Optimizing Nitrogen Supplementation: Timing Strategies to Mitigate Waterlogging Stress in Winter- and Spring-Type Canola
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Waterlogging, and Fertilizer Treatments
2.2. Stomatal Conductance, SPAD, and Photochemical Quantum Yield of Photosystem II
2.3. Shoot Element Analysis
2.4. Statistics Analysis
3. Results
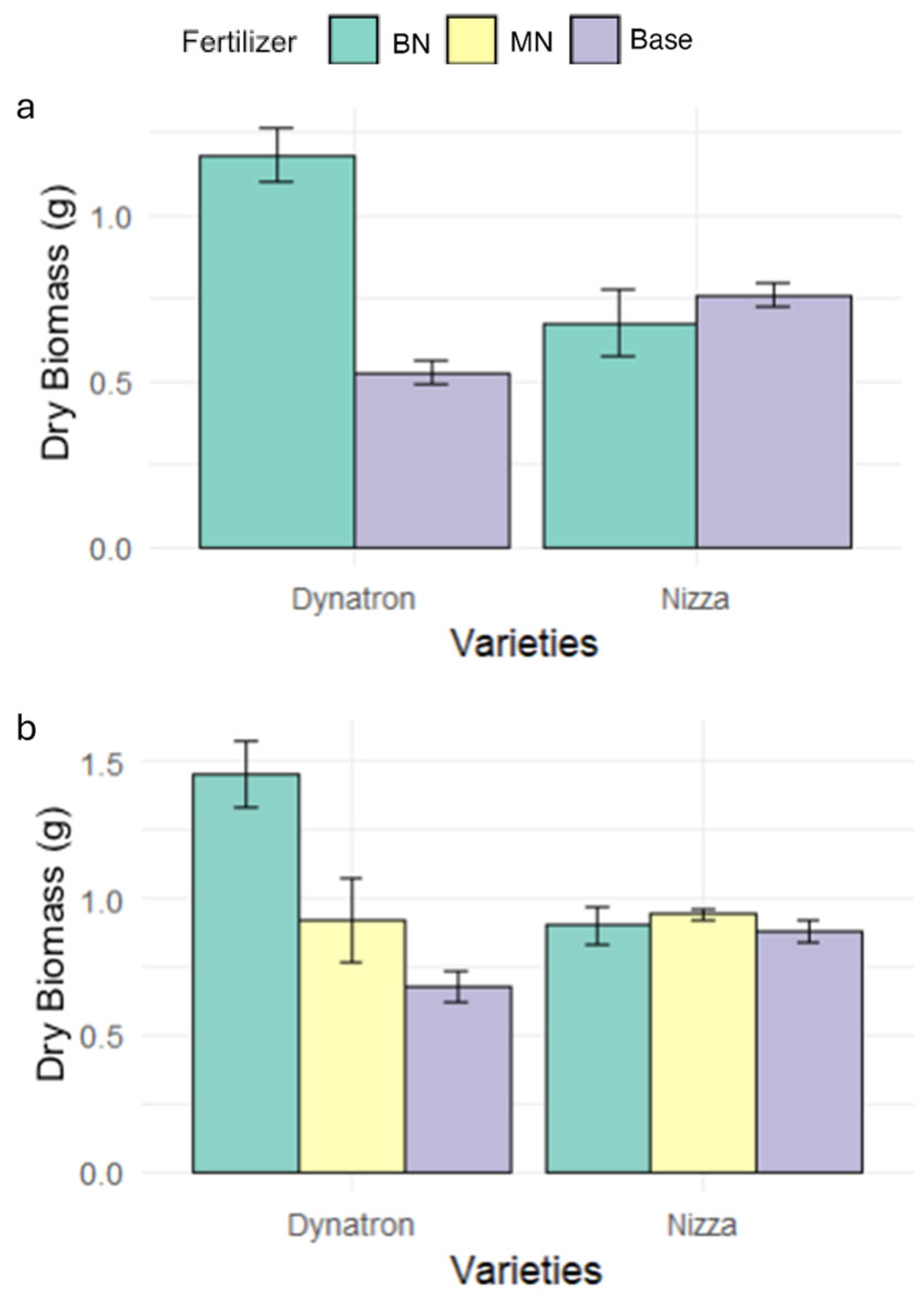
3.1. SPAD Value, Stomatal Conductance, Photochemical Quantum Yield of Photosystem II, and Biomass
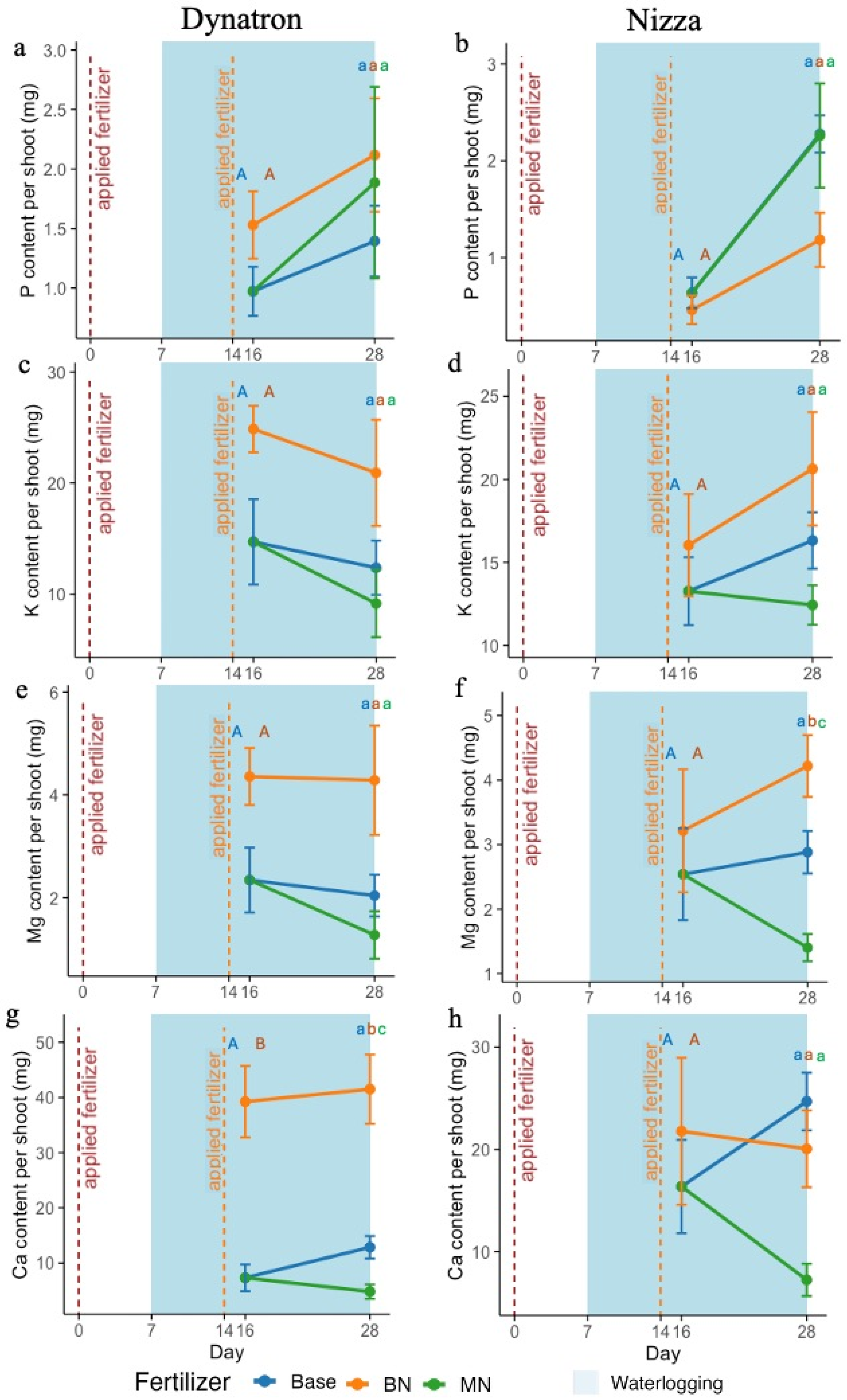
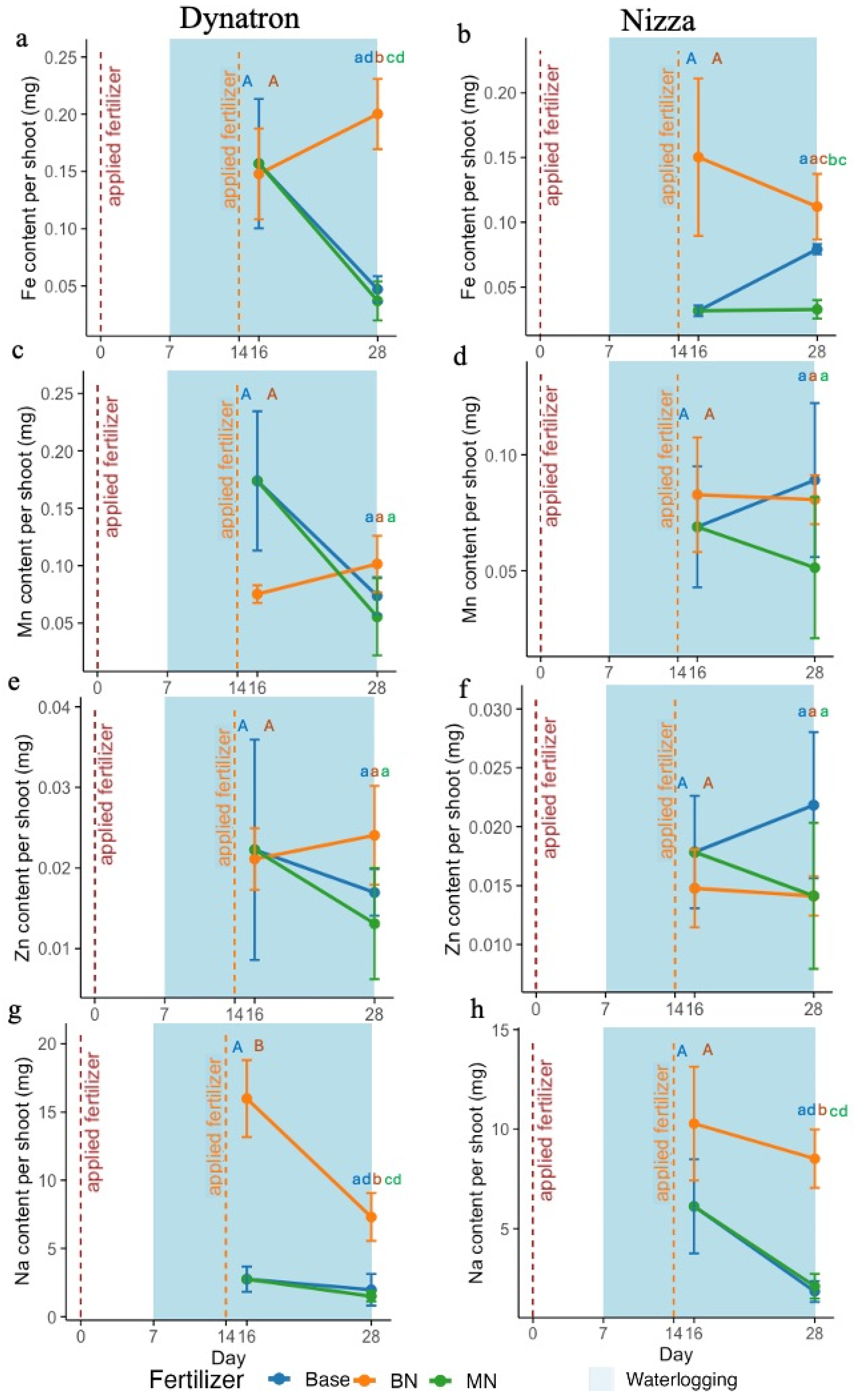
3.2. Shoot Nutrient Contents Under Waterlogging
4. Discussion
4.1. Waterlogging Alleviation by Nitrogen Is Partially Linked to Photosynthetic Mechanisms and the Nature of Winter or Spring Characteristics in Canola
4.2. BN Alleviates Waterlogging Stress, Highly Linked to K, Mg, Ca, and P Regulations in Plants
4.3. Nitrogen-Induced Waterlogging Alleviation in Canola Is Not Related to Toxic Element Accumulations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HRZ | Australia’s high-rainfall zone |
| PhilPS2 | Photochemical quantum yield of photosystem II |
| SPAD | Soil plant analysis development (SPAD) value |
References
- McCaskill, M.R.; Riffkin, P.; Pearce, A.; Christy, B.; Norton, R.; Speirs, A.; Clough, A.; Midwood, J.; Merry, A.; Suraweera, D.; et al. Soil-test critical values for wheat (Triticum aestivum) and canola (Brassica napus) in the high-rainfall cropping zone of southern Australia. Crop Pasture Sci. 2020, 71, 959–975. [Google Scholar] [CrossRef]
