Microwave Treatment for Citrus Huanglongbing Control: Pathogen Elimination and Metabolomic Analysis
Abstract
1. Introduction
2. Results
2.1. Detection of Candidatus Liberibacter Asiaticus in Citrus
2.2. The Tolerance of Citrus to Microwave
2.3. Microwave Treatment for the Elimination of CLas
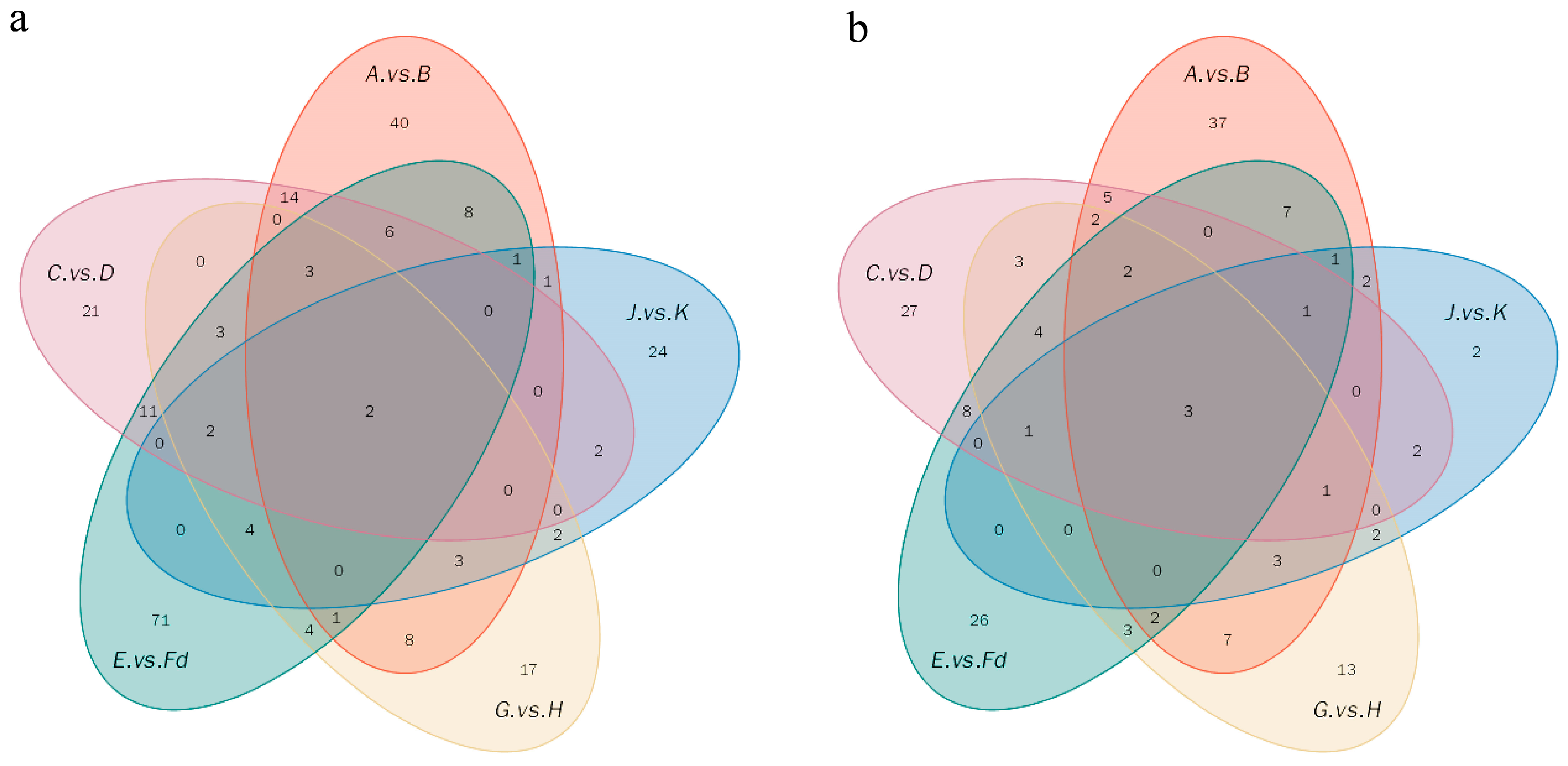
2.4. The Effect of Microwave Treatment on the Metabolites of Citrus
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Microwave Treatment of Citrus Plants
4.3. Real-Time Fluorescence Quantitative PCR (qPCR) Detection of CLas
4.4. Metabolomics Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, L.; Ke, F.; Nie, Z.; Wang, P.; Xu, J. Citrus Genetic Engineering for Disease Resistance: Past, Present and Future. Int. J. Mol. Sci. 2019, 20, 5256. [Google Scholar] [CrossRef]
- Mubeen, M.; Bakhtawar, F.; Iftikhar, Y.; Shakeel, Q.; Sajid, A.; Iqbal, R.; Aljowaie, R.M.; Chaudhary, T. Biological and molecular characterization of citrus bent leaf viroid. Heliyon 2024, 10, e28209. [Google Scholar] [CrossRef]
- Bove, J. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- da Graça, J.V.; Douhan, G.W.; Halbert, S.E.; Keremane, M.L.; Lee, R.F.; Vidalakis, G.; Zhao, H. Huanglongbing: An overview of a complex pathosystem ravaging the world’s citrus. J. Integr. Plant Biol. 2016, 58, 373–387. [Google Scholar] [CrossRef]
- Jagoueix, S.; Bove, J.M.; Garnier, M. The phloem-limited bacterium of greening disease of citrus is a member of the alpha subdivision of the Proteobacteria. Int. J. Syst. Bacteriol. 1994, 44, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Zhang, L.; Coaker, G.; Ma, W.; He, S.; Wang, N. Citrus CsACD2 Is a Target of Candidatus Liberibacter Asiaticus in Huanglongbing Disease. Plant Physiol. 2020, 184, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Jagoueix, S.; Bové, J.M.; Garnier, M. PCR detection of the two ‘Candidatus’ Liberobacter species associated with greening disease of citrus. Mol. Cell. Probes 1996, 10, 43–50. [Google Scholar] [CrossRef]
- Teixeira, D.D.; Danet, J.L.; Eveillard, S.; Martins, E.C.; Junior, W.C.J.; Yamamoto, P.T.; Lopes, S.A.; Bassanezi, R.B.; Ayres, A.J.; Saillard, C.; et al. Citrus huanglongbing in São Paulo State, Brazil: PCR detection of the ‘Candidatus’ Liberibacter species associated with the disease. Mol. Cell. Probes 2005, 19, 173–179. [Google Scholar] [CrossRef]
