Biotechnological Advances in Sanguinarine and Chelerythrine Production from Plume Poppy (Macleaya cordata): A Gene Editing Perspective
Abstract
1. Introduction
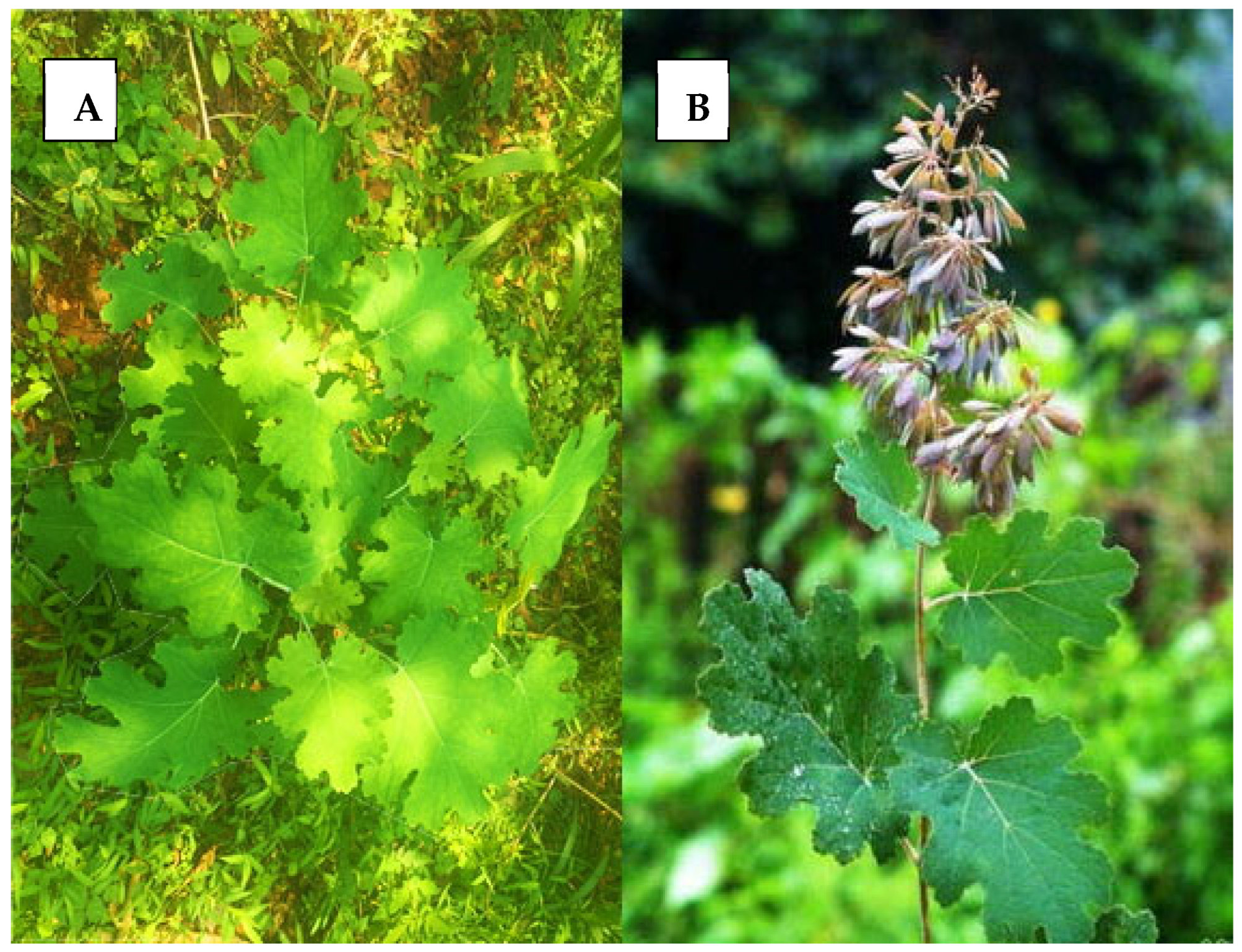
2. Botanical Description of Macleaya cordata (Plume Poppy)
3. Sanguinarine and Chelerythrine Biosynthesis and Applications
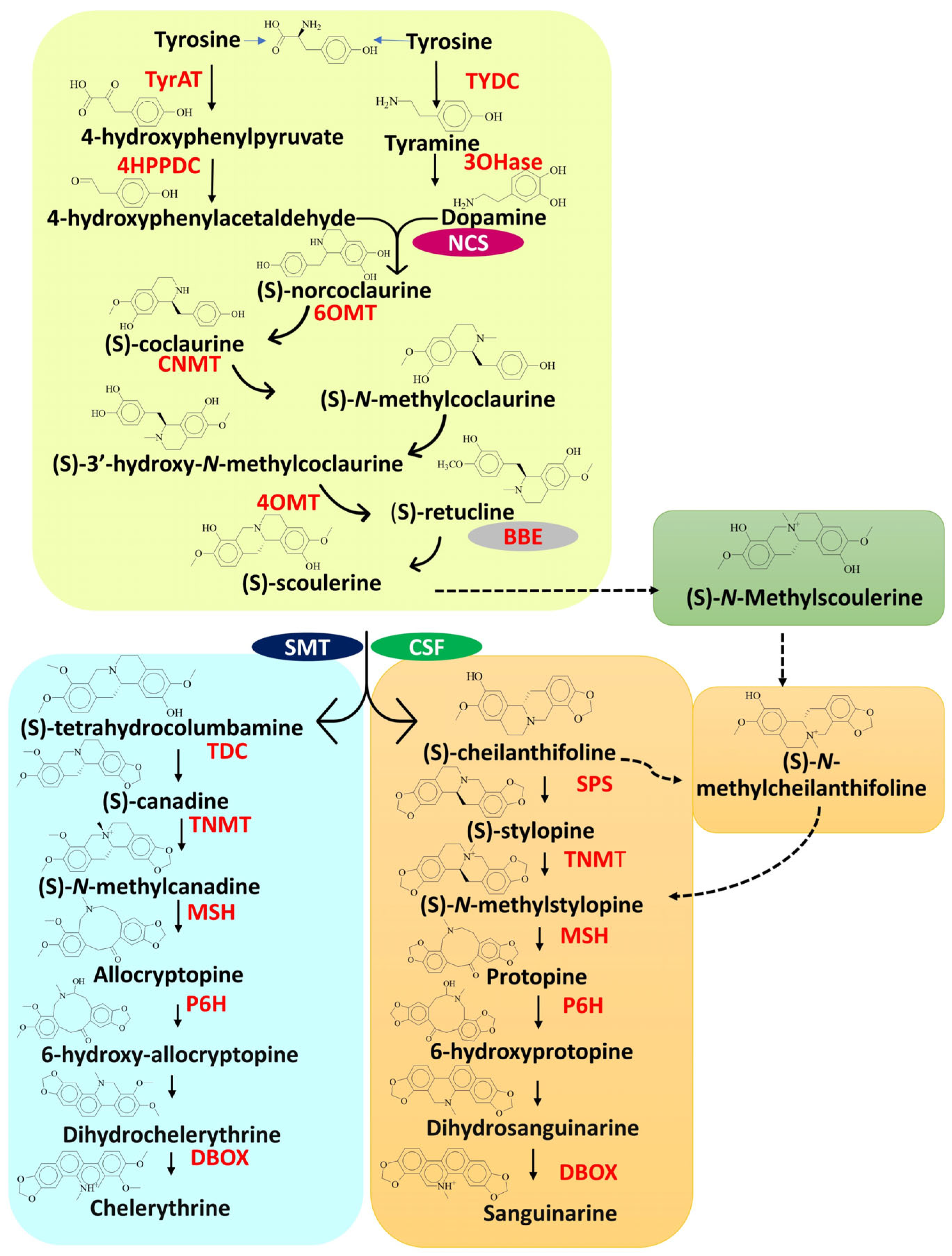
3.1. Biosynthesis of Sanguinarine and Chelerythrine
3.2. Applications of Sanguinarine and Chelerythrine
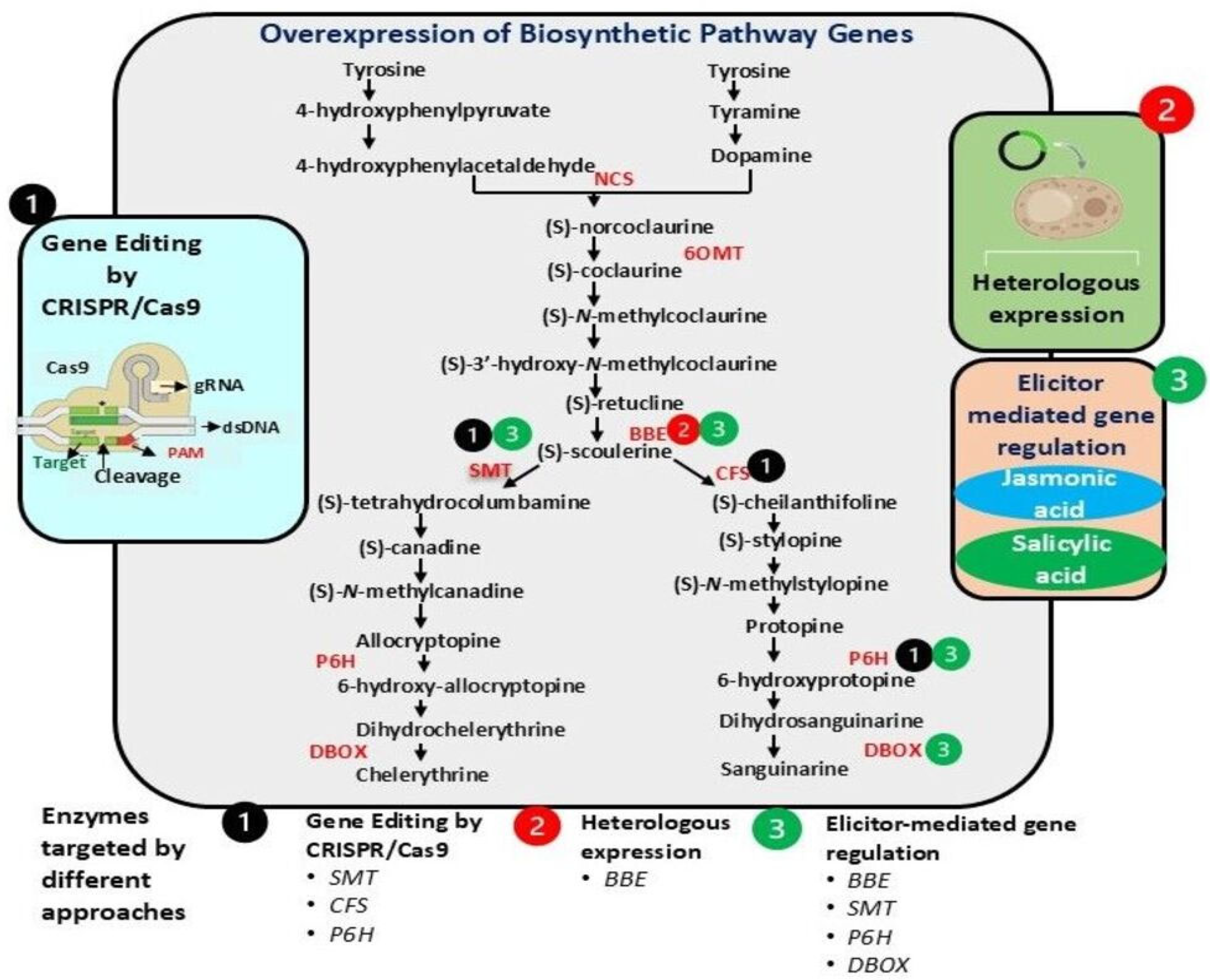
4. Transgenic Approaches for Enhancing Sanguinarine and Chelerythrine Production
4.1. Gene Editing Using CRISPR/Cas9
4.2. Overexpression of Key Biosynthetic Genes
4.3. Enhancing BIAs, Alkaloid Delivery, and Stability
4.4. Metabolic Engineering and Pathway Diversification
5. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Yang, Y.; Sun, Y.; Wang, Z.; Yin, M.; Sun, R.; Xue, L.; Huang, X.; Wang, C.; Yan, X.; Li, W.; et al. Full-Length Transcriptome and Metabolite Analysis Reveal Reticuline Epimerase-Independent Pathways for Benzylisoquinoline Alkaloids Biosynthesis in Sinomenium acutum. Front. Plant Sci. 2022, 13, 1086335. [Google Scholar] [CrossRef]
- Aghaali, Z.; Naghavi, M.R. Developing Benzylisoquinoline Alkaloid-Enriched Opium Poppy via CRISPR-Directed Genome Editing: A Review. BMC Plant Biol. 2024, 24, 700. [Google Scholar] [CrossRef]
- Lee, E.J.; Hagel, J.M.; Facchini, P.J. Role of the Phloem in the Biochemistry and Ecophysiology of Benzylisoquinoline Alkaloid Metabolism. Front. Plant Sci. 2013, 4, 51735. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Liu, Y.C.; Huang, J.L.; Liu, X.; Bin; Qing, Z.X.; Zeng, J.G.; Liu, Z.Y. Medicinal Plants of the Genus Macleaya (Macleaya cordata, Macleaya microcarpa): A Review of Their Phytochemistry, Pharmacology, and Toxicology. Phyther. Res. 2018, 32, 19–48. [Google Scholar] [CrossRef]
- Zuo, Z.; Zheng, Y.; Liang, Z.; Liu, Y.; Tang, Q.; Liu, X.; Zhao, Z.; Zeng, J. Tissue-Specific Metabolite Profiling of Benzylisoquinoline Alkaloids in the Root of Macleaya cordata by Combining Laser Microdissection with Ultra-High-Performance Liquid Chromatography/Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2017, 31, 397–410. [Google Scholar] [CrossRef]
- Laines-Hidalgo, J.I.; Muñoz-Sánchez, J.A.; Loza-Müller, L.; Vázquez-Flota, F. An Update of the Sanguinarine and Benzophenanthridine Alkaloids’ Biosynthesis and Their Applications. Molecules 2022, 27, 1378. [Google Scholar] [CrossRef]
- Huang, P.; Cheng, P.; Sun, M.; Liu, X.; Qing, Z.; Liu, Y.; Yang, Z.; Liu, H.; Li, C.; Zeng, J.; et al. Systemic Review of Macleaya cordata Genetics, Biosynthesis of Active Ingredients and Functions. Med. Plant Biol. 2024, 3, e020. [Google Scholar] [CrossRef]
- Kosina, P.; Gregorova, J.; Gruz, J.; Vacek, J.; Kolar, M.; Vogel, M.; Roos, W.; Naumann, K.; Simanek, V.; Ulrichova, J. Phytochemical and Antimicrobial Characterization of Macleaya cordata Herb. Fitoterapia 2010, 81, 1006–1012. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Huang, P.; Ma, Y.; Qing, Z.; Tang, Q.; Cao, H.; Cheng, P.; Zheng, Y.; Yuan, Z.; et al. The Genome of Medicinal Plant Macleaya cordata Provides New Insights into Benzylisoquinoline Alkaloids Metabolism. Mol. Plant 2017, 10, 975–989. [Google Scholar] [CrossRef]
