GsCYP93D1, a Cytochrome P450 Gene from Wild Soybean, Mediates the Regulation of Plant Alkaline Tolerance and ABA Sensitivity
Abstract
1. Introduction
2. Results
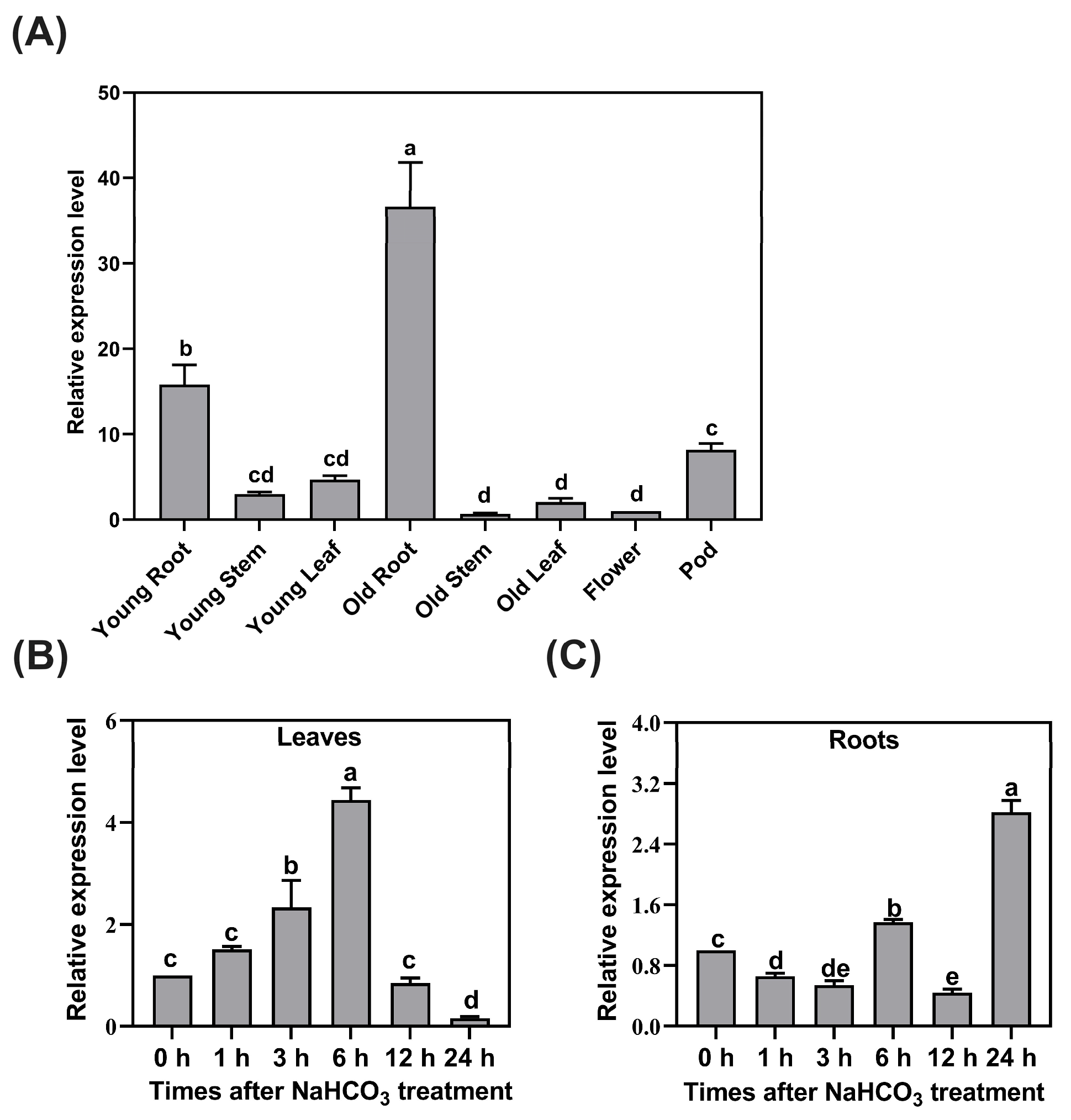
2.1. Spatial and Temporal Expression Patterns of GsCYP93D1 in Wild Soybean
2.2. GsCYP93D1 Enhanced Alkaline Tolerance in Arabidopsis
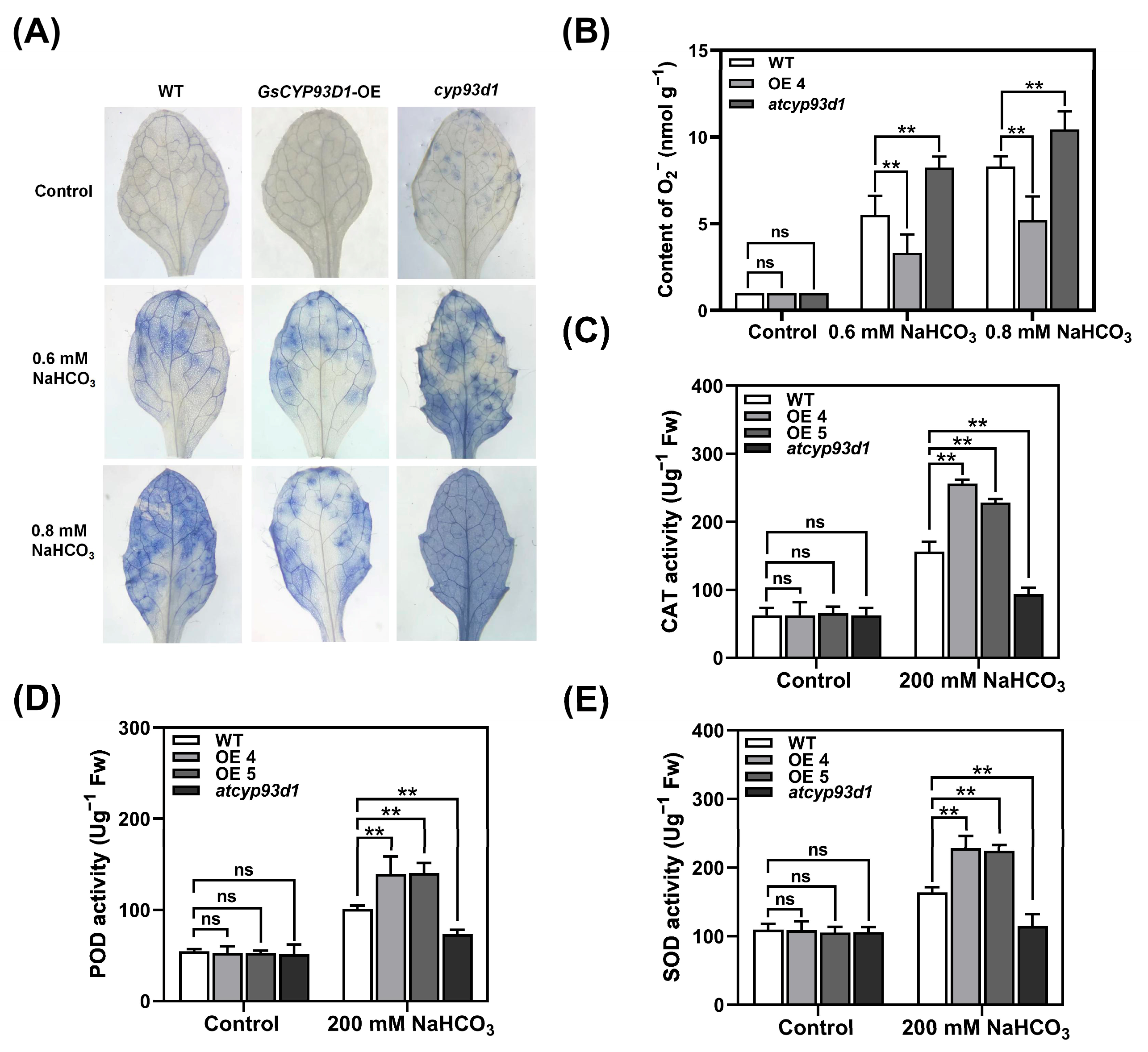
2.3. Modulation of Redox Homeostasis by GsCYP93D1 Confers Stress Resilience
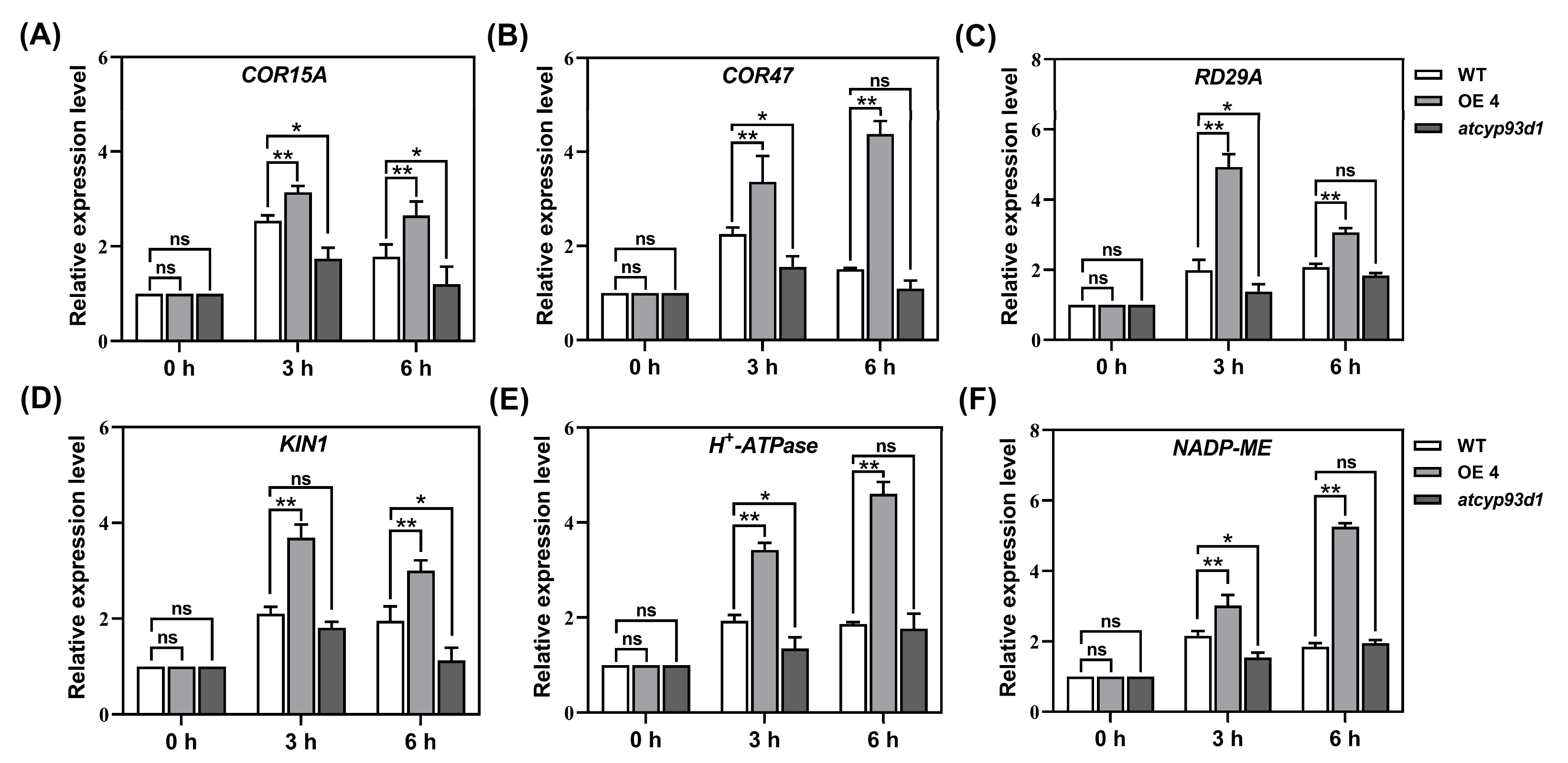
2.4. GsCYP93D1 Regulated the Expression Levels of Stress Responsive Genes
2.5. GsCYP93D1 Enhanced Alkaline Tolerance in Hairy Roots of Soybean
2.6. GsCYP93D1 Enhanced ABA Sensitivity in Arabidopsis
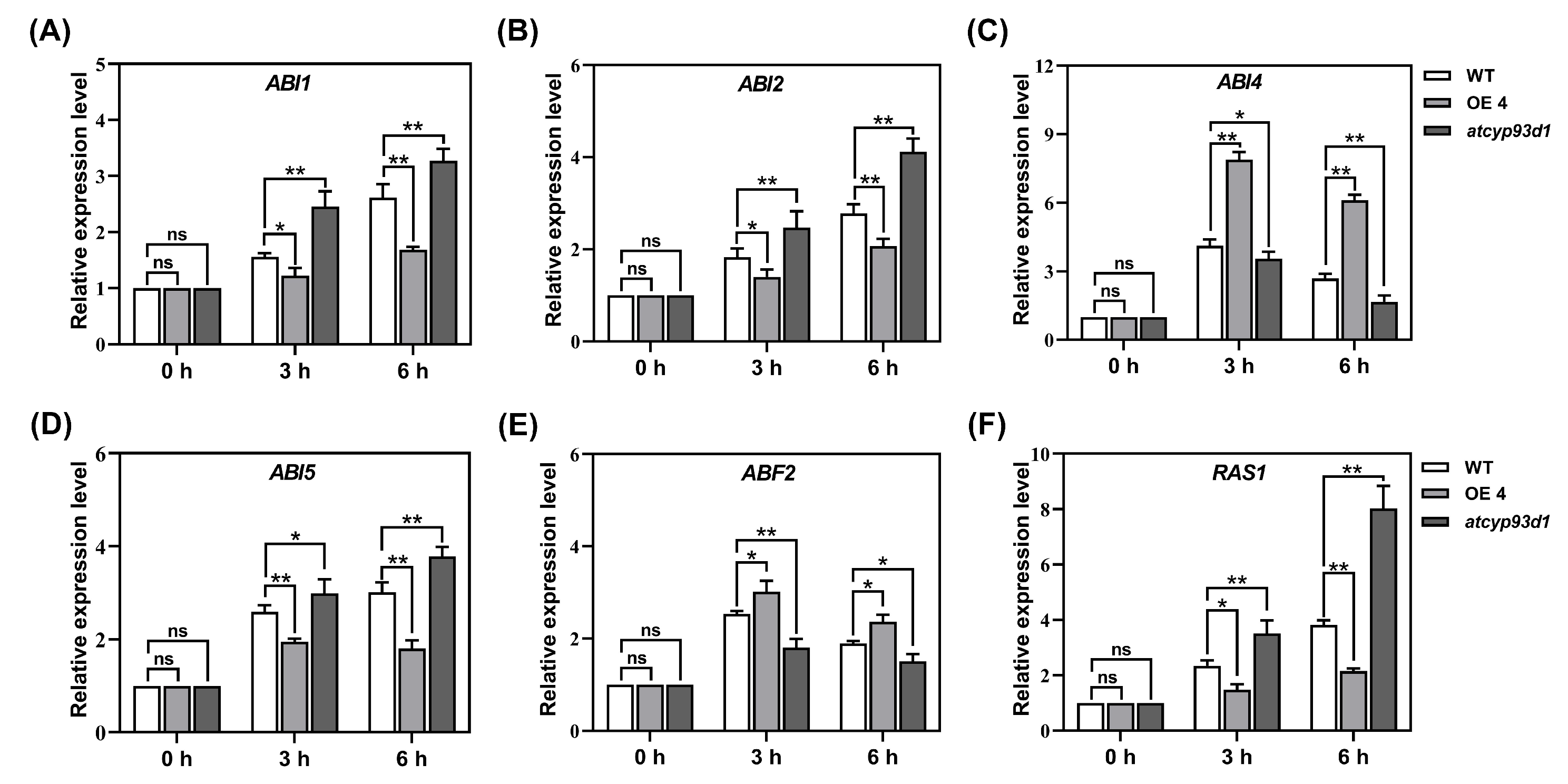
2.7. GsCYP93D1 Altered Expression Patterns of ABA Signal-Related Genes
3. Discussion
4. Materials and Methods
4.1. Materials and Growing Conditions
4.2. Analysis of Tissue Localization and Expression Characteristics Under Alkaline Stress
4.3. Generation of GsCYP93D1 Transgenic Plants
4.4. Functional Analysis of Transgenic Arabidopsis Under Alkaline Stress
4.5. NBT Staining and Superoxide Anion Determination of Transgenic Arabidopsis Under Alkali Stress
4.6. Alkaline Stress Responses in Hairy Roots of Transgenic Soybean Lines
4.7. Analysis of GsCYP93D1-Regulated Gene Expression in Response to Alkaline Stress
4.8. Functional Characterization of Transgenic Arabidopsis Under ABA Treatment
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CYPs | Cytochrome P450 enzymes |
| qRT-PCR | Quantitative real-time PCR |
| ROS | Reactive oxygen species |
| ABA | Abscisic acid |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| CAT | Catalase |
| MDA | Malondialdehyde |
| NBT | Nitroblue tetrazolium |
| O2− | Superoxide anion |
| WT | Wild type |
References
- Yang, S.; Xu, Y.; Tang, Z.; Jin, S.; Yang, S. The Impact of Alkaline Stress on Plant Growth and Its Alkaline Resistance Mechanisms. Int. J. Mol. Sci. 2024, 25, 13719. [Google Scholar] [CrossRef]