- Zhang, H.; Turner, N.C.; Poole, M.L.; Simpson, N. Crop production in the high rainfall zones of southern Australia—Potential, constraints and opportunities. Aust. J. Exp. Agric. 2006, 46, 1035–1049. [Google Scholar] [CrossRef]
- Shaw, R.E.; Meyer, W.S.; McNeill, A.; Tyerman, S.D. Waterlogging in Australian agricultural landscapes: A review of plant responses and crop models. Crop Pasture Sci. 2013, 64, 549–562. [Google Scholar] [CrossRef]
- Simpson, N.L.; Brennan, R.F.; Anderson, W.K. Grain yield increases in wheat and barley to nitrogen applied after transient waterlogging in the high rainfall cropping zone of western Australia. J. Plant Nutr. 2016, 39, 974–992. [Google Scholar] [CrossRef]
- MacEwan, R.J.; Crawford, D.M.; Newton, P.J.; Clune, T.S. High clay contents, dense soils, and spatial variability are the principal subsoil constraints to cropping the higher rainfall land in south-eastern Australia. Soil Res. 2010, 48, 150–166. [Google Scholar] [CrossRef]
- Riffkin, P.; Potter, T.; Kearney, G. Yield performance of late-maturing winter canola (Brassica napus L.) types in the High Rainfall Zone of southern Australia. Crop Pasture Sci. 2012, 63, 17–32. [Google Scholar] [CrossRef]
- Sprague, S.J.; Kirkegaard, J.A.; Graham, J.M.; Dove, H.; Kelman, W.M. Crop and livestock production for dual-purpose winter canola (Brassica napus) in the high-rainfall zone of south-eastern Australia. Field Crops Res. 2014, 156, 30–39. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, C.; Pang, J.; Niu, Y.; Liu, H.; Zhang, W.; Zhou, M. Genome-wide association study reveals quantitative trait loci for waterlogging-triggered adventitious roots and aerenchyma formation in common wheat. Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef]