- Lei, Q.; Liu, H.; Peng, Y.; Xiao, P. In Silico Target Fishing and Pharmacological Profiling for the Isoquinoline Alkaloids of Macleaya cordata (Bo Luo Hui). Chin. Med. 2015, 10, 37. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, Y.; Liu, W.; Liu, X.; Liu, F.; Huang, P.; Zhu, P.; Chen, J.; Shi, M.; Guo, F.; et al. Integration of Transcriptome, Proteome and Metabolism Data Reveals the Alkaloids Biosynthesis in Macleaya cordata and Macleaya microcarpa. PLoS ONE 2013, 8, e53409. [Google Scholar] [CrossRef]
- Lei, F.; Liu, X.; Huang, H.; Fu, S.; Zou, K.; Zhang, S.; Zhou, L.; Zeng, J.; Liu, H.; Jiang, L.; et al. The Macleaya cordata Symbiont: Revealing the Effects of Plant Niches and Alkaloids on the Bacterial Community. Front. Microbiol. 2021, 12, 681210. [Google Scholar] [CrossRef]
- Sun, M.; Liu, X.; Liu, W.; Xu, Z.; Wang, Y.; Zhou, L.; Huang, P.; Zeng, J. The Effects of Protopine 6-Hydroxylase (P6H) Overexpression on Benzylisoquinoline Alkaloids in Macleaya cordata. Plant Cell Tissue Organ Cult. 2022, 148, 429–437. [Google Scholar] [CrossRef]
- Acharjee, S.; Kumar, R.; Kumar, N. Role of Plant Biotechnology in Enhancement of Alkaloid Production from Cell Culture System of Catharanthus Roseus: A Medicinal Plant with Potent Anti-Tumor Properties. Ind. Crops Prod. 2022, 176, 114298. [Google Scholar] [CrossRef]
- Ziegler, J.; Facchini, P.J. Alkaloid Biosynthesis: Metabolism and Trafficking. Annu. Rev. Plant Biol. 2008, 59, 735–769. [Google Scholar] [CrossRef]
- Huang, P.; Liu, W.; Xu, M.; Jiang, R.; Xia, L.; Wang, P.; Li, H.; Tang, Z.; Zheng, Q.; Zeng, J. Modulation of Benzylisoquinoline Alkaloid Biosynthesis by Overexpression Berberine Bridge Enzyme in Macleaya cordata. Sci. Rep. 2018, 8, 17988. [Google Scholar] [CrossRef]
- Hagel, J.M.; Facchini, P.J. Benzylisoquinoline Alkaloid Metabolism: A Century of Discovery and a Brave New World. Plant Cell Physiol. 2013, 54, 647–672. [Google Scholar] [CrossRef]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Molecular Targets and Anticancer Potential of Sanguinarine—A Benzophenanthridine Alkaloid. Phytomedicine 2017, 34, 143–153. [Google Scholar] [CrossRef]
- Fu, C.; Guan, G.; Wang, H. The Anticancer Effect of Sanguinarine: A Review. Curr. Pharm. Des. 2018, 24, 2760–2764. [Google Scholar] [CrossRef]
- Zhao, Z.M.; Shang, X.F.; Lawoe, R.K.; Liu, Y.Q.; Zhou, R.; Sun, Y.; Yan, Y.F.; Li, J.C.; Yang, G.Z.; Yang, C.J. Anti-Phytopathogenic Activity and the Possible Mechanisms of Action of Isoquinoline Alkaloid Sanguinarine. Pestic. Biochem. Physiol. 2019, 159, 51–58. [Google Scholar] [CrossRef]
- Niu, X.F.; Zhou, P.; Li, W.F.; Xu, H.B. Effects of Chelerythrine, a Specific Inhibitor of Cyclooxygenase-2, on Acute Inflammation in Mice. Fitoterapia 2011, 82, 620–625. [Google Scholar] [CrossRef]
- Fan, L.; Fan, Y.; Liu, L.; Tao, W.; Shan, X.; Dong, Y.; Li, L.; Zhang, S.; Wang, H. Chelerythrine Attenuates the Inflammation of Lipopolysaccharide-Induced Acute Lung Inflammation Through NF-ΚB Signaling Pathway Mediated by Nrf2. Front. Pharmacol. 2018, 9, 384399. [Google Scholar] [CrossRef]
