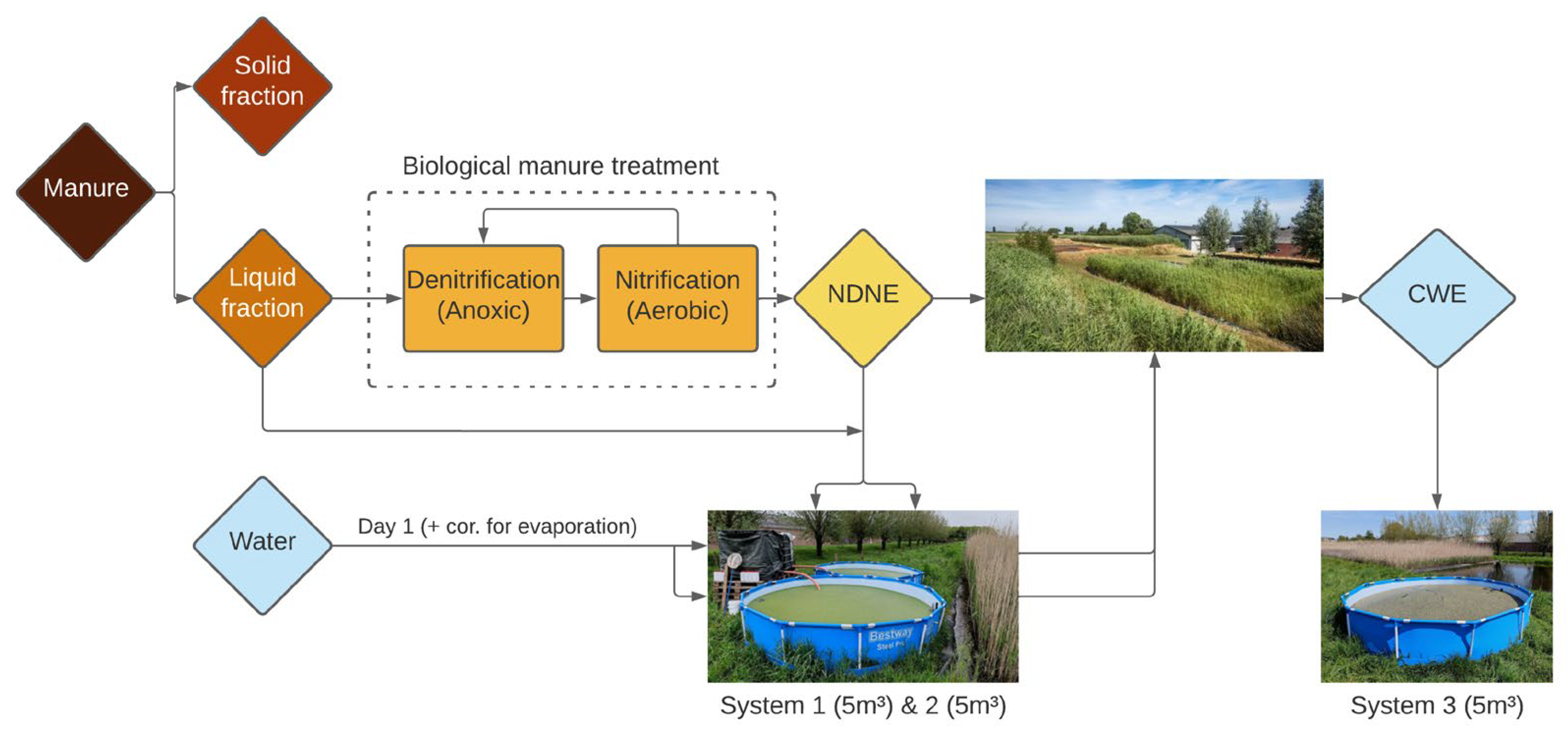
3.1.1. Partially Treated Pig Manure Is a Potential Nutrient Source for Protein-Rich Duckweed Biomass
The duckweed treatment systems S1 and S2 were both fertilised weekly with a mixture of the liquid fraction (LF) and nitrification–denitrification effluent (NDNE) from pig manure processing.