- Fukao, T.; Barrera-Figueroa, B.E.; Juntawong, P.; Peña-Castro, J.M. Submergence and Waterlogging Stress in Plants: A Review Highlighting Research Opportunities and Understudied Aspects. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Ploschuk, R.A.; Miralles, D.J.; Colmer, T.D.; Ploschuk, E.L.; Striker, G.G. Waterlogging of Winter Crops at Early and Late Stages: Impacts on Leaf Physiology, Growth and Yield. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Elzenga, J.T.M.; van Veen, H. Waterlogging and Plant Nutrient Uptake. In Waterlogging Signalling and Tolerance in Plants; Mancuso, S., Shabala, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 23–35. [Google Scholar]
- Norton, R.M. Nitrogen management to optimise canola production in Australia. Crop Pasture Sci. 2016, 67, 419–438. [Google Scholar] [CrossRef]
- Sylvester-Bradley, R.; Riffkin, P.; O’Leary, G. Designing resource-efficient ideotypes for new cropping conditions: Wheat (Triticum aestivum L.) in the High Rainfall Zone of southern Australia. Field Crops Res. 2012, 125, 69–82. [Google Scholar] [CrossRef]
- Nguyen, L.T.T.; Osanai, Y.; Anderson, I.C.; Bange, M.P.; Braunack, M.; Tissue, D.T.; Singh, B.K. Impacts of waterlogging on soil nitrification and ammonia-oxidizing communities in farming system. Plant Soil 2018, 426, 299–311. [Google Scholar] [CrossRef]