- Chen, N.; Qi, Y.; Ma, X.; Xiao, X.; Liu, Q.; Xia, T.; Xiang, J.; Zeng, J.; Tang, J. Rediscovery of Traditional Plant Medicine: An Underestimated Anticancer Drug of Chelerythrine. Front. Pharmacol. 2022, 13, 906301. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Cheng, H.; Li, Y.; Zhou, G. Chelerythrine, a Major Ingredient Isolated from Macleaya cordata (Willd.) R. Br. (Papaveraceae), Inhibits Fluconazole-Resistant Candida albicans Biofilms. J. Herb. Med. 2023, 42, 100752. [Google Scholar] [CrossRef]
- Gou, Y.; Li, D.; Zhao, M.; Li, M.; Zhang, J.; Zhou, Y.; Xiao, F.; Liu, G.; Ding, H.; Sun, C.; et al. Intein-Mediated Temperature Control for Complete Biosynthesis of Sanguinarine and Its Halogenated Derivatives in Yeast. Nat. Commun. 2024, 15, 5238. [Google Scholar] [CrossRef]
- Wang, F.; Yin, Y.; Yang, M.; Chen, J.; Fu, C.; Huang, K. Effects of Combined Supplementation of Macleaya cordata Extract and Benzoic Acid on the Growth Performance, Immune Responses, Antioxidant Capacity, Intestinal Morphology, and Microbial Composition in Weaned Piglets. Front. Vet. Sci. 2021, 8, 708597. [Google Scholar] [CrossRef]
- Sun, M.; Zhong, X.; Zhou, L.; Liu, W.; Song, R.; Huang, P.; Zeng, J. CRISPR/Cas9 Revolutionizes Macleaya cordata Breeding: A Leap in Sanguinarine Biosynthesis. Hortic. Res. 2024, 11, uhae024. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.; Zhang, C.; Lv, L.; Gao, B.; Li, M. Antibiotics Review Research Progress on Antibacterial Activities and Mechanisms of Natural Alkaloids: A Review. Antibiotics 2021, 10, 25. [Google Scholar] [CrossRef]
- Liu, Z.H.; Wang, W.M.; Zhang, Z.; Sun, L.; Wu, S.C. Natural Antibacterial and Antivirulence Alkaloids From Macleaya cordata Against Methicillin-Resistant Staphylococcus aureus. Front. Pharmacol. 2022, 13, 813172. [Google Scholar] [CrossRef]
- Shi, Y.; Zhong, L.; Chen, K.; Fan, Y.; Xie, K.; Zhang, J.; Dai, J.; Hu, Y. Sanguinarine Attenuates Hydrogen Peroxide-Induced Toxicity in Liver of Monopterus Albus: Role of Oxidative Stress, Inflammation and Apoptosis. Fish Shellfish Immunol. 2022, 125, 190–199. [Google Scholar] [CrossRef]
- Singh, C.K.; Kaur, S.; George, J.; Nihal, M.; Hahn, M.C.P.; Scarlett, C.O.; Ahmad, N. Molecular Signatures of Sanguinarine in Human Pancreatic Cancer Cells: A Large Scale Label-Free Comparative Proteomics Approach. Oncotarget 2015, 6, 10335. [Google Scholar] [CrossRef]
- Gaziano, R.; Moroni, G.; Buè, C.; Miele, M.T.; Sinibaldi-Vallebona, P.; Pica, F. Antitumor Effects of the Benzophenanthridine Alkaloid Sanguinarine: Evidence and Perspectives. World J. Gastrointest. Oncol. 2016, 8, 30. [Google Scholar] [CrossRef]
- Sun, Q.; Li, W.; Li, H.; Wang, X.; Wang, Y.; Niu, X. Preparation, Characterization and Anti-Ulcer Efficacy of Sanguinarine Loaded Solid Lipid Nanoparticles. Pharmacology 2017, 100, 14–24. [Google Scholar] [CrossRef]
- Jeng, J.H.; Wu, H.L.; Lin, B.R.; Lan, W.H.; Chang, H.H.; Ho, Y.S.; Lee, P.H.; Wang, Y.J.; Wang, J.S.; Chen, Y.J.; et al. Antiplatelet Effect of Sanguinarine Is Correlated to Calcium Mobilization, Thromboxane and CAMP Production. Atherosclerosis 2007, 191, 250–258. [Google Scholar] [CrossRef]
