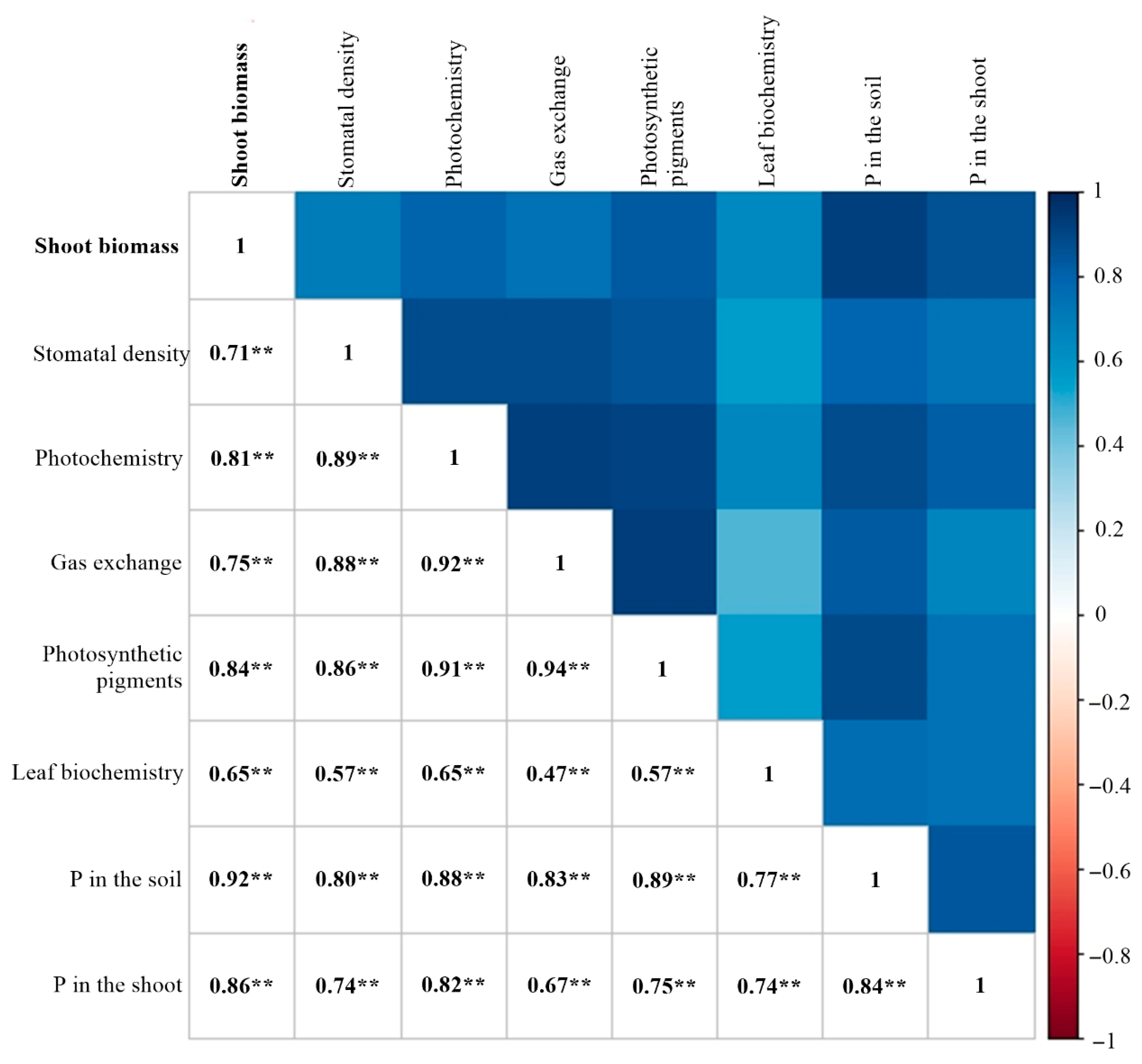
2.5. Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Cultivation Conditions, Plant Material, Experimental Design, and Treatments
4.2. Physiological Assessments
4.3. Biochemical Assessments
4.4. Phosphorus Content in the Soil and Shoot
4.5. Shoot Biomass
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bordonal, R.O.; Carvalho, J.L.N.; Lal, R.; Figueiredo, E.B.; Oliveira, B.G.; La Scala, N. Sustainability of sugarcane production in Brazil: A review. Agron. Sustain. Dev. 2018, 38, 13. [Google Scholar] [CrossRef]
- Companhia Nacional de Abastecimento. Acompanhamento da Safra Brasileira de Cana-de-Açúcar: Safra 2023/2024—4° Levantamento. Available online: https://www.conab.gov.br/component/k2/item/download/52732_1ba60aad418c90b0a86daf409a3703a5 (accessed on 21 May 2024).
- Guignard, M.S.; Leitch, A.R.; Acquisti, C.; Eizaguirre, C.; Elser, J.J.; Hessen, D.O.; Jeyasingh, P.D.; Neiman, M.; Richardson, A.E.; Soltis, P.S.; et al. Impacts of nitrogen and phosphorus: From genomes to natural ecosystems and agriculture. Front. Ecol. Evol. 2017, 5, 70. [Google Scholar] [CrossRef]
- Lizcano-Toledo, R.; Reyes-Mantín, M.P.; Celi, L.; Fenández-Ondoño, E. Phosphorus dynamics in the soil–plant–environment relationship in cropping systems: A review. Appl. Sci. 2021, 11, 11133. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier: London, UK, 2012. [Google Scholar]
- Teixeira, W.G.; Sousa, R.T.X.; Korndörfer, G.H. Response of sugarcane to doses of phosphorus provided by organomineral fertilizer. Biosci. J. 2014, 30, 1729–1736. [Google Scholar]
- Albuquerque, A.W.; Sá, L.A.; Rodrigues, W.A.R.; Moura, A.B.; Oliveira Filho, M.S. Growth and yield of sugarcane as a function of phosphorus doses and forms of application. Rev. Bras. Eng. Agric. Ambient. 2016, 20, 29–35. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal; Artmed: Porto Alegre, Brazil, 2017. [Google Scholar]
- Silva, A.M.S.; Oliveira, E.C.A.; Willadino, L.G.; Freire, F.J.; Rocha, A.T. Corrective phosphate application as a practice for reducing oxidative stress and increasing productivity in sugarcane. Cienc. Agron. 2019, 50, 188–196. [Google Scholar] [CrossRef]
- Preston, C.L.; Diaz, D.A.R.; Mengel, D.B. Corn response to long-term phosphorus fertilizer application rate and placement with strip-tillage. Agron. J. 2019, 111, 841–850. [Google Scholar] [CrossRef]
- White, P.J.; Brown, P.H. Plant nutrition for sustainable development and global health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef]
- White, P.J.; Hammond, J.P. Phosphorus nutrition of terrestrial plants. In The Ecophysiology of Plant-Phosphorus Interactions; White, P.J., Hammond, J.P., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 51–81. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.H.; Prochnow, L.I.; Cantarella, H. Recent developments of fertilizer production and use to improve nutrient efficiency and minimize environmental impacts. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 267–322. [Google Scholar] [CrossRef]
- Caione, G.; Prado, R.M.; Campos, C.N.S.; Moda, L.R.; Vasconcelos, R.L.; Pizauro Júnior, J.M. Response of sugarcane in a red ultisol to phosphorus rates, phosphorus sources, and filter cake. Sci. World J. 2015, 2015, 405970. [Google Scholar] [CrossRef]
- Borges, B.M.M.N.; Abdala, D.B.; Souza, M.F.; Viglio, L.M.; Coelho, M.J.A.; Pavinato, P.S.; Franco, H.C.J. Organomineral phosphate fertilizer from sugarcane byproduct and its effects on soil phosphorus availability and sugarcane yield. Geoderma 2019, 339, 20–30. [Google Scholar] [CrossRef]
- Novais, R.F.; Smyth, T.J.; Nunes, F.N. Fósforo. In Fertilidade do Solo; Novais, R.F., Alvarez, V.V.H., Barros, N.F., Fontes, R.L.F., Cantarutti, R.B., Neves, J.C.L., Eds.; Sociedade Brasileira de Ciência do Solo: Viçosa, Brazil, 2007; pp. 471–550. [Google Scholar]
- Asomaning, S.K. Processes and factors affecting phosphorus sorption in soils. In Sorption in 2020s; Kyzas, G., Lazaridis, N., Eds.; IntechOpen: London, UK, 2020; pp. 1–15. [Google Scholar]
- Hanyabui, E.; Apori, S.O.; Frimpong, K.A.; Atiah, K.; Abindaw, T.; Ali, M.; Asiamah, J.Y.; Byalebeka, J. Phosphorus sorption in tropical soils. AIMS Agric. Food 2020, 5, 599–616. [Google Scholar] [CrossRef]
- Roy, E.D.; Richards, P.D.; Martinelli, L.A.; Della Coletta, L.; Lins, S.R.M.; Vazquez, F.F.; Willig, E.; Spera, S.A.; VanWey, L.K.; Porder, S. The phosphorus cost of agricultural intensification in the tropics. Nat. Plants 2016, 2, 2–7. [Google Scholar] [CrossRef]
- Billah, M.; Matiullah, K.; Bano, A.; Hassan, T.U.; Munir, A.; Gurmani, A.R. Phosphorus and phosphate solubilizing bacteria: Keys for sustainable agriculture. Geomicrobiol. J. 2019, 36, 904–916. [Google Scholar] [CrossRef]
- Shukla, A.K. Ecology and Diversity of Plant Growth Promoting Rhizobacteria in Agricultural Landscape. In PGPR Amelioration in Sustainable Agriculture: Food Security and Environmental Management; Singh, A.K., Kumar, A., Singh, P.K., Eds.; Woodhead Publishing: Duxford, UK, 2019; pp. 1–14. [Google Scholar]
- Rosa, P.A.L.; Mortinho, E.S.; Jalal, A.; Galindo, F.S.; Buzetti, S.; Fernandes, G.C.; Barco Neto, M.; Pavinato, P.S.; Teixeira Filho, M.C.M. Inoculation with growth-promoting bacteria associated with the reduction of phosphate fertilization in sugarcane. Front. Environ. Sci. 2020, 8, 32. [Google Scholar] [CrossRef]
- Rosa, P.A.L.; Galindo, F.S.; Oliveira, C.E.S.; Jalal, A.; Mortinho, E.S.; Fernandes, G.C.; Marega, E.M.R.; Buzetti, S.; Teixeira Filho, M.C.M. Inoculation with plant growth-promoting bacteria to reduce phosphate fertilization requirement and enhance technological quality and yield of sugarcane. Microorganisms 2022, 10, 192. [Google Scholar] [CrossRef]
- Meena, R.S.; Meena, V.S.; Meena, S.K.; Verma, J.P. The needs of healthy soils for a healthy world. J. Clean. Prod. 2015, 102, 560–561. [Google Scholar] [CrossRef]