- Rasowo, B.A.; Khamis, F.M.; Mohamed, S.A.; Ajene, I.J.; Aidoo, O.F.; Ombura, L.; Sétamou, M.; Ekesi, S.; Borgemeister, C. African Citrus Greening Disease in East Africa: Incidence, Severity, and Distribution Patterns. J. Econ. Entomol. 2019, 112, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Sivager, G.; Calvez, L.; Bruyere, S.; Boisne-Noc, R.; Brat, P.; Gros, O.; Ollitrault, P.; Morillon, R. Specific Physiological and Anatomical Traits Associated with Polyploidy and Better Detoxification Processes Contribute to Improved Huanglongbing Tolerance of the Persian Lime Compared with the Mexican Lime. Front. Plant Sci. 2021, 12, 685679. [Google Scholar] [CrossRef]
- Bassanezi, R.B.; Lopes, S.A.; de Miranda, M.P.; Wulff, N.A.; Volpe, H.X.L.; Ayres, A.J. Overview of citrus Huanglongbing spread and management strategies in Brazil. Trop. Plant Pathol. 2020, 45, 251–264. [Google Scholar] [CrossRef]
- Zhou, C. The status of citrus Huanglongbing in China. Trop. Plant Pathol. 2020, 45, 279–284. [Google Scholar] [CrossRef]
- Hao, G.; Stover, E.; Gupta, G. Overexpression of a Modified Plant Thionin Enhances Disease Resistance to Citrus Canker and Huanglongbing (HLB). Front. Plant Sci. 2016, 7, 1078. [Google Scholar] [CrossRef]
- Zou, X.; Jiang, X.; Xu, L.; Lei, T.; Peng, A.; He, Y.; Yao, L.; Chen, S. Transgenic citrus expressing synthesized cecropin B genes in the phloem exhibits decreased susceptibility to Huanglongbing. Plant Mol. Biol. 2017, 93, 341–353. [Google Scholar] [CrossRef]
- Tavano, E.; Erpen, L.; Aluisi, B.; Harakava, R.; Lopes, J.; Vieira, M.-L.; Piedade, S.; Mendes, B.; Mourão Filho, F. Sweet orange genetic transformation with the attacin A gene under the control of phloem-specific promoters and inoculation with Candidatus Liberibacter asiaticus. J. Hortic. Sci. Biotechnol. 2018, 94, 210–219. [Google Scholar] [CrossRef]
- Kosmiatin, M.; Martasari, C.; Yunimar; Akhdiya, A.; Husni, A. In vitro selection to increase Huanglongbing tolerance of citrus derived from in vitro breeding. IOP Conf. Ser. Earth Environ. Sci. 2020, 457, 012080. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Ma, J.; Zhao, D.; Wang, Y.; Yan, L.; Wu, L.; He, Y. Controlling citrus Huanglongbing based on soil remediation and biocontrol. Eur. J. Plant Pathol. 2024, 169, 379–393. [Google Scholar] [CrossRef]
- Zhao, P.; Yang, H.; Sun, Y.; Zhang, J.; Gao, K.; Wu, J.; Zhu, C.; Yin, C.; Chen, X.; Liu, Q.; et al. Targeted MYC2 stabilization confers citrus Huanglongbing resistance. Science 2025, 388, 191–198. [Google Scholar] [CrossRef]
- Lopes, S.A.; Frare, G.F.; Bertolini, E.; Cambra, M.; Fernandes, N.G.; Ayres, A.J.; Marin, D.R.; Bové, J.M. Liberibacters Associated with Citrus Huanglongbing in Brazil: ‘Candidatus Liberibacter asiaticus’ Is Heat Tolerant, ‘Ca. L. americanus’ Is Heat Sensitive. Plant Dis. 2009, 93, 257–262. [Google Scholar] [CrossRef]