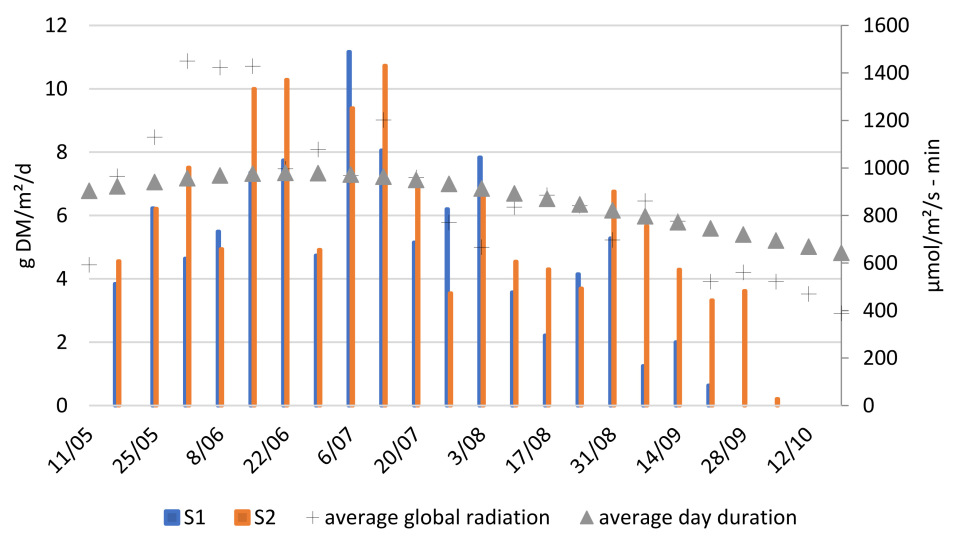
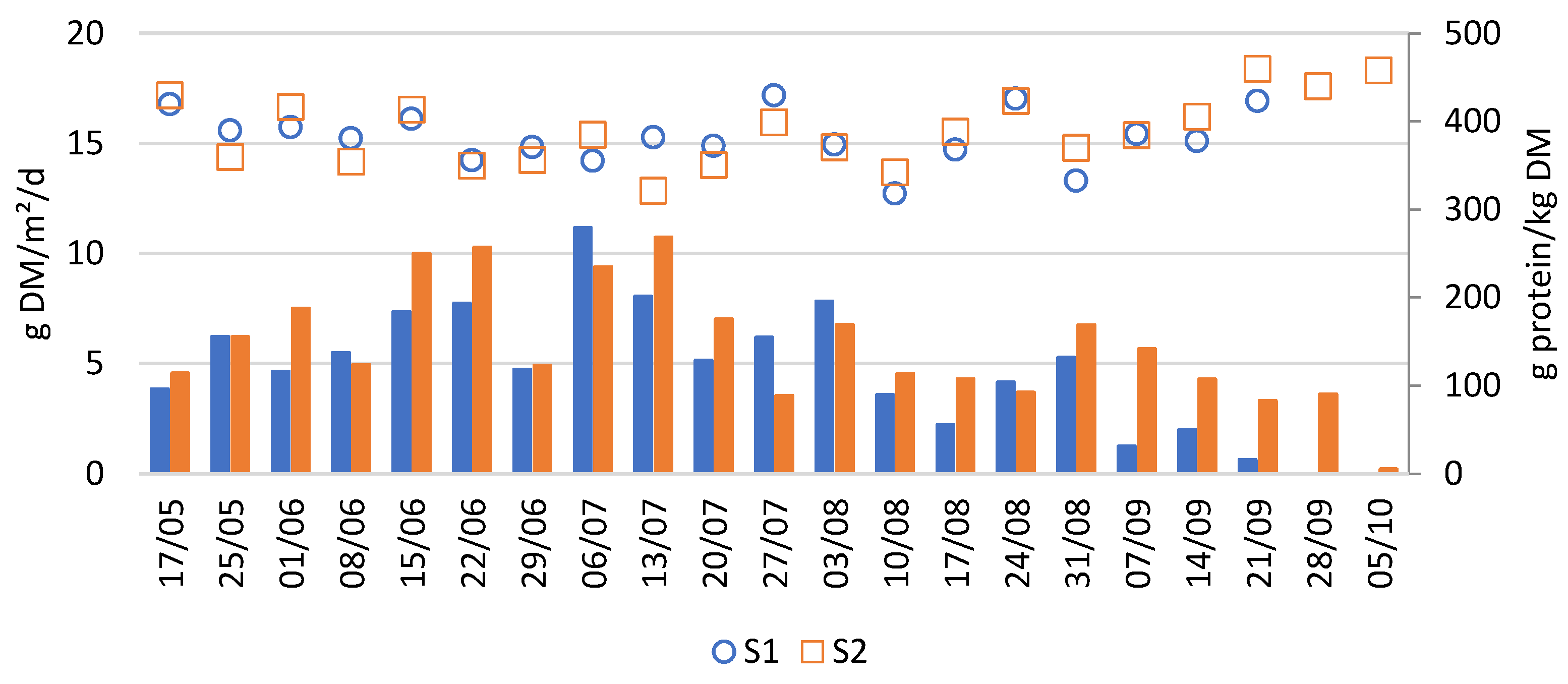
Figure 3 reports the biomass (dry matter) and protein production of these systems over the growing season. The average weekly biomass productivity of both S1 and S2 was 5.5 ± 2.6 g DM/m
2/day. This yield is consistent with previous research in Flanders using similar nutrient sources, which reported an average biomass productivity of 6.1 ± 2.5 g DM/m
2/day [
3].
