Differences in the Response of Invasive Solidago canadensis and Native Imperata cylindrica to Glyphosate
Abstract
1. Introduction
2. Results
2.1. Plant Mortality Rate in Glyphosate and Competition Treatments
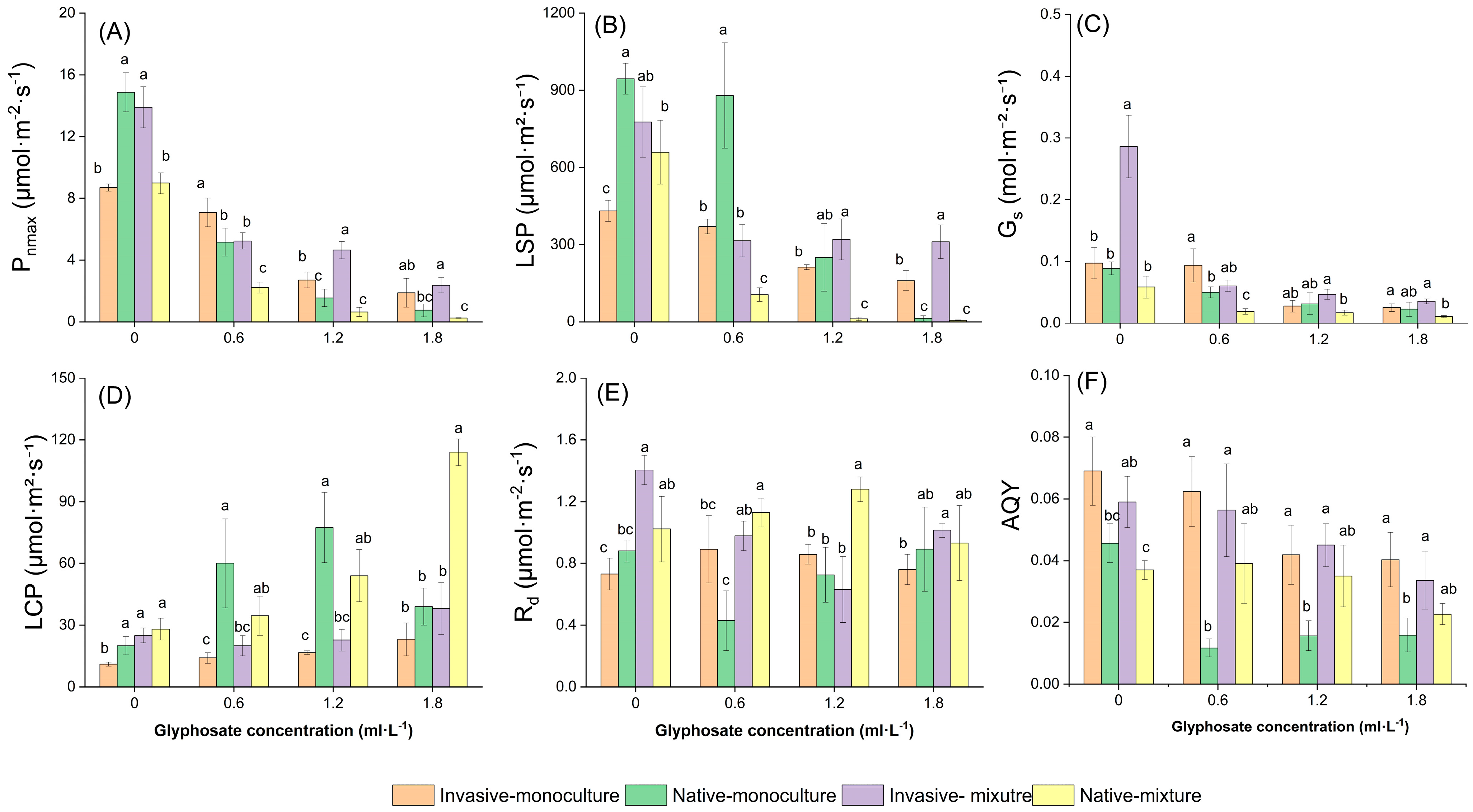
2.2. Response of Photosynthetic Capacity to Glyphosate Application and Competition
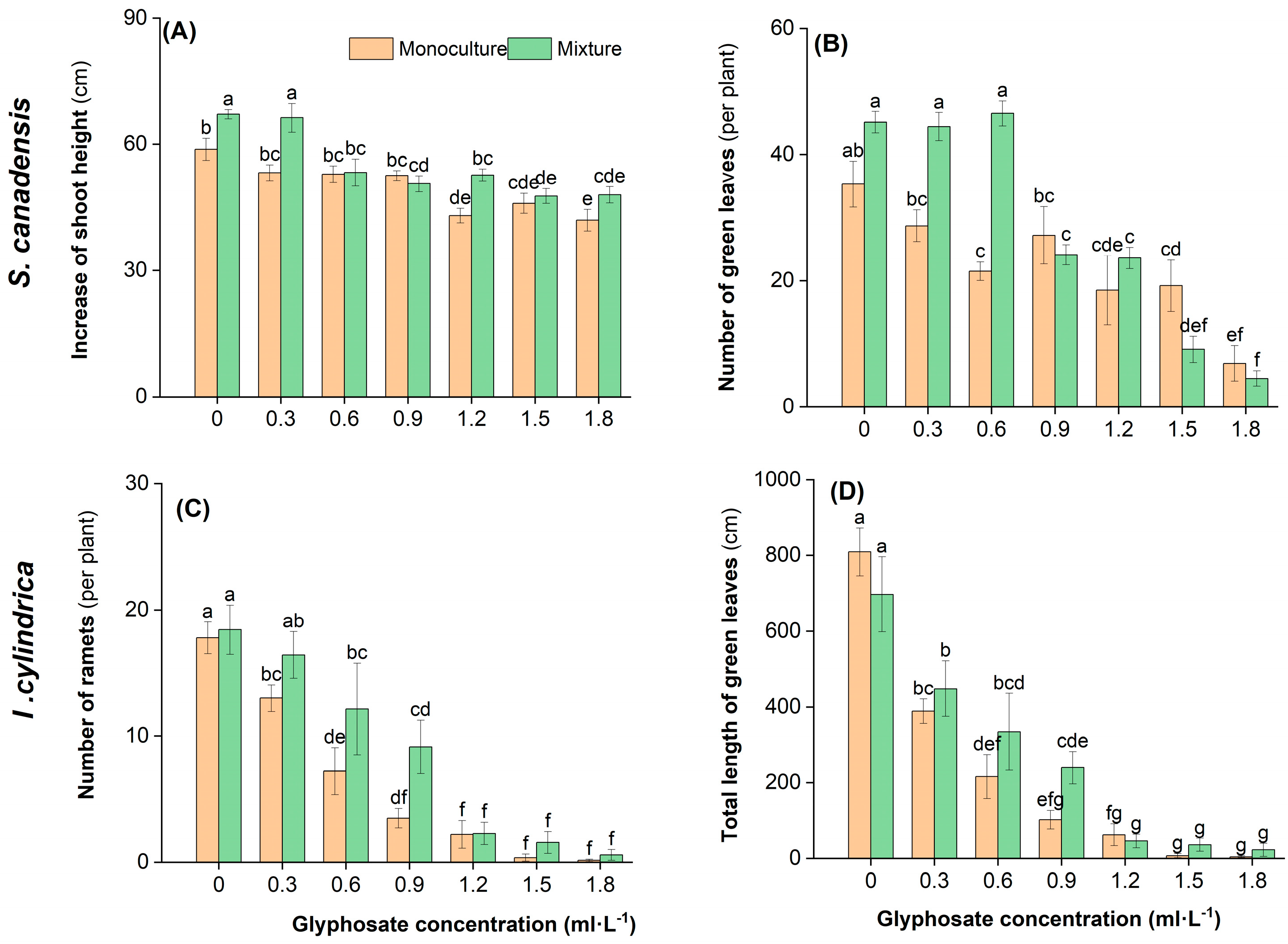
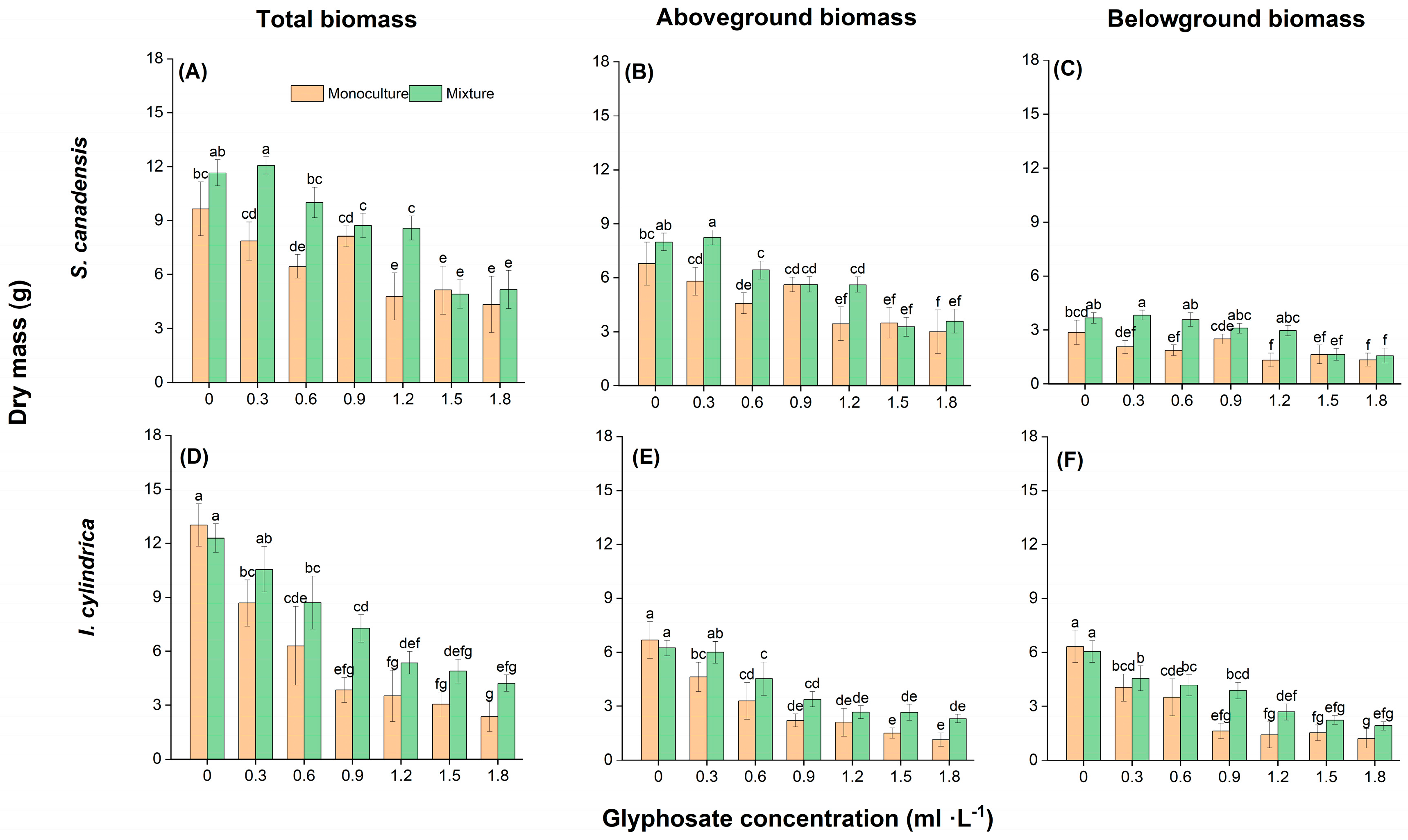
2.3. Plant Growth Response to Glyphosate and Competition
3. Discussion
3.1. High Tolerance of S. canadensis to Glyphosate
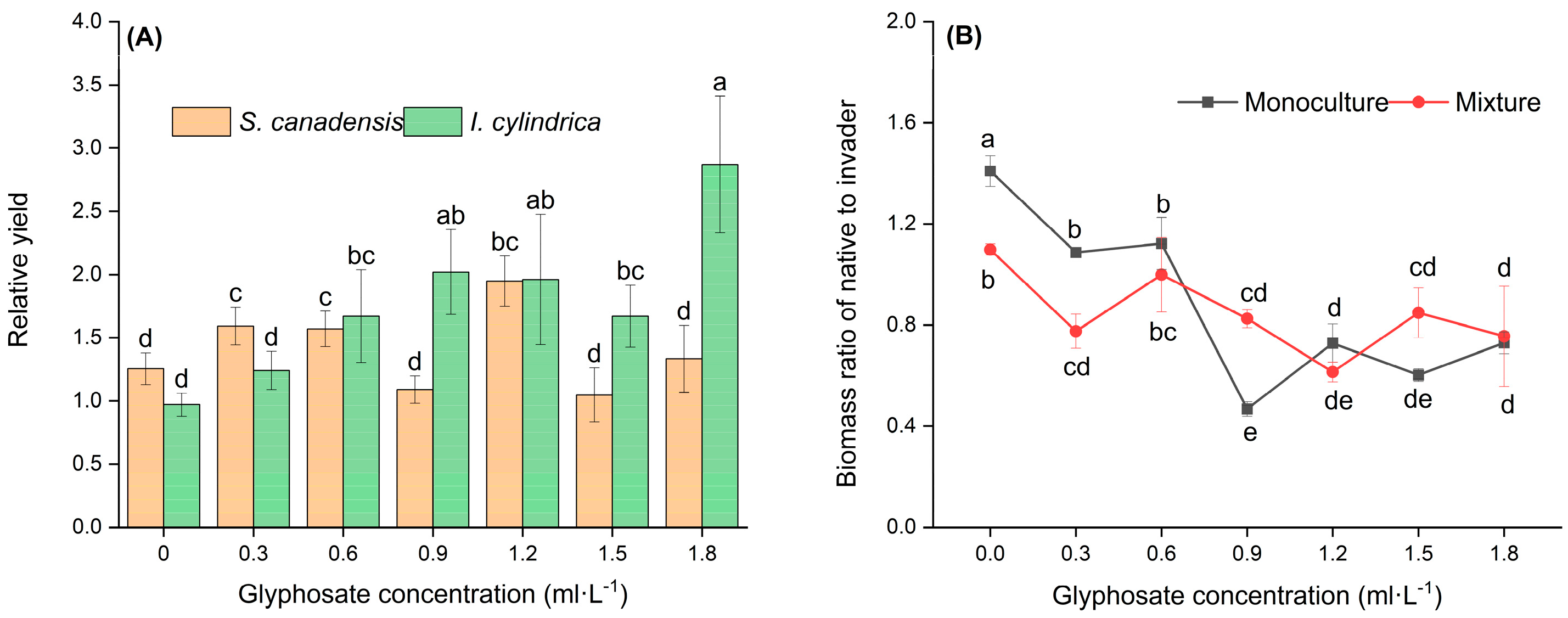
3.2. Glyphosate Treatment Altered Competition Between S. canadensis and I. cylindrica
3.3. Potential Approaches for Alleviating Nontarget Effects of Herbicides
4. Materials and Methods
4.1. Seedling Culture
4.2. Competition and Glyphosate Treatments
4.3. Photosynthesis Measurements
4.4. Measurement of Mortality, Growth, and Competition Effects
4.5. Data Analysis and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Vantarová, K.H.; Eliáš, P., Jr.; Jiménez-Ruiz, J.; Tokarska-Guzik, B.; Cires, E. Biological invasions in the twenty-first century: A global risk. Biologia 2023, 78, 1211–1218. [Google Scholar] [CrossRef]
- Pejchar, L.; Mooney, H.A. Invasive species, ecosystem services and human well-being. Trends Ecol. Evol. 2009, 24, 497–504. [Google Scholar] [CrossRef]
- Simberloff, D.; Martin, J.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef]
- Diagne, C.; Leroy, B.; Vaissière, A.C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.; Bradshaw, C.J.A.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Simberloff, D. We can eliminate invasions or live with them. Successful management projects. Biol. Invasions 2009, 11, 149157. [Google Scholar] [CrossRef]
- Olszańska, A.; Solarz, W.; Najberek, K. To kill or not to kill—Practitioners’ opinions on invasive alien species management as a step towards enhancing control of biological invasions. Environ. Sci. Policy. 2016, 58, 107–116. [Google Scholar] [CrossRef]
- Wagner, V.; Antunes, P.M.; Irvine, M.; Nelson, C.R. Herbicide usage for invasive non-native plant management in wildland areas of North America. J. Appl. Ecol. 2017, 54, 198–204. [Google Scholar] [CrossRef]
- Mingione, J.E.; Coon, J.J.; Miller, J.R. Evaluating native plant community characteristics after restoration efforts in invaded tallgrass prairies. Rangel. Ecol. Manag. 2025, 98, 134–145. [Google Scholar] [CrossRef]