- Ghatrehsamani, S.; Abdulridha, J.; Balafoutis, A.; Zhang, X.; Ehsani, R.; Ampatzidis, Y. Development and evaluation of a mobile thermotherapy technology for in-field treatment of Huanglongbing (HLB) affected trees. Biosyst. Eng. 2019, 182, 1–15. [Google Scholar] [CrossRef]
- Banik, S.; Bandyopadhyay, S.; Ganguly, S. Bioeffects of microwave—A brief review. Bioresour. Technol. 2003, 87, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Vardaxis, N.J.; Hoogeveen, M.M.; Boon, M.E.; Hair, C.G. Sporicidal activity of chemical and physical tissue fixation methods. J. Clin. Pathol. 1997, 50, 429–433. [Google Scholar] [CrossRef]
- Kipriyanov, F.A.; Savinykh, P.A.; Isupov, A.Y.; Plotnikova, Y.A.; Medvedeva, N.A.; Belozerova, S.V. Prospects for the use of microwave energy in grain crop seeding. J. Water Land. Dev. 2021, 49, 74–78. [Google Scholar] [CrossRef]
- Luan, D.L.; Yu, H.X.; Hu, M.M.; Bai, X.Y.; Wang, Y.F. Underlying mechanism of the microwave non-thermal effect as additional microbial inactivation on Clostridium sporogenes at pasteurization temperature level. J. Food Eng. 2025, 387, 112337. [Google Scholar] [CrossRef]
- Barkhade, T.; Nigam, K.; Ravi, G. Investigating the effects of microwave plasma on bacterial cell structures, viability, and membrane integrity. Sci. Rep. 2025, 15, 18052. [Google Scholar] [CrossRef]
- Maynaud, G.; Baudoin, E.; Bourillon, J.; Duponnois, R.; Cleyet-Marel, J.-C.; Brunel, B. Short-term effect of 915-MHz microwave treatments on soil physicochemical and biological properties. Eur. J. Soil. Sci. 2019, 70, 443–453. [Google Scholar] [CrossRef]
- Zhang, R.S.; An, S.Z.; Shi, C.; Kasidaer. Germicidal Efficacy of Microwave Treatment Against Endophyte and Its Effects on the Germinating Vigor of Achnatherum inebrians. J. Nucl. Agric. Sci. 2016, 30, 1792–1797. [Google Scholar]
- Zhang, R.S.; An, S.Z.; Shi, C.; Kasidaer; Wang, T. Germicidal Efficacy of Microwave on Endophyte and Its Effects on the Germinating Vigor of Elymus dahuricus. Chin. J. Grassl. 2016, 38, 100–104. [Google Scholar]
- ZHang, J.T.; Liu, J.F.; Liang, J.Y.; Liu, G.B.; Zheng, Y.Q.; Xu, M.R. Effect of microwave heat treatment in eliminating “Candidatus Liberibacter asiaticus” on periwinkle infected with Citrus Huanglongbing. Trans. Chin. Soc. Agric. Eng. 2024, 40, 211–218. [Google Scholar]