- Kumar, A.; Maurya, B.R.; Raghuwanshi, R.; Meena, V.S.; Islam, M.T. Co-inoculation with Enterobacter and Rhizobacteria on yield and nutrient uptake by wheat (Triticum aestivum L.) in the alluvial soil under Indo-Gangetic plain of India. J. Plant Growth Regul. 2017, 36, 608–617. [Google Scholar] [CrossRef]
- Shen, H.; He, X.; Liu, Y.; Chen, Y.; Tang, J.; Guo, T. A complex inoculant of N2-fixing, P- and K-solubilizing bacteria from a purple soil improves the growth of kiwifruit (Actinidia chinensis) plantlets. Front. Microbiol. 2016, 7, 841. [Google Scholar] [CrossRef]
- Kalayu, G. Phosphate solubilizing microorganisms: Promising approach as biofertilizers. Int. J. Agron. 2019, 2019, 4917256. [Google Scholar] [CrossRef]
- Saeid, A.; Prochownik, E.; Dobrowolska-Iwanek, J. Phosphorus solubilization by Bacillus species. Molecules 2018, 23, 2897. [Google Scholar] [CrossRef]
- Sarmah, R.; Sarma, A.K. Phosphate Solubilizing Microorganisms: A Review. Commun. Soil Sci. Plant Anal. 2023, 54, 1306–1315. [Google Scholar] [CrossRef]
- Elhaissoufi, W.; Khourchi, S.; Ibnyasser, A.; Ghoulam, C.; Rchiad, Z.; Zeroual, Y.; Lyamlouli, K.; Bargaz, A. Phosphate solubilizing rhizobacteria could have a stronger influence on wheat root traits and aboveground physiology than rhizosphere P solubilization. Front. Plant Sci. 2020, 11, 979. [Google Scholar] [CrossRef]
- Cheng, Y.; Yuan, J.; Wang, G.; Hu, Z.; Luo, W.; Zhao, X.; Guo, Y.; Ji, X.; Hu, W.; Li, M. Phosphate-solubilizing bacteria improve the antioxidant enzyme activity of Potamogeton crispus L. and enhance the remediation effect on Cd-contaminated sediment. J. Hazard. Mater. 2024, 470, 134305. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. J. Soil Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar] [CrossRef]
- Shi, Z.; Pan, F.; Kong, X.; Lang, J.; Ye, M.; Wu, Q.; Wang, G.; Han, L.; Zhou, N. Effects of inoculation with phosphate solubilizing bacteria on the physiology, biochemistry, and expression of genes related to the protective enzyme system of Fritillaria taipaiensis P.Y. Li. Phyton-Int. J. Exp. Bot. 2024, 93, 247–260. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, X.; Kim, M.-S.; Kornyeyev, D.A.; Holaday, S.; Paré, P.W. Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant J. 2008, 56, 264–273. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Gao, J.; Pan, S.; Wang, G. Bacillus subtilis-regulation of stomatal movement and instantaneous water use efficiency in Vicia faba. Plant Growth Regul. 2016, 78, 43–55. [Google Scholar] [CrossRef]
- Majid, M.; Ali, M.; Shahzad, K.; Ahmad, F.; Ikram, R.M.; Ishtiaq, M.; Alaraidh, I.A.; Al-Hashimi, A.; Ali, H.M.; Zarei, T.; et al. Mitigation of osmotic stress in cotton for the improvement in growth and yield through inoculation of rhizobacteria and phosphate solubilizing bacteria coated diammonium phosphate. Sustainability 2020, 12, 10456. [Google Scholar] [CrossRef]
- Fonseca, M.C.; Bossolani, J.W.; Oliveira, S.L.; Moretti, L.G.; Portugal, J.R.; Scudeletti, D.; Oliveira, E.F.; Crusciol, C.A.C. Bacillus subtilis inoculation improves nutrient uptake and physiological activity in sugarcane under drought stress. Microorganisms 2022, 10, 809. [Google Scholar] [CrossRef]
- Rampazzo, P.E.; Marcos, F.C.C.; Cipriano, M.A.P.; Marchiori, P.E.R.; Freitas, S.S.; Machado, E.C.; Nascimento, L.C.; Brochi, M.; Ribeiro, R.V. Rhizobacteria improve sugarcane growth and photosynthesis under well-watered conditions. Ann. Appl. Biol. 2018, 172, 309–320. [Google Scholar] [CrossRef]
- Aye, P.P.; Pinjai, P.; Tawornpruek, S. Effect of phosphorus solubilizing bacteria on soil available phosphorus and growth and yield of sugarcane. Walailak J. Sci. Technol. 2021, 18, 10754. [Google Scholar] [CrossRef]
- Santos, H.L.; Silva, G.F.; Carnietto, M.R.A.; Oliveira, L.C.; Nogueira, C.H.C.; Silva, M.A. Bacillus velezensis associated with organomineral fertilizer and reduced phosphate doses improves soil microbial-chemical properties and biomass of sugarcane. Agronomy 2022, 12, 2701. [Google Scholar] [CrossRef]
- Abdallah, D.B.; Frikha-Gargouri, O.; Tounsi, S. Rizhospheric competence, plant growth promotion and biocontrol efficacy of Bacillus amyloliquefaciens subsp. plantarum strain 32a. Biol. Control 2018, 124, 61–67. [Google Scholar] [CrossRef]
- Bayisa, R.A. Bacillus velezensis AR1 mediated plant nourishing through solubilization of hardly soluble phosphorus nutrient sources. Cogent Food Agric. 2023, 9, 2276561. [Google Scholar] [CrossRef]
- Iqbal, Z.; Ahmad, M.; Raza, M.A.; Hilger, T.; Rasche, F. Phosphate Solubilizing Bacillus sp. modulate soil exoenzyme activities and improve wheat growth. Microb. Ecol. 2024, 87, 31. [Google Scholar] [CrossRef]
- Suleman, M.; Yasmin, S.; Rasul, M.; Yahya, M.; Atta, B.M.; Mirza, M.S. Phosphate solubilizing bacteria with glucose dehydrogenase gene for phosphorus uptake and beneficial effects on wheat. PLoS ONE 2018, 13, e0204408. [Google Scholar] [CrossRef]
- Emami, S.; Alikhani, H.A.; Pourbabaee, A.A.; Etesami, H.; Motasharezadeh, B.; Sarmadian, F. Consortium of endophyte and rhizosphere phosphate solubilizing bacteria improves phosphorous use efficiency in wheat cultivars in phosphorus deficient soils. Rhizosphere 2020, 14, 100196. [Google Scholar] [CrossRef]
- Setlow, P. Mechanisms which contribute to the long-term survival of spores of Bacillus species. J. Appl. Bacteriol. 1994, 76, 49S–60S. [Google Scholar] [CrossRef]
- Nicholson, W.L.; Munakata, N.; Horneck, G.; Melosh, H.J.; Setlow, P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 2000, 64, 548–572. [Google Scholar] [CrossRef] [PubMed]
- Leser, T.D.; Knarreborg, A.; Worm, J. Germination and outgrowth of Bacillus subtilis and Bacillus licheniformis spores in the gastrointestinal tract of pigs. J. Appl. Microbiol. 2008, 104, 1025–1033. [Google Scholar] [CrossRef]
- Kalidas-Singh, S.; Thakuria, D. Seedling root-dip in phosphorus and biofertilizer added soil slurry method of nutrient management for transplanted rice in acid soil. J. Soil Sci. Plant Nutr. 2018, 18, 921–938. [Google Scholar] [CrossRef]