- Weidenhamer, J.D.; Callaway, R.M. Direct and indirect effects of invasive plants on soil chemistry and ecosystem function. J. Chem. Ecol. 2010, 36, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Schwoerer, T.; Morton, J.M.; Little, J. Changes in perceived risk and ecosystem services after herbicide use on an aquatic invader. Ecosyst. People 2024, 20, 2381628. [Google Scholar] [CrossRef]
- Rinella, M.J.; Maxwell, B.D.; Fay, P.K.; Weaver, T.; Sheley, R.L. Control effort exacerbates invasive-species problem. Ecol. Appl. 2009, 19, 155–162. [Google Scholar] [CrossRef]
- LaBar, C.C.; Schultz, C.B. Investigating the role of herbicides in controlling invasive grasses in prairie habitats: Effects on non-target butterflies. Nat. Areas J. 2012, 32, 177–189. [Google Scholar] [CrossRef]
- Mikulyuk, A.; Kujawa, E.; Nault, M.E.; Van Egeren, S.; Wagner, K.I.; Barton, M.; Hauxwell, J.; Zanden, M.J.V.; Kevan, P.G. Is the cure worse than the disease? Comparing the ecological effects of an invasive aquatic plant and the herbicide treatments used to control it. Facets 2020, 5, 353–366. [Google Scholar] [CrossRef]
- Londo, J.P.; Bautista, N.S.; Sagers, C.L.; Lee, E.H.; Watrud, L.S. Glyphosate drift promotes changes in fitness and transgene gene flow in canola (Brassica napus) and hybrids. Ann. Bot. 2010, 106, 957–965. [Google Scholar] [CrossRef]
- Mateos-Naranjo, E.; Perez-Martin, A. Effects of sub-lethal glyphosate concentrations on growth and photosynthetic performance of non-target species Bolboschoenus maritimus. Chemosphere 2013, 93, 2631–2638. [Google Scholar] [CrossRef]
- Neumann, G.; Kohls, S.; Landsberg, E.; Stock-Oliveira Souza, K.; Yamada, T.; Römheld, V. Relevance of glyphosate transfer to non-target plants via the rhizosphere. J. Plant Dis. Prot. 2006, 20, 963–969. [Google Scholar]
- Tesfamariam, T.; Bott, S.; Cakmak, I.; Römheld, V.; Neumann, G. Glyphosate in the rhizosphere—Role of waiting times and different glyphosate binding forms in soils for phytotoxicity to non-target plants. Eur. J. Agron. 2009, 31, 126–132. [Google Scholar] [CrossRef]
- McManamen, C.; Nelson, C.R.; Wagner, V. Timing of seeding after herbicide application influences rates of germination and seedling biomass of native plants used for grassland restoration. Restor Ecol. 2018, 26, 1137–1148. [Google Scholar] [CrossRef]
- Carlson, A.M.; Gorchov, D.L. Effects of herbicide on the invasive Biennial Alliaria petiolata (Garlic Mustard) and initial responses of native plants in a Southwestern Ohio forest. Restor. Ecol. 2004, 12, 559–567. [Google Scholar] [CrossRef]
