Seed Size-Number Trade-Off Exists in Graminoids but Not in Forbs or Legumes: A Study from 11 Common Species in Alpine Steppe Communities
Abstract
1. Introduction
- Does a trade-off between seed size and number exist in a multispecies natural alpine steppe community?
- Are the trade-off patterns different in different functional groups?
- What is the role of limiting resources in determining seed size and number trade-offs?
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Site
4.2. Experimental Design and Seed Trait Measurements
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PICs | phylogenetically independent contrasts |
| PCA | principal components analysis |
References
- Bostock, S.J.; Benton, R.A. The reproductive strategies of five perennial compositae. J. Ecol. 1979, 67, 91–107. [Google Scholar] [CrossRef]
- Chen, R.; Shi, C.; Zhang, L.; Tu, C.; Weiner, J. Potential role of kin selection in the transition from vegetative to reproductive allocation in plants. J. Plant Ecol. 2023, 16, rtad025. [Google Scholar] [CrossRef]
- Mironchenko, A.; Kozłowski, J. Optimal allocation patterns and optimal seed mass of a perennial plant. J. Theor. Biol. 2014, 354, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Roach, D.A. Plant Life Histories: Ecology, Phylogeny, and Evolution; Cambridge University Press: Cambridge, UK, 1999; p. 313. ISBN 0521574951. [Google Scholar]
- Adler, P.B.; Salguero-Gómez, R.; Compagnoni, A.; Hsu, J.S.; Ray-Mukherjee, J.; Mbeau-Ache, C.; Franco, M. Functional traits explain variation in plant life history strategies. Proc. Natl. Acad. Sci. USA. 2013, 111, 740–745. [Google Scholar] [CrossRef]
- Grotkopp, E.; Rejmánek, M.; Rost, T.L. Toward a causal explanation of plant invasiveness: Seedling growth and life-history strategies of 29 pine (Pinus) species. Am. Nat. 2002, 159, 396–419. [Google Scholar] [CrossRef]
- Saatkamp, A.; Cochrane, A.; Commander, L.; Guja, L.K.; Jimenez-Alfaro, B.; Larson, J.; Nicotra, A.; Poschlod, P.; Silveira, F.A.O.; Cross, A.T.; et al. A research agenda for seed-trait functional ecology. New Phytol. 2018, 221, 1764–1775. [Google Scholar] [CrossRef]