- Iqbal, A.; Song, M.; Shah, Z.; Alamzeb, M.; Iqbal, M. Integrated use of plant residues, phosphorus and beneficial microbes improve hybrid maize productivity in semiarid climates. Acta Ecol. Sin. 2019, 39, 348–355. [Google Scholar] [CrossRef]
- Adnan, M.; Fahad, S.; Zamin, M.; Shah, S.; Mian, I.A.; Danish, S.; Zafar-ul-Hye, M.; Battaglia, M.L.; Naz, R.M.M.; Saeed, B.; et al. Coupling phosphate-solubilizing bacteria with phosphorus supplements improve maize phosphorus acquisition and growth under lime induced salinity stress. Plants 2020, 9, 900. [Google Scholar] [CrossRef]
- Biswas, S.S.; Biswas, D.R.; Ghosh, A.; Sarkar, A.; Das, A.; Roy, T. Phosphate solubilizing bacteria inoculated low-grade rock phosphate can supplement P fertilizer to grow wheat in sub-tropical inceptisol. Rhizosphere 2022, 23, 100556. [Google Scholar] [CrossRef]
- Singh, B.; Pandey, R. Differences in root exudation among phosphorus-starved genotypes of maize and green gram and its relationship with phosphorus uptake. J. Plant Nutr. 2003, 26, 2391–2401. [Google Scholar] [CrossRef]
- Beauregard, M.S.; Hamel, C.; Nayyar, A.; St-Arnaud, M. Long-term phosphorus fertilization impacts soil fungal and bacterial diversity but not AM fungal community in alfalfa. Microb. Ecol. 2010, 59, 379–389. [Google Scholar] [CrossRef]
- Grafe, M.; Goers, M.; von Tucher, S.; Baum, C.; Zimmer, D.; Leinweber, P.; Vestergaard, G.; Kublik, S.; Schloter, M.; Schulz, S. Bacterial potentials for uptake, solubilization and mineralization of extracellular phosphorus in agricultural soils are highly stable under different fertilization regimes. Environ. Microbiol. Rep. 2018, 10, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Kertesz, M.A.; Feng, G. Phosphorus forms affect the hyphosphere bacterial community involved in soil organic phosphorus turnover. Mycorrhiza 2019, 29, 351–362. [Google Scholar] [CrossRef]
- Widdig, M.; Schleuss, P.M.; Weig, A.R.; Guhr, A.; Biederman, L.A.; Borer, E.T.; Crawley, M.J.; Kirkman, K.P.; Seabloom, E.W.; Wragg, P.D.; et al. Nitrogen and phosphorus additions alter the abundance of phosphorus-solubilizing bacteria and phosphatase activity in grassland soils. Front. Environ. Sci. 2019, 7, 185. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, G.; Chen, H.; Chen, C.; Wang, J.; Ai, S.; Wei, D.; Li, D.; Ma, B.; Tang, C.; et al. Long-term nutrient inputs shift soil microbial functional profiles of phosphorus cycling in diverse agroecosystems. ISME J. 2020, 14, 757–770. [Google Scholar] [CrossRef]
- Mander, C.; Wakelin, S.; Young, S.; Condron, L.; O’Callaghan, M. Incidence and diversity of phosphate-solubilising bacteria are linked to phosphorus status in grassland soils. Soil Biol. Biochem. 2012, 44, 93–101. [Google Scholar] [CrossRef]
- Long, X.E.; Yao, H.; Huang, Y.; Wei, W.; Zhu, Y.G. Phosphate levels influence the utilisation of rice rhizodeposition carbon and the phosphate-solubilising microbial community in a paddy soil. Soil Biol. Biochem. 2018, 118, 103–114. [Google Scholar] [CrossRef]
- Zhao, K.; Penttinen, P.; Zhang, X.P.; Ao, X.L.; Liu, M.K.; Yu, X.M.; Chen, Q. Maize rhizosphere in Sichuan, China, hosts plant growth promoting Burkholderia cepacia with phosphate solubilizing and antifungal abilities. Microbiol. Res. 2014, 169, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.M.; Diaz, P.A.E.; Lobo, L.L.; Rigobelo, E.C. Use of plant growth-promoting rhizobacteria in maize and sugarcane: Characteristics and applications. Front. Sustain. Food Syst. 2020, 4, 136. [Google Scholar] [CrossRef]
- Steele, M.R.; Gitelson, A.A.; Rundquist, D.C. A comparison of two techniques for nondestructive measurement of chlorophyll content in grapevine leaves. Agron. J. 2008, 100, 779–782. [Google Scholar] [CrossRef]
- Silva, M.A.; Santos, C.M.; Vitorino, H.S.; Rhein, A.F.L. Pigmentos fotossintéticos e índice SPAD como descritores de intensidade do estresse por deficiência hídrica em cana-de-açúcar. Biosci. J. 2014, 30, 173–181. [Google Scholar]
- Houborg, R.; Fisher, J.B.; Skidmore, A.K. Advances in remote sensing of vegetation function and traits. Int. J. Appl. Earth Obs. Geoinf. 2015, 43, 1–6. [Google Scholar] [CrossRef]
- Chou, S.; Chen, B.; Chen, J.; Wang, M.; Wang, S.; Croft, H.; Shi, Q. Estimation of leaf photosynthetic capacity from the photochemical reflectance index and leaf pigments. Ecol. Indic. 2019, 107, 105867. [Google Scholar] [CrossRef]
- Urbonaviciute, A.; Samuoliene, G.; Sakalauskaite, J.; Duchovskis, P.; Brazaityte, A.; Siksnianiene, J.B.; Ulinskaite, R.; Sabajeviene, G.; Baranauskis, K. The effect of elevated CO2 concentrations on leaf carbohydrate, chlorophyll contents and photosynthesis in radish. Pol. J. Environ. Stud. 2006, 15, 921–925. [Google Scholar]
- Lokstein, H.; Renger, G.; Götze, J.P. Photosynthetic light-harvesting (antenna) complexes—Structures and functions. Molecules 2021, 26, 3378. [Google Scholar] [CrossRef] [PubMed]
- Siefermann, D.; Yamamoto, H.Y. Properties of NADPH and oxygen-dependent zeaxanthin epoxidation in isolated chloroplasts: A transmembrane model for the violaxanthin cycle. Arch. Biochem. Biophys. 1975, 171, 70–77. [Google Scholar] [CrossRef]
- Bhutta, M.A.; Bibi, A.; Ahmad, N.H.; Kanwal, S.; Amjad, Z.; Rehman, H.; Farooq, U.; Khalid, M.N.; Nayab, S.F. Molecular mechanisms of photoinhibition in plants: A review. Sarhad J. Agric. 2023, 39, 340–345. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: A survival strategy. Appl. Microbiol. Biotechnol. 2018, 102, 7821–7835. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Triphathi, P.; Chandra, A. Isolation and molecular characterization of plant growth-promoting Bacillus spp. and their impact on sugarcane (Saccharum spp. Hybrids) growth and tolerance towards drought stress. Acta Physiol. Plant. 2018, 40, 199. [Google Scholar] [CrossRef]
- Chaudhary, P.; Khati, P.; Chaudhary, A.; Gangola, S.; Sharma, A. Bioinoculation using indigenous Bacillus spp. improves growth and yield of Zea mays under the influence of nanozeolite. 3 Biotech 2021, 11, 11. [Google Scholar] [CrossRef]