- Peterson, P.G.; Merrett, M.F.; Fowler, S.V.; Barrett, D.P.; Paynter, Q. Comparing biocontrol and herbicide for managing an invasive non-native plant species: Efficacy, non-target effects and secondary invasion. J. Appl. Ecol. 2020, 57, 1876–1884. [Google Scholar] [CrossRef]
- Heckman, R.W.; McColley, C.; Slater, M.N.; Carr, D.E. The role of community composition in grassland response to two methods of exotic forb removal. Weed Res. 2017, 57, 44–53. [Google Scholar] [CrossRef]
- Cornish, P.S.; Burgin, S. Residual effects of glyphosate herbicide in ecological restoration. Restor. Ecol. 2005, 13, 695–702. [Google Scholar] [CrossRef]
- Marrs, R.H.; Frost, A.J.; Plant, R.A. Effect of mecoprop drift on some plant species of conservation interest when grown in standardized mixtures in microcosms. Environ. Pollut. 1991, 73, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Kurniadie, D.; Widianto, R.; Widayat, D.; Umiyati, U.; Nasahi, C.; Kato-Noguchi, H. Herbicide-resistant invasive plant species Ludwigia decurrens Walter. Plants 2021, 10, 1973. [Google Scholar] [CrossRef] [PubMed]
- Ice, D. Resistance to Three Common Herbicides in Chameleon Plant (Houttuynia cordata Thunb.), a Highly Invasive Exotic Species. Master’s Thesis, Purdue University, West Lafayette, IN, USA, 2022. [Google Scholar]
- Crone, E.E.; Marler, M.; Pearson, D.E. Non-target effects of broadleaf herbicide on a native perennial forb: A demographic framework for assessing and minimizing impacts. J. Appl. Ecol. 2009, 46, 673–682. [Google Scholar] [CrossRef]
- Cheng, J.; Li, J.; Zhang, Z.; Lu, H.; Chen, G.; Yao, B.; Dong, Y.; Ma, L.; Yuan, X.; Xu, J.; et al. Autopolyploidy-driven range expansion of a temperate-originated plant to pan-tropic under global change. Ecol. Monogr. 2021, 91, e01445. [Google Scholar] [CrossRef]
- Szymura, M.; Świerszcz, S.; Szymura, T.H. Restoration of ecologically valuable grassland on sites degraded by invasive Solidago: Lessons from a 6-year experiment. Land Degrad. Dev. 2022, 33, 1985–1998. [Google Scholar] [CrossRef]
- Lin, H.; Chen, L.; Li, J. Multiple introductions and distinct. genetic groups of canada goldenrod (Solidago canadensis) in China revealed by genomic single-nucleotide polymorphisms. Plants 2023, 12, 1734. [Google Scholar] [CrossRef]
- Wang, C.Y.; Jiang, K.; Liu, J.; Zhou, J.W.; Wu, B.D. Moderate and heavy Solidago canadensis L. invasion are associated with decreased taxonomic diversity but increased functional diversity of plant communities in East China. Ecol. Eng. 2018, 112, 55–64. [Google Scholar] [CrossRef]
- Szymura, M.; Szymura, T.H. Interactions between alien goldenrods (Solidago and Euthamia species) and comparison with native species in Central Europe. Flora 2016, 218, 51–61. [Google Scholar] [CrossRef]