Weekly production rates showed some variability, partly due to inconsistencies in the harvesting method. Since only part of the duckweed was harvested each week and the remaining fraction was not precisely controlled, slight differences in post-harvest coverage may have affected the weekly productivities. Nevertheless, a clear trend in duckweed productivity can be observed. As shown in
Figure 3, duckweed biomass production showed a steep increase during early summer, peaking between 15/06 and 13/07, followed by a slower decline until complete die-off in late September. The peak productivity during summer is likely explained by favourable weather conditions, particularly high global radiation and long daylight hours (
Figure A1).
Duckweed growth ceased on 21/09 in S1 and 28/09 in S2, resulting in growing seasons of 134 and 141 days, respectively, which are shorter than the anticipated 175-day growing season for temperate Belgian conditions. Although an earlier start (e.g., March–April) could have extended the growing period, the early growth cessation is unexpected given that environmental conditions remained suitable throughout September (
Figure A1). A longer growing season should have been achievable under these conditions [
3]. Therefore, potential causes of this early production halt are further investigated in
Section 3.2.
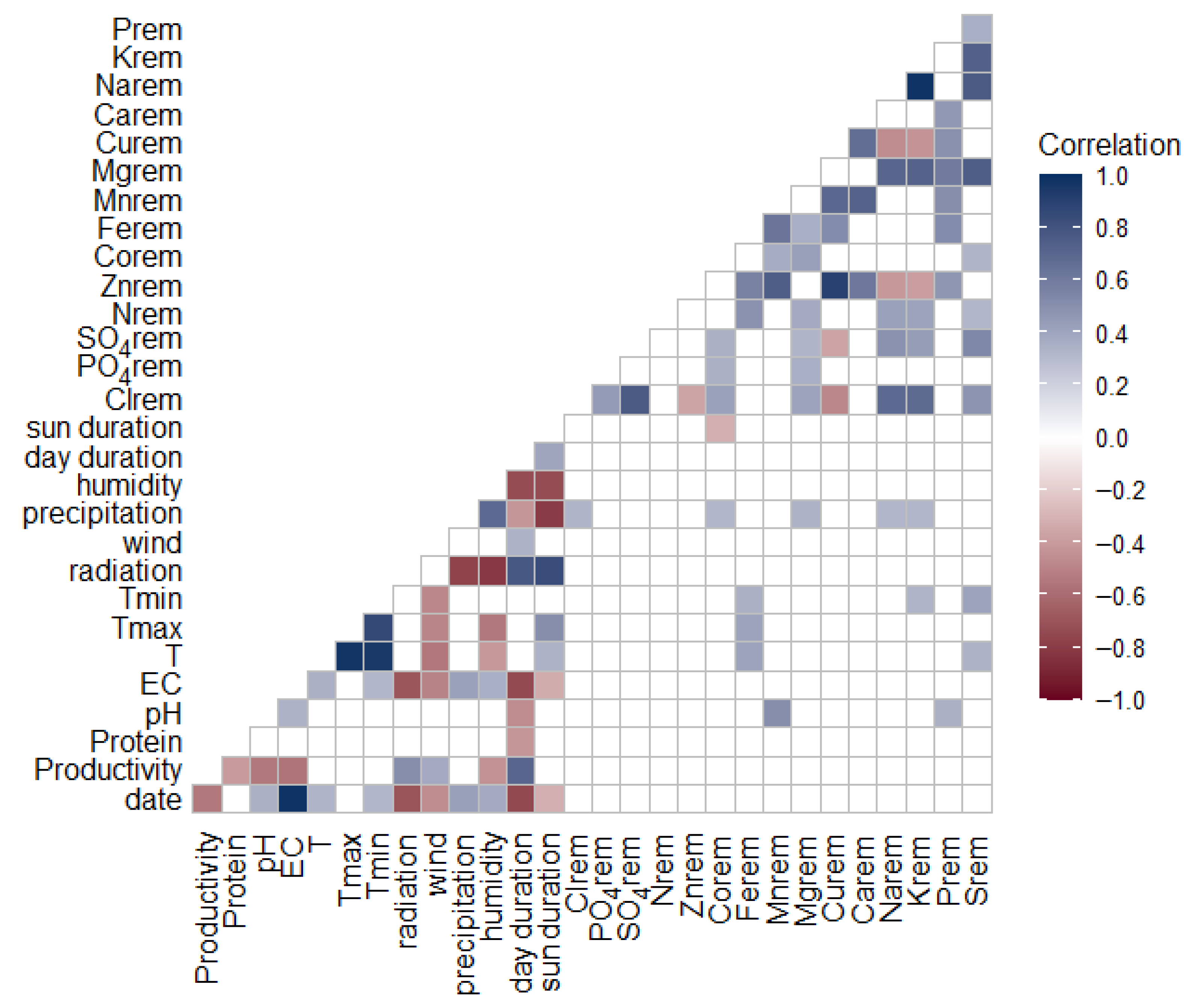
Figure 3 also shows that the protein concentration of the harvested duckweed fluctuated between 30 and 45% throughout the growing season. These values position duckweed as a promising high-quality protein feedstock. However, if duckweed is to be used as a reliable protein source in feed formulations, it is crucial that its protein concentration remains stable over time. To investigate which factors may explain the observed fluctuations in protein content, a Spearman correlation analysis was performed. This analysis revealed a significant negative correlation between protein concentration and total biomass productivity (Spearman’s r = –0.4,
p = 0.01), suggesting a trade-off between yield and protein concentration. Additionally, the correlation plot (
Figure A3) indicated other significant negative associations between protein content and both day duration and iron (Fe) concentration in the medium. However, these relationships are likely indirect, mediated through their influence on productivity. To the best of our knowledge, this inverse relationship between duckweed biomass productivity and protein content has not been previously reported in the literature.