- Wollmer, A.-C.; Pitann, B.; Mühling, K.-H. Timing of Waterlogging Is Crucial for the Development of Micronutrient Deficiencies or Toxicities in Winter Wheat and Rapeseed. J. Plant Growth Regul. 2019, 38, 824–830. [Google Scholar] [CrossRef]
- Fernando, D.R.; Lynch, J.P. Manganese phytotoxicity: New light on an old problem. Ann. Bot. 2015, 116, 313–319. [Google Scholar] [CrossRef]
- Li, J.; Jia, Y.; Dong, R.; Huang, R.; Liu, P.; Li, X.; Wang, Z.; Liu, G.; Chen, Z. Advances in the Mechanisms of Plant Tolerance to Manganese Toxicity. Int. J. Mol. Sci. 2019, 20, 5096. [Google Scholar] [CrossRef]
- Li, G.; Wu, J.; Kronzucker, H.J.; Li, B.; Shi, W. Physiological and molecular mechanisms of plant-root responses to iron toxicity. J. Plant Physiol. 2024, 297, 154257. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, A.; Chen, S.; Chen, Y. Genotypic Variability in Root Morphological Traits in Canola (Brassica napus L.) at the Seedling Stage. Crops 2025, 5, 18. [Google Scholar] [CrossRef]
- Arifuzzaman, M.; Horvath, D.; Rahman, M. Transcriptome Analysis Suggests Cytokinin and Gibberellin Signaling May Account for Differences Between Spring and Winter Canola (Brassica napus L.) Root Development. J. Plant Biol. 2022, 65, 531–547. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, C.; Harrison, M.T.; Zhou, M. Nitrogen Fertilization Alleviates Barley (Hordeum vulgare L.) Waterlogging. Agronomy 2024, 14, 1712. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, D.; Lin, X. Effects of Waterlogging on Nitrogen Accumulation and Alleviation of Waterlogging Damage by Application of Nitrogen Fertilizer and Mixtalol in Winter Rape (Brassica napus L.). J. Plant Growth Regul. 1997, 16, 47–53. [Google Scholar] [CrossRef]