- Chaudhary, P.; Khati, P.; Gangola, S.; Kumar, A.; Kumar, R.; Sharma, A. Impact of nanochitosan and Bacillus spp. on health, productivity and defence response in Zea mays under field condition. 3 Biotech 2021, 11, 237. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Hafeez, A.; Saliha, A.; Javed, M.A.; Sumaira; Afridi, M.S.; Dawoud, T.M.; Almaary, K.S.; Muresan, C.C.; Marc, R.A.; et al. Bacillus thuringiensis PM25 ameliorates oxidative damage of salinity stress in maize via regulating growth, leaf pigments, antioxidant defense system, and stress responsive gene expression. Front. Plant Sci. 2022, 13, 921668. [Google Scholar] [CrossRef]
- Tahir, M.; Khalid, U.; Ijaz, M.; Shah, G.M.; Naeem, M.A.; Shahid, M.; Mahmood, K.; Ahmad, N.; Kareem, F. Combined application of bio-organic phosphate and phosphorus solubilizing bacteria (Bacillus strain MWT 14) improve the performance of bread wheat with low fertilizer input under an arid climate. Braz. J. Microbiol. 2018, 49, 15–24. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.M.; Kim, K.M.; Lee, I.J. Thermotolerance effect of plant growth-promoting Bacillus cereus SA1 on soybean during heat stress. BMC Microbiol. 2020, 20, 175. [Google Scholar] [CrossRef]
- Crafts-Brandner, S.J. Phosphorus nutrition influence on leaf senescence in soybean. Plant Physiol. 1992, 98, 1128–1132. [Google Scholar] [CrossRef]
- Singh, S.K.; Badgujar, G.; Reddy, V.R.; Fleisher, D.H.; Bunce, J.A. Carbon dioxide diffusion across stomata and mesophyll and photo-biochemical processes as affected by growth CO2 and phosphorus nutrition in cotton. J. Plant Physiol. 2013, 170, 801–813. [Google Scholar] [CrossRef]
- Singh, S.K.; Reddy, V.R. Response of carbon assimilation and chlorophyll fluorescence to soybean leaf phosphorus across CO2: Alternative electron sink, nutrient efficiency and critical concentration. J. Photoch. Photobio. B Biol. 2015, 151, 276–284. [Google Scholar] [CrossRef]
- Hong-Hai, L.; Merope, T.M.; Ya-Li, Z.; Wang-Feng, Z. Combining gas exchange and chlorophyll a fluorescence measurements to analyze the photosynthetic activity of drip-irrigated cotton under different soil water deficits. J. Integr. Agric. 2016, 15, 1256–1266. [Google Scholar] [CrossRef]
- Wang, S.K.; Zhuang, S.; Zhang, M.; Yang, F.; Meng, Q. Overexpression of a tomato carotenoid ε-hydroxylase gene (SILUT1) improved the drought tolerance of transgenic tobacco. J. Plant Physiol. 2018, 170, 235–245. [Google Scholar] [CrossRef]
- Melis, A. Photosystem-II damage and repair cycle in chloroplasts: What modulates the rate of photodamage in vivo? Trends Plant Sci. 1999, 4, 1360–1385. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.N.; Siqueira-Silva, A.I.; Souza, J.P.; Kuki, K.N.; Pereira, E.G. Acclimation responses of macaw palm seedlings to contrasting light environments. Sci. Rep. 2018, 8, 15300. [Google Scholar] [CrossRef] [PubMed]
- Stirbet, A.; Lazár, D.; Kromdijk, J.; Govindjee, G. Chlorophyll a fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Guidi, L.; Calatayud, A. Non-invasive tools to estimate stress-induced changes in photosynthetic performance in plants inhabiting Mediterranean areas. Environ. Exp. Bot. 2014, 103, 42–52. [Google Scholar] [CrossRef]
- Schimpl, F.C.; Ribeiro, R.V.; Pereira, L.; Rodrigues, H.S.; Mazzafera, P. Photochemical responses to abrupt and gradual chilling treatments in eucalyptus species. Theor. Exp. Plant Physiol. 2018, 30, 9–17. [Google Scholar] [CrossRef]
- Samaniego-Gámez, B.Y.; Garruña, R.; Tun-Suárez, J.M.; Kantun-Can, J.; Reyes-Ramírez, A.; Cervanes-Díaz, L. Bacillus spp. inoculation improves photosystem II efficiency and enhances photosynthesis in pepper plants. Chil. J. Agric. Res. 2016, 76, 409–416. [Google Scholar] [CrossRef]
- Samaniego-Gámez, B.Y.; Garruña, R.; Tun-Suárez, J.M.; Moreno-Valenzuela, O.A.; Reyes-Ramírez, A.; Valle-Gough, R.E.; Ail-Catzim, C.E.; Toscano-Palomar, L. Healthy photosynthetic mechanism suggests ISR elicited by Bacillus spp. in Capsicum chinense plants infected with PepGMV. Pathogens 2021, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lou, K.; Li, C. Growth and photosynthetic efficiency promotion of sugar beet (Beta vulgaris L.) by endophytic bacteria. Photosynth. Res. 2010, 105, 5–13. [Google Scholar] [CrossRef]
- Marcos, F.C.C.; Iório, R.P.F.; Silveira, A.P.D.; Ribeiro, R.V.; Machado, E.C.; Lagôa, A.M.M.A. Endophytic bacteria affect sugarcane physiology without changing plant growth. Bragantia 2016, 75, 256. [Google Scholar] [CrossRef]
- Ozfidan-Konakci, C.; Arikan, B.; Alp-Turgut, F.N.; Balci, M.; Yildiztugay, E. Halotolerant plant growth-promoting bacteria, Bacillus pumilus modulates water status, chlorophyll fluorescence kinetics and antioxidant balance in salt and/or arsenic-exposed wheat. Environ. Res. 2023, 231, 116089. [Google Scholar] [CrossRef]
- Singh, S.K.; Reddy, V.R. Combined effects of phosphorus nutrition and elevated carbon dioxide concentration on chlorophyll fluorescence, photosynthesis and nutrient efficiency of cotton. J. Plant Nutr. Soil Sci. 2014, 177, 892–902. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V. Nonphotochemical Chlorophyll Fluorescence Quenching: Mechanism and Effectiveness in Protecting Plants from Photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching: A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the saturation pulse method. Heinz Walz GmbH 2008, 1, 27–35. [Google Scholar]
- Murchie, E.H.; Ruban, A.V. Dynamic non-photochemical quenching in plants: From molecular mechanism to productivity. Plant J. 2020, 101, 885–896. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Reinoso, A.D.; Ligarreto-Moreno, G.A.; Restrepo-Díaz, H. Physiological and biochemical expressions of a determinated growth common bean genotype (Phaseolus vulgaris L.) to water deficit stress periods. J. Anim. Plant Sci. 2018, 28, 119–127. [Google Scholar]