- Zhou, X.; Li, C.; Li, H.; Shi, Q. The competition–dispersal trade-off exists in forbs but not in graminoids: A case study from multispecies alpine grassland communities. Ecol. Evol. 2019, 9, 1403–1409. [Google Scholar] [CrossRef]
- Geritz, S.A.H. Evolutionarily Stable Seed Polymorphism and Small-Scale Spatial Variation in Seedling Density. Am. Nat. 1995, 146, 685–707. [Google Scholar] [CrossRef]
- Kirkby, M.J. The theory of island biogeography. Geogr. J. 1968, 134, 592. [Google Scholar] [CrossRef]
- Westoby, M. A leaf-height-seed (LHS) plant ecology strategy scheme. Plant Soil 1998, 199, 213–227. [Google Scholar] [CrossRef]
- Barthlott, W. Epidermal and seed surface characters of plants: Systematic applicability and some evolutionary aspects. Nord. J. Bot. 1981, 1, 345–355. [Google Scholar] [CrossRef]
- Metz, J.; Liancourt, P.; Kigel, J.; Harel, D.; Sternberg, M.; Tielbörger, K. Plant survival in relation to seed size along environmental gradients: A long-term study from semi-arid and Mediterranean annual plant communities. J. Ecol. 2010, 98, 697–704. [Google Scholar] [CrossRef]
- Agren, J. Seed Size and Number in Rubus Chamaemorus: Between-Habitat Variation, and Effects of Defoliation and Supplemental Pollination. J. Ecol. 1989, 77, 1080–1092. [Google Scholar] [CrossRef]
- Bogdziewicz, M.; Acuña, M.A.; Andrus, R.; Ascoli, D.; Bergeron, Y.; Brveiller, D.; Boivin, T.; Bonal, R.; Caignard, T.; Cailleret, M.; et al. Linking seed size and number to trait syndromes in trees. Glob. Ecol. Biogeogr. 2023, 32, 683–694. [Google Scholar] [CrossRef]
- Bufford, J.L.; Hulme, P.E. Seed size–number trade-offs are absent in the introduced range for three congeneric plant invaders. J. Ecol. 2021, 109, 3849–3860. [Google Scholar] [CrossRef]
- Qiu, T.; Andrus, R.; Aravena, M.-C.; Ascoli, D.; Bergeron, Y.; Berretti, R.; Berveiller, D.; Bogdziewicz, M.; Boivin, T.; Bonal, R.; et al. Limits to reproduction and seed size-number trade-offs that shape forest dominance and future recovery. Nat. Commun. 2022, 13, 2381. [Google Scholar] [CrossRef]
- Leishman, M.R.; Wright, I.J.; Moles, A.T.; Westoby, M. The evolutionary ecology of seed size. In Seeds: The Ecology of Regeneration in Plant Communities; CABI: Wallingford, UK, 2000; pp. 31–57. [Google Scholar] [CrossRef]