- Cocuzza, G.E.M.; Alberto, U.; Hernández-Suárez, E.; Siverio, F.; Di Silvestro, S.; Tena, A.; Carmelo, R. A review on Trioza erytreae (African citrus psyllid), now in mainland Europe, and its potential risk as vector of huanglongbing (HLB) in citrus. J. Pest Sci. 2017, 90, 1–17. [Google Scholar] [CrossRef]
- Grafton-Cardwell, E.E.; Stelinski, L.L.; Stansly, P.A. Biology and management of Asian citrus psyllid, vector of the huanglongbing pathogens. Annu. Rev. Entomol. 2013, 58, 413–432. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Freed, S.; Jin, F.L.; Akmal, M.; Mehmood, M. Monitoring of insecticide resistance in Diaphorina citri Kuwayama (Hemiptera: Psyllidae) from citrus groves of Punjab, Pakistan. Crop Prot. 2016, 86, 62–68. [Google Scholar] [CrossRef]
- Siriwardhana, P.H.A.P.; Gunawardena, B.W.A. An innovative approach: Supply of electric current to control Weligama Coconut Leaf Wilt Disease-Phytoplasma. Plant Sci. Feed 2014, 4, 19–23. [Google Scholar]
- Fatemeh, A.; Vahid, N.; Faezeh, G.; Faribors, M.; Seyyed, N.N. Biological effects of weak electromagnetic field on healthy and infected lime (Citrus aurantifolia) trees with phytoplasma. Sci. World J. 2012, 716929. [Google Scholar] [CrossRef]
- Jia, Z.C.; Ehsani, R.; Zheng, J.Q.; Xu, L.Y.; Zhou, H.P.; Ding, R. Heating characteristics and field control effect of rapid citrus huanglongbing steam heat treatment. Trans. Chin. Soc. Agric. Eng. 2017, 33, 219–225. [Google Scholar]
- Zhang, J.T.; Chen, H.; Wen, S.; Li, S.H.; Deng, X.L.; Lan, Y.B. Experiment on temperature field distribution characteristics of citrus huanglongbing hot air rapid treatment. Trans. Chin. Soc. Agric. Eng. 2017, 33, 267–277. [Google Scholar]
- Hoffman, M.T.; Doud, M.S.; Williams, L.; Zhang, M.Q.; Ding, F.; Stover, E.; Hall, D.; Zhang, S.; Jones, L.; Gooch, M.; et al. Heat treatment eliminates ‘Candidatus Liberibacter asiaticus’ from infected citrus trees under controlled conditions. Phytopathology 2013, 103, 15–22. [Google Scholar] [CrossRef]
- Doud, M.M.; Wang, Y.; Hoffman, M.T.; Latza, C.L.; Luo, W.; Armstrong, C.M.; Gottwald, T.R.; Dai, L.; Luo, F.; Duan, Y. Solar thermotherapy reduces the titer of Candidatus Liberibacter asiaticus and enhances canopy growth by altering gene expression profiles in HLB-affected citrus plants. Hortic. Res. 2017, 4, 17054. [Google Scholar] [CrossRef] [PubMed]
- Crifò, T.; Puglisi, I.; Petrone, G.; Recupero, G.R.; Lo Piero, A.R. Expression analysis in response to low temperature stress in blood oranges: Implication of the flavonoid biosynthetic pathway. Gene 2011, 476, 1–9. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Sales, C.; Beltrán, J.; Gómez-Cadenas, A.; Arbona, V. Activation of Secondary Metabolism in Citrus Plants Is Associated to Sensitivity to Combined Drought and High Temperatures. Front. Plant Sci. 2016, 7, 1954. [Google Scholar] [CrossRef]