- Costa-Santos, M.; Mariz-Ponte, N.; Dias, M.C.; Moura, L.; Marques, G.; Santos, C. Effect of Bacillus spp. and Brevibacillus sp. on the photosynthesis and redox status of Solanum lycopersicum. Horticulturae 2021, 7, 24. [Google Scholar] [CrossRef]
- Carvalho Neta, S.J.; Araújo, V.L.V.P.; Fracetto, F.L.C.; Silva, C.C.G.; Souza, E.R.; Silva, W.R.; Lumini, E.; Fracetto, G.G.M. Growth-promoting bacteria and arbuscular mycorrhizal fungus enhance maize tolerance to saline stress. Microbiol. Res. 2024, 284, 127708. [Google Scholar] [CrossRef]
- McAdam, S.A.M.; Brodribb, T.J. Stomatal innovation and the rise of seed plants. Ecol. Lett. 2012, 15, 1–8. [Google Scholar] [CrossRef]
- Wong, S.C.; Cowan, I.R.; Farquhar, G.D. Stomatal conductance correlates with photosynthetic capacity. Nature 1979, 282, 424–426. [Google Scholar] [CrossRef]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sugano, S.S.; Shimada, T.; Hara-Nishimura, I. Enhancement of leaf photosynthetic capacity through increased stomatal density in Arabidopsis. New Phytol. 2013, 198, 757–764. [Google Scholar] [CrossRef]
- Sadoka, K.; Yamori, W.; Shimada, T.; Sugano, S.S.; Hara-Nishimura, I.; Tanaka, Y. Higher stomatal density improves photosynthetic induction and biomass production in Arabidopsis under fluctuating light. Front. Plant Sci. 2020, 11, 589603. [Google Scholar] [CrossRef]
- Cappellari, L.R.; Santoro, M.V.; Reinoso, H.; Travaglia, C.; Giordano, W.; Banchio, E. Anatomical, morphological, and phytochemical effects of inoculation with plant growth-promoting rhizobacteria on peppermint (Mentha piperita). J. Chem. Ecol. 2015, 41, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; Geronimo, G.Z.; Santos, H.L. Genetic and morpho-physiological differentiation of sugarcane genotypes under drought stress. Int. J. Agric. Biol. 2020, 24, 311–318. [Google Scholar]
- Ku, S.; Edwards, G. Oxygen inhibition of photosynthesis: II. Kinetic characteristics as affected by temperature. Plant Physiol. 1977, 59, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.; Wang, P.; Li, Y.S.; Wang, G.; Liu, P.; Khan, A. Leaf gas exchange, phosphorus uptake, growth and yield responses of cotton cultivars to different phosphorus rates. Photosynthetica 2018, 56, 1414–1421. [Google Scholar] [CrossRef]
- Kimura, H.; Hashimoto-Sugimoto, M.; Iba, K.; Terashima, I.; Yamori, W. Improved stomatal opening enhances photosynthetic rate and biomass production in fluctuating light. J. Exp. Bot. 2020, 71, 2339–2350. [Google Scholar] [CrossRef]
- Naseem, H.; Bano, A. Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J. Plant Interact. 2014, 4, 689–701. [Google Scholar] [CrossRef]
- Zheng, W.; Zeng, S.; Bais, H.; LaManna, J.M.; Hussey, D.S.; Jacobson, D.L.; Jin, Y. Plant growth-promoting rhizobacteria (PGPR) reduce evaporation and increase soil water retention. Water Resour. Res. 2018, 54, 3673–3687. [Google Scholar] [CrossRef]
- Díaz-Cornejo, S.; Otero, M.C.; Banerjee, A.; Gordillo-Fuenzalida, F. Biological properties of exopolysaccharides produced by Bacillus spp. Microbiol. Res. 2023, 268, 127276. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Amby, D.B.; Hegelund, J.N.; Fimognari, L.; Groβinsky, D.K.; Westergaard, J.C.; Müller, R.; Moelbak, L.; Liu, F.; Roitsch, T. Bacillus licheniformis FMCH001 increases water use efficiency via growth stimulation in both normal and drought conditions. Front. Plant Sci. 2020, 11, 297. [Google Scholar] [CrossRef]
- Almeida, L.C.O.; Santos, H.L.; de Castro Nogueira, C.; Carnietto, M.R.A.; da Silva, G.F.; Boaro, C.S.F.; de Almeida Silva, M. Plant Growth-Promoting Bacteria Enhance Survival, Growth, and Nutritional Content of Sugarcane Propagated through Pre-Sprouted Seedlings under Water Deficit. Agriculture 2024, 14, 189. [Google Scholar] [CrossRef]
- Glick, B.R. Introduction to Plant Growth-Promoting Bacteria. In Beneficial Plant-Bacterial Interactions, 2nd ed.; Glick, B.R., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–37. [Google Scholar] [CrossRef]
- Smith, B.N. Photosynthesis, respiration and growth. In Handbook of Photosynthesis; Pessarakli, M., Ed.; CRC Press: London, UK, 2004; pp. 671–677. [Google Scholar]
- Murchie, E.H.; Pinto, M.; Horton, P. Agriculture and the new challenges for photosynthesis research. New Phytol. 2009, 181, 532–552. [Google Scholar] [CrossRef]
- Reynolds, M.; Foulkes, M.J.; Slafer, G.A.; Berry, P.; Parry, M.A.; Snape, J.W.; Angus, W.J. Raising yield potential in wheat. J. Exp. Bot. 2009, 60, 1899–1918. [Google Scholar] [CrossRef]
- Chytyk, C.; Hucl, P.; Gray, G. Leaf photosynthetic properties and biomass accumulation of selected western Canadian spring wheat cultivars. Can. J. Plant Sci. 2011, 91, 305–314. [Google Scholar] [CrossRef]
- Rodrigues, J.D.; Jadoski, C.J.; Fagan, E.B.; Ono, E.O.; Soares, L.H.; Dourado Neto, D. Fisiologia da Produção de Cana-de-Açúcar; ANDREI: São Paulo, Brazil, 2018. [Google Scholar]
- Hossain, M.A.; Burritt, D.J.; Fujita, M. Proline and glycine betaine modulate cadmium-induced oxidative stress tolerance in plants. In Plant-Environmental Interaction: Response and Approaches to Mitigate Stress; Azooz, M.M., Ahmad, P., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 97–123. [Google Scholar] [CrossRef]
- Anjum, N.; Thangavel, P.; Rasheed, F.; Masood, A.; Pirasteh-Anosheh, H.; Khan, N. Osmolytes: Efficient oxidative stress-busters in plants. In Plant Stress Physiology; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Wiley: Hoboken, NJ, USA, 2023; pp. 1–30. [Google Scholar] [CrossRef]
- Less, H.; Galili, G. Principal transcriptional programs regulating plant amino acid metabolism in response to abiotic stresses. Plant Physiol. 2008, 147, 316–330. [Google Scholar] [CrossRef] [PubMed]
- Chiappero, J.; Cappellari, L.D.R.; Alderete, L.G.S.; Palermo, T.B.; Banchio, E. Plant growth-promoting rhizobacteria improve the antioxidant status in Mentha piperita grown under drought stress leading to an enhancement of plant growth and total phenolic content. Ind. Crops Prod. 2019, 139, 111553. [Google Scholar] [CrossRef]