- Moles, A.T.; Ackerly, D.D.; Webb, C.O.; Tweddle, J.C.; Dickie, J.B.; Pitman, A.J.; Westoby, M. Factors that shape seed mass evolution. Proc. Natl. Acad. Sci. USA 2005, 102, 10540–10544. [Google Scholar] [CrossRef]
- Moles, A.T.; Westoby, M. Seed size and plant strategy across the whole life cycle. Oikos 2006, 113, 91–105. [Google Scholar] [CrossRef]
- Schupp, E.W.; Howe, H.F.; Augspurger, C.K.; Levey, D.J. Arrival and survival in tropical treefall gaps. Ecology 1989, 70, 562–564. [Google Scholar] [CrossRef]
- Venable, D.L.; Rees, M. The scaling of seed size. J. Ecol. 2009, 97, 27–31. [Google Scholar] [CrossRef]
- Abbas, A.M.; Rubio-Casal, A.E.; De Cires, A.; Figueroa, E.M.; Pickart, A.J.; Castillo, J.M. Burial effects on seed germination and seedling emergence of two halophytes of contrasting seed size. Plant Ecol. Divers. 2020, 13, 339–349. [Google Scholar] [CrossRef]
- Han, T.; Lu, H.; Ren, H.; Wang, J.; Song, G.; Hui, D.; Guo, Q.; Zhu, S. Are reproductive traits of dominant species associated with specific resource allocation strategies during forest succession in southern China? Ecol. Indic. 2019, 102, 538–546. [Google Scholar] [CrossRef]
- Westoby, M.; Leishman, M.; Lord, J.; Poorter, H.; Schoen, D.J. Comparative ecology of seed size and dispersal. Phil. Trans. R. Soc. Lond B. 1996, 351, 1309–1318. [Google Scholar] [CrossRef]
- Venable, D.L.; Brown, J.S. The selective interactions of dispersal, dormancy, and seed size as adaptations for reducing risk in variable environments. Am. Nat. 1988, 131, 360–384. [Google Scholar] [CrossRef]
- Moles, A.T.; Ackerly, D.D.; Tweddle, J.C.; Dickie, J.B.; Smith, R.; Leishman, M.R.; Mayfield, M.M.; Pitman, A.; Wood, J.T.; Westoby, M. Global patterns in seed size. Glob. Ecol. Biogeogr. 2006, 16, 109–116. [Google Scholar] [CrossRef]
- Parciak, W. Environmental variation in seed number, size, and dispersal of a fleshy-fruited plant. Ecology 2002, 83, 780–793. [Google Scholar] [CrossRef][Green Version]
- Venable, D.L.; Lawlor, L. Delayed germination and dispersal in desert annuals: Escape in space and time. Oecologia 1980, 46, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Ashman, T.-L.; Knight, T.M.; Steets, J.A.; Amarasekare, P.; Burd, M.; Campbell, D.R.; Dudash, M.R.; Johnston, M.O.; Mazer, S.J.; Mitchell, R.J.; et al. Pollen limitation of plant reproduction: Ecological and evolutionary causes and consequences. Ecology 2004, 85, 2408–2421. [Google Scholar] [CrossRef]