- Lou, G.; Wang, J.; Hu, J.; Gan, Q.; Peng, C.; Xiong, H.; Huang, Q. Sanguinarine: A Double-Edged Sword of Anticancer and Carcinogenesis and Its Future Application Prospect. Anticancer Agents Med. Chem. 2021, 21, 2100–2110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xie, J.; Wang, G.; Zhang, G.; Yang, H. Anti-Osteoporosis Activity of Sanguinarine in Preosteoblast MC3T3-E1 Cells and an Ovariectomized Rat Model. J. Cell. Physiol. 2018, 233, 4626–4633. [Google Scholar] [CrossRef]
- de Souza Silva, M.S.; dos Santos, M.L.M.F.; da Silva, A.M.; França, W.W.M.; Araújo, S.B.; da Silva, R.L.; do Nascimento, W.R.C.; da Silva Santos, N.P.; da Cruz Filho, I.J.; de Azevedo Albuquerque, M.C.P.; et al. Sanguinarine: An Alkaloid with Promising in Vitro and in Vivo Antiparasitic Activity against Different Developmental Stages of Schistosoma Mansoni and in Silico Pharmacokinetic Properties (ADMET). Parasitol. Res. 2024, 123, 143. [Google Scholar] [CrossRef]
- Croaker, A.; King, G.J.; Pyne, J.H.; Anoopkumar-Dukie, S.; Liu, L. Sanguinaria Canadensis: Traditional Medicine, Phytochemical Composition, Biological Activities and Current Uses. Int. J. Mol. Sci. 2016, 17, 1414. [Google Scholar] [CrossRef]
- Jana, J.; Mondal, S.; Bhattacharjee, P.; Sengupta, P.; Roychowdhury, T.; Saha, P.; Kundu, P.; Chatterjee, S. Chelerythrine down Regulates Expression of VEGFA, BCL2 and KRAS by Arresting G-Quadruplex Structures at Their Promoter Regions. Sci. Rep. 2017, 7, 40706. [Google Scholar] [CrossRef]
- Wei, Q.; Zhao, M.; Li, X. Extraction of Chelerythrine and Its Effects on Pathogenic Fungus Spore Germination. Pharmacogn. Mag. 2017, 13, 600. [Google Scholar] [CrossRef]
- Wei, Q.H.; Cui, D.Z.; Liu, X.F.; Chai, Y.Y.; Zhao, N.; Wang, J.Y.; Zhao, M. In Vitro Antifungal Activity and Possible Mechanisms of Action of Chelerythrine. Pestic. Biochem. Physiol. 2020, 164, 140–148. [Google Scholar] [CrossRef]
- Zheng, W.; Qiu, L.; Wang, R.; Feng, X.; Han, Y.; Zhu, Y.; Chen, D.; Liu, Y.; Jin, L.; Li, Y. Selective Targeting of PPARγ by the Natural Product Chelerythrine with a Unique Binding Mode and Improved Antidiabetic Potency. Sci. Rep. 2015, 5, 12222. [Google Scholar] [CrossRef] [PubMed]
- Vrba, J.; Doležel, P.; Vičar, J.; Modrianský, M.; Ulrichová, J. Chelerythrine and Dihydrochelerythrine Induce G1 Phase Arrest and Bimodal Cell Death in Human Leukemia HL-60 Cells. Toxicol. Vitr. 2008, 22, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Pu, Q.; Wang, R.; Gu, Y.; He, L.; Du, X.; Tang, G.; Han, D. Antibacterial Activity and Mechanism of Chelerythrine against Streptococcus agalactiae. Front. Vet. Sci. 2024, 11, 1408376. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Yao, J.Y.; Zhou, Z.M.; Shen, J.Y.; Ru, H.S.; Liu, X.L. Activity of the Chelerythrine, a Quaternary Benzo[c]Phenanthridine Alkaloid from Chelidonium majus L. on Dactylogyrus intermedius. Parasitol. Res. 2011, 109, 247–252. [Google Scholar] [CrossRef]
- Li, W.F.; Hao, D.J.; Fan, T.; Huang, H.M.; Yao, H.; Niu, X.F. Protective Effect of Chelerythrine against Ethanol-Induced Gastric Ulcer in Mice. Chem. Biol. Interact. 2014, 208, 18–27. [Google Scholar] [CrossRef]
- Chmura, S.J.; Dolan, M.E.; Cha, A.; Mauceri, H.J.; Kufe, D.W.; Weichselbaum, R.R. In vitro and in vivo activity of protein kinase C inhibitor chelerythrine chloride induces tumor cell toxicity and growth delay in vivo. Clin. Cancer Res. 2000, 6, 737–742. [Google Scholar]