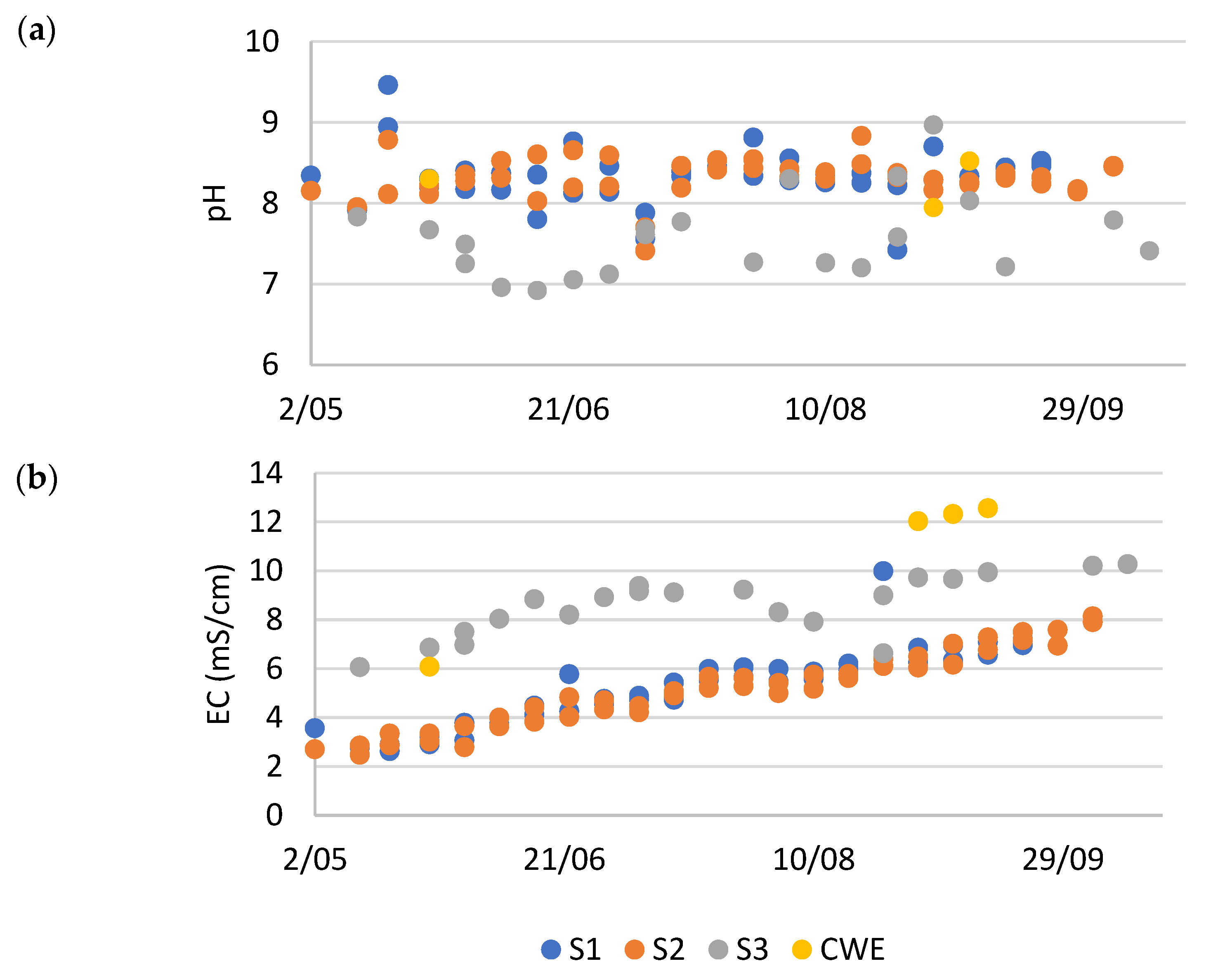
Table 3 extrapolates the productivity and protein content to a larger scale. It is apparent that the duckweed in S3, fertilized with effluent from the constructed wetland, showed very poor to no growth and had a considerable low protein concentration. Without any prior processing of the effluent, this medium is not suitable for duckweed cultivation because of its too high electrical conductivity (EC) (
Figure A2).