- Madsen, J.D. Resource allocation at the individual plant level. Aquat. Bot. 1991, 41, 67–86. [Google Scholar] [CrossRef]
- Germain, R.M.; Gilbert, B. Hidden responses to environmental variation: Maternal effects reveal species niche dimensions. Ecol. Lett. 2014, 17, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Lebrija-Trejos, E.; Lobato, M.-C.C.; Sternberg, M. Reproductive traits and seed dynamics at two environmentally contrasting annual plant communities: From fieldwork to theoretical expectations. Isr. J. Ecol. Evol. 2011, 57, 73–90. [Google Scholar] [CrossRef]
- Catling, A.A.; Mayfield, M.M.; Dwyer, J.M. Individual vital rates respond differently to local-scale environmental variation and neighbour removal. J. Ecol. 2024, 112, 1369–1382. [Google Scholar] [CrossRef]
- Cheplick, G.P. Plasticity of seed number, mass, and allocation in clones of the perennial grass amphibromus scabrivalvis. Int. J. Plant Sci. 1995, 156, 522–529. [Google Scholar] [CrossRef]
- Dong, B.-C.; van Kleunen, M.; Yu, F.-H. Context-Dependent Parental Effects on Clonal Offspring Performance. Front. Plant Sci. 2018, 9, 1824. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, A.; Eriksson, O. A comparative study of seed number, seed size, seedling size and recruitment in grassland plants. Oikos 2000, 88, 494–502. [Google Scholar] [CrossRef]
- Wang, X.; Ge, W.; Zhang, M.; Fernández-Pascual, E.; Moles, A.; Saatkamp, A.; Rosbakh, S.; Bu, H.; Panahi, P.; Ma, M. Large and non-spherical seeds are less likely to form a persistent soil seed bank. Proc. R Soc. B. 2024, 291, 2023–2764. [Google Scholar] [CrossRef]
- Thürig, B.; Körner, C.; Stöcklin, J. Seed production and seed quality in a calcareous grassland in elevated CO2. Glob. Change Biol. 2003, 9, 873–884. [Google Scholar] [CrossRef]
- Abbas, A.M.; Mancilla-Leytón, J.M.; Castillo, J.M. Can camels disperse seeds of the invasive tree Prosopis juliflora? Weed Res. 2018, 58, 221–228. [Google Scholar] [CrossRef]
- Abbas, A.M.; Al-Kahtani, M.; Mousa, M.A.; Badry, M.O.; Hassaneen, A.S.A.; Ezzat-Ahmed, A.; Mancilla-Leytón, J.M.; Castillo, J.M. Endozoochory by Goats of Two Invasive Weeds with Contrasted Propagule Traits. Sustainability 2020, 12, 5450. [Google Scholar] [CrossRef]
- Guo, H.; Mazer, S.J.; Du, G. Geographic variation in seed mass within and among nine species of Pedicularis (Orobanchaceae): Effects of elevation, plant size and seed number per fruit. J. Ecol. 2010, 98, 1232–1242. [Google Scholar] [CrossRef]
- Cochrane, A.; Yates, C.J.; Hoyle, G.L.; Nicotra, A.B. Will among-population variation in seed traits improve the chance of species persistence under climate change? Glob. Ecol. Biogeogr. 2015, 24, 12–24. [Google Scholar] [CrossRef]
- Vandvik, V.; Klanderud, K.; Meineri, E.; Måren, I.E.; Töpper, J. Seed banks are biodiversity reservoirs: Species–area relationships above versus below ground. Oikos 2016, 125, 218–228. [Google Scholar] [CrossRef]
- Leishman, M.R.; Murray, B.R. The relationship between seed size and abundance in plant communities: Model predictions and observed patterns. Oikos 2001, 94, 151–161. [Google Scholar] [CrossRef]
- Parker, V.T.; Ingalls, S.B. Seed size–seed number trade-offs: Influence of seed size on the density of fire-stimulated persistent soil seed banks. Am. J. Bot. 2022, 109, 486–493. [Google Scholar] [CrossRef]
- Lázaro, A.; Larrinaga, A.R. A multi-level test of the seed number/size trade-off in two Scandinavian communities. PLoS ONE 2018, 13, e0201175. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.C.; Fretwell, S.D. The optimal balance between size and number of offspring. Am. Nat. 1974, 108, 499–506. [Google Scholar] [CrossRef]
- Leishman, M.R. Does the seed size/number trade-off model determine plant community structure? An assessment of the model mechanisms and their generality. Oikos 2001, 93, 294–302. [Google Scholar] [CrossRef]
- Sadras, V.O. Evolutionary aspects of the trade-off between seed size and number in crops. Field Crop. Res. 2007, 100, 125–138. [Google Scholar] [CrossRef]
- Turnbull, L.A.; Crawley, M.J.; Rees, M. Are plant populations seed-limited? A review of seed sowing experiments. Oikos 2000, 88, 225–238. [Google Scholar] [CrossRef]
- Dani, K.G.S.; Kodandaramaiah, U. Plant and Animal Reproductive Strategies: Lessons from Offspring Size and Number Tradeoffs. Front. Ecol. Evol. 2017, 5, 38. [Google Scholar] [CrossRef]