- Nunes, F.N.; Cantarutti, R.B.; Novais, R.F.; Silva, I.R.; Tótola, M.R.; Ribeiro, B.N. Atividade de fosfatases em gramíneas forrageiras em resposta à disponibilidade de fósforo no solo e à altura de corte das plantas. Rev. Bras. Cienc. Solo 2008, 32, 1899–1909. [Google Scholar] [CrossRef][Green Version]
- Bozzo, G.G.; Dunn, E.L.; Plaxton, W.C. Differential synthesis of phosphate-starvation inducible purple acid phosphatase isozymes in tomato (Lycopersicon esculentum) suspension cells and seedlings. Plant Cell Environ. 2006, 29, 303–313. [Google Scholar] [CrossRef]
- Xu Cheng, J.; Min Huang, L.; Chen, C.; Wang, J.; Xian Long, X. Effective lead immobilization by phosphate rock solubilization mediated by phosphate rock amendment and phosphate solubilizing bacteria. Chemosphere 2019, 237, 124540. [Google Scholar] [CrossRef] [PubMed]
- Rfaki, A.; Zennouhi, O.; Aliyat, F.Z.; Nassiri, L.; Ibijbijen, J. Isolation, selection and characterization of root-associated rock phosphate solubilizing bacteria in Moroccan wheat (Triticum aestivum L.). Geomicrobiol. J. 2020, 37, 230–241. [Google Scholar] [CrossRef]
- Bargaz, A.; Elhaissoufi, W.; Khourchi, S.; Benmrid, B.; Borden, K.A.; Rchiad, Z. Benefits of phosphate solubilizing bacteria on belowground crop performance for improved crop acquisition of phosphorus. Microbiol. Res. 2021, 252, 126842. [Google Scholar] [CrossRef]
- Malhotra, H.; Sharma, S.; Pandey, R. Phosphorus nutrition: Plant growth in response to deficiency and excess. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2018; pp. 171–190. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G.; Takagaki, M. Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production. In Photosynthesis and Respiration, 2nd ed.; Yamori, W., Ed.; Academic Press: London, UK, 2020; pp. 197–206. [Google Scholar]
- Tahir, H.A.; Gu, Q.; Wu, H.; Raza, W.; Hanif, A.; Wu, L.; Colman, M.V.; Gao, X. Plant growth promotion by volatile organic compounds produced by Bacillus subtilis SYST2. Front. Microbiol. 2017, 8, 171. [Google Scholar] [CrossRef]
- Silva, L.I.; Pereira, M.C.; Carvalho, A.M.X.; Buttrós, V.H.; Pasqual, M.; Dória, J. Phosphorus-Solubilizing Microorganisms: A Key to Sustainable Agriculture. Agriculture 2023, 13, 462. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, S.; He, B.; Sun, M.; Li, J.; Peng, R.; Sun, L.; Wang, X.; Cai, Y.; Wang, H.; et al. Phosphate-solubilising bacteria promote horticultural plant growth through phosphate solubilisation and phytohormone regulation. N. Z. J. Crop Hortic. Sci. 2024, 52, 125–140. [Google Scholar] [CrossRef]
- Zubair, M.; Hanif, A.; Farzand, A.; Sheikh, T.M.M.; Khan, A.R.; Suleman, M.; Ayaz, M.; Gao, X. Genetic screening and expression analysis of psychrophilic Bacillus sp. reveal their potential to alleviate cold stress and modulate phytohormones in wheat. Microorganisms 2019, 7, 337. [Google Scholar] [CrossRef]
- Safirzadeh, S.; Chorom, M.; Enayatizamir, N. Effect of phosphate solubilising bacteria (Enterobacter cloacae) on phosphorus uptake efficiency in sugarcane (Saccharum officinarum L.). Soil Res. 2019, 57, 333–341. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araújo-Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa Solos: Brasília, Brazil, 2018. [Google Scholar]
- Patrício, F.R.A.; Almeida, I.M.G.; Santos, A.S.; Cabral, O.; Tessarioli Neto, J.; Sinigaglia, C.; Beriam, L.O.S.; Rodrigues Neto, J. Avaliação da solarização do solo para o controle de Ralstonia solanacearum. Fitopatol. Bras. 2005, 30, 475–481. [Google Scholar] [CrossRef]
- Vitti, G.C.; Luz, P.H.C.; Altran, W.S. Nutrição e Adubação. In Cana-de-Açúcar: Do Plantio à Colheita; Santos, F., Bórem, A., Eds.; UFV: Viçosa, Brazil, 2015; pp. 49–79. [Google Scholar]
- Braga Junior, R.L.C.; Landel, M.G.A.; Silva, D.N.; Bidóia, M.A.P.; Silva, T.N.; Silva, V.H.P.; Luz, A.M.; Anjos, I.A. Censo Varietal IAC: Região Centro-Sul—Safra 2019/20; Technical Bulletin 225; Instituto Agronômico de Campinas: Campinas, Brazil, 2021. [Google Scholar]
- van Raij, B.; Cantarella, H.; Quaggio, J.A.; Furlani, A.M.C. Recomendações de Adubação e Calagem Para o Estado de São Paulo; Instituto Agronômico de Campinas: Campinas, Brazil, 1997. [Google Scholar]
- Mazumdar, R.K.; Chakider, B.P.; Mukherjee, S.K. Selection and classification of mango root stocks in the nursery stage. Acta Hortic. 1969, 24, 101–106. [Google Scholar] [CrossRef]
- Schreiber, J.; Bilger, W.; Hormann, H.; Neubauer, C. Chlorophyll fluorescence as a diagnostic tool: Basics and some aspects of practical relevance. In Photosynthesis: A Comprehensive Treatise; Raghavendra, A.S., Ed.; Cambridge University Press: Cambridge, UK, 1998; pp. 320–336. [Google Scholar]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Biochem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Yemm, E.W.; Cocking, E.C. The determination of amino acids with ninhydrin. Analyst 1955, 80, 209–213. [Google Scholar] [CrossRef]
- Ozawa, K.; Osaki, M.; Matisui, H.; Honma, M.; Tadano, T. Purification and properties of acid phosphatase secreted from lupin roots under phosphorus-deficiency conditions. J. Soil Sci. Plant Nutr. 1995, 41, 461–469. [Google Scholar] [CrossRef]
- van Raij, B.; Quaggio, J.A.; Cantarella, H.; Abreu, C.A. Os métodos de análise química do sistema IAC de análise de solo no contexto nacional. In Análise Química Para Avaliação da Fertilidade de Solos Tropicais; van Raij, B., Andrade, J.C., Cantarella, H., Quaggio, J.A., Eds.; Instituto Agronômico de Campinas: Campinas, Brazil, 2001; pp. 5–39. [Google Scholar]
- Malavolta, E.; Vitti, G.C.; Oliveira, A.S. Avaliação do Estado Nutricional de Plantas: Princípios e Aplicações, 2nd ed.; Potafós: Piracicaba, Brazil, 1997. [Google Scholar]
- McCray, J.M.; Mylavarapu, R. Sugarcane Nutrient Management Using Leaf Analysis; SS-AGR-259; University of Florida—Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 2010; Available online: http://edis.ifas.ufl.edu/sc076 (accessed on 29 June 2025).