- Wang, J.; Song, Y.; Zhang, N.; Li, N.; Liu, C.; Wang, B. Using Liposomes to Alleviate the Toxicity of Chelerythrine, a Natural PKC Inhibitor, in Treating Non-Small Cell Lung Cancer. Front. Oncol. 2021, 11, 658543. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, Y.; Zhang, L.; Zhang, J.; Wei, X. Chelerythrine Chloride from Macleaya cordata Induces Growth Inhibition and Apoptosis in Human Gastric Cancer BGC-823 Cells. Acta Pharm. Sin. B 2012, 2, 464–471. [Google Scholar] [CrossRef]
- Al-Mamoori, F.; Qasem, A.M.A.; Al-Mamoori, F.; Qasem, A.M.A. Alkaloids as New Leads for Neurodegenerative Diseases. In Medicinal Plants—Chemical, Biochemical, and Pharmacological Approaches; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Zhu, H.; Li, C.; Gao, C. Applications of CRISPR–Cas in Agriculture and Plant Biotechnology. Nat. Rev. Mol. Cell Biol. 2020, 21, 661–677. [Google Scholar] [CrossRef]
- Hassan, M.M.; Zhang, Y.; Yuan, G.; De, K.; Chen, J.G.; Muchero, W.; Tuskan, G.A.; Qi, Y.; Yang, X. Construct Design for CRISPR/Cas-Based Genome Editing in Plants. Trends Plant Sci. 2021, 26, 1133–1152. [Google Scholar] [CrossRef]
- Alagoz, Y.; Gurkok, T.; Zhang, B.; Unver, T. Manipulating the Biosynthesis of Bioactive Compound Alkaloids for Next-Generation Metabolic Engineering in Opium Poppy Using CRISPR-Cas 9 Genome Editing Technology. Sci. Rep. 2016, 6, 30910. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, G.; Cheng, C.; Lei, L.; Sun, J.; Xu, Y.; Deng, C.; Dai, Z.; Yang, Z.; Chen, X.; et al. Establishment of an Agrobacterium-Mediated Genetic Transformation and CRISPR/Cas9-Mediated Targeted Mutagenesis in Hemp (Cannabis sativa L.). Plant Biotechnol. J. 2021, 19, 1979–1987. [Google Scholar] [CrossRef]
- Xu, Z.; Xia, L.; Sun, M.; Huang, P.; Zeng, J. Effects of Codon Optimization, N-Terminal Truncation and Gene Dose on the Heterologous Expression of Berberine Bridge Enzyme. World J. Microbiol. Biotechnol. 2022, 38, 77. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, Y.; Duan, Y.; Deng, D.; Gao, Q.; Shen, Q.; Fang, W.; Zhu, X.; Chen, Y.; Cao, Y.; et al. The Effects of Overexpressing UDPGlycosyltransferases Genes on the Plant Response to Abiotic Stress a Metaanalysis. Beverage Plant Res. 2023, 3, 28. [Google Scholar] [CrossRef]
- Wen, Y.; Liao, Y.; Tang, Y.; Zhang, H.; Zhang, J.; Liao, Z. Metabolic Effects of Elicitors on the Biosynthesis of Tropane Alkaloids in Medicinal Plants. Plants 2023, 12, 3050. [Google Scholar] [CrossRef]
- Inui, T.; Tamura, K.I.; Fujii, N.; Morishige, T.; Sato, F. Overexpression of Coptis Japonica Norcoclaurine 6- O -Methyltransferase Overcomes the Rate-Limiting Step in Benzylisoquinoline Alkaloid Biosynthesis in Cultured Eschscholzia californica. Plant Cell Physiol. 2007, 48, 252–262. [Google Scholar] [CrossRef]
- Moyano, E.; Jouhikainen, K.; Tammela, P.; Palazón, J.; Cusidó, R.M.; Piñol, M.T.; Teeri, T.H.; Oksman-Caldentey, K.M. Effect of Pmt Gene Overexpression on Tropane Alkaloid Production in Transformed Root Cultures of Datura metel and Hyoscyamus muticus. J. Exp. Bot. 2003, 54, 203–211. [Google Scholar] [CrossRef]