- Lönnberg, K.; Eriksson, O. Rules of the seed size game: Contests between large-seeded and small-seeded species. Oikos 2013, 122, 1080–1084. [Google Scholar] [CrossRef]
- Germain, R.M.; Grainger, T.N.; Jones, N.T.; Gilbert, B. Maternal provisioning is structured by species’ competitive neighborhoods. Oikos 2019, 128, 45–53. [Google Scholar] [CrossRef]
- Harper, J.L.; Lovell, P.H.; Moore, K.G. The Shapes and Sizes of Seeds. Annu. Rev. Ecol. Syst. 1970, 1, 327–356. [Google Scholar] [CrossRef]
- Venable, D.L. Bet hedging in a guild of desert annuals. Ecology 2007, 88, 1086–1090. [Google Scholar] [CrossRef]
- Bellés-Sancho, P.; Beukes, C.; James, E.K.; Pessi, G. Nitrogen-Fixing Symbiotic Paraburkholderia Species: Current Knowledge and Future Perspectives. Nitrogen 2023, 4, 135–158. [Google Scholar] [CrossRef]
- Kang, X.; Zhou, J.; Abuman; Liu, Y.; Zhang, S.; Liu, W.; Bu, H.; Qi, W. Regional gradients in intraspecific seed mass variation are associated with species biotic attributes and niche breadth. AoB Plants 2022, 14, plac013. [Google Scholar] [CrossRef]
- Venable, D.L. Size-Number Trade-Offs and the Variation of Seed Size with Plant Resource Status. Am. Nat. 1992, 140, 287–304. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Kim, J.G. The optimal balance between sexual and asexual reproduction in variable environments: A systematic review. J. Ecol. Environ. 2016, 40, 12. [Google Scholar] [CrossRef]
- Hutchings, M.J. Differential foraging for resources, and structural plasticity in plants. Trends Ecol. Evol. 1988, 3, 200–204. [Google Scholar] [CrossRef]
- Xiao, Y.; Tang, J.; Qing, H.; Zhou, C.; Kong, W.; An, S. Trade-offs among growth, clonal, and sexual reproduction in an invasive plant Spartina alterniflora responding to inundation and clonal integration. Hydrobiologia 2011, 658, 353–363. [Google Scholar] [CrossRef]
- Lei, S.A. Benefits and Costs of Vegetative and Sexual Reproduction in Perennial Plants: A Review of Literature. J. Ariz. Nev. Acad. Sci. 2010, 42, 9–14. [Google Scholar] [CrossRef]
- Parvinen, K. Metapopulation dynamics and the evolution of dispersal. In Complex Population Dynamics: Nonlinear Modeling in Ecology, Epidemiology and Genetics; World Scientific Publishing: Hackensack, NJ, USA, 2007; pp. 77–107. [Google Scholar] [CrossRef]