- Świerszcz, S.; Szymura, M.; Wolski, K.; Szymura, T.H. Comparison of methods for restoring meadows invaded by Solidago species. Pol. J. Environ. Stud. 2017, 26, 1251–1258. [Google Scholar] [CrossRef]
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef]
- Richmond, M.E. Glyphosate: A review of its global use, environmental impact, and potential health effects on humans and other species. J. Environ. Stud. Sci. 2018, 8, 416–434. [Google Scholar] [CrossRef]
- Ye, X.Q.; Ma, R.X.; Lei, S.T.; Wu, M.; Yu, F.H. Maintained nutrient accumulation in invasive Solidago canadensis in response to competition: Competition tolerance and nutrient accumulation. Flora 2022, 295, 152136. [Google Scholar] [CrossRef]
- Yanniccari, M.; Tambussi, E.; Istilart, C.; Castro, A.M. Glyphosate effects on gas exchange and chlorophyll fluorescence responses of two Lolium perenne L. biotypes with differential herbicide sensitivity. Plant Physiol. Biochem. 2012, 57, 210–217. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Baucom, R.S. Evolutionary and ecological insights from herbicide-resistant weeds: What have we learned about plant adaptation, and what is left to uncover? New Phytol. 2019, 223, 68–82. [Google Scholar] [CrossRef]
- Onen, H.; Farooq, S.; Gunal, H.; Ozaslan, C.; Erdem, H. Higher tolerance to abiotic stresses and soil types may accelerate common ragweed (Ambrosia artemisiifolia) invasion. Weed Sci. 2017, 65, 115–127. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Jagodziński, A.M. Low impact of disturbance on ecological success of invasive tree and shrub species in temperate forests. Plant Ecol. 2018, 219, 1369–1380. [Google Scholar] [CrossRef]
- Hodgins, K.A.; Guggisberg, A.; Nurkowski, K.; Rieseberg, L.H. Genetically based trait differentiation but lack of trade-offs between stress tolerance and performance in introduced Canada Thistle. Plant Commun. 2020, 1, 100116. [Google Scholar] [CrossRef]
- Loubet, I.; Caddoux, L.; Fontaine, S.; Michel, S.; Pernin, F.; Barrès, B.; Le Corre, V.; Délye, C. A high diversity of mechanisms endows ALS-inhibiting herbicide resistance in the invasive common ragweed (Ambrosia artemisiifolia L.). Sci. Rep. 2021, 11, 19904. [Google Scholar] [CrossRef]
- Zenni, R.D.; Bailey, J.K.; Simberloff, D. Rapid evolution and range expansion of an invasive plant are driven by provenance-environment interactions. Ecol. Lett. 2014, 17, 727–735. [Google Scholar] [CrossRef]