- AOAC. Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2016. [Google Scholar]
- Hair, J.F.; Black, W.C., Jr.; Babin, B.J.; Anderson, R.E.; Tatham, R.L. Análise Multivariada de Dados, 6th ed.; Bookman: Porto Alegre, Brazil, 2009. [Google Scholar]
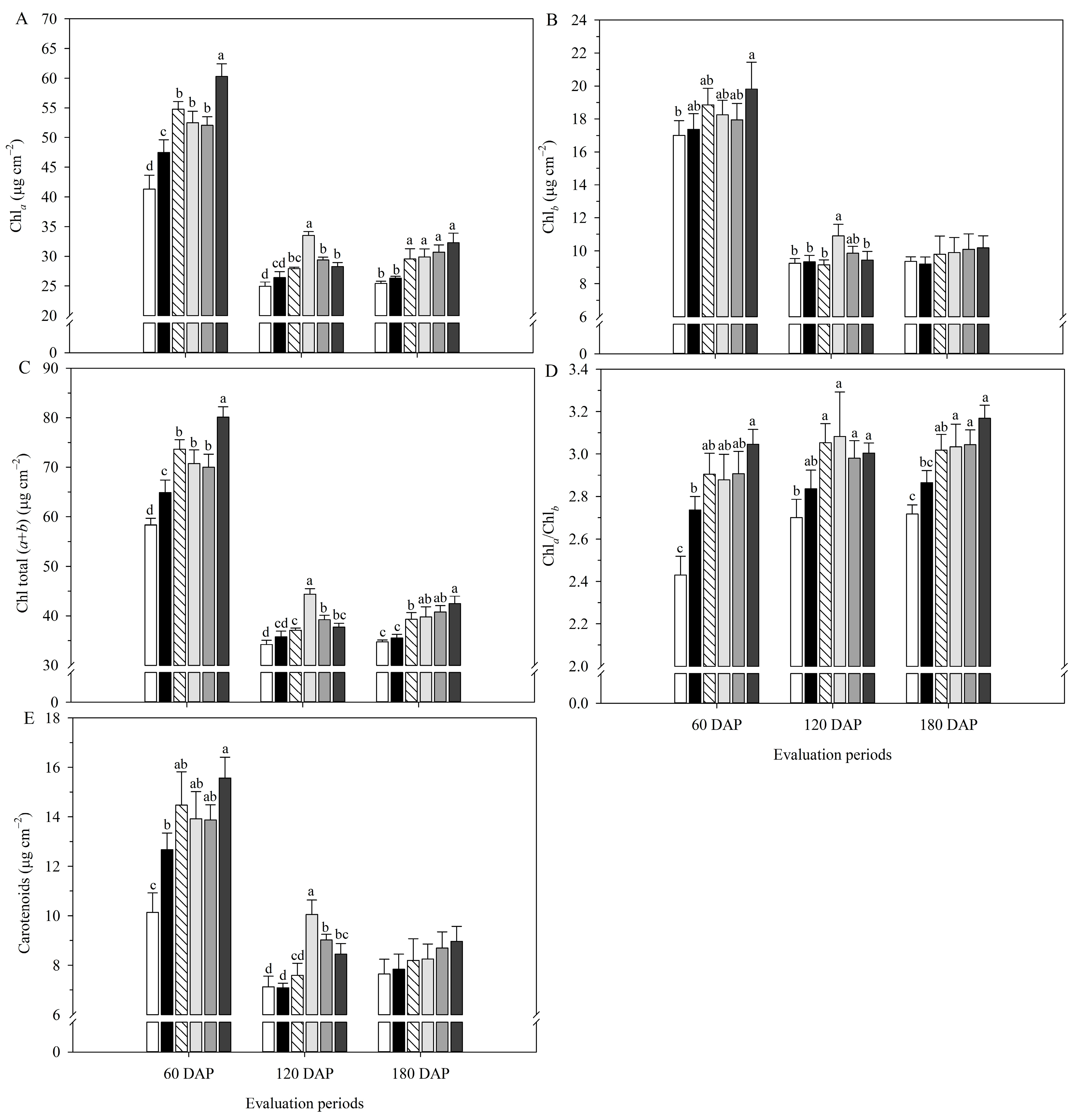
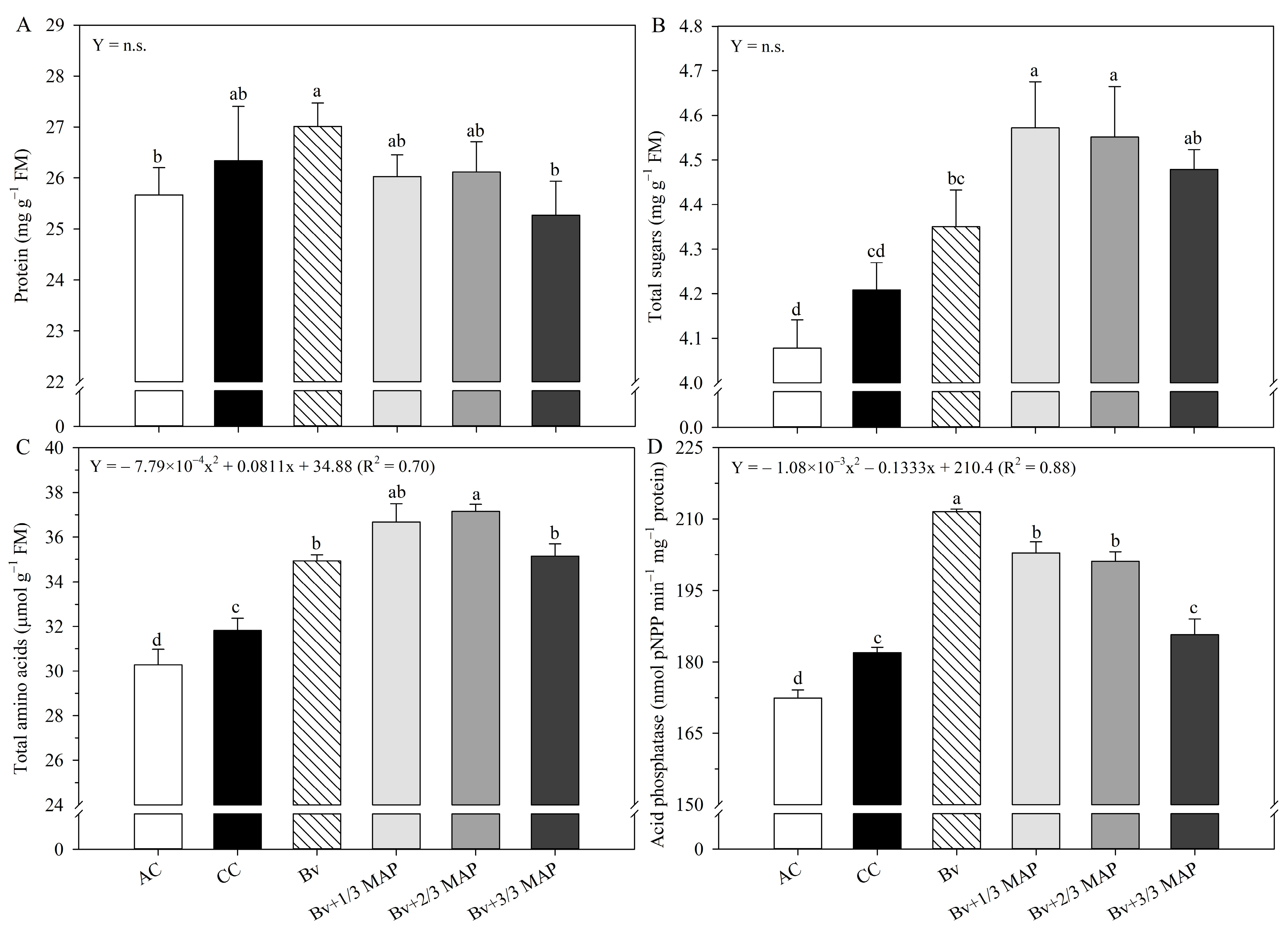
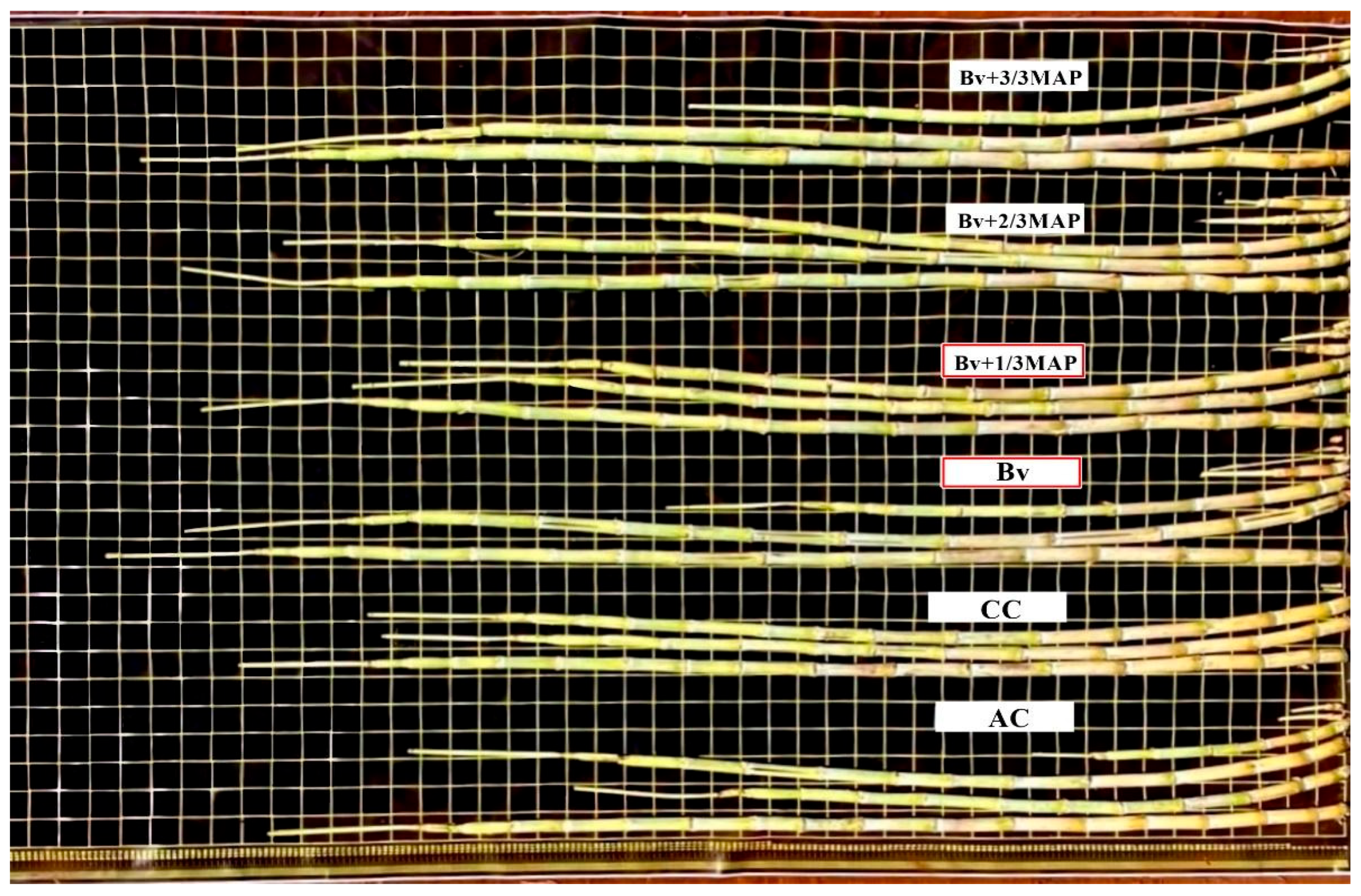
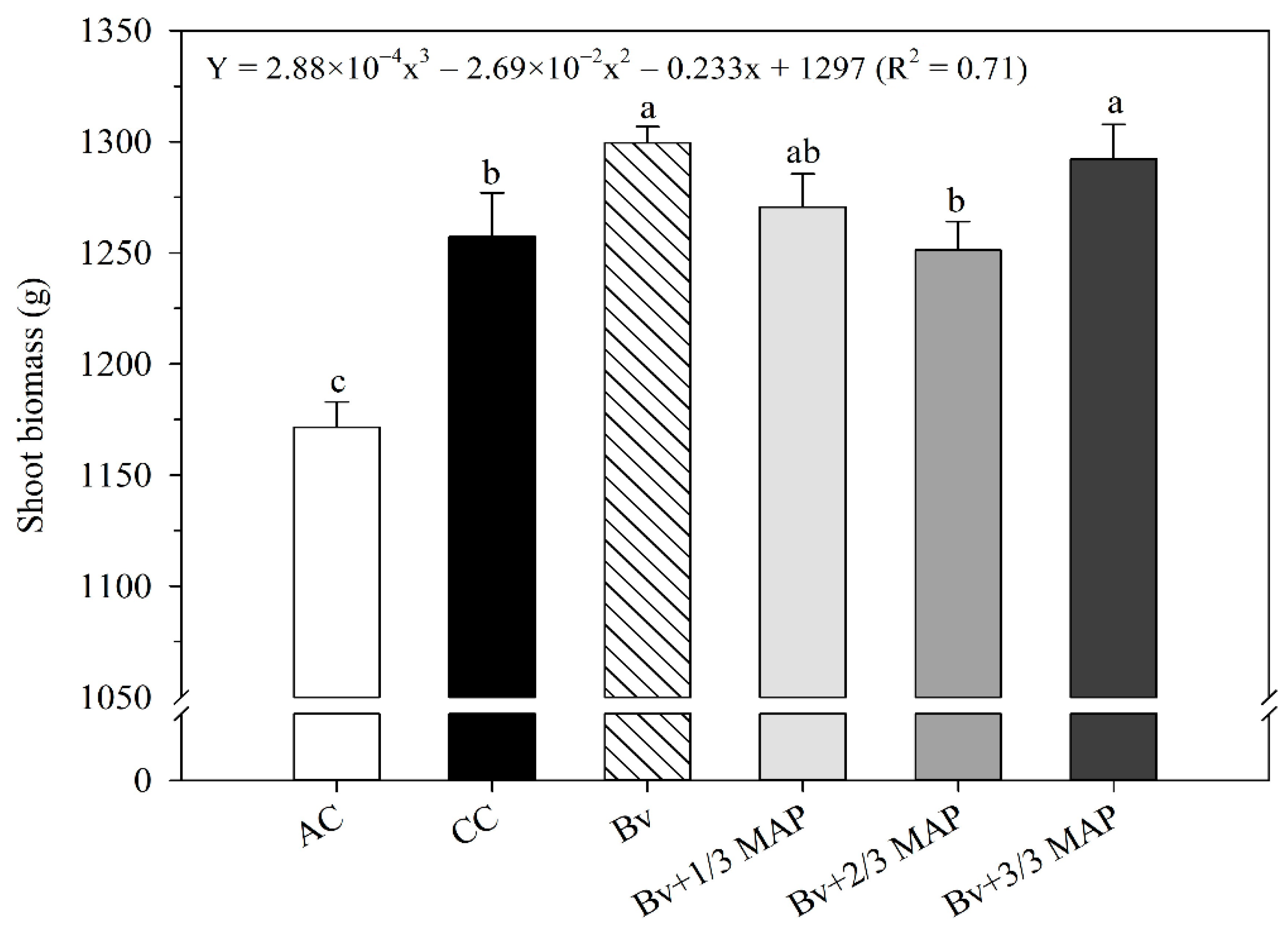


| Treatments | P in the Soil (mg dm–3) | P Accumulated in Sugarcane (g plant−1) |
|---|---|---|
| AC | 24.95 d | 0.62 c |
| CC | 33.30 c | 0.72 b |
| Bv | 42.02 a | 0.80 a |
| Bv + 1/3 MAP | 39.22 ab | 0.72 b |
| Bv + 2/3 MAP | 37.00 bc | 0.75 ab |
| Bv + 3/3 MAP | 41.01 a | 0.75 ab |
| C.V. (%) | 6.61 | 5.10 |
| Regression | Y = 2.5 × 10–5×3 − 2.21 × 10–3×2 − 0.0389x + 42.02 (R2 = 0.70) | Y = −1 × 10–6x3 + 1.18 × 10–4x2 − 5.52 × 10–3x + 0.798 (R2 = 0.70) |
| Groups of Variables | Explanation Percentage (%) |
|---|---|
| Stomatal density | 72.98 |
| Photochemistry | 84.64 |
| Gas exchange | 79.07 |
| Photosynthetic pigments | 82.32 |
| Leaf biochemistry | 98.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luiz Santos, H.; Ferreira da Silva, G.; Rodrigues Alves Carnietto, M.; Silva, G.F.d.; Nascimento Fernandes, C.; Ferreira, L.d.S.; de Almeida Silva, M. Improving Sugarcane Biomass and Phosphorus Fertilization Through Phosphate-Solubilizing Bacteria: A Photosynthesis-Based Approach. Plants 2025, 14, 2732. https://doi.org/10.3390/plants14172732