- Asai, T.; Matsukawa, T.; Kajiyama, S. Metabolomic analysis of primary metabolites in citrus leaf during defense responses. J. Biosci. Bioeng. 2017, 123, 376–381. [Google Scholar] [CrossRef]
- Köllner, T.G.; Held, M.; Lenk, C.; Hiltpold, I.; Turlings, T.C.; Gershenzon, J.; Degenhardt, J. A maize (E)-beta-caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most American maize varieties. Plant Cell 2008, 20, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Munnik, T.; Testerink, C. Plant phospholipid signaling: “in a nutshell”. J. Lipid Res. 2009, 50, S260–S265. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Greer, M.S.; Weselake, R.J. Plant phospholipase A: Advances in molecular biology, biochemistry, and cellular function. Biomol. Concepts 2013, 4, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Saito, K. Roles of lipids as signaling molecules and mitigators during stress response in plants. Plant J. 2014, 79, 584–596. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, J.; Wu, G. Glycine metabolism in animals and humans: Implications for nutrition and health. Amino Acids 2013, 45, 463–477. [Google Scholar] [CrossRef]
- Li, W.B.; Hartung, J.S.; Levy, L. Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus huanglongbing. J. Microbiol. Methods 2006, 66, 104–115. [Google Scholar] [CrossRef]



| Citrus Group | Microwave Power (W) | Duration (s) | Highest Temperature (°C) | Citrus Grade * After 90 Days |
|---|---|---|---|---|
| 1 | 1500 | 30 | 62 | 3 |
| 2 | 25 | 59 | 3 | |
| 3 | 20 | 57 | 3 | |
| 4 | 15 | 54 | 2 | |
| 5 | 1000 | 30 | 58 | 3 |
| 6 | 25 | 56 | 3 | |
| 7 | 20 | 54 | 2 | |
| 8 | 15 | 53 | 2 | |
| 9 | 500 | 30 | 55 | 2 |
| 10 | 25 | 54 | 2 | |
| 11 | 20 | 52 | 1 | |
| 12 | 15 | 50 | 1 | |
| 13 | 250 | 30 | 45 | 1 |
| 14 | 25 | 40 | 1 | |
| 15 | 20 | 36 | 1 | |
| 16 | 15 | 32 | 1 |
| Citrus Num. | Treatment | Before Treatment | After Treatment | Percent Change of CLas Titer (%) ** | |||
|---|---|---|---|---|---|---|---|
| CT of CLas | Titer of CLas | CT of CLas | Titer of CLas | ||||
| M1 | Microwave treatment * | 500 W, 20 s | 25.9 | 2.81 × 105 | 33.8 | 1.57 × 103 | −99.44 |
| M2 | 19.4 | 2.01 × 107 | 29.1 | 3.44 × 104 | −99.83 | ||
| M3 | 30.5 | 1.37 × 104 | 34.3 | 1.13 × 103 | −91.75 | ||
| M4 | 27.6 | 9.20 × 104 | 34.8 | 8.14 × 102 | −99.12 | ||
| M5 | 32.3 | 4.20 × 103 | 33.9 | 1.47 × 103 | −65.03 | ||