- Zhu, Y.; Bao, A.; Dong, M.; Huang, Z. Effects of increasing water or nutrient supplies on reproduction trade-offs in the natural populations of clonal plant Hedysarum leave. J. Plant Ecol. 2007, 31, 658–664. [Google Scholar] [CrossRef]
- Cook, B.I.; Wolkovich, E.M.; Parmesan, C. Divergent responses to spring and winter warming drive community level flowering trends. Proc. Natl. Acad. Sci. USA 2012, 109, 9000–9005. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Y.; Guo, X.; Ning, D.; Zhou, X.; Feng, J.; Yuan, M.M.; Liu, S.; Guo, J.; Gao, Z.; et al. Reduction of microbial diversity in grassland soil is driven by long-term climate warming. Nat. Microbiol. 2022, 7, 1054–1062. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, Z.; Zhang, P.; Li, H.; Chu, C.; Li, X.; Du, G. Different categories of biodiversity explain productivity variation after fertilization in a Tibetan alpine meadow community. Ecol. Evol. 2017, 7, 3464–3474. [Google Scholar] [CrossRef]
- Li, K.; Liu, X.; Song, L.; Gong, Y.; Lu, C.; Yue, P.; Tian, C.; Zhang, F. Response of alpine grassland to elevated nitrogen deposition and water supply in China. Oecologia 2015, 177, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Niu, K.; Luo, Y.; Choler, P.; Du, G. The role of biomass allocation strategy in diversity loss due to fertilization. Basic Appl. Ecol. 2007, 9, 485–493. [Google Scholar] [CrossRef]
- Niu, K.; Schmid, B.; Choler, P.; Du, G. Relationship between Reproductive Allocation and Relative Abundance among 32 Species of a Tibetan Alpine Meadow: Effects of Fertilization and Grazing. PLoS ONE 2012, 7, e35448. [Google Scholar] [CrossRef]
- Wang, X.; Alvarez, M.; Donohue, K.; Ge, W.; Cao, Y.; Liu, K.; Du, G.; Bu, H. Elevation filters seed traits and germination strategies in the eastern Tibetan Plateau. Ecography 2020, 44, 242–254. [Google Scholar] [CrossRef]
- Bu, H.Y.; Jia, P.; Qi, W.; Liu, K.; Xu, D.H.; Ge, W.J.; Wang, X.J. The effects of phylogeny, life-history traits and altitude on the carbon, nitrogen, and phosphorus contents of seeds across 203 species from an alpine meadow. Plant Ecol. 2018, 219, 737–748. [Google Scholar] [CrossRef]