Luiz Santos H, Ferreira da Silva G, Rodrigues Alves Carnietto M, Silva GFd, Nascimento Fernandes C, Ferreira LdS, de Almeida Silva M. Improving Sugarcane Biomass and Phosphorus Fertilization Through Phosphate-Solubilizing Bacteria: A Photosynthesis-Based Approach. Plants. 2025; 14(17):2732. https://doi.org/10.3390/plants14172732
Chicago/Turabian StyleLuiz Santos, Hariane, Gustavo Ferreira da Silva, Melina Rodrigues Alves Carnietto, Gustavo Ferreira da Silva, Caio Nascimento Fernandes, Lusiane de Sousa Ferreira, and Marcelo de Almeida Silva. 2025. "Improving Sugarcane Biomass and Phosphorus Fertilization Through Phosphate-Solubilizing Bacteria: A Photosynthesis-Based Approach" Plants 14, no. 17: 2732. https://doi.org/10.3390/plants14172732
APA StyleLuiz Santos, H., Ferreira da Silva, G., Rodrigues Alves Carnietto, M., Silva, G. F. d., Nascimento Fernandes, C., Ferreira, L. d. S., & de Almeida Silva, M. (2025). Improving Sugarcane Biomass and Phosphorus Fertilization Through Phosphate-Solubilizing Bacteria: A Photosynthesis-Based Approach. Plants, 14(17), 2732. https://doi.org/10.3390/plants14172732