- Wang, S.; Liao, Z.Y.; Cao, P.; Schmid, M.W.; Zhang, L.; Bi, J.; Endriss, S.B.; Zhao, Y.; Parepa, M.; Hu, W.Y.; et al. General-purpose genotypes and evolution of higher plasticity in clonality underlie knotweed invasion. New Phytol. 2025, 246, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Xue, L.; Cheng, J.; Yang, X.; Xie, H.; Song, X.; Qiang, S. Polyploidization-driven differentiation of freezing tolerance in Solidago canadensis. Plant Cell Environ. 2020, 43, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Powles, S. Metabolism-based herbicide resistance and cross-resistance in crop weeds: A threat to herbicide sustainability and global crop production. Plant Physiol. 2014, 166, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Yang, H. Metabolism and detoxification of pesticides in plants. Sci. Total Environ. 2021, 790, 148034. [Google Scholar] [CrossRef] [PubMed]
- Van Kleunen, M.; Weber, E.; Fischer, M. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 2010, 13, 235–245. [Google Scholar] [CrossRef]
- Gallagher, R.V.; Randall, R.P.; Leishman, M.R. Trait differences between naturalized and invasive plant species independent of residence time and phylogeny. Conserv. Biol. 2015, 29, 360–369. [Google Scholar] [CrossRef]
- Song, Y.B.; Yu, F.H.; Keser, L.H.; Dawson, W.; Fischer, M.; Dong, M.; van Kleunen, M. United we stand, divided we fall: A meta-analysis of experiments on clonal integration and its relationship to invasiveness. Oecologia 2013, 171, 317–327. [Google Scholar] [CrossRef]
- Wang, Y.J.; Müller-Schärer, H.; van Kleunen, M.; Cai, A.M.; Zhang, P.; Yan, R.; Dong, B.C.; Yu, F.H. Invasive alien plants benefit more from clonal integration in heterogeneous environments than natives. New Phytol. 2017, 216, 1072–1078. [Google Scholar] [CrossRef]
- Roiloa, S.R.; Yu, F.H.; Barreiro, R. Effects of glyphosate application on physiologically integrated clones of the invasive plant Carpobrotus edulis. Diversity 2022, 14, 47. [Google Scholar] [CrossRef]
- Hu, D.; Jiang, X.Q.; Dai, Z.C.; Chen, D.Y.; Zhang, Y.; Qi, S.S.; Du, D.L. Arbuscular mycorrhizal fungi enhance the capacity of invasive Sphagneticola trilobata to tolerate herbicides. Chinese J. Plant Ecol. 2024, 48, 651–659. [Google Scholar] [CrossRef]
- Möhrle, K.; Reyes-Aldana, H.E.; Kollmann, J.; Teixeira, L.H. Suppression of an invasive native plant species by designed grassland communities. Plants 2021, 10, 775. [Google Scholar] [CrossRef] [PubMed]
- Byun, C.; Kettenring, K.M.; Tarsa, E.E.; de Blois, S. Applying ecological principles to maximize resistance to invasion in restored plant communities. Ecol. Eng. 2023, 190, 106926. [Google Scholar] [CrossRef]