- Fang, S.; Hou, X.; Liang, X. Response mechanisms of plants under saline-alkali stress. Front. Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef]
- Shaban, A.S.; Safhi, F.A.; Fakhr, M.A.; Pruthi, R.; Abozahra, M.S.; El-Tahan, A.M.; Subudhi, P.K. Comparison of the morpho-physiological and molecular responses to salinity and alkalinity stresses in rice. Plants 2023, 13, 60. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y. How do plants maintain pH and ion homeostasis under saline-alkali stress? Front. Plant Sci. 2023, 14, 1217193. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Chen, J.-G.; Gao, Y.-Q.; Li, X.; Chao, Z.-F.; Chen, Z.-R.; Li, Q.-Q.; Han, M.-L.; Wang, Y.-L.; Wang, Y.-F. AtHKT1 drives adaptation of Arabidopsis thaliana to salinity by reducing floral sodium content. PLoS Genet. 2017, 13, e1007086. [Google Scholar] [CrossRef]
- Wang, X.; Ajab, Z.; Liu, C.; Hu, S.; Liu, J.; Guan, Q. Overexpression of transcription factor SlWRKY28 improved the tolerance of Populus davidiana× P. bolleana to alkaline salt stress. BMC Genet. 2020, 21, 103. [Google Scholar] [CrossRef]
- Nie, W.; Gong, B.; Wen, D.; Qiao, P.; Guo, H.; Shi, Q. Brassinosteroid Enhances Cucumber Stress Tolerance to NaHCO3 by Modulating Nitrogen Metabolism, Ionic Balance and Phytohormonal Response. Plants 2024, 14, 80. [Google Scholar] [CrossRef]
- Singh, P.; Choudhary, K.K.; Chaudhary, N.; Gupta, S.; Sahu, M.; Tejaswini, B.; Sarkar, S. Salt stress resilience in plants mediated through osmolyte accumulation and its crosstalk mechanism with phytohormones. Front. Plant Sci. 2022, 13, 1006617. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, C.; Cui, C.; Song, J.; Ji, G.; Sun, N.; Qi, S.; Li, J.; Xu, Z.; Zhang, H. Physiological and Molecular Responses of Poplar to Salt Stress and Functional Analysis of PagGRXC9 to Salt Tolerance. Tree Physiol. 2025, 45, tpaf039. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Feng, Z.; Xu, Y.; Xie, Z.; Yang, Y.; Lu, G.; Jin, Y.; Wang, M.; Liu, M.; Yang, H.; Li, W. Overexpression of abscisic acid biosynthesis gene OsNCED3 enhances survival rate and tolerance to alkaline stress in rice seedlings. Plants 2024, 13, 1713. [Google Scholar] [CrossRef]
- Kushiro, T.; Okamoto, M.; Nakabayashi, K.; Yamagishi, K.; Kitamura, S.; Asami, T.; Hirai, N.; Koshiba, T.; Kamiya, Y.; Nambara, E. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: Key enzymes in ABA catabolism. EMBO J. 2004, 23, 1647–1656. [Google Scholar] [CrossRef]
- Liu, X.-L.; Zhang, H.; Jin, Y.-Y.; Wang, M.-M.; Yang, H.-Y.; Ma, H.-Y.; Jiang, C.-J.; Liang, Z.-W. Abscisic acid primes rice seedlings for enhanced tolerance to alkaline stress by upregulating antioxidant defense and stress tolerance-related genes. Plant Soil 2019, 438, 39–55. [Google Scholar] [CrossRef]
- Liu, X.; Xie, X.; Zheng, C.; Wei, L.; Li, X.; Jin, Y.; Zhang, G.; Jiang, C.-J.; Liang, Z. RNAi-mediated suppression of the abscisic acid catabolism gene OsABA8ox1 increases abscisic acid content and tolerance to saline–alkaline stress in rice (Oryza sativa L.). Crop J. 2022, 10, 354–367. [Google Scholar] [CrossRef]
- Mizutani, M.; Sato, F. Unusual P450 reactions in plant secondary metabolism. Arch. Biochem. Biophys. 2011, 507, 194–203. [Google Scholar] [CrossRef]
- Schuler, M.A.; Werck-Reichhart, D. Functional genomics of P450s. Annu. Rev. Plant Biol. 2003, 54, 629–667. [Google Scholar] [CrossRef]
- Lv, X.; Zhao, X.; Wang, F.; Wang, H.; Zhang, Y.; Ruan, B.; Dong, G.; Yu, Y.; Wu, L.; Chen, F. Rice Cytochrome P450 Protein CYP71P1 Is Required for Heat Stress Tolerance by Regulating Serotonin Biosynthesis and ROS Homeostasis. Plants 2025, 14, 1072. [Google Scholar] [CrossRef]