| M6 | 31.5 | 7.11 × 103 | 34.1 | 1.29 × 103 | −81.87 | ||
| M7 | 500 W, 15 s | 21.2 | 2.73 × 104 | 28.5 | 1.83 × 103 | −93.29 | |
| M8 | 16.8 | 4.74 × 107 | 25.4 | 1.50 × 106 | −96.84 | ||
| M9 | 31.6 | 8.52 × 103 | 33.6 | 3.34 × 103 | −60.80 | ||
| M10 | 25.1 | 1.71 × 105 | 31.7 | 2.02 × 104 | −88.21 | ||
| M11 | 32.1 | 2.84 × 103 | 33.5 | 1.26 × 103 | −55.47 | ||
| M12 | 28.3 | 5.62 × 104 | 32.2 | 8.69 × 103 | −84.53 | ||
| M13 | 250 W, 30 s | 32.2 | 2.33 × 103 | 33.1 | 1.34 × 103 | −42.38 | |
| M14 | 17.9 | 5.45 × 106 | 24.4 | 8.55 × 105 | −84.31 | ||
| M15 | 28.0 | 6.17 × 104 | 32.6 | 1.39 × 104 | −77.52 | ||
| M16 | 30.7 | 4.39 × 103 | 32.2 | 1.63 × 103 | −62.94 | ||
| M17 | 20.6 | 7.69 × 105 | 26.3 | 1.40 × 105 | −81.78 | ||
| M18 | 25.9 | 5.38 × 104 | 31.8 | 1.43 × 104 | −73.47 | ||
| M19 | 250 W, 25 s | 18.4 | 2.24 × 106 | 23.3 | 5.92 × 105 | −73.57 | |
| M20 | 31.2 | 5.27 × 103 | 32.4 | 3.15 × 103 | −40.27 | ||
| M21 | 28.3 | 3.10 × 104 | 31.0 | 1.17 × 104 | −62.39 | ||
| M22 | 24.2 | 2.77 × 105 | 28.8 | 8.67 × 104 | −68.71 | ||
| M23 | 25.5 | 1.48 × 105 | 29.3 | 5.84 × 104 | −60.54 | ||
| M24 | 32.3 | 6.22 × 103 | 33.1 | 3.95 × 103 | −36.55 | ||
| M25 | 250 W, 20 s | 32.1 | 6.54 × 103 | 32.8 | 4.68 × 103 | −28.41 | |
| M26 | 28.1 | 4.54 × 104 | 30.0 | 2.56 × 104 | −43.54 | ||
| M27 | 26.1 | 2.93 × 105 | 28.5 | 1.52 × 105 | −48.26 | ||
| M28 | 25.4 | 2.16 × 105 | 27.1 | 9.97 × 104 | −53.86 | ||
| M29 | 32.5 | 4.47 × 103 | 33.1 | 3.42 × 103 | −23.43 | ||
| M30 | 25.6 | 2.94 × 105 | 28.2 | 1.81 × 105 | −39.50 | ||
| M31 | 250 W, 15 s | 27.1 | 1.08 × 105 | 28.4 | 8.07 × 104 | −25.28 | |
| M32 | 30.9 | 3.31 × 103 | 31.4 | 2.62 × 103 | −20.94 | ||
| M33 | 24.5 | 7.64 × 105 | 26.3 | 5.18 × 105 | −32.15 | ||
| M34 | 31.8 | 3.28 × 103 | 32.7 | 2.69 × 103 | −18.10 | ||
| M35 | 18.8 | 3.05 × 106 | 21.5 | 1.97 × 106 | −35.57 | ||
| M36 | 21.0 | 5.39 × 105 | 22.6 | 4.02 × 105 | −25.44 | ||
| N1 | NO treatment (Control group) | 32.2 | 4.49 × 103 | 27.4 | 1.05 × 105 | 2238.65 | |
| N2 | 27.7 | 8.62 × 104 | 25.7 | 3.21 × 105 | 271.88 | ||
| N3 | 28.2 | 6.21 × 104 | 26.5 | 1.90 × 105 | 205.38 | ||
| N4 | 24.1 | 9.17 × 105 | 21.4 | 5.40 × 106 | 488.90 | ||
| N5 | 29.8 | 3.01 × 104 | 25.9 | 2.81 × 105 | 832.57 | ||
| N6 | 23.8 | 1.12 × 106 | 23.1 | 1.77 × 106 | 58.36 | ||
| Compared Groups | Num. of Total Ident. * | Num. of Total Sig. ** | Num. of Sig. Up *** | Num. of Sig. Down **** |
|---|---|---|---|---|
| A vs. B | 1747 | 160 | 74 | 86 |
| C vs. D | 123 | 31 | 92 | |
| E vs. Fd | 174 | 43 | 131 | |
| G vs. H | 95 | 40 | 55 | |
| J vs. K | 59 | 28 | 31 |