- McCaskill, M.; Armstrong, R.; Zhou, M. Nutrition Strategies to Mitigate Yield Losses Following Waterlogging—Lessons from Southern Environments. Available online: https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2023/08/nutrition-strategies-to-mitigate-yield-losses-following-waterlogging-lessons-from-southern-environments (accessed on 22 November 2024).
- Habibzadeh, F.; Sorooshzadeh, A.; Pirdashti, H.; Modarres-Sanavy, S.A.M. Alleviation of waterlogging damage by foliar application of nitrogen compounds and tricyclazole in canola. Aust. J. Crop Sci. 2013, 7, 401–406. [Google Scholar]
- Hussain, M.A.; Naeem, A.; Pitann, B.; Mühling, K.H. Foliar-applied sulfur is supplemental to soil application in alleviating waterlogging stress through the mitigation of oxidative stress and ion homeostasis in rapeseed (Brassica napus L.). Plant Stress 2024, 13, 100529. [Google Scholar] [CrossRef]
- Men, S.; Chen, H.; Chen, S.; Zheng, S.; Shen, X.; Wang, C.; Yang, Z.; Liu, D. Effects of supplemental nitrogen application on physiological characteristics, dry matter and nitrogen accumulation of winter rapeseed (Brassica napus L.) under waterlogging stress. Sci. Rep. 2020, 10, 10201. [Google Scholar] [CrossRef] [PubMed]
- Soil Quality Australia. Fact Sheets Waterlogging. Available online: https://www.soilquality.org.au/factsheets/waterlogging (accessed on 9 July 2025).