- Webb, C.O.; Ackerly, D.D.; McPeek, M.A.; Donoghue, M.J. Phylogenies and Community Ecology. Annu. Rev. Ecol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
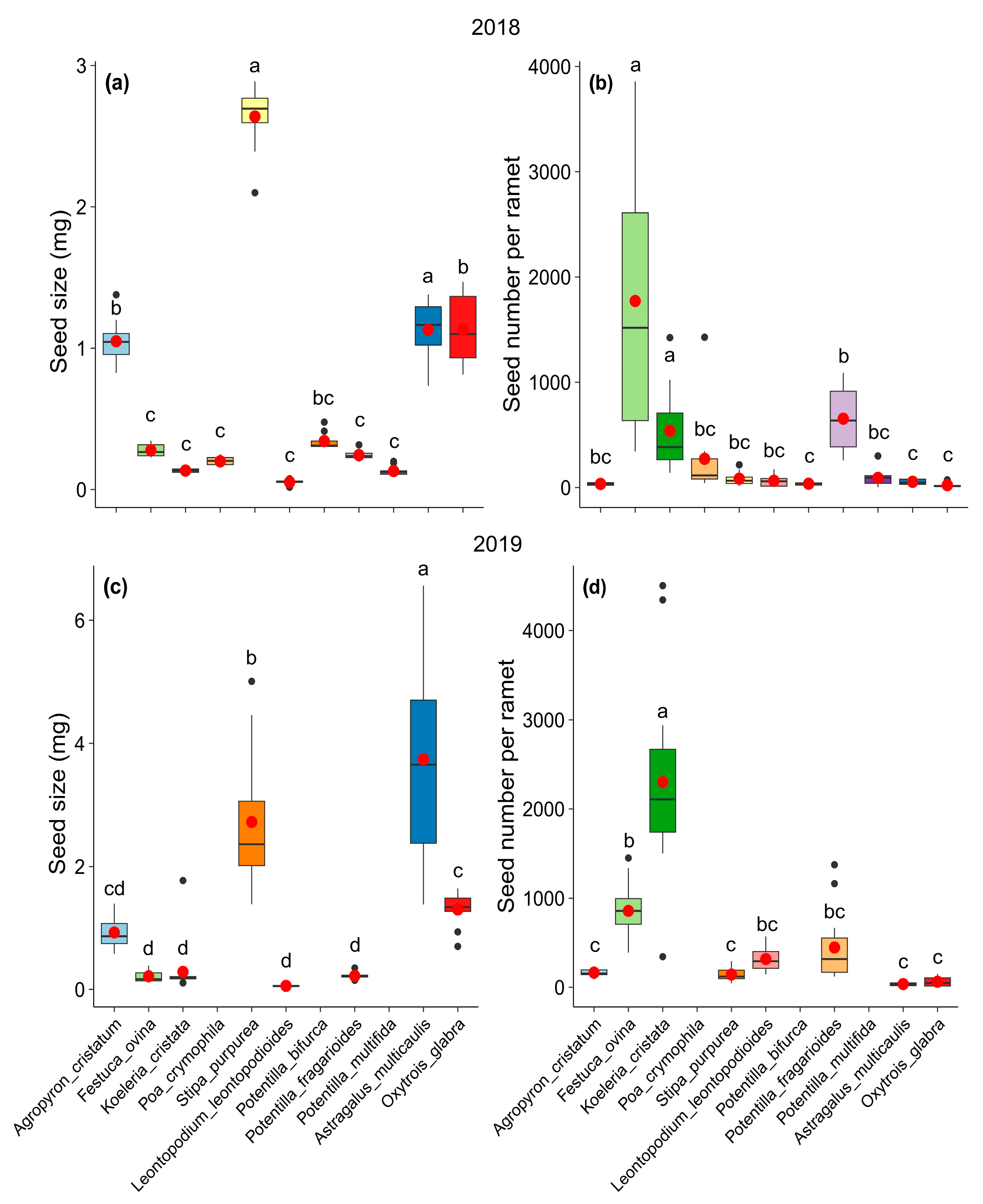
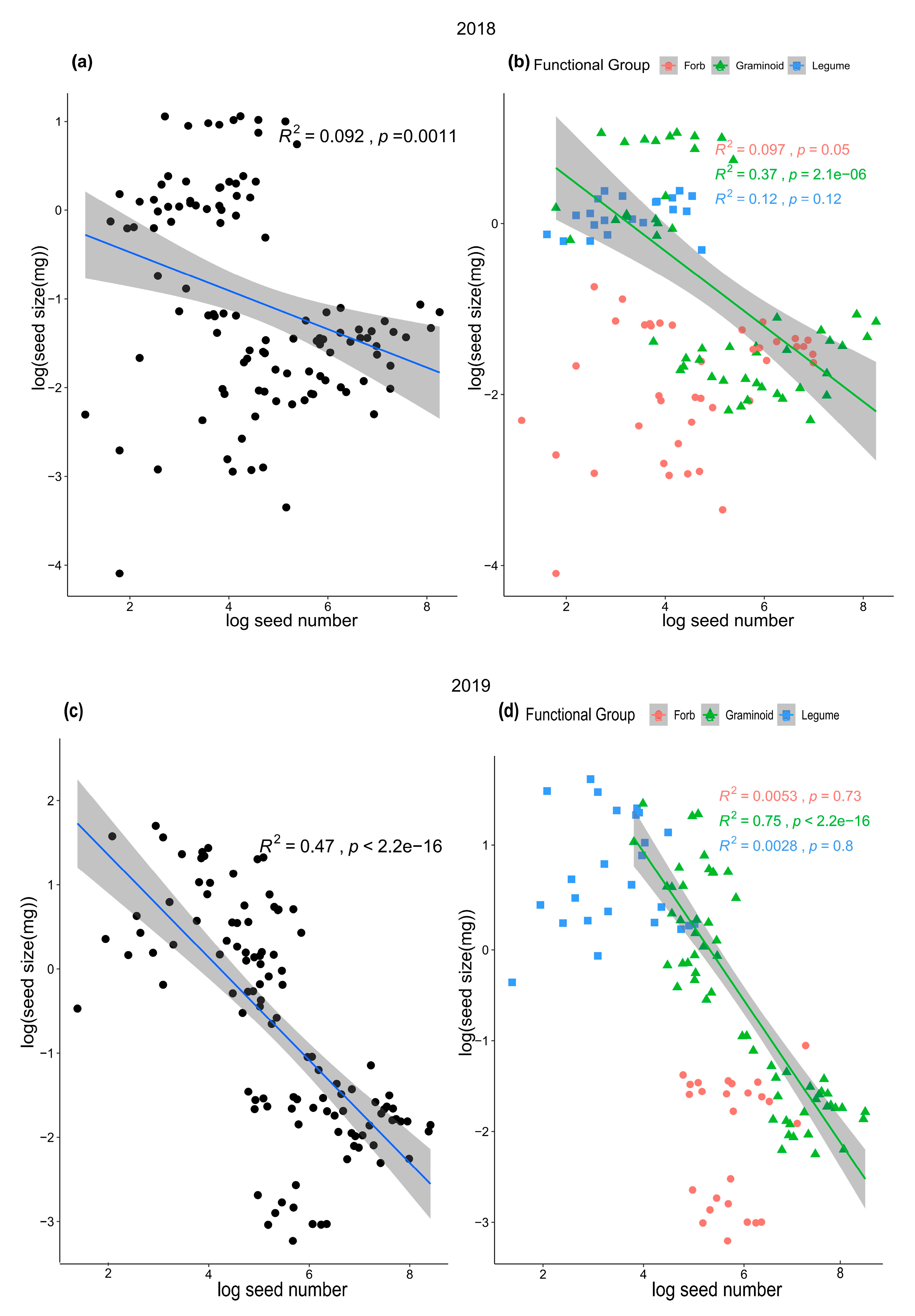
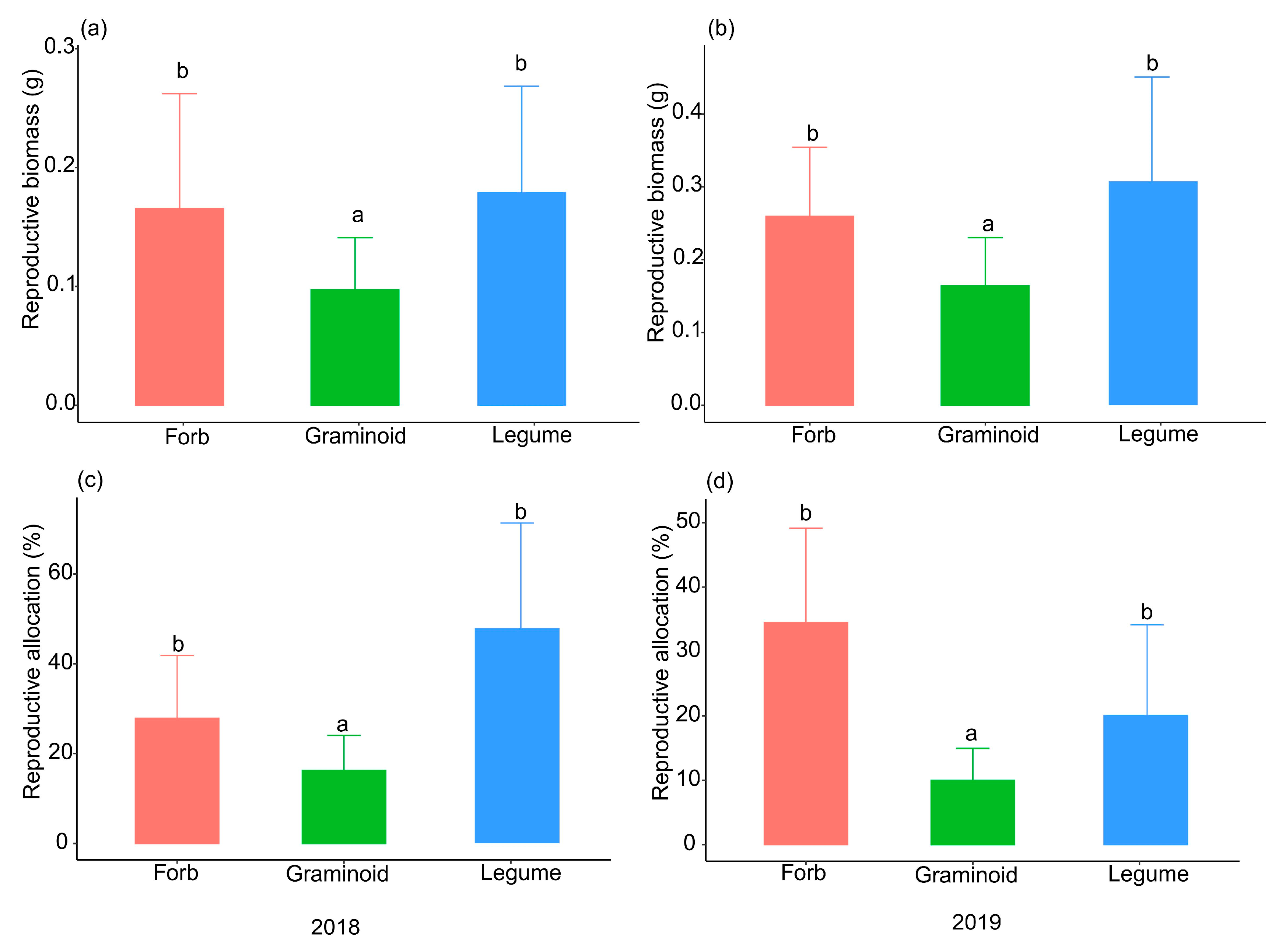
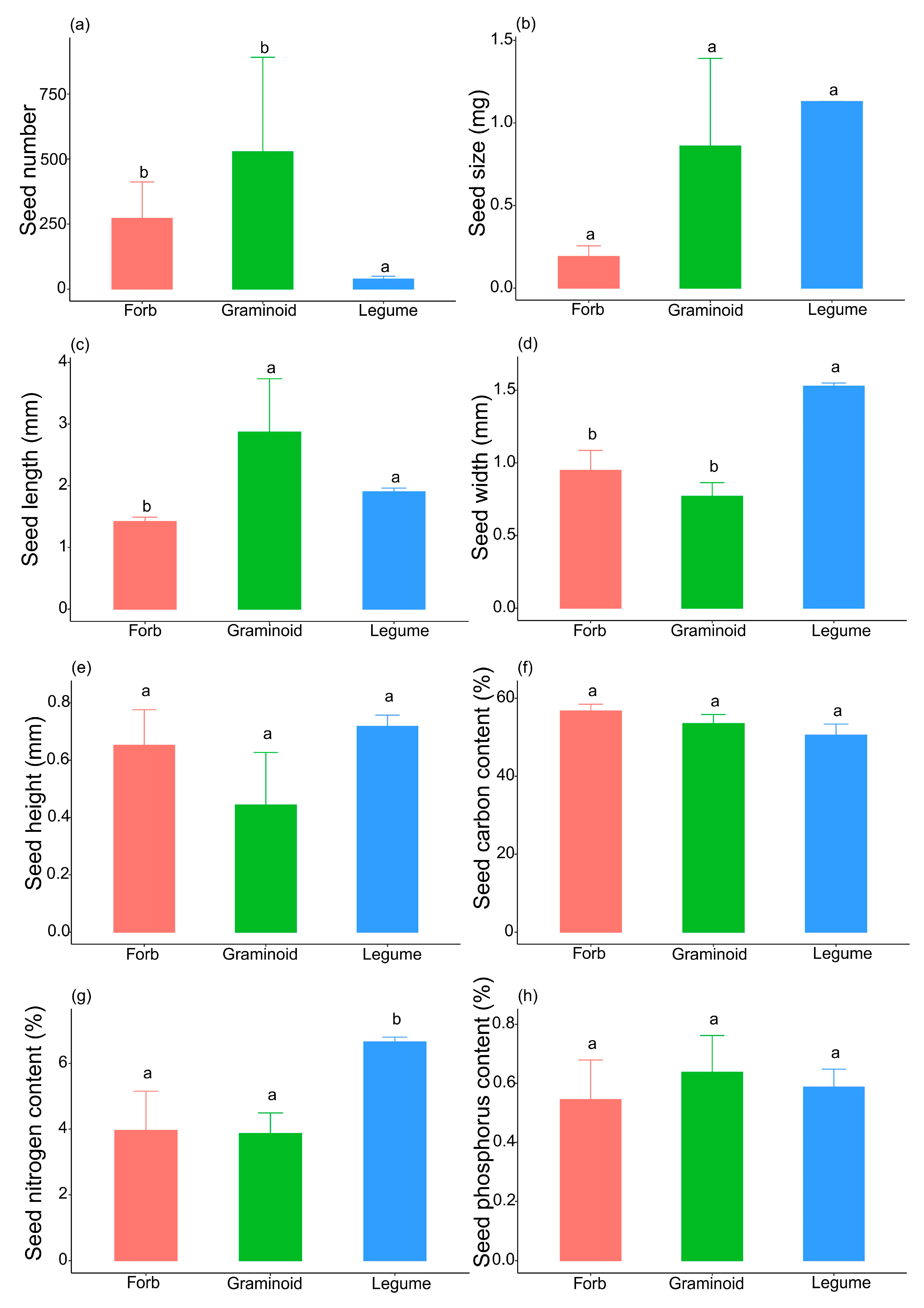
| Species | Functional Group | Seed Size (mg) | Seed Number | Reproduction Type |
|---|---|---|---|---|
| Agropyron cristatum | graminoid | 0.9879 ± 0.16 | 98 ± 19.82 | sexual + clonal |
| Festuca ovina | graminoid | 0.2458 ± 0.04 | 1315 ± 255.69 | sexual + clonal |
| Koeleria cristata | graminoid | 0.2102 ± 0.02 | 1420 ± 579.41 | sexual + clonal |
| Poa crymophila | graminoid | 0.2 ± 0.03 | 273 ± 193.10 | sexual + clonal |
| Stipa purpurea | graminoid | 2.68 ± 0.67 | 113 ± 65.58 | sexual + clonal |
| Leontopodium leontopodioides | forb | 0.0547 ± 0.02 | 191 ± 54.92 | sexual |
| Potentilla bifurca | forb | 0.3431 ± 0.07 | 36 ± 16.44 | sexual |
| Potentilla fragarioides | forb | 0.2315 ± 0.03 | 551 ± 308.05 | sexual |
| Potentilla multifida | forb | 0.131 ± 0.03 | 91 ± 82.93 | sexual |
| Astragalus multicaulis | legume | 2.435 ± 1.3 | 45 ± 33.91 | sexual |
| Oxytrois glabra | legume | 1.216 ± 0.25 | 42 ± 23.05 | sexual |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Duan, R.; Long, J.; Bu, H. Seed Size-Number Trade-Off Exists in Graminoids but Not in Forbs or Legumes: A Study from 11 Common Species in Alpine Steppe Communities. Plants 2025, 14, 2730. https://doi.org/10.3390/plants14172730
Zhou X, Duan R, Long J, Bu H. Seed Size-Number Trade-Off Exists in Graminoids but Not in Forbs or Legumes: A Study from 11 Common Species in Alpine Steppe Communities. Plants. 2025; 14(17):2730. https://doi.org/10.3390/plants14172730
Chicago/Turabian StyleZhou, Xiaolong, Ronghua Duan, Jian Long, and Haiyan Bu. 2025. "Seed Size-Number Trade-Off Exists in Graminoids but Not in Forbs or Legumes: A Study from 11 Common Species in Alpine Steppe Communities" Plants 14, no. 17: 2730. https://doi.org/10.3390/plants14172730
APA StyleZhou, X., Duan, R., Long, J., & Bu, H. (2025). Seed Size-Number Trade-Off Exists in Graminoids but Not in Forbs or Legumes: A Study from 11 Common Species in Alpine Steppe Communities. Plants, 14(17), 2730. https://doi.org/10.3390/plants14172730