| Num. | Polarity Mode (m/z) | Metabolite Name | Molecular Formula | Compound Type | A vs. B | C vs. D | E vs. Fd | G vs. H | J vs. K | Up or Down |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | Beta-caryophyllene | C15H24 | Lipids and lipid-like molecules | 0 * | 1 ** | 1 | 1 | 1 | up |
| 2 | − | LPI 16:0 | C25H49O12P | 1 | 1 | 1 | 1 | 0 | up | |
| 3 | − | LPI 18:3 | C27H47O12P | 1 | 1 | 0 | 1 | 1 | up | |
| 4 | − | 2-Methoxyestradiol | C19H26O3 | 1 | 1 | 1 | 1 | 1 | down | |
| 5 | − | Bornyl acetate | C12H20O2 | 1 | 1 | 1 | 0 | 1 | down | |
| 6 | + | Enoxolone | C30H46O4 | 1 | 1 | 1 | 1 | 0 | down | |
| 7 | + | Methylmalonate | C4H6O4 | Organic acids and derivatives | 1 | 1 | 1 | 1 | 1 | down |
| 8 | + | 2-Oxoadipic acid | C6H8O5 | 1 | 1 | 1 | 1 | 1 | down | |
| 9 | − | 5′-S-methyl-5′-thioadenosine | C11H15N5O3S | Nucleosides, nucleotides, and analogues | 1 | 1 | 1 | 1 | 1 | down |
| 10 | − | Gentiopicrin | C16H20O9 | Others | 1 | 1 | 1 | 1 | 1 | down |
| 11 | − | Kaempferol-3-Galactoside-6″- Rhamnoside-3‴-Rhamnoside | C33H40O19 | 0 | 1 | 1 | 1 | 1 | down | |
| 12 | − | 2-{[(1-benzothiophen-3-ylmethyl)- 3-imino]methyl}phenol | C16H13NOS | 1 | 1 | 1 | 1 | 0 | down | |
| 13 | + | (6E)-7-(2H-1,3-benzodioxol-5-yl)-1- (piperidin-1-yl)hept-6-en-1-one | C19H25NO3 | 1 | 1 | 1 | 1 | 0 | down | |
| 14 | + | 4-oxo-4-[(1-phenylethyl)amino]but-2- 5-enoic acid | C12H13NO3 | 1 | 1 | 1 | 1 | 0 | down | |
| 15 | + | 6-methylpyrimido [4,5-d]pyrimidin-4- 7-amine | C7H7N5 | 0 | 1 | 1 | 1 | 1 | down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Li, Y.; Li, G.; Wu, Y.; Mao, J.; Lin, J.; Diao, M.; Huang, Z. Microwave Treatment for Citrus Huanglongbing Control: Pathogen Elimination and Metabolomic Analysis. Plants 2025, 14, 2712. https://doi.org/10.3390/plants14172712
Chen X, Li Y, Li G, Wu Y, Mao J, Lin J, Diao M, Huang Z. Microwave Treatment for Citrus Huanglongbing Control: Pathogen Elimination and Metabolomic Analysis. Plants. 2025; 14(17):2712. https://doi.org/10.3390/plants14172712
Chicago/Turabian StyleChen, Xianrui, Yunyun Li, Gen Li, Yanling Wu, Junru Mao, Jiasheng Lin, Mengxue Diao, and Zhimin Huang. 2025. "Microwave Treatment for Citrus Huanglongbing Control: Pathogen Elimination and Metabolomic Analysis" Plants 14, no. 17: 2712. https://doi.org/10.3390/plants14172712
APA StyleChen, X., Li, Y., Li, G., Wu, Y., Mao, J., Lin, J., Diao, M., & Huang, Z. (2025). Microwave Treatment for Citrus Huanglongbing Control: Pathogen Elimination and Metabolomic Analysis. Plants, 14(17), 2712. https://doi.org/10.3390/plants14172712