Duckweed cultivated in systems S1 and S2, fertilized with partially treated pig manure (LF and NDNE), showed good productivity, comparable to previous findings by Devlamynck et al. (
Table 3) [
3]. Biomass yields were lower than those reported in pilot systems operating under warmer climatic conditions, such as in the Spanish study (17 tonnes DM/ha/year) and the Brazilian study (68 tonnes DM/ha/year) [
1,
5]. Nonetheless, even within the temperate Belgian climate, the duckweed protein yield obtained in this study suggests strong potential as an alternative to conventional protein sources. For reference, Brazil’s 2023 soybean production averaged 3.4 tonnes/ha with a crude protein content of approximately 35.8% [
35,
36], corresponding to an average protein yield of about 1.2 tonnes/ha/year. Notably, the annual protein yield achieved in S1 and S2 exceeded this value by a factor of 2.2 and 2.8, respectively. Therefore, considering the significant potential for further optimization of these systems, discussed in
Section 3.2, duckweed cultivation in such setups emerges as a promising local alternative to soybean protein production.
3.1.2. Duckweed Growth Resulted in Substantial Nutrient Removal
In addition to its agronomical performance, the environmental performance of the duckweed treatment system was evaluated, focusing specifically on its nutrient removal capacity. In plant-based systems, nutrients are removed via three main processes, which are sedimentation, plant uptake, and microbiological processes like nitrification–denitrification [
1,
37]. Additionally, dependent on the pH and temperature of the growing medium, nutrient losses can also occur via volatilization, particularly of ammonia.
By weekly sampling of the growing medium, both before and after fertilization, along with nutrient analysis of the harvested duckweed, the overall system removal and the specific removal through duckweed harvesting could be calculated. The removal by plant uptake could not be determined as not all of the duckweed biomass covering the system was harvested every week. The average overall and specific removals for S1 and S2 together are shown in
Table 4.
The variation within removal rates, presented in
Table 4, is substantial, which may be attributed to the inherent random variation characteristic of biological systems, as has been observed in previous research by Devlamynck et al. [
4]. However, negative overall system removal rates were occasionally observed when medium nutrient concentrations measured just before harvesting were higher than those measured after fertilisation one week earlier. This could be attributed to inconsistent mixing of the system prior to sampling or nutrient release from the sediment during mixing before sampling. While these negative values were included in the calculation of overall nutrient removal, they were excluded from the calculation of the specific nutrient removal attributable to duckweed harvest.
Seasonal variation in removal rates was also apparent, but no consistent trends could be observed from the weekly measured nutrient concentrations in the medium. Spearman correlation analysis did not show any significant correlation between nutrient removal and productivity or harvest date. Only pH, T and precipitation had a small positive correlation with the system removal of certain nutrients, as presented in
Figure A5.
Despite the high variability, nutrient removal rates observed in S1 and S2 were generally consistent with those reported in previous studies. For nitrogen (N), an average removal rate of 1.2 g/m
2/day was recorded, closely aligning with previous findings by Devlamynck et al., who reported N removal rates of 1.1 g/m
2/day under similar wastewater conditions and weather patterns [
3,
4]. The nitrogen removal attributable to harvest in this study also fell within the range of N uptake previously observed (327 ± 107 and 264 ± 123 mg N/m
2/day) [
3,
4]. The proportion of total nitrogen removed through harvesting was higher in this study (48 ± 74%) compared to the previous research (27% and 24% [
3,
4]).
For phosphorus (P), previous studies reported system removal rates of 0.13 and 0.37 g/m
2/day, with P uptake rates of 67 ± 26 and 58 ± 31 mg P/m
2/day and relative uptake percentages between 17% and 39% [
3,
4]. In the current study, similar system removal (0.13 g/m
2/day) and uptake rates (72 ± 40 mg/m
2/d) were achieved, but with a notably higher proportion of phosphorus removed via harvest (97 ± 136%). This suggests that nearly all P removal was attributed to duckweed uptake, in contrast to earlier studies where sedimentation played a more prominent role. A plausible explanation is that phosphorus resuspension from the sediment during water mixing prior to sampling may have elevated the measured concentrations (both before and after fertilization), thereby possibly underestimating the actual system removal. Consequently, the contribution of non-uptake pathways, such as sedimentation, may have been underestimated, resulting in a possible overestimation of the harvest-based share. Moreover, the harvest-based removal percentages shown in
Table 4 should not be directly compared to the relative uptake values reported by Devlamynck et al. [
3,
4], as these were estimated using average concentrations and reflect total uptake rather than removal through harvesting.