- Heitz, T.; Widemann, E.; Lugan, R.; Miesch, L.; Ullmann, P.; Désaubry, L.; Holder, E.; Grausem, B.; Kandel, S.; Miesch, M. Cytochromes P450 CYP94C1 and CYP94B3 catalyze two successive oxidation steps of plant hormone jasmonoyl-isoleucine for catabolic turnover. J. Biol. Chem. 2012, 287, 6296–6306. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yuan, J.; Qin, L.; Shi, W.; Xia, G.; Liu, S. TaCYP81D5, one member in a wheat cytochrome P450 gene cluster, confers salinity tolerance via reactive oxygen species scavenging. Plant Biotechnol. J. 2020, 18, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Seebeck, T.; Schrenker, D.; Yu, O. CYP709B3, a cytochrome P450 monooxygenase gene involved in salt tolerance in Arabidopsis thaliana. BMC Plant Biol. 2013, 13, 169. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Peng, M.; Luo, Q.; Jiang, M.; Zhang, X.; Zong, X.; Meng, F.; Li, Y. Isolation and detection of transcript-derived fragments (TDFs) in NaCl-stressed black locust (Robinia pseudoacacia L.) using cDNA-AFLP analysis. Acta Physiol. Plant. 2015, 37, 168. [Google Scholar] [CrossRef]
- Seitz, C.; Eder, C.; Deiml, B.; Kellner, S.; Martens, S.; Forkmann, G. Cloning, functional identification and sequence analysis of flavonoid 3′-hydroxylase and flavonoid 3′, 5′-hydroxylase cDNAs reveals independent evolution of flavonoid 3′, 5′-hydroxylase in the Asteraceae family. Plant Mol. Biol. 2006, 61, 365–381. [Google Scholar] [CrossRef]
- Guo, P.; Bai, G.; Carver, B.; Li, R.; Bernardo, A.; Baum, M. Transcriptional analysis between two wheat near-isogenic lines contrasting in aluminum tolerance under aluminum stress. Mol. Genet. Genom. 2007, 277, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Yu, Y.; DuanMu, H.; Chen, C.; Duan, X.; Zhu, P.; Chen, R.; Li, Q.; Zhu, Y.; Ding, X. A novel Glycine soja homeodomain-leucine zipper (HD-Zip) I gene, Gshdz4, positively regulates bicarbonate tolerance and responds to osmotic stress in Arabidopsis. BMC Plant Biol. 2016, 16, 184. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Li, Y.; Lv, D.-K.; Bai, X.; Ji, W.; Cai, H.; Wang, A.-X.; Zhu, Y.-M. Alkaline-stress response in Glycine soja leaf identifies specific transcription factors and ABA-mediated signaling factors. Funct. Integr. Genom. 2011, 11, 369–379. [Google Scholar] [CrossRef]
- Pandian, B.A.; Sathishraj, R.; Djanaguiraman, M.; Prasad, P.V.; Jugulam, M. Role of cytochrome P450 enzymes in plant stress response. Antioxidants 2020, 9, 454. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Rao, S.; Dai, G.; Chen, J. Genome-Wide Identification of the CYP78A Gene Family in Lycium and Functional Characterization of LrCYP78A5. Plants 2025, 14, 1152. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, L.; Chen, R.; Ma, Q.; Ma, D.; Liu, X. Genome-wide identification of the cytochrome P450 family and analysis of CYP regarding salt tolerance in Medicago sativa L. Grass Res. 2023, 3, 21. [Google Scholar] [CrossRef]
- Wu, J.; Fang, Y.; Xu, L.; Jin, X.; iqbal, A.; Nisa, Z.-u.; Ali, N.; Chen, C.; Shah, A.A.; Gatasheh, M.K. The Glycine soja cytochrome P450 gene GsCYP82C4 confers alkaline tolerance by promoting reactive oxygen species scavenging. Physiol. Plant. 2024, 176, e14252. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.-L.; Zhang, R.-X.; Yuan, H.-Y.; Wang, M.-M.; Yang, H.-Y.; Ma, H.-Y.; Liu, D.; Jiang, C.-J.; Liang, Z.-W. Root Damage under Alkaline Stress Is Associated with Reactive Oxygen Species Accumulation in Rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 1580. [Google Scholar] [CrossRef]
- Sun, K.; Fang, H.; Chen, Y.; Zhuang, Z.; Chen, Q.; Shan, T.; Khan, M.K.R.; Zhang, J.; Wang, B. Genome-wide analysis of the cytochrome P450 gene family involved in salt tolerance in Gossypium hirsutum. Front. Plant Sci. 2021, 12, 685054. [Google Scholar] [CrossRef]
- Sun, X.L.; Sun, M.Z.; Jia, B.W.; Qin, Z.W.; Yang, K.J.; Chen, C.; Yu, Q.Y.; Zhu, Y.M. A Glycine soja methionine sulfoxide reductase B5a interacts with the Ca2+/CAM-binding kinase GsCBRLK and activates ROS signaling under carbonate alkaline stress. Plant J. 2016, 86, 514–529. [Google Scholar] [CrossRef]
- Li, M.; Guo, P.; Nan, N.; Ma, A.; Liu, W.; Wang, T.-J.; Yun, D.-J.; Xu, Z.-Y. Plasma membrane-localized H+-ATPase OsAHA3 functions in saline–alkaline stress tolerance in rice. Plant Cell Rep. 2024, 43, 9. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cheng, Y.; Zhang, X.; Guan, Q.; Nishiuchi, S.; Hase, K.; Takano, T. Expression of an NADP-malic enzyme gene in rice (Oryza sativa. L) is induced by environmental stresses; over-expression of the gene in Arabidopsis confers salt and osmotic stress tolerance. Plant Mol. Biol. 2007, 64, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cheng, J.; Sun, X.; Zhao, T.; Li, M.; Wang, Q.; Li, S.; Xin, H. Overexpression of VaWRKY14 increases drought tolerance in Arabidopsis by modulating the expression of stress-related genes. Plant Cell Rep. 2018, 37, 1159–1172. [Google Scholar] [CrossRef]
- Cai, J.; Liu, T.; Li, Y.; Ow, D.W. A C-terminal fragment of Arabidopsis OXIDATIVE STRESS 2 can play a positive role in salt tolerance. Biochem. Biophys. Res. Commun. 2021, 556, 23–30. [Google Scholar] [CrossRef]
- Liu, A.; Yu, Y.; Duan, X.; Sun, X.; Duanmu, H.; Zhu, Y. GsSKP21, a Glycine soja S-phase kinase-associated protein, mediates the regulation of plant alkaline tolerance and ABA sensitivity. Plant Mol. Biol. 2015, 87, 111–124. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Hou, Z.-H.; Zhang, Y.; Li, Z.-Y.; Chen, J.; Zhou, Y.-B.; Chen, M.; Fu, J.-D.; Ma, Y.-Z.; Zhang, H. A soybean EF-Tu family protein GmEF8, an interactor of GmCBL1, enhances drought and heat tolerance in transgenic Arabidopsis and soybean. Int. J. Biol. Macromol. 2022, 205, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Ketehouli, T.; Zhou, Y.-G.; Dai, S.-Y.; Carther, K.F.I.; Sun, D.-Q.; Li, Y.; Nguyen, Q.V.H.; Xu, H.; Wang, F.-W.; Liu, W.-C. A soybean calcineurin B-like protein-interacting protein kinase, GmPKS4, regulates plant responses to salt and alkali stresses. J. Plant Physiol. 2021, 256, 153331. [Google Scholar] [CrossRef]
- Pirredda, M.; Fañanás-Pueyo, I.; Oñate-Sánchez, L.; Mira, S. Seed longevity and ageing: A review on physiological and genetic factors with an emphasis on hormonal regulation. Plants 2023, 13, 41. [Google Scholar] [CrossRef]
- Li, S.; Yuan, J.; Zhou, F.; Liu, Y.; Xie, H.; Jia, W.; Chao, Y.; Han, L. Modulating ABA-dependent growth and development by overexpressing cytochrome P450 ABA 8′-hydroxylase in Medicago truncatula. Environ. Exp. Bot. 2025, 229, 106060. [Google Scholar] [CrossRef]
- Degenhardt, B.; Gimmler, H.; Hose, E.; Hartung, W. Effect of alkaline and saline substrates on ABA contents, distribution and transport in plant roots. Plant Soil 2000, 225, 83–94. [Google Scholar] [CrossRef]
- Jung, C.; Seo, J.S.; Han, S.W.; Koo, Y.J.; Kim, C.H.; Song, S.I.; Nahm, B.H.; Do Choi, Y.; Cheong, J.-J. Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. Plant Physiol. 2008, 146, 323–324. [Google Scholar] [CrossRef]
- Nisa, Z.-u.; Wang, Y.; Ali, N.; Chen, C.; Zhang, X.; Jin, X.; Yu, L.; Jing, L.; Chen, C.; Elansary, H.O. Strigolactone signaling gene from soybean GmMAX2a enhances the drought and salt-alkaline resistance in Arabidopsis via regulating transcriptional profiles of stress-related genes. Funct. Integr. Genom. 2023, 23, 216. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A. Assay of Catalases and Peroxidases; Wiley: Hoboken, NJ, USA, 1955; Volume 2, pp. 764–775. [Google Scholar] [CrossRef]
- Asada, K. Production and scavenging of active oxygen in photosynthesis. In Photo-Inhibition; Elsevier: Amsterdam, The Netherlands, 1987. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Yang, K.; Wang, Y.; Yang, L.; Hu, L.; Liu, R.; Shi, Z. Melatonin facilitates lateral root development by coordinating PAO-derived hydrogen peroxide and Rboh-derived superoxide radical. Free Radic. Biol. Med. 2019, 143, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Kereszt, A.; Li, D.; Indrasumunar, A.; Nguyen, C.D.; Nontachaiyapoom, S.; Kinkema, M.; Gresshoff, P.M. Agrobacterium rhizogenes-mediated transformation of soybean to study root biology. Nat. Protoc. 2007, 2, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Landi, M. Commentary to: “Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds” by Hodges et al., Planta (1999) 207: 604–611. Planta 2017, 245, 1067. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, X.; Liu, X.; Wang, Y.; Li, Y.; Hussain, S.; Jing, X.; Chen, S.; Wang, S. AtAUEs, a Small Family of ABA Up-Regulated EAR Motif-Containing Proteins Regulate ABA Responses in Arabidopsis. Plants 2024, 13, 3282. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Dai, J.; Xu, N.; Zhou, W.; Xu, L.; Pang, Q.; Duanmu, H.; Li, H. GsCYP93D1, a Cytochrome P450 Gene from Wild Soybean, Mediates the Regulation of Plant Alkaline Tolerance and ABA Sensitivity. Plants 2025, 14, 2623. https://doi.org/10.3390/plants14172623
Chen C, Dai J, Xu N, Zhou W, Xu L, Pang Q, Duanmu H, Li H. GsCYP93D1, a Cytochrome P450 Gene from Wild Soybean, Mediates the Regulation of Plant Alkaline Tolerance and ABA Sensitivity. Plants. 2025; 14(17):2623. https://doi.org/10.3390/plants14172623
Chicago/Turabian StyleChen, Chao, Jianyue Dai, Nuo Xu, Wanying Zhou, Liankun Xu, Qiuying Pang, Huizi Duanmu, and Haiying Li. 2025. "GsCYP93D1, a Cytochrome P450 Gene from Wild Soybean, Mediates the Regulation of Plant Alkaline Tolerance and ABA Sensitivity" Plants 14, no. 17: 2623. https://doi.org/10.3390/plants14172623
APA StyleChen, C., Dai, J., Xu, N., Zhou, W., Xu, L., Pang, Q., Duanmu, H., & Li, H. (2025). GsCYP93D1, a Cytochrome P450 Gene from Wild Soybean, Mediates the Regulation of Plant Alkaline Tolerance and ABA Sensitivity. Plants, 14(17), 2623. https://doi.org/10.3390/plants14172623