- Golivets, M.; Wallin, K.F. Neighbour tolerance, not suppression, provides competitive advantage to non-native plants. Ecol. Lett. 2018, 21, 745–759. [Google Scholar] [CrossRef]
- Castro-Díez, P.; Pauchard, A.; Traveset, A.; Vilà, M. Linking the impacts of plant invasion on community functional structure and ecosystem properties. J. Veg. Sci. 2016, 27, 1233–1242. [Google Scholar] [CrossRef]
- Woch, M.W.; Kapusta, P.; Stanek, M.; Zubek, S.; Stefanowicz, A.M. Functional traits predict resident plant response to Reynoutria japonica invasion in riparian and fallow communities in southern Poland. AoB Plants 2021, 13, plab035. [Google Scholar] [CrossRef]
- Ye, X.Q.; Yan, Y.N.; Wu, M.; Yu, F.H. High capacity of nutrient accumulation by invasive Solidago canadensis in a coastal grassland. Front. Plant Sci. 2019, 10, 575. [Google Scholar] [CrossRef]
- Hutchinson, R.A.; Fremier, A.K.; Viers, J.H. Interaction of restored hydrological connectivity and herbicide suppresses dominance of a floodplain invasive species. Restor. Ecol. 2020, 28, 1551–1560. [Google Scholar] [CrossRef]
- Huang, Q.Q.; Fan, Z.W.; Li, X.X.; Wang, Y.; Liu, Y.; Shen, Y.D. Effects of nutrient addition and clipping on biomass production of invasive and native annual Asteraceae plants. Weed Res. 2018, 58, 318–326. [Google Scholar] [CrossRef]
- Simberloff, D. The role of propagule pressure in biological invasions. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 81–102. [Google Scholar] [CrossRef]
- Murillo, R.D.A.; Wagner, V. Propagule pressure and soil disturbance diminish plant community resistance to invasion across habitat types. J. Veg. Sci. 2025, 36, e70033. [Google Scholar] [CrossRef]
- Damgaard, C.; Strandberg, B.; Mathiassen, S.K.; Kudsk, P. The effect of nitrogen and glyphosate on survival and colonisation of perennial grass species in an agro-ecosystem: Does the relative importance of survival decrease with competitive ability? PLoS ONE 2013, 8, e60992. [Google Scholar] [CrossRef] [PubMed]
- Maestre, F.T.; Callaway, R.M.; Valladares, F.; Lortie, C.J. Refining the stress-gradient hypothesis Refining the stress-gradient hypothesis for competition and facilitation in plant communities. J. Ecol. 2009, 97, 199–205. [Google Scholar] [CrossRef]
- Spake, R.; Soga, M.; Catford, J.A.; Eigenbrod, F. Applying the stress-gradient hypothesis to curb the spread of invasive bamboo. J. Appl. Ecol. 2021, 58, 1993–2003. [Google Scholar] [CrossRef]
- Smith, A.L.; Kanjithanda, R.M.; Hayashi, T.; French, J.; Milner, R.N.C. Reducing herbicide input and optimizing spray method can minimize nontarget impacts on native grassland plant species. Ecol. Appl. 2023, 33, e2864. [Google Scholar] [CrossRef]
- Ye, X.Q.; Meng, J.L.; Ma, R.X.; Liang, J.L.; Wu, M.; Man, R.Z.; Yu, F.H. Winter leaf phenology differences facilitate selective control of an invasive plant species by herbicide. NeoBiota 2024, 96, 67–87. [Google Scholar] [CrossRef]
- Davies, K.W.; Clenet, D.R.; Madsen, M.D.; Brown, V.S.; Ritchie, A.L.; Svejcar, L.N. Activated carbon seed technologies: Innovative solutions to assist in the restoration and revegetation of invaded drylands. J. Environ. Manag. 2024, 371, 123281. [Google Scholar] [CrossRef] [PubMed]
- Svejcar, L.N.; Martyn, T.E.; Edlund, H.R.; Davies, K.W. A Test of activated carbon and soil seed enhancements for improved sub-shrub and grass seedling survival with and without herbicide application. Plants 2024, 13, 3074. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; McCarthy, B. A new index of interspecific competition for replacement and additive designs. Ecol. Res. 2001, 16, 29–40. [Google Scholar] [CrossRef]
- Ye, M.; Liu, S.; Liu, Q.; Ma, J.; Wang, X.; Bao, L.; Yang, Z.; Li, K. Effects of two herbicides on Solidago canadensis L. J. Anhui Sci. Tech. Univ. 2008, 22, 7–11, (In Chinese with English abstract). [Google Scholar]
- Xu, J.; Lv, Y.; Liu, X.; Wei, Q.; Qi, Z.; Yang, S.; Liao, L. A general non-rectangular hyperbola equation for photosynthetic light response curve of rice at various leaf ages. Sci. Rep. 2019, 9, 9909. [Google Scholar] [CrossRef] [PubMed]
- de Wit, C.T. On Competition; Pudoc: Wageningen, The Netherlands, 1960; pp. 1–82. [Google Scholar]
| Glyphosate Treatments (ml·L−1) | S. canadensis Monoculture | S. canadensis Mixture | I. cylindrica Monoculture | I. cylindrica Mixture |
|---|---|---|---|---|
| 0 | 0.0 ± 0.0 bA | 0.0 ± 0.0 bA | 0.0 ± 0.0 cA | 0.0 ± 0.0 cA |
| 0.3 | 0.0 ± 0.0 bA | 0.0 ± 0.0 bA | 0.0 ± 0.0 cA | 0.0 ± 0.0 cA |
| 0.6 | 14.3 ± 10.7 bA | 0.0 ± 0.0 bB | 14.3 ± 9.2 bA | 14.3 ± 9.2 cA |
| 0.9 | 7.1 ± 4.6 bB | 7.1 ± 2.1 bB | 39.3 ± 12.0 bA | 7.1 ± 7.1 cB |
| 1.2 | 25.0 ± 14.4 abB | 0.0 ± 0.0 bC | 71.4 ± 12.7 aA | 50.0 ± 15.4 bA |
| 1.5 | 21.4 ± 8.5 abC | 50.0 ± 10.9 aB | 89.3 ± 7.4 aA | 57.1 ± 14.9 abB |
| 1.8 | 46.4 ± 14.9 aB | 64.3 ± 14.3 aB | 92.9 ± 7.1 aA | 85.7 ± 9.2 aA |
| Treatments | Species | Photosynthetic Parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| Pnmax | LSP | GS | LCP | Rd | AQY | |||
| Glyphosate (G) | S. canadensis | F | 31.1 | 11.4 | 20.985 | 2.1 | 2.1 | 1.0 |
| P | 0.000 ** | 0.000 ** | 0.000 ** | 0.047 * | 0.146 | 0.423 | ||