- Huang, P.; Xia, L.; Zhou, L.; Liu, W.; Wang, P.; Qing, Z.; Zeng, J. Influence of Different Elicitors on BIA Production in Macleaya cordata. Sci. Rep. 2021, 11, 619. [Google Scholar] [CrossRef]
- Huang, P.; Xia, L.; Liu, W.; Jiang, R.; Liu, X.; Tang, Q.; Xu, M.; Yu, L.; Tang, Z.; Zeng, J. Hairy Root Induction and Benzylisoquinoline Alkaloid Production in Macleaya cordata. Sci. Rep. 2018, 8, 11986. [Google Scholar] [CrossRef]
- Yang, P.; Qi, Y.; Huang, B.; Huang, X. The ARF Gene Family in the Medicinal Plant Macleaya cordata: Genome-Wide Survey and Gene Expression Profiling. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Wink, M.; Schimmer, O. Molecular Modes of Action of Defensive Secondary Metabolites. In Functions and Biotechnology of Plant Secondary Metabolites, 2nd ed.; Wiley: Hoboken, NJ, USA, 2010; Volume 39, pp. 21–161. [Google Scholar] [CrossRef]
- Manoharan, R.; Nair, C.S.; Eissa, N.; Cheng, H.; Ge, P.; Ren, M.; Jaleel, A. Therapeutic Potential of Solanum Alkaloids with Special Emphasis on Cancer: A Comprehensive Review. Drug Des. Dev. Ther. 2024, 18, 3063–3074. [Google Scholar] [CrossRef] [PubMed]
- Gilani, A.H. Atta-ur-Rahman Trends in Ethnopharmacology. J. Ethnopharmacol. 2005, 100, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Chen, Y.; Chen, J.; Liao, H.; Li, Y.; Ma, Y. Jatrorrhizine: A Review of Sources, Pharmacology, Pharmacokinetics and Toxicity. Front. Pharmacol. 2022, 12, 783127. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Harwansh, R.K.; Bhattacharyya, S. Bioavailability of Herbal Products: Approach Toward Improved Pharmacokinetics. In Evidence-Based Validation of Herbal Medicine; Elsevier: Amsterdam, The Netherlands, 2015; pp. 217–245. [Google Scholar] [CrossRef]
- Pignatello, R.; Cianciolo, S.; Giuffrida, A.K. Drug Delivery Systems for the Controlled Delivery of Berberine. In Novel Drug Delivery Systems for Phytoconstituents; Taylor & Francis: Abingdon, UK, 2019; pp. 283–300. [Google Scholar] [CrossRef]
- Dastmalchi, M.; Chang, L.; Chen, R.; Yu, L.; Chen, X.; Hagel, J.M.; Facchini, P.J. Purine Permease-Type Benzylisoquinoline Alkaloid Transporters in Opium Poppy. Plant Physiol. 2019, 181, 916–933. [Google Scholar] [CrossRef]
- Shitan, N.; Dalmas, F.; Dan, K.; Kato, N.; Ueda, K.; Sato, F.; Forestier, C.; Yazaki, K. Characterization of Coptis Japonica CjABCB2, an ATP-Binding Cassette Protein Involved in Alkaloid Transport. Phytochemistry 2013, 91, 109–116. [Google Scholar] [CrossRef]
- Angelova, S.; Buchheim, M.; Frowitter, D.; Schierhorn, A.; Roos, W. Overproduction of Alkaloid Phytoalexins in California Poppy Cells Is Associated with the Co-Expression of Biosynthetic and Stress-Protective Enzymes. Mol. Plant 2010, 3, 927–939. [Google Scholar] [CrossRef]
- Zhan, X.; Chen, Z.; Chen, R.; Shen, C. Environmental and Genetic Factors Involved in Plant Protection-Associated Secondary Metabolite Biosynthesis Pathways. Front. Plant Sci. 2022, 13, 877304. [Google Scholar] [CrossRef]
- Deloache, W.C.; Russ, Z.N.; Narcross, L.; Gonzales, A.M.; Martin, V.J.J.; Dueber, J.E. An Enzyme-Coupled Biosensor Enables (S)-Reticuline Production in Yeast from Glucose. Nat. Chem. Biol. 2015, 11, 465–471. [Google Scholar] [CrossRef]
- Vavricka, C.J.; Yoshida, T.; Kuriya, Y.; Takahashi, S.; Ogawa, T.; Ono, F.; Agari, K.; Kiyota, H.; Li, J.; Ishii, J.; et al. Mechanism-Based Tuning of Insect 3,4-Dihydroxyphenylacetaldehyde Synthase for Synthetic Bioproduction of Benzylisoquinoline Alkaloids. Nat. Commun. 2019, 10, 2015. [Google Scholar] [CrossRef]
- Jeyasri, R.; Muthuramalingam, P.; Karthick, K.; Shin, H.; Choi, S.H.; Ramesh, M. Methyl Jasmonate and Salicylic Acid as Powerful Elicitors for Enhancing the Production of Secondary Metabolites in Medicinal Plants: An Updated Review. Plant Cell Tissue Organ Cult. 2023, 153, 447–458. [Google Scholar] [CrossRef]
- Tanahashi, T.; Zenk, M.H. Elicitor Induction and Characterization of Microsomal Protopine-6-Hydroxylase, the Central Enzyme in Benzophenanthridine Alkaloid Biosynthesis. Phytochemistry 1990, 29, 1113–1122. [Google Scholar] [CrossRef]
- Gurkok, T.; Turktas, M.; Parmaksiz, I.; Unver, T. Transcriptome Profiling of Alkaloid Biosynthesis in Elicitor Induced Opium Poppy. Plant Mol. Biol. Rep. 2015, 33, 673–688. [Google Scholar] [CrossRef]
- Winzer, T.; Kern, M.; King, A.J.; Larson, T.R.; Teodor, R.I.; Donninger, S.L.; Li, Y.; Dowle, A.A.; Cartwright, J.; Bates, R.; et al. Morphinan Biosynthesis in Opium Poppy Requires a P450-Oxidoreductase Fusion Protein. Science 2015, 349, 309–312. [Google Scholar] [CrossRef]
- Birchfield, A.S.; McIntosh, C.A. Metabolic Engineering and Synthetic Biology of Plant Natural Products—A Minireview. Curr. Plant Biol. 2020, 24, 100163. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, X.; Liu, T.; Wang, Y.; Ahmed, N.; Li, Z.; Jiang, H. Synthetic Biology of Plant Natural Products: From Pathway Elucidation to Engineered Biosynthesis in Plant Cells. Plant Commun. 2021, 2, 100229. [Google Scholar] [CrossRef]
- Patra, B.; Schluttenhofer, C.; Wu, Y.; Pattanaik, S.; Yuan, L. Transcriptional Regulation of Secondary Metabolite Biosynthesis in Plants. Biochim. Biophys. Acta-Gene Regul. Mech. 2013, 1829, 1236–1247. [Google Scholar] [CrossRef]
- Yamada, Y.; Sato, F. Transcription Factors in Alkaloid Engineering. Biomolecules 2021, 11, 1719. [Google Scholar] [CrossRef]
- Fukushima, A.; Kusano, M.; Redestig, H.; Arita, M.; Saito, K. Integrated Omics Approaches in Plant Systems Biology. Curr. Opin. Chem. Biol. 2009, 13, 532–538. [Google Scholar] [CrossRef]
- Diamond, A.; Desgagné-Penix, I. Metabolic Engineering for the Production of Plant Isoquinoline Alkaloids. Plant Biotechnol. J. 2016, 14, 1319–1328. [Google Scholar] [CrossRef]
- Narcross, L.; Bourgeois, L.; Fossati, E.; Burton, E.; Martin, V.J.J. Mining Enzyme Diversity of Transcriptome Libraries through DNA Synthesis for Benzylisoquinoline Alkaloid Pathway Optimization in Yeast. ACS Synth. Biol. 2016, 5, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Dellas, N.; Thomas, S.T.; Manning, G.; Noel, J.P. Discovery of a Metabolic Alternative to the Classical Mevalonate Pathway. eLife 2013, 2, e00672. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.; Smolke, C.D. Engineering a Microbial Biosynthesis Platform for de Novo Production of Tropane Alkaloids. Nat. Commun. 2019, 10, 3634. [Google Scholar] [CrossRef]
- O’Connor, S.E. Engineering of Secondary Metabolism. Annu. Rev. Genet. 2015, 49, 71–94. [Google Scholar] [CrossRef]
- Deng, X.; Zhao, L.; Fang, T.; Xiong, Y.; Ogutu, C.; Yang, D.; Vimolmangkang, S.; Liu, Y.; Han, Y. Investigation of Benzylisoquinoline Alkaloid Biosynthetic Pathway and Its Transcriptional Regulation in Lotus. Hortic. Res. 2018, 5, 29. [Google Scholar] [CrossRef]
- Wang, Y.; Tappertzhofen, N.; Méndez-Sánchez, D.; Bawn, M.; Lyu, B.; Ward, J.M.; Hailes, H.C. Design and Use of de Novo Cascades for the Biosynthesis of New Benzylisoquinoline Alkaloids. Angew. Chem. Int. Ed. 2019, 58, 10120–10125. [Google Scholar] [CrossRef]
- Watkins, J.L.; Facchini, P.J. Compartmentalization at the Interface of Primary and Alkaloid Metabolism. Curr. Opin. Plant Biol. 2022, 66, 102186. [Google Scholar] [CrossRef]
| Target Gene/Purpose in Macleaya cordata (Plume Poppy) | Gene Manipulation Technique | Specialized Metabolites | References |
|---|---|---|---|
| Knockout of SMT | CRISPR/Cas9 mediated disruption of chelerythrine biosynthesis | Elevated sanguinarine levels by 3.29-fold | [27] |
| Upregulated expression of BBE | Endogenous expression of plume poppy BBE, a crucial enzyme for converting (S)-reticuline to (S)-scoulerine | Sanguinarine and chelerythrine were slightly reduced due to feedback inhibition of plume poppy BBE overexpression | [16] |
| Overexpression of BBE | Optimization and heterologous expression of a gene of plume poppy BBE | Elevated (S)-scoulerine, key precursor of chelerythrine yield by 58 times higher than the original level | [55] |
| Upregulated expression of P6H and DBOX | Elicitor-mediated gene regulation | Elevated sanguinarine and chelerythrine content by 10 and 14-fold, respectively | [60] |
| Upregulated expression of P6H | Over expression of plume poppy P6H | Elevated sanguinarine and chelerythrine production | [13] |
| Upregulated expression of P6H and DBOX | Induction of hairy roots | Elevated sanguinarine production | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rather, B.A.; Xu, W.; Tantray, A.Y.; Mahajan, M.; Sun, H.; Cong, H.; Jiang, X.; Khan, M.I.R.; Qiao, F. Biotechnological Advances in Sanguinarine and Chelerythrine Production from Plume Poppy (Macleaya cordata): A Gene Editing Perspective. Plants 2025, 14, 2667. https://doi.org/10.3390/plants14172667
Rather BA, Xu W, Tantray AY, Mahajan M, Sun H, Cong H, Jiang X, Khan MIR, Qiao F. Biotechnological Advances in Sanguinarine and Chelerythrine Production from Plume Poppy (Macleaya cordata): A Gene Editing Perspective. Plants. 2025; 14(17):2667. https://doi.org/10.3390/plants14172667
Chicago/Turabian StyleRather, Bilal A., Wujun Xu, Aadil Yousuf Tantray, Moksh Mahajan, Huapeng Sun, Hanqing Cong, Xuefei Jiang, M. Iqbal R. Khan, and Fei Qiao. 2025. "Biotechnological Advances in Sanguinarine and Chelerythrine Production from Plume Poppy (Macleaya cordata): A Gene Editing Perspective" Plants 14, no. 17: 2667. https://doi.org/10.3390/plants14172667
APA StyleRather, B. A., Xu, W., Tantray, A. Y., Mahajan, M., Sun, H., Cong, H., Jiang, X., Khan, M. I. R., & Qiao, F. (2025). Biotechnological Advances in Sanguinarine and Chelerythrine Production from Plume Poppy (Macleaya cordata): A Gene Editing Perspective. Plants, 14(17), 2667. https://doi.org/10.3390/plants14172667