- Nurbaiti, S.; Milasari, A.; Arif Wibowo, W.; Nilamsari, E.; Rachmawati, D. Assessing Foliar Chlorophyll Content with SPAD-502 Chlorophyll Meter: A Comparison with Spectrophotometer Method in Various Plants. J. Ris. Biol. Dan Apl. 2025, 7, 50–56. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Qi, D.; Wu, Q. Physiological Characteristics, Lint Yield, and Nitrogen Use Efficiency of Cotton Under Different Nitrogen Application Rates and Waterlogging Durations. J. Soil Sci. Plant Nutr. 2023, 23, 5594–5607. [Google Scholar] [CrossRef]
- Wu, J.-D.; Li, J.-C.; Wei, F.-Z.; Wang, C.-Y.; Zhang, Y.; Sun, G. Effects of nitrogen spraying on the post-anthesis stage of winter wheat under waterlogging stress. Acta Physiol. Plant. 2014, 36, 207–216. [Google Scholar] [CrossRef]
- Manik, S.M.N.; Pengilley, G.; Dean, G.; Field, B.; Shabala, S.; Zhou, M. Soil and Crop Management Practices to Minimize the Impact of Waterlogging on Crop Productivity. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Tan, X.; Xu, H.; Khan, S.; Equiza, M.A.; Lee, S.H.; Vaziriyeganeh, M.; Zwiazek, J.J. Plant water transport and aquaporins in oxygen-deprived environments. J. Plant Physiol. 2018, 227, 20–30. [Google Scholar] [CrossRef]
- Olorunwa, O.J.; Adhikari, B.; Brazel, S.; Shi, A.; Popescu, S.C.; Popescu, G.V.; Barickman, T.C. Growth and Photosynthetic Responses of Cowpea Genotypes under Waterlogging at the Reproductive Stage. Plants 2022, 11, 2315. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Q.; Wang, Z.; Wang, L.; Li, X.; Fan, Z.; Zhang, Y.; Li, J.; Gao, X.; Shi, J.; et al. Effects of Nitrogen Fertilizer on Photosynthetic Characteristics, Biomass, and Yield of Wheat under Different Shading Conditions. Agronomy 2021, 11, 1989. [Google Scholar] [CrossRef]
- Xu, X.; Du, X.; Wang, F.; Sha, J.; Chen, Q.; Tian, G.; Zhu, Z.; Ge, S.; Jiang, Y. Effects of Potassium Levels on Plant Growth, Accumulation and Distribution of Carbon, and Nitrate Metabolism in Apple Dwarf Rootstock Seedlings. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Rawat, J.; Pandey, N.; Saxena, J. Role of Potassium in Plant Photosynthesis, Transport, Growth and Yield. In Role of Potassium in Abiotic Stress; Iqbal, N., Umar, S., Eds.; Springer Nature: Singapore, 2022; pp. 1–14. [Google Scholar]
- Fang, X.Z.; Liu, X.X.; Zhu, Y.X.; Ye, J.Y.; Jin, C.W. The K+ and NO3− Interaction Mediated by NITRATE TRANSPORTER1.1 Ensures Better Plant Growth under K+-Limiting Conditions. Plant Physiol. 2020, 184, 1900–1916. [Google Scholar] [CrossRef]
- Sahrawat, K.L. Factors Affecting Nitrification in Soils. Commun. Soil Sci. Plant Anal. 2008, 39, 1436–1446. [Google Scholar] [CrossRef]
- Motasim, A.M.; Samsuri, A.W.; Nabayi, A.; Akter, A.; Haque, M.A.; Abdul Sukor, A.S.; Adibah, A.M. Urea application in soil: Processes, losses, and alternatives—A review. Discov. Agric. 2024, 2, 42. [Google Scholar] [CrossRef]
- Pandey, C.B.; Kumar, U.; Kaviraj, M.; Minick, K.J.; Mishra, A.K.; Singh, J.S. DNRA: A short-circuit in biological N-cycling to conserve nitrogen in terrestrial ecosystems. Sci. Total Environ. 2020, 738, 139710. [Google Scholar] [CrossRef] [PubMed]
- Hawkesford, M.J.; Cakmak, I.; Coskun, D.; De Kok, L.J.; Lambers, H.; Schjoerring, J.K.; White, P.J. Chapter 6—Functions of macronutrients. In Marschner’s Mineral Nutrition of Plants, 4th ed.; Rengel, Z., Cakmak, I., White, P.J., Eds.; Academic Press: San Diego, CA, USA, 2023; pp. 201–281. [Google Scholar]
- Zhang, Y.; Chen, X.; Geng, S.; Zhang, X. A review of soil waterlogging impacts, mechanisms, and adaptive strategies. Front. Plant Sci. 2025, 16. [Google Scholar] [CrossRef]
- Haq, S.I.U.; Tariq, F.; Sama, N.U.; Jamal, H.; Mohamed, H.I. Role of autophagy in plant growth and adaptation to salt stress. Planta 2025, 261, 49. [Google Scholar] [CrossRef]
- Guo, Y.; Ren, G.; Zhang, K.; Li, Z.; Miao, Y.; Guo, H. Leaf senescence: Progression, regulation, and application. Mol. Hortic. 2021, 1, 5. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, S.; Wan, S.; Li, X. The Significance of Calcium in Photosynthesis. Int. J. Mol. Sci. 2019, 20, 1353. [Google Scholar] [CrossRef]
- Boem, F.H.G.; Lavado, R.S.; Porcelli, C.A. Note on the effects of winter and spring waterlogging on growth, chemical composition and yield of rapeseed. Field Crops Res. 1996, 47, 175–179. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Lu, H.; Liu, Y.; Mao, C. Phosphate Uptake and Transport in Plants: An Elaborate Regulatory System. Plant Cell Physiol. 2021, 62, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.L.; Zheng, Z.M. Relationship between plant nitrogen and phosphorus accumulations in a canola crop as affected by nitrogen management under ample phosphorus supply conditions. Can. J. Plant Sci. 2016, 96, 853–866. [Google Scholar] [CrossRef]
- Langan, P.; Bernád, V.; Walsh, J.; Henchy, J.; Khodaeiaminjan, M.; Mangina, E.; Negrão, S. Phenotyping for waterlogging tolerance in crops: Current trends and future prospects. J. Exp. Bot. 2022, 73, 5149–5169. [Google Scholar] [CrossRef]
- Wu, J.; Song, Y.; Wan, G.-Y.; Sun, L.-Q.; Wang, J.-X.; Zhang, Z.-S.; Xiang, C.-B. Boosting crop yield and nitrogen use efficiency: The hidden power of nitrogen-iron balance. New Crops 2025, 2, 100047. [Google Scholar] [CrossRef]
- Iu, K.L.; Pulford, I.D.; Duncan, H.J. Influence of waterlogging and lime or organic matter additions on the distribution of trace metals in an acid soil. Plant Soil 1981, 59, 317–326. [Google Scholar] [CrossRef]
- Jones, J.D. Iron Availability and Management Considerations: A 4R Approach. Crops Soils 2020, 53, 32–37. [Google Scholar] [CrossRef]
- Singh, S.P.; Setter, T.L. Effect of Waterlogging on Element Concentrations, Growth and Yield of Wheat Varieties Under Farmer’s Sodic Field Conditions. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 87, 513–520. [Google Scholar] [CrossRef]
- Zahra, N.; Hafeez, M.B.; Shaukat, K.; Wahid, A.; Hasanuzzaman, M. Fe toxicity in plants: Impacts and remediation. Physiol. Plant. 2021, 173, 201–222. [Google Scholar] [CrossRef]
- Green, M.S.; Etherington, J.R. Oxidation of Ferrous Iron by Rice (Oryza sativa L.) Roots: A Mechanism for Waterlogging Tolerance? J. Exp. Bot. 1977, 28, 678–690. [Google Scholar] [CrossRef]
- Li, J.; Tian, J.; Zhou, M.; Tian, J.; Liang, C. Research progress on the physiological and molecular mechanisms underlying soybean aluminum resistance. New Crops 2025, 2, 100034. [Google Scholar] [CrossRef]
- Shu-jie, Z.; Xing, L.; Xiao-jia, H.U.; Chang-bing, Y.U.; Li-hua, X.I.E.; Yin-shui, L.I. Effects of waterlogging on growth and nutrient uptake in oilseed rape seedlings. Chin. J. Oil Crop Sci. 2013, 35, 650–657. [Google Scholar] [CrossRef]
- Waldren, S.; Etherington, J.R.; Davies, M.S. Comparative Studies of Plant Growth and Distribution in Relation to Waterlogging: XIV. Iron, Manganese, Calcium and Phosphorus Concentrations in Leaves and Roots of Geum rivale L. and G. urbanum L. Grown in Waterlogged Soil. New Phytol. 1987, 106, 689–696. [Google Scholar] [CrossRef]
- Khabaz-Saberi, H.; Setter, T.L.; Waters, I. Waterlogging Induces High to Toxic Concentrations of Iron, Aluminum, and Manganese in Wheat Varieties on Acidic Soil. J. Plant Nutr. 2006, 29, 899–911. [Google Scholar] [CrossRef]

| Varieties | Waterlogging Treatment | Fertilizer Treatment |
|---|---|---|
| Dynatron | Waterlogging | Base fertilizer |
| Base fertilizer + 0.6 g of urea 1 week before waterlogging (Day 0) | ||
| Base fertilizer + 0.6 g of urea on day 14 during the middle of waterlogging (7 days after waterlogging) | ||
| Control | Base fertilizer | |
| Nizza | Waterlogging | Base fertilizer |
| Base fertilizer + 0.6 g of urea 1 week before waterlogging (Day 0) | ||
| Base fertilizer + 0.6 g of urea on day 14 during the middle of waterlogging (7 days after waterlogging) | ||
| Control | Base fertilizer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Sharmita, O.; Siddique, A.B.; Shabala, S.; Zhou, M.; Zhao, C. Optimizing Nitrogen Supplementation: Timing Strategies to Mitigate Waterlogging Stress in Winter- and Spring-Type Canola. Plants 2025, 14, 2641. https://doi.org/10.3390/plants14172641
Zhao H, Sharmita O, Siddique AB, Shabala S, Zhou M, Zhao C. Optimizing Nitrogen Supplementation: Timing Strategies to Mitigate Waterlogging Stress in Winter- and Spring-Type Canola. Plants. 2025; 14(17):2641. https://doi.org/10.3390/plants14172641
Chicago/Turabian StyleZhao, Haochen, Onusha Sharmita, Abu Bakar Siddique, Sergey Shabala, Meixue Zhou, and Chenchen Zhao. 2025. "Optimizing Nitrogen Supplementation: Timing Strategies to Mitigate Waterlogging Stress in Winter- and Spring-Type Canola" Plants 14, no. 17: 2641. https://doi.org/10.3390/plants14172641
APA StyleZhao, H., Sharmita, O., Siddique, A. B., Shabala, S., Zhou, M., & Zhao, C. (2025). Optimizing Nitrogen Supplementation: Timing Strategies to Mitigate Waterlogging Stress in Winter- and Spring-Type Canola. Plants, 14(17), 2641. https://doi.org/10.3390/plants14172641