| I. cylindrica | F | 44.1 | 18.9 | 10.399 | 2.2 | 1.5 | 3.3 | |
| P | 0.000 ** | 0.000 ** | 0.000 ** | 0.026 * | 0.25 | 0.046 * | ||
| Competition (C) | S. canadensis | F | 7.9 | 8.8 | 8.05 | 3.5 | 4.5 | 1.7 |
| P | 0.013 * | 0.009 * | 0.012 * | 0.081 | 0.05 | 0.213 | ||
| I. cylindrica | F | 10.9 | 17.8 | 7.8 | 0.03 | 19.8 | 11.5 | |
| P | 0.005 ** | 0.001 ** | 0.013 * | 0.874 | 0.009 ** | 0.004 ** | ||
| G × C | S. canadensis | F | 6.1 | 2.5 | 8.986 | 0.64 | 4.1 | 0.8 |
| P | 0.006 ** | 0.094 | 0.001 ** | 0.602 | 0.065 | 0.513 | ||
| I. cylindrica | F | 2.3 | 2.9 | 0.417 | 2.6 | 0.5 | 1.7 | |
| P | 0.112 | 0.066 | 0.743 | 0.086 | 0.656 | 0.204 | ||
| S. canadensis | ||||||
|---|---|---|---|---|---|---|
| Treatments | Significance | Total biomass | Aboveground biomass | Belowground biomass | Shoot height | Number of green leaves |
| Glyphosate (G) | F | 20.4 | 20.2 | 11.8 | 18.0 | 11.2 |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Competition (C) | F | 69.7 | 82.6 | 22.4 | 18.5 | 0.6 |
| P | <0.001 | <0.001 | <0.001 | <0.001 | 0.439 | |
| G × C | F | 2.9 | 0.5 | 2.9 | 3.0 | 1.6 |
| P | 0.011 | 0.03 | 0.013 | 0.01 | 0.151 | |
| I. cylindrica | ||||||
| Treatments | Significance | Total biomass | Aboveground biomass | Belowground biomass | Number of ramets | Total length of green leaves |
| Glyphosate (G) | F | 37.7 | 29.1 | 36.9 | 50.8 | 52.523 |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Competition (C) | F | 37.8 | 32.2 | 36.9 | 13.2 | 1.379 |
| P | <0.001 | <0.001 | <0.001 | <0.001 | 0.244 | |
| G × C | F | 7.8 | 5.6 | 8.7 | 5.8 | 1.254 |
| P | <0.001 | <0.001 | <0.001 | <0.001 | 0.288 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, X.; Gu, C.; Meng, J.; Wu, M. Differences in the Response of Invasive Solidago canadensis and Native Imperata cylindrica to Glyphosate. Plants 2025, 14, 2640. https://doi.org/10.3390/plants14172640
Ye X, Gu C, Meng J, Wu M. Differences in the Response of Invasive Solidago canadensis and Native Imperata cylindrica to Glyphosate. Plants. 2025; 14(17):2640. https://doi.org/10.3390/plants14172640
Chicago/Turabian StyleYe, Xiaoqi, Chunfeng Gu, Jinliu Meng, and Ming Wu. 2025. "Differences in the Response of Invasive Solidago canadensis and Native Imperata cylindrica to Glyphosate" Plants 14, no. 17: 2640. https://doi.org/10.3390/plants14172640
APA StyleYe, X., Gu, C., Meng, J., & Wu, M. (2025). Differences in the Response of Invasive Solidago canadensis and Native Imperata cylindrica to Glyphosate. Plants, 14(17), 2640. https://doi.org/10.3390/plants14172640