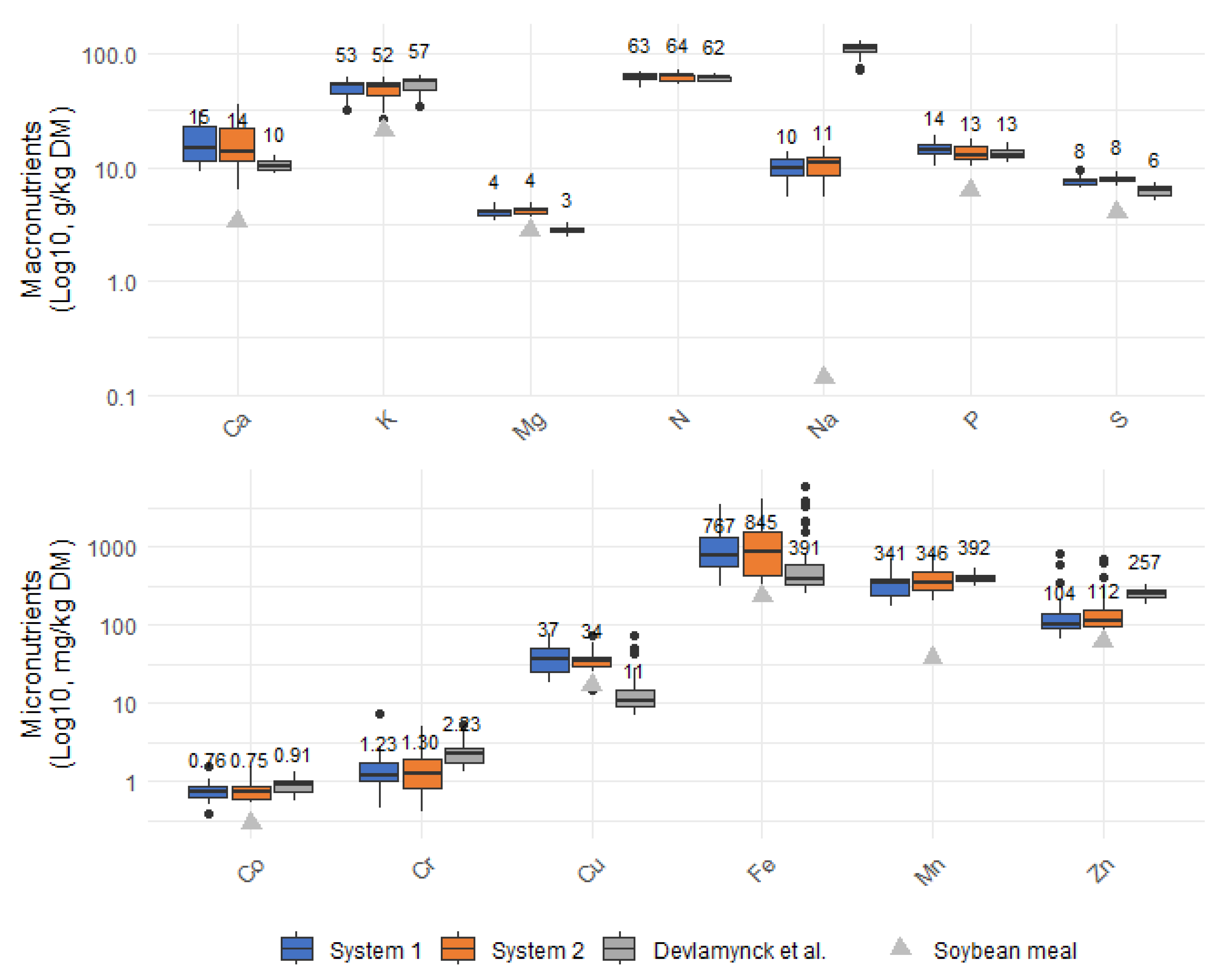
Although the harvest-based share of total phosphorus (P) removal may have been overestimated, the opposite pattern was observed for highly soluble elements such as potassium (K) and sodium (Na), which are typically removed primarily through plant uptake. In this study, the relatively low calculated harvest-based removal percentages for K and Na likely resulted from high variability in the weekly system removal estimates. The K removal via harvest in the present study (255 ± 144 mg/m
2/day) was comparable to previously reported plant uptake rates (233 ± 85 mg/m
2/day), while the overall K removal was considerably higher and more variable [
3,
4]. Other elements considerably removed via harvesting included calcium (Ca), manganese (Mn), magnesium (Mg), and sulphur (S), all showing harvest-based removal rates comparable with uptake values reported by Devlamynck et al. [
3,
4].
Sedimentation is expected to account for a substantial share of nutrient and metal removal in duckweed treatment ponds, particularly for phosphorus and heavy metals. At the end of the monitoring period, sedimentation was assessed in both systems, and only a small amount of settled solids was measured (4.5–5.25 mL/L), corresponding to an estimated sediment depth of only ~0.2–0.3 cm. This limited accumulation is probably due to repeated resuspension of solids during weekly mixing, which hindered stable sediment formation. Future studies should consider operating undisturbed systems to better evaluate the sedimentation dynamics and accurately distinguish between removal pathways.
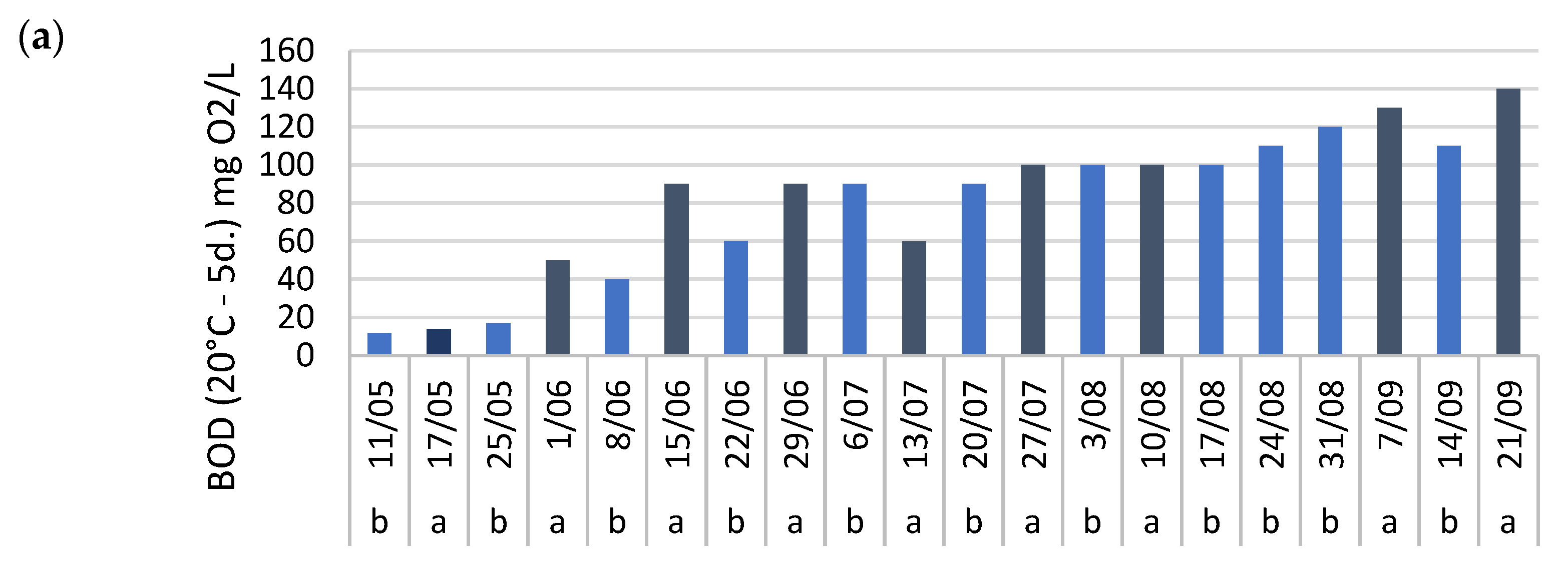
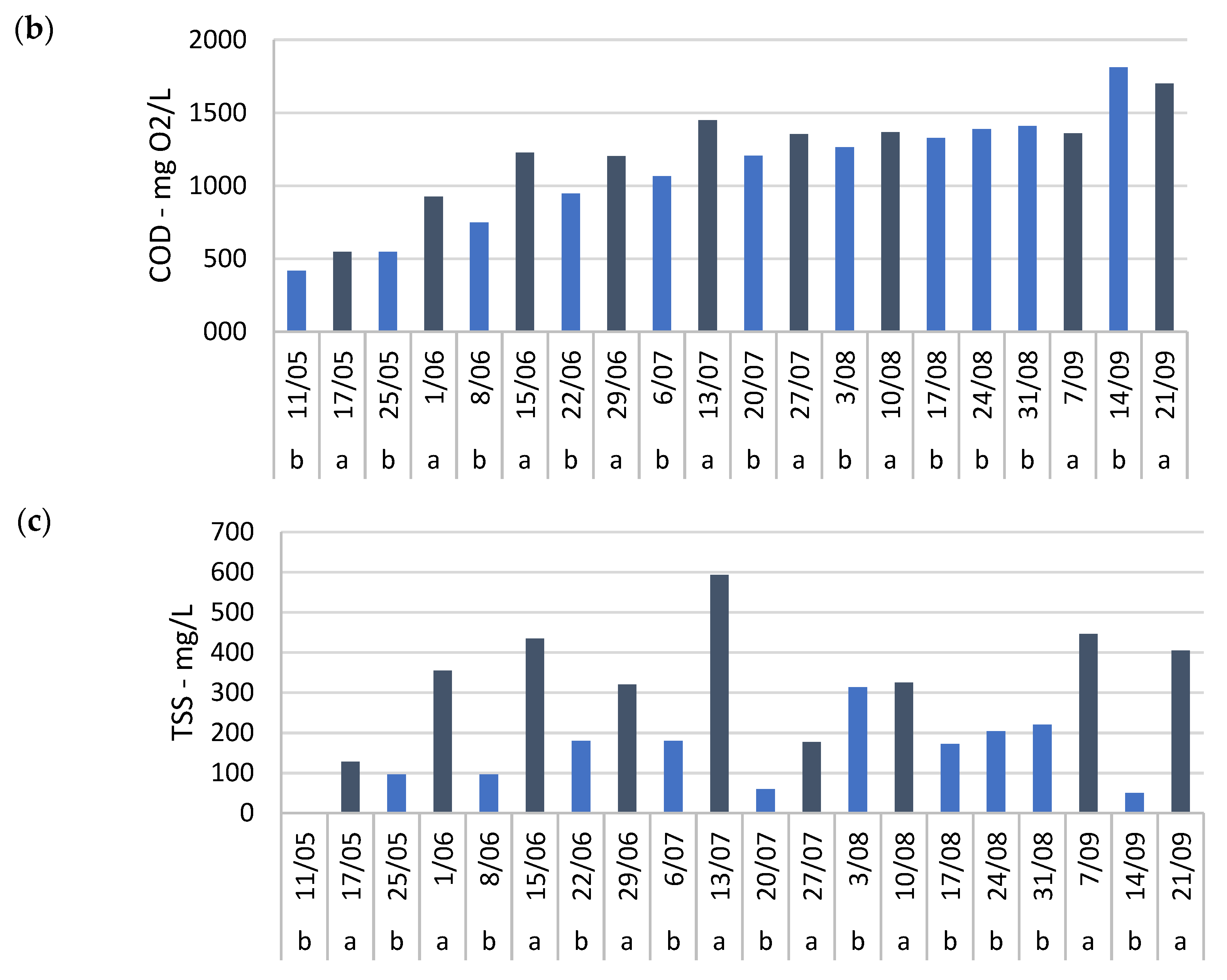
Mixing also complicated the assessment of BOD, COD, and TSS removal in system 1, where these parameters were monitored. Although some fluctuation was observed between individual measurements, a consistent pattern was observed, particularly during the first half of the growing season. TSS concentrations were generally lower one week after fertilization compared to before (
Figure A6c). Similar trends were observed for BOD and COD (
Figure A6a,b), suggesting positive removal throughout this period. However, as with most nutrients, the calculated seasonal average removals of BOD, COD, and TSS showed large standard deviations (
Table 4). Furthermore, both BOD and COD concentrations increased substantially over time, from 12 mg/L and 418 mg/L O
2 on May 11th to 108 mg/L and 1612 mg/L O
2 by September 28th, respectively (
Figure A6a,b). The observed low BOD/COD ratio (<0.1) indicates that most of the organic matter was not readily biodegradable, limiting microbial breakdown and explaining the low removal efficiency. In contrast, systems with higher organic loading have demonstrated effective BOD, COD, and TSS removal in duckweed-based treatments [
38,
39,
40], likely due to increased microbial activity supported by the duckweed layer, which enhances oxygen availability and provides surfaces for microbial colonization [
38,
39].
Compared to other wastewater treatment technologies, such as reed-based constructed wetlands, the monitored duckweed system demonstrated a competitive environmental performance. For instance, Meers et al. [
7] reported average nitrogen and phosphorus removal rates of 0.89 g N/m
2/day and 1.4–2.7 g P/m
2/day, respectively, in a full-scale constructed wetland treating similar pig manure effluent. While these systems are effective, they typically yield low-nutrient biomass and are harvested infrequently. Duckweed systems, on the other hand, not only facilitate efficient nutrient capture but also frequently generate nutrient-rich biomass suitable for valorisation. Constructed wetlands may, however, provide a valuable complementary post-treatment step, particularly for the removal of organic matter via sedimentation, adsorption, and microbial degradation processes that may be limited in duckweed systems under low BOD/COD conditions.
Additionally, Zimmo et al. compared duckweed and algae-based treatment systems and found that algae systems achieved higher overall nitrogen removal rates [
37]. However, under conditions of low organic loading, which are more comparable to our study, duckweed was responsible for a greater proportion of nitrogen assimilation, resulting in higher nitrogen recovery into biomass [
37]. For phosphorus, they observed that organic loading had little influence on total phosphorus removal in either system [
37]. Interestingly, the highest phosphorus removal occurred in the duckweed-based system [
37].