Silicon Accumulation and Photosynthetic Capacity of Dendrocalamus brandisii in Response to Sodium Silicate Foliar Application Across Vegetative Phenological Stages
Abstract
1. Introduction
2. Results
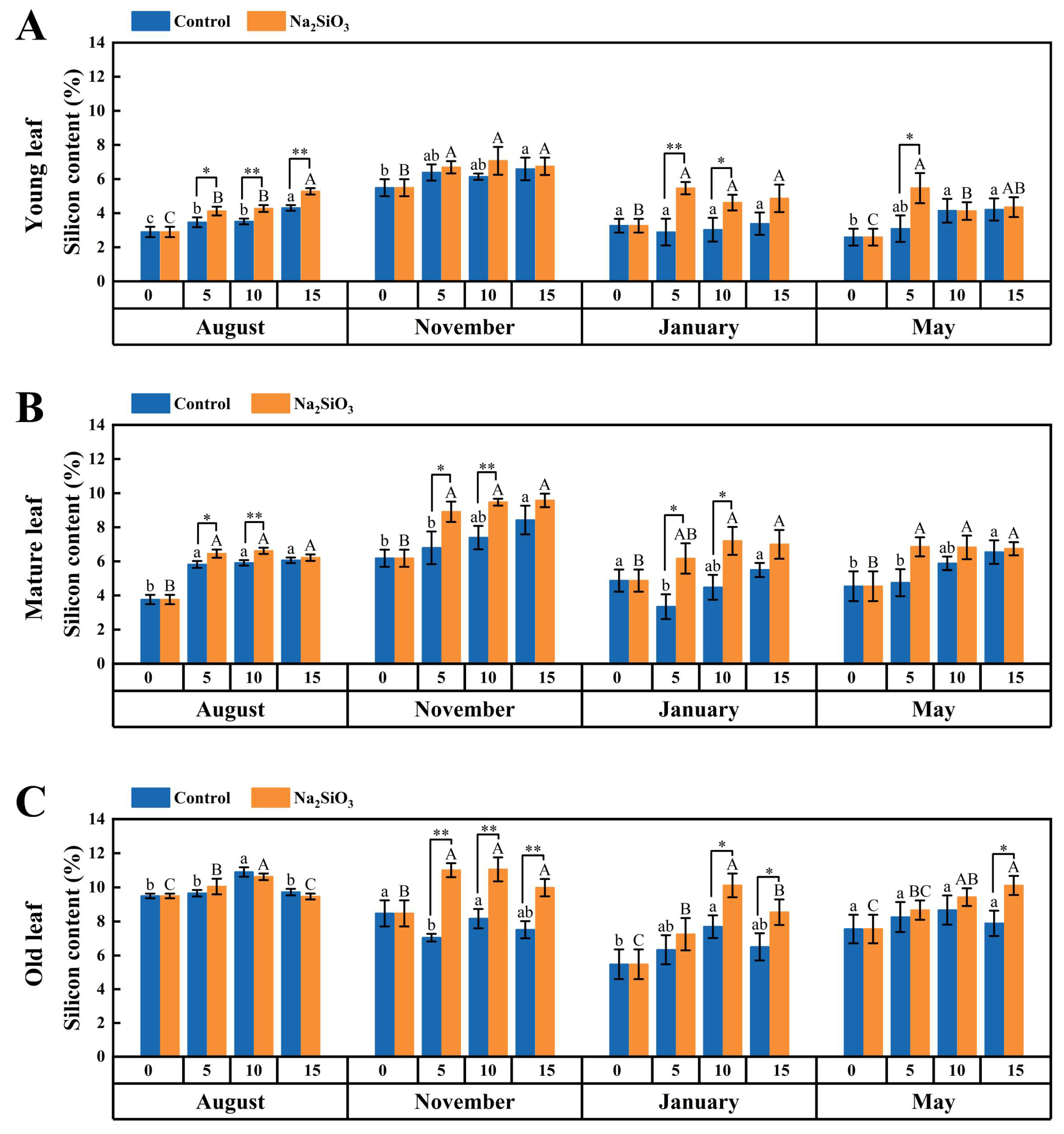
2.1. Silicon Content in D. brandisii Leaves Under Treatment at Different Phenological Stages
2.2. Net Photosynthetic Rate (Pn) of D. brandisii Leaves Under Treatment at Different Phenological Stages
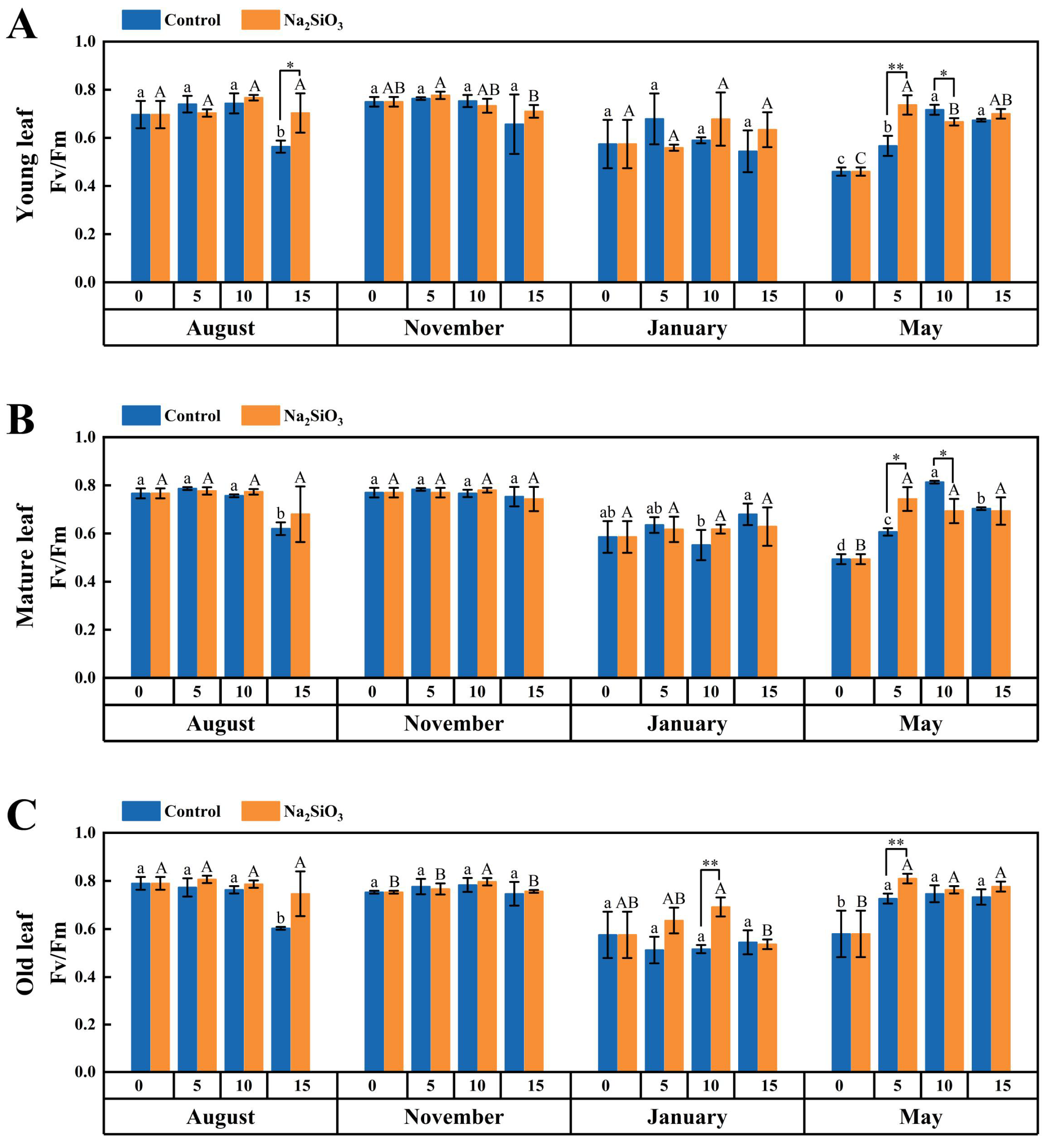
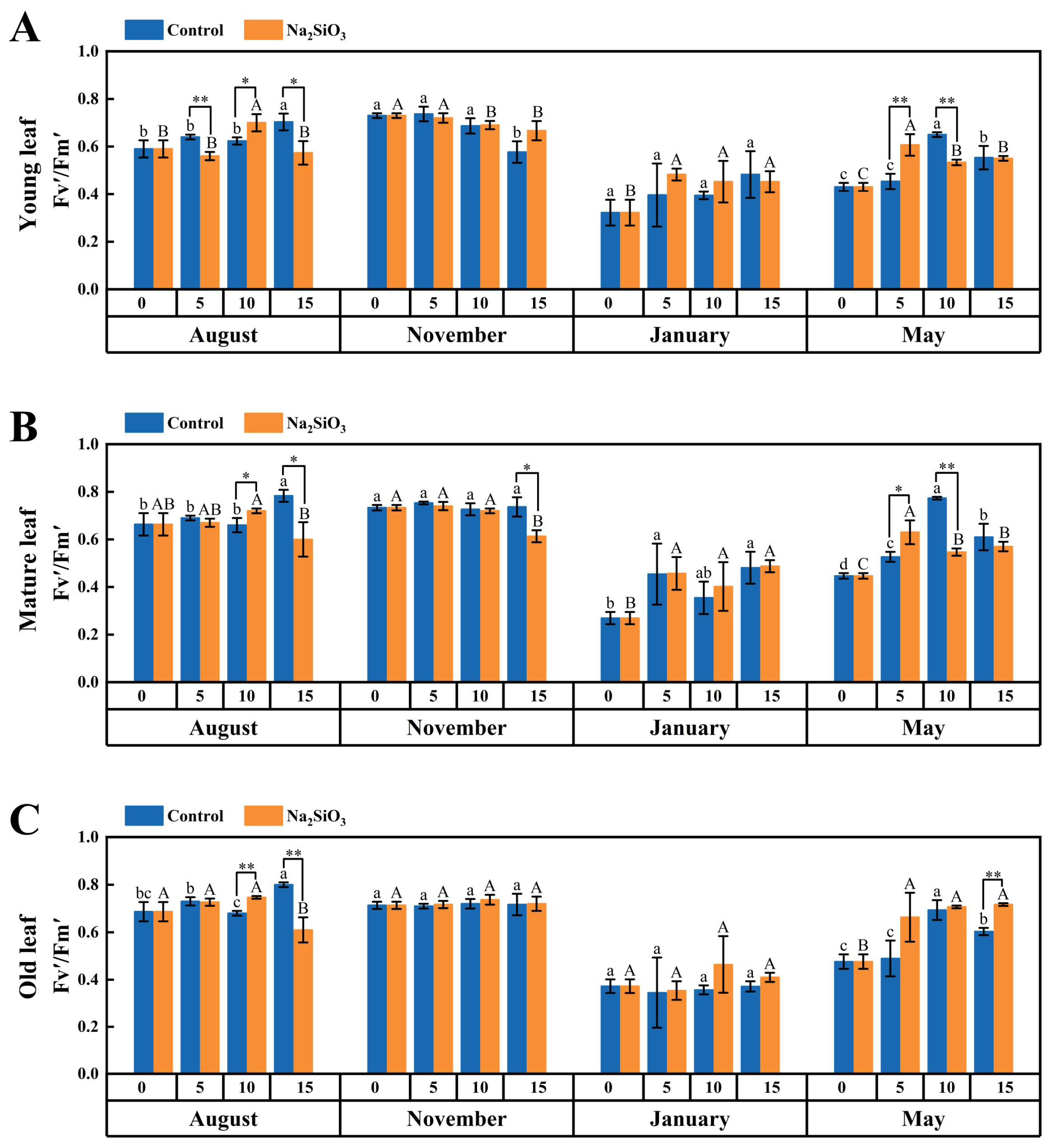
2.3. Chlorophyll Fluorescence Rate in D. brandisii Leaves Under Treatment at Different Phenological Stages
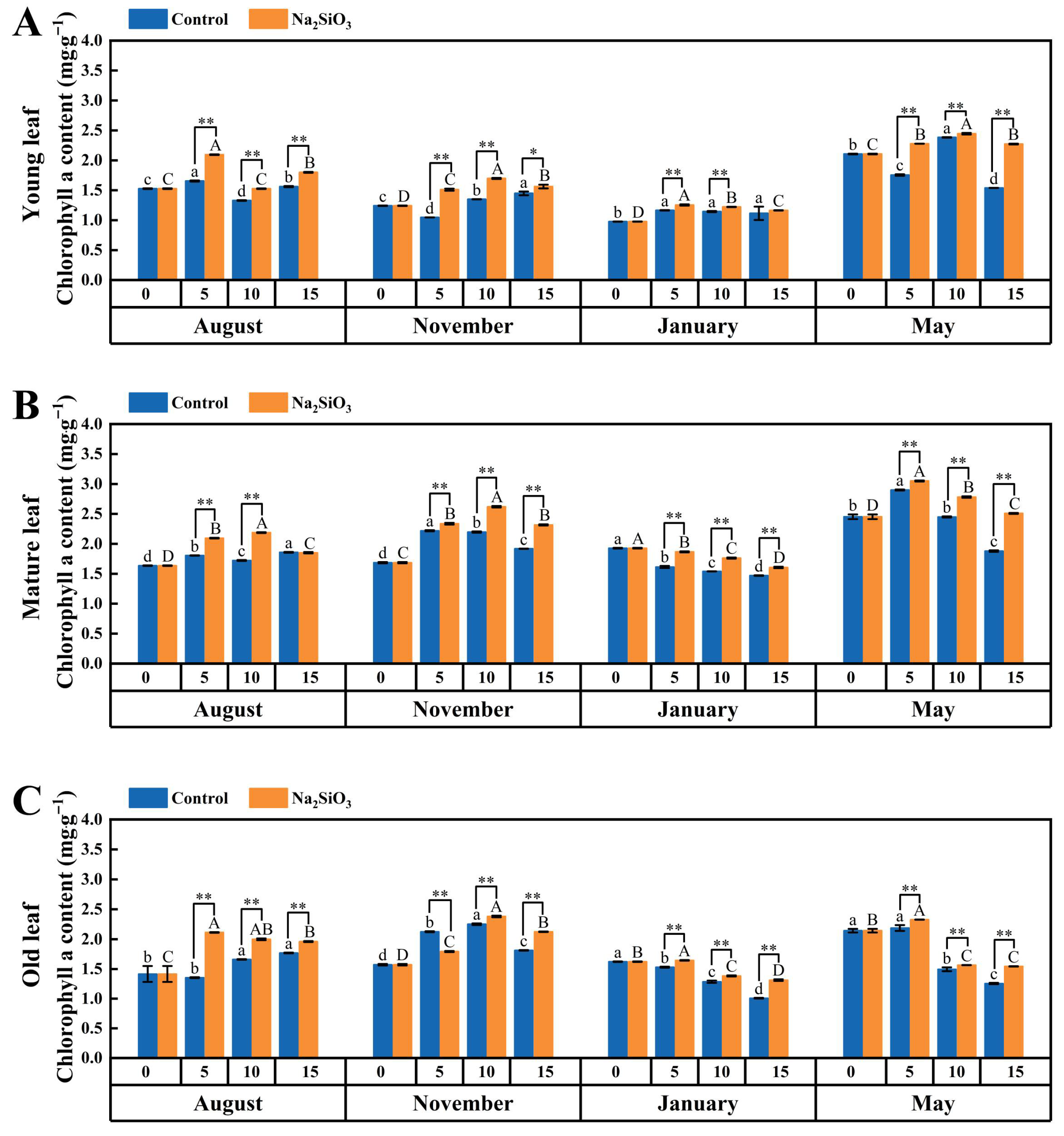
2.4. Photosynthetic Pigments of D. brandisii Leaves under Treatment at Different Phenological Stages
2.4.1. Chlorophyll a
2.4.2. Chlorophyll b
2.4.3. Total Chlorophyll
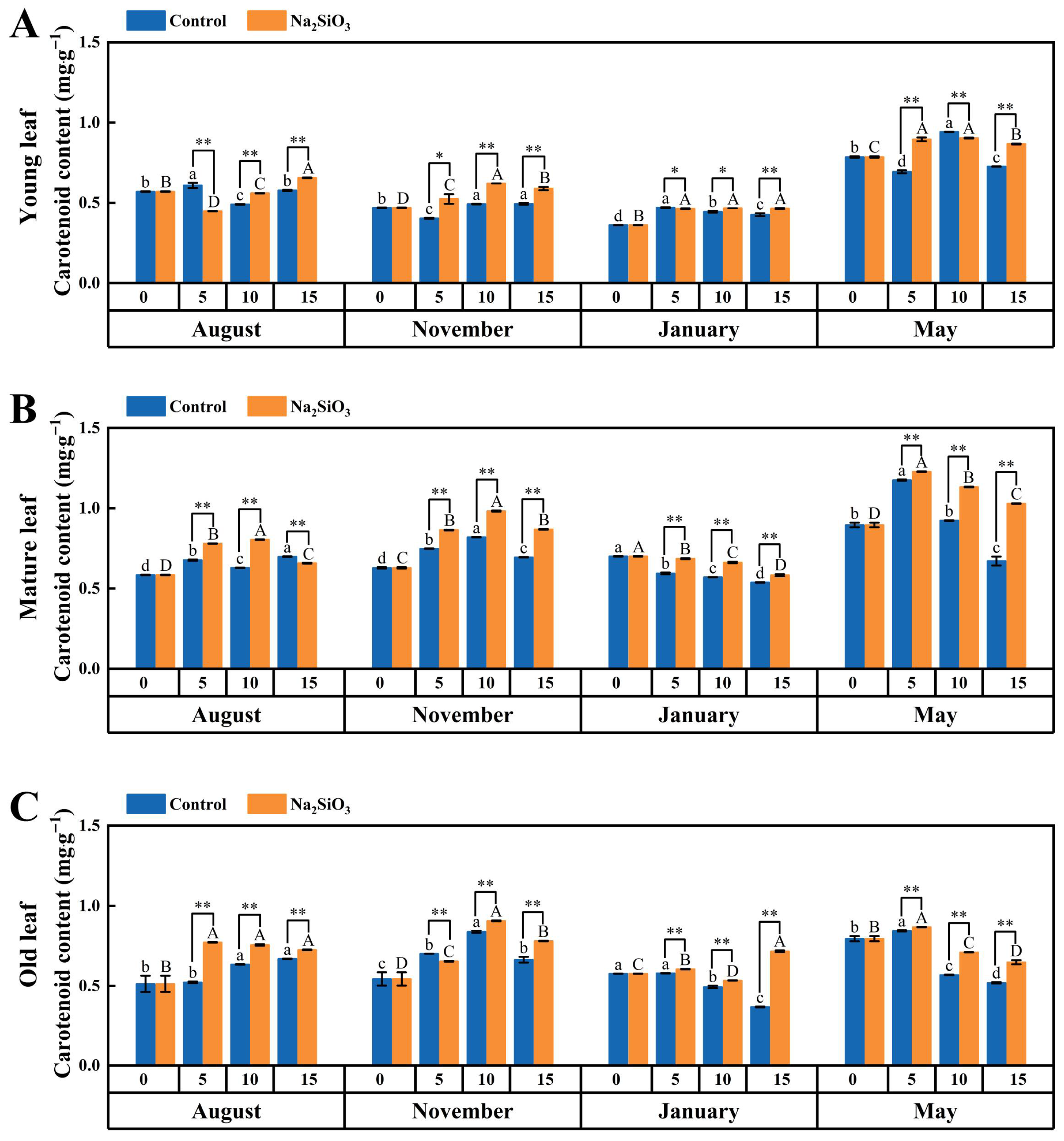
2.4.4. Carotenoid
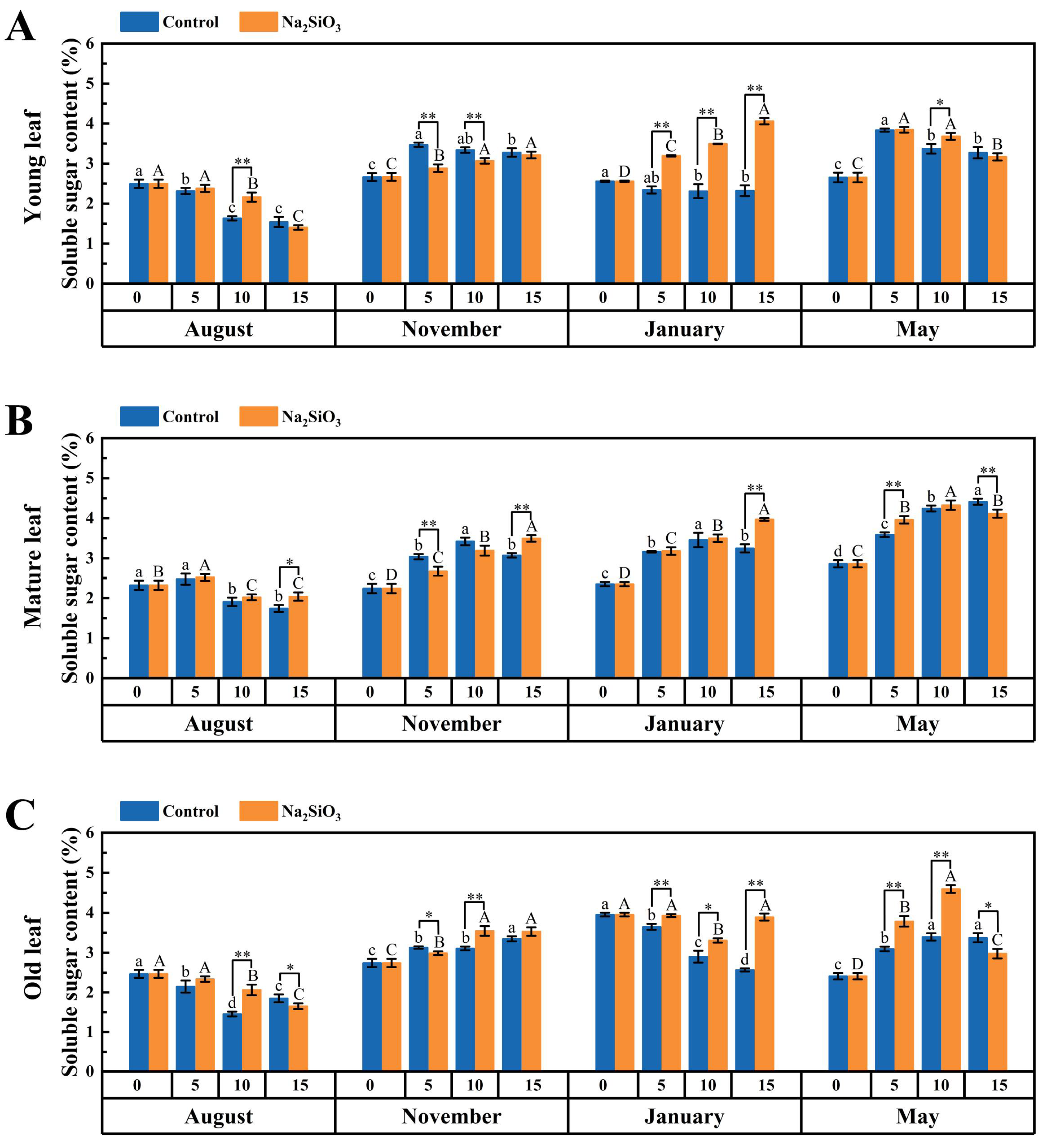
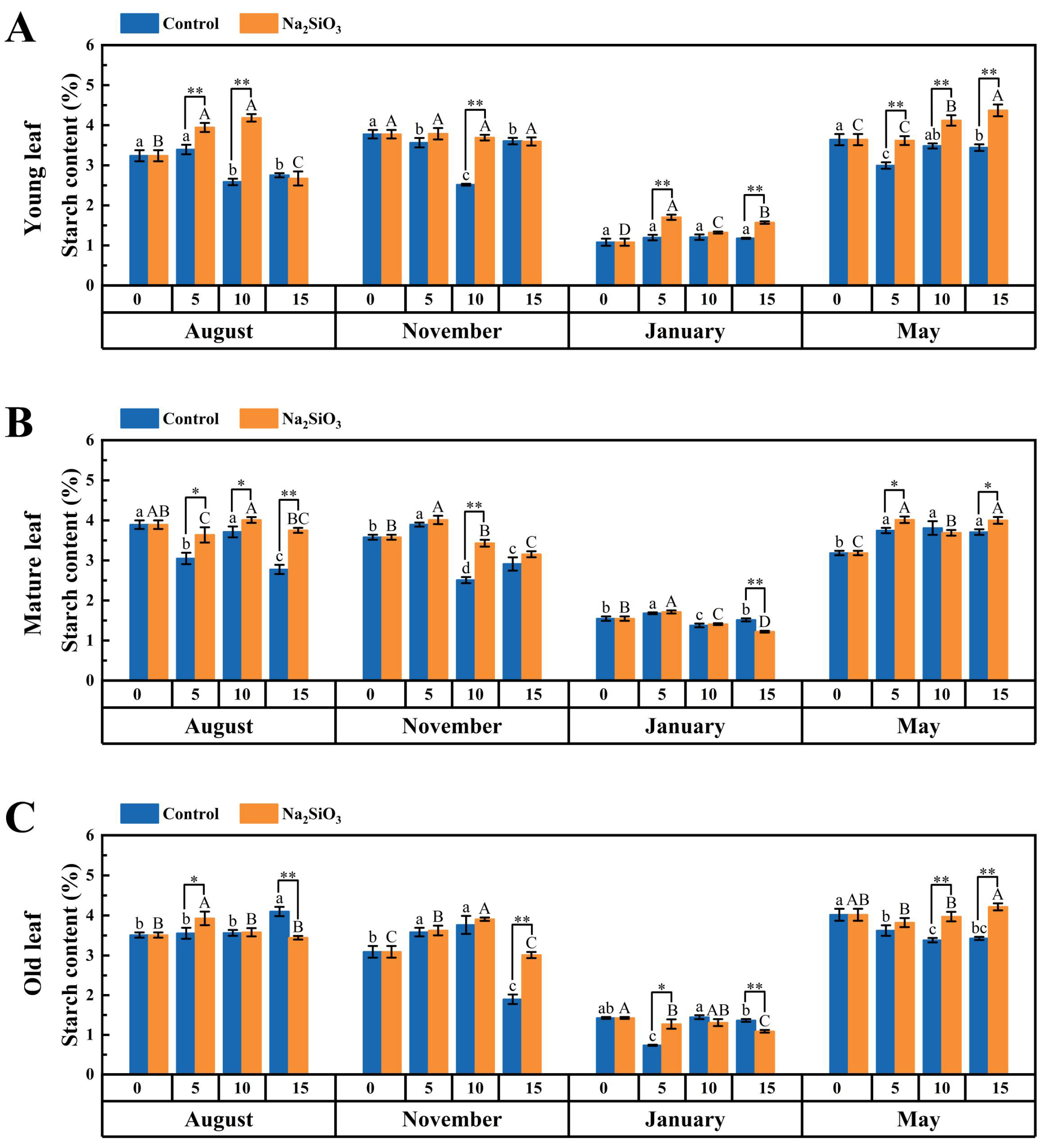
2.5. Photoassimilate Accumulation in D. brandisii Leaves Under Treatment at Different Phenological Stages
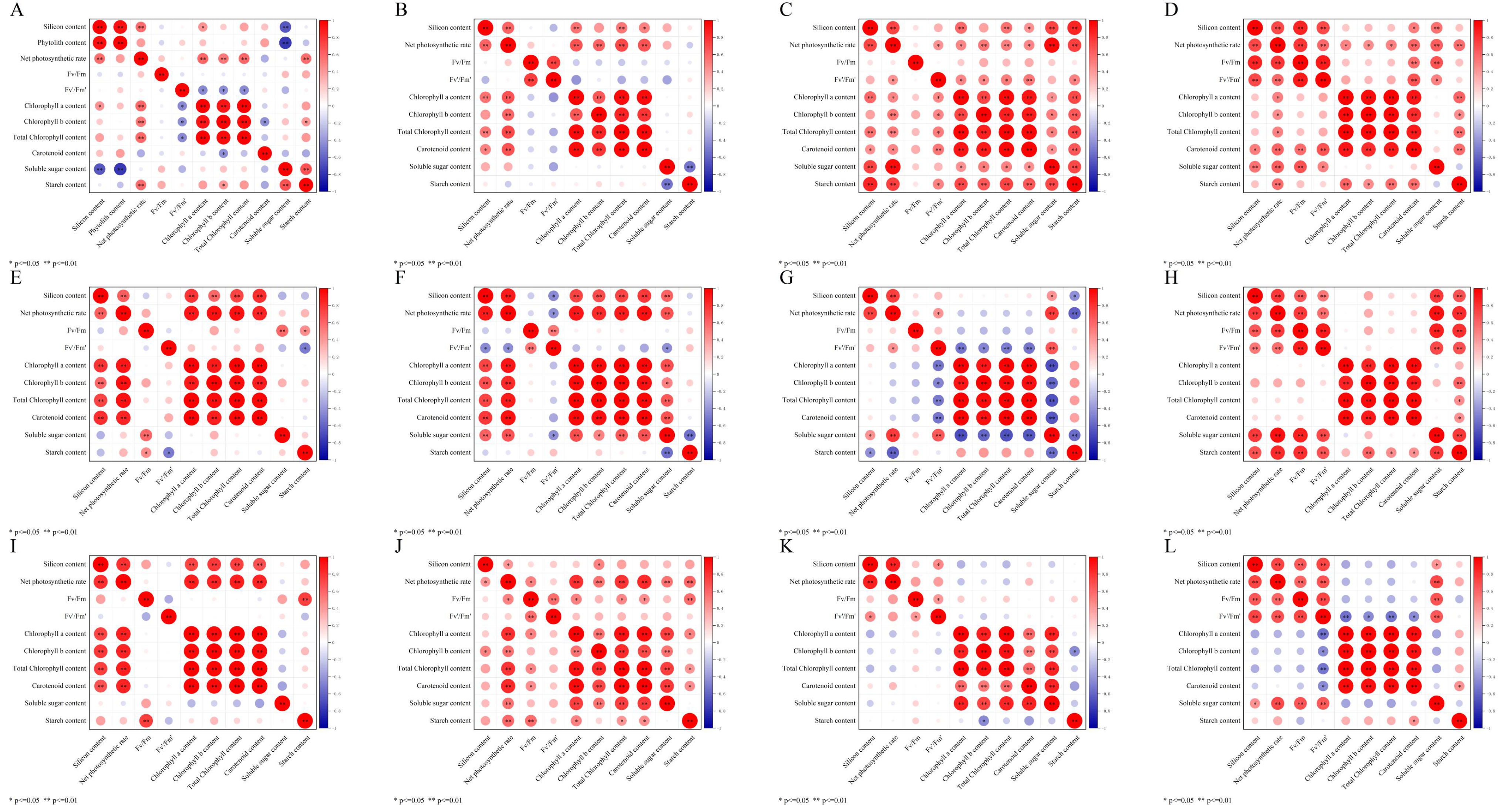
2.6. Correlation Analysis
3. Discussion
3.1. Effect of SS on Silicon Content in D. brandisii Leaves at Different Phenological Stages
3.2. Effect of SS on Photosynthesis in D. brandisii Leaves at Different Phenological Stages
3.3. Effect of SS on Photoassimilate Accumulation in D. brandisii Leaves at Different Phenological Stages
4. Materials and Methods
4.1. Sample Collection and Preparation
4.2. Experimental Methods
4.2.1. Determination of Silicon Content
4.2.2. Determination of Net Photosynthetic Rate (Pn)
4.2.3. Determination of Chlorophyll Fluorescence Rate
4.2.4. Determination of Photosynthetic Pigment
4.2.5. Determination of Soluble Sugar and Starch Content
4.2.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manivannan, A.; Soundararajan, P.; Jeong, B.R. Editorial: Silicon: A “Quasi-Essential”. Element’s role in plant physiology and development. Front. Plant Sci. 2023, 14, 1157185. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y. Biostimulants in horticulture. Sci. Hortic. 2015, 196, 1–134. [Google Scholar] [CrossRef]
- Xu, R.; Huang, J.; Guo, H.; Wang, C.; Zhan, H. Functions of silicon and phytolith in higher plants. Plant Signal Behav. 2023, 18, 2198848. [Google Scholar] [CrossRef]
- Yang, M.; Chen, S.; Chao, K.; Ji, C.; Shi, Y. Effects of nano silicon fertilizer on the lodging resistance characteristics of wheat basal second stem node. BMC Plant Biol. 2024, 24, 54. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Functions and transport of silicon in plants. Cell Mol. Life Sci. 2008, 65, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Quigley, K.M.; Anderson, T.M. Leaf silica concentration in Serengeti grasses increases with watering but not clipping: Insights from a common garden study and literature review. Front. Plant Sci. 2014, 5, 568. [Google Scholar] [CrossRef] [PubMed]
- Deren, C.W.; Datnoff, L.E.; Snyder, G.H. Variable silicon content of rice cultivars grown on everglades histosols. J. Plant Nutr. 1992, 15, 2363–2368. [Google Scholar] [CrossRef]
- Huang, H.; Xu, L.; Bokhtiar, S.M.; Srivastava, M.; Li, Y.; Yang, L. Effect of calcium silicate fertilizer on soil characteristics, sugarcane nutrients and its yield parameters. J. South. Agricult. 2011, 42, 756–759. [Google Scholar] [CrossRef]
- Ke, Y.; Huang, X.; Zhang, Z.; Xiao, C.; Wu, L.; Li, Y.; Jian, H.; Wu, Y. Effect of silicon fertilizer on nitrogen, phosphorus and potassium nutrition of rice and analysis of reasons for increasing production. Guangdong Agricult Sci. 1997, 5, 25–27. [Google Scholar] [CrossRef]
- Pei, F.; Dong, C.; Chen, W.; Yang, Y.; Fang, Q.; Duan, J.; Huang, P.; Wang, H. Preparation and the effect of nano-silicon fertilizer on the growth of Amaranth. Horticult Seed. 2015, 6, 12–17. [Google Scholar] [CrossRef]
- Detmann, K.C.; Araújo, W.; Martins, S.; Sanglard, L.; Reis, J.; Detmann, E.; Rodrigues, F.; Nunes-Nesi, A.; Fernie, A.; DaMatta, F. Silicon nutrition increases grain yield, which, in turn, exerts a feed-forward stimulation of photosynthetic rates via enhanced mesophyll conductance and alters primary metabolism in rice. New Phytol. 2012, 196, 752–762. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, G.; Huo, Z.; Xiao, Y.; Xiong, F.; Zhang, H.; Dai, Q. Effects of application of nitrogen combined with silicon on the photosynthesis and activities of nitrogen metabolic enzyme of rice leaf. J Yangzhou Univ. (Agric. Life Sci Ed.). 2010, 31, 44–49. [Google Scholar]
- Jones, L.; Milne, A.; Sanders, J. Tabashir: An opal of plant origin. Science 1966, 151, 464–466. [Google Scholar] [CrossRef]
- Zhan, H.; Li, J.; Niu, Z.; Li, M.; Wang, C.; Wang, S. Silicon variation and phytolith morphology in different organs of Dendrocalamus brandisii (Munro) Kurz (Bambusoideae). Braz. J. Bot. 2019, 42, 529–541. [Google Scholar] [CrossRef]
- Niu, Z.; He, W.; Wang, C.; Zhang, L.; Zhan, H.; Wang, S. The changes in phytolith morphology in culms of Dendrocalamus giganteus. J. Bamboo Res. 2016, 35, 9–14+25. [Google Scholar]
- Motomura, H.; Fujii, T.; Suzuki, M. Silica deposition in relation to ageing of leaf tissues in Sasa veitchii (Carriere) Rehder (Poaceae: Bambusoideae). Ann. Bot. 2004, 93, 235–248. [Google Scholar] [CrossRef]
- Zhu, F.; Niu, Z.; Li, J.; Yu, L.; Wang, S.; Wang, C.; Zhan, H. Variation of the phytolith content and morphology of Dendrocalamus giganteus at different phenological periods. J. Southwest. For. Univ. 2022, 42, 71–77. [Google Scholar]
- Li, D.; Wang, Z.; Zhu, Z.; Xia, N.; Jia, L.; Guo, Z.; Yang, G.; Stapleton, C. Bambuseae. In Flora of China; Wu, Z., Raven, P., Hong, D., Eds., Science Press: Beijing, China, 2006. [Google Scholar]
- Xu, R.; He, H.; Guo, J.; Zhu, F.; Wang, S.; Dai, C.; Zheng, X.; Xie, D.; Li, H.; Wang, C.; et al. Characteristics of silicon and phytolith distribution in bamboo (Ferrocalamus strictus): Variations between different organs and ages. Rev. Palaeobot. Palynol. 2023, 311, 104817. [Google Scholar] [CrossRef]
- Zhang, L.; Zhan, H.; Niu, Z.; Wang, C.; Wang, S. Effects of exogenous silicon on silicon, chlorophyll, and soluble sugar contents in seedling leaf blades of Dendrocalamus brandisii. J. Plant Nutr. Fert. 2016, 45, 101–106. [Google Scholar]
- Rotmuenwai, N.; Aryuyo, K.; Kruethaworn, N.; Wattananit, W.; Yookongkaew, N. Exploring silica accumulation in bamboo leaves: A study on phytolith morphology and epidermal patterning in the tropical giant bamboo Dendrocalamus copelandii. Ann. Bot. 2025, 135, 757–768. [Google Scholar] [CrossRef]
- Hodson, M. The development of phytoliths in plants and its influence on their chemistry and isotopic composition: Implications for palaeoecology and archaeology. J. Archaeol. Sci. 2016, 68, 62–69. [Google Scholar] [CrossRef]
- Liu, J.; Lin, S.; Xu, P.; Wang, X.; Bai, J. Effects of exogenous silicon on the activities of antioxidant enzymes and lipid peroxidation in chilling-stressed cucumber leaves. ASC 2009, 8, 1075–1086. [Google Scholar] [CrossRef]
- Habibi, G. Effect of foliar-applied silicon on photochemistry, antioxidant capacity, and growth in maize plants subjected to chilling stress. Acta Agric. Slov. 2016, 107, 33–43. [Google Scholar] [CrossRef]
- Guerriero, G.; Deshmukh, R.; Sonah, H.; Sergeant, K.; Hausman, J.F.; Lentzen, E.; Valle, N.; Siddiqui, S.; Exley, C. Identification of the aquaporin gene family in Cannabis sativa and evidence for the accumulation of silicon in its tissues. Plant Sci. 2019, 287, 110167. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Chen, L.; Liang, R.; Huang, S.; Li, X.; Huang, B.; Luo, H.; Zhang, M.; Wang, X.; Zhu, H. The role of light in regulating plant growth, development and sugar metabolism: A review. Front. Plant Sci. 2025, 15, 1507628. [Google Scholar] [CrossRef]
- Alnusaire, T.; Abdulmajeed, A.; ALrashidi, A.; Alharbi, B.; Alharbi, K.; Alayafi, A.; Alghanem, S.; Alsudays, I.; Soliman, M. Synergistic Effects of Nano-Silica and Biofertilizers on Growth, Physiological Performance, and Essential Oil Production in Satureja montana. J. Soil. Sci. Plant Nutr. 2025, 25, 2948–2961. [Google Scholar] [CrossRef]
- Tárraga, W.; Picco, A.; Longo, G. Understanding protein adsorption on silica mesoporous materials through thermodynamic simulations. Surf. Interfaces 2024, 52, 104870. [Google Scholar] [CrossRef]
- Su, S.; Yang, C.; Rao, X.; Li, X. Progress on effects of silicon on plant stress resistance. J. Huazhong Agric. Univ. 2022, 41, 160–168. [Google Scholar] [CrossRef]
- Li, N.; Wang, K.; Lv, Y.; Zhang, Z.; Cao, B.; Chen, Z.; Xu, K. Silicon enhanced the resistance of Chinese cabbage (Brassica rapa L. ssp. pekinensis) to ofloxacin on the growth, photosynthetic characteristics and antioxidant system. Plant Physiol. Biochem. 2022, 175, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Xu, K.; Shi, J.; Xin, G.; Liu, C.; Li, X. Effects of silicon on growth, photosynthesis and transpiration of tomato. J. Plant Nutr. Fert. 2013, 19, 354–360. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, H.; Fu, Y.; Li, P.; Li, S.; Gao, Y. Regulatory effects of silicon nanoparticles on the growth and photosynthesis of cotton seedlings under salt and low-temperature dual stress. BMC Plant Biol. 2023, 23, 504. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, D.; Luo, H.; Yao, Y.; Wang, Z.; Tsutomu, M.; Tian, X. Effects of exogenous silicon on the pollination and fertility characteristics of hybrid rice under heat stress during anthesis. J. Appl. Ecol. 2013, 24, 3113–3122. [Google Scholar]
- Harizanova, A.; Koleva-Valkova, L. Effect of silicon on photosynthetic rate and the chlorophyll fluorescence parameters at hydroponically grown cucumber plants under salinity stress. J. Cent. Eur. Agric. 2019, 20, 953–960. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Isayenkov, S.V. The Physiological and Molecular Mechanisms of Silicon Action in Salt Stress Amelioration. Plants 2024, 13, 525. [Google Scholar] [CrossRef]
- Tobiasz-Salach, R.; Stadnik, B.; Mazurek, M.; Buczek, J.; Leszczyńska, D. Foliar Application of Silicon Influences the Physiological and Epigenetic Responses of Wheat Grown Under Salt Stress. Int. J. Mol. Sci. 2024, 25, 13297. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.L.; Muneer, S.; Kim, Y.H.; Al-Rawahi, A.; Al-Harrasi, A. Silicon and Salinity: Crosstalk in Crop-Mediated Stress Tolerance Mechanisms. Front. Plant Sci. 2019, 10, 1429. [Google Scholar] [CrossRef]
- Jat, M.; Ray, M.; Ahmad, M.A.; Prakash, P. Unravelling the photosynthetic dynamics and fluorescence parameters under ameliorative effects of 24-epibrassinolide in wheat (Triticum aestivum L.) grown under heat stress regime. Sci. Rep. 2024, 14, 30745. [Google Scholar] [CrossRef]
- Song, X.; Mo, F.; Yan, M.; Zhang, X.; Zhang, B.; Huang, X.; Huang, D.; Pan, Y.; Verma, K.; Li, Y. Effect of Smut Infection on the Photosynthetic Physiological Characteristics and Related Defense Enzymes of Sugarcane. Life 2022, 12, 1201. [Google Scholar] [CrossRef]
- Nowakowska, J.; Dang, M.; Kiełtyk, P.; Niemczyk, M.; Malewski, T.; Szulc, W.; Rutkowska, B.; Borowik, P.; Oszako, T. Silicon Modifies Photosynthesis Efficiency and hsp Gene Expression in European Beech (Fagus sylvatica) Seedlings Exposed to Drought Stress. Genes 2024, 15, 1233. [Google Scholar] [CrossRef]
- Bai, R.; Liu, H.; Liu, Y.; Yong, J. Effects of foliar application of magnesium fertilizer on photosynthesis and growth in grapes. Agronomy 2024, 14, 2659. [Google Scholar] [CrossRef]
- Arnao, M.; Hernández-Ruiz, J. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J. Pineal Res. 2009, 46, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Kamran, M.; Ding, R.; Meng, X.; Wang, H.; Ahmad, I.; Fahad, S.; Han, Q. Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. PeerJ 2019, 7, e7793. [Google Scholar] [CrossRef]
- Huang, S.; Fan, T.; Liu, M.; Chen, R.; Liang, C.; Cheng, W.; Chen, Y.; Xue, S.; Yang, X. Effects of plastic film residues on photophysiological characteristics and biomass accumulation of soybean (Glycine max). Chin. J. Eco Agric. 2021, 29, 979–990. [Google Scholar] [CrossRef]
- Sadak, M.; Sekara, A.; Al-Ashkar, I.; Habib-Ur-Rahman, M.; Skalicky, M.; Brestic, M.; Kumar, A.; Sabagh, A.; Abdelhamid, M. Exogenous aspartic acid alleviates salt stress-induced decline in growth by enhancing antioxidants and compatible solutes while reducing reactive oxygen species in wheat. Front. Plant Sci. 2022, 13, 987641. [Google Scholar] [CrossRef]
- Sun, D.; Lu, X.; Hu, Y.; Li, W.; Duan, Y.; Pang, Z.; Hu, H. Research progress of silica nanoparticle effects on the growth and development of plants. Chin. J. Trop. Crops. 2019, 40, 2300–2311. [Google Scholar]
- Kim, Y.H.; Khan, A.; Waqas, M.; Lee, I.J. Silicon Regulates Antioxidant Activities of Crop Plants under Abiotic-Induced Oxidative Stress: A Review. Front. Plant Sci. 2017, 8, 510. [Google Scholar] [CrossRef] [PubMed]
- Muneer, S.; Park, Y.; Manivannan, A.; Soundararajan, P.; Jeong, B. Physiological and proteomic analysis in chloroplasts of Solanum lycopersicum L. under silicon efficiency and salinity stress. Int. J. Mol. Sci. 2014, 15, 21803–21824. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, Q.; Ding, C.; Huang, Y.; Liao, J.; Chen, T.; Feng, S.; Zhou, L.; Zhang, Z.; Chen, Y.; et al. Effect of Low Temperature on Chlorophyll Biosynthesis and Chloroplast Biogenesis of Rice Seedlings during Greening. Int. J. Mol. Sci. 2020, 21, 1390. [Google Scholar] [CrossRef]
- Lv, C.; Huang, B. Effects of boron on membrane lipid peroxidation and endogenous protective systems in leaves of Eucalyptus grandis × Eucalyptus urophylla under low temperature. J. Trop. Subtr. Bot. 2003, 2, 217–222. [Google Scholar]
- Scofield, G.; Ruuska, S.; Aoki, N.; Lewis, D.; Tabe, L.; Jenkins, C. Starch storage in the stems of wheat plants: Localization and temporal changes. Ann. Bot. 2009, 103, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Dong, Q.; You, C.; Zhai, H.; Hao, Y. Expression analysis and functional characterization of apple MdVHP1 gene reveals its involvement in Na(+), malate and soluble sugar accumulation. Plant Physiol. Biochem. 2011, 49, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Koch, K. Carbohydrate-modulated gene expression in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 509–540. [Google Scholar] [CrossRef] [PubMed]
- Boriboonkaset, T.; Theerawitaya, C.; Yamada, N.; Pichakum, A.; Supaibulwatana, K.; Cha-Um, S.; Takabe, T.; Kirdmanee, C. Regulation of some carbohydrate metabolism-related genes, starch and soluble sugar contents, photosynthetic activities and yield attributes of two contrasting rice genotypes subjected to salt stress. Protoplasma 2013, 250, 1157–1167. [Google Scholar] [CrossRef]
- Sulpice, R.; Pyl, E.T.; Ishihara, H.; Trenkamp, S.; Steinfath, M.; Witucka-Wall, H.; Gibon, Y.; Usadel, B.; Poree, F.; Piques, M.; et al. Starch as a major integrator in the regulation of plant growth. Proc. Natl. Acad. Sci. USA 2009, 106, 10348–10353. [Google Scholar] [CrossRef]
- Irfan, M.; Maqsoo, M.; Rehman, H.; Mahboob, W.; Sarwar, N.; Hafeez, O.; Hussain, S.; Ercisli, S.; Akhtar, M.; Aziz, T. Silicon nutrition in plants under water-deficit conditions: Overview and prospects. Water 2023, 15, 739. [Google Scholar] [CrossRef]
- Iqbal, S.; Balal, R.; Seleiman, M.; Mattia, M.; Chater, J.; Shahid, M. Silicon and Potassium-Induced Modulations in Leaf Carbohydrate Metabolism Confer Freezing Tolerance in Satsuma Mandarin. Silicon 2024, 16, 5135–5146. [Google Scholar] [CrossRef]
- Hussain, S.; Mumtaz, M.; Manzoor, S.; Li, S.; Ahmed, I.; Skalicky, M.; Brestic, M.; Rastogi, A.; Ulhassan, Z.; Shafiq, I.; et al. Foliar application of silicon improves growth of soybean by enhancing carbon metabolism under shading conditions. Plant Physiol. Biochem. 2021, 159, 43–52. [Google Scholar] [CrossRef]
- Jeandet, P.; Formela-Luboińska, M.; Labudda, M.; Morkunas, I. The Role of Sugars in Plant Responses to Stress and Their Regulatory Function during Development. Int. J. Mol. Sci. 2022, 23, 5161. [Google Scholar] [CrossRef]
- Klančnik, K.; Vogel-Mikuš, K.; Gaberščik, A. Silicified structures affect leaf optical properties in grasses and sedge. J. Photochem. Photobiol. B 2014, 130, 1–10. [Google Scholar] [CrossRef]
- Frew, A.; Weston, L.; Reynolds, O.; Gurr, G. The role of silicon in plant biology: A paradigm shift in research approach. Ann. Bot. 2018, 121, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Gao, D.; Chen, J.; Luo, S. Probing the mechanisms of silicon-mediated pathogen resistance. Plant Signal Behav. 2009, 4, 1–3. [Google Scholar] [CrossRef]
- Cao, B.; Ma, Q.; Zhao, Q.; Wang, L.; Xu, K. Effects of silicon on absorbed light allocation, antioxidant enzymes and ultrastructure of chloroplasts in tomato leaves under simulated drought stress. Scientia Hortic. 2015, 194, 53–62. [Google Scholar] [CrossRef]
- Shi, W.; Li, J.; Zhan, H.; Yu, L.; Wang, C.; Wang, S. Relation between Water Storage and Photoassimilate Accumulation of Neosinocalamus affinis with Phenology. Forests. 2023, 14, 531. [Google Scholar] [CrossRef]
- GB/T2677.3-1993; Fibrous Raw Material—Determination of Ash. Standards Press of China: Qinhuangdao, China, 1993.
- GB/T 7978-1987; Pulps—Determination of Alcohol-Silicon Dioxide. Standards Press of China: Qinhuangdao, China, 1987.
- Maxwell, K.; Johnson, G. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescenc. Biochim. Biophys. Acta, Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Zou, Q. Guide to Plant Physiology Experiment; China Agriculture Press: Beijing, China, 2008. [Google Scholar]
- Lichtenthaler, H. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Glassop, D.; Roessner, U.; Bacic, A.; Bonnett, G. Changes in the sugarcane metabolome with stem development. Are they related to sucrose accumulation? Plant Cell Physiol. 2007, 48, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.; Hamilto, J.; Rebers, P.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]



| Sample Timing | Phenological Stage | Phenology of Bamboo Clump |
|---|---|---|
| August 2022 Summer/rainy season | Shooting | Shoot buds continued forming and developing. Shoots generated out of the ground constantly. |
| November 2022 Autumn/dry season | Late shooting | Shooting completed, and most finished height growth. |
| January 2023 Winter/dry season | Dormancy | New culms ceased growing and the bamboo clumps entered dormancy. |
| May 2023 Spring/dry season | Branching and leafing | Branches and leaves extended simultaneously, and shoot buds started sprouting at the bases of 1- and 2-year culms. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Huang, L.; Yu, L.; Zhu, F.; Chang, J.; Li, M.; Wang, S.; Wang, C.; Zhan, H. Silicon Accumulation and Photosynthetic Capacity of Dendrocalamus brandisii in Response to Sodium Silicate Foliar Application Across Vegetative Phenological Stages. Plants 2025, 14, 2624. https://doi.org/10.3390/plants14172624
Yang Y, Huang L, Yu L, Zhu F, Chang J, Li M, Wang S, Wang C, Zhan H. Silicon Accumulation and Photosynthetic Capacity of Dendrocalamus brandisii in Response to Sodium Silicate Foliar Application Across Vegetative Phenological Stages. Plants. 2025; 14(17):2624. https://doi.org/10.3390/plants14172624
Chicago/Turabian StyleYang, Yuntao, Lei Huang, Lixia Yu, Fangwei Zhu, Ju Chang, Maobiao Li, Shuguang Wang, Changming Wang, and Hui Zhan. 2025. "Silicon Accumulation and Photosynthetic Capacity of Dendrocalamus brandisii in Response to Sodium Silicate Foliar Application Across Vegetative Phenological Stages" Plants 14, no. 17: 2624. https://doi.org/10.3390/plants14172624
APA StyleYang, Y., Huang, L., Yu, L., Zhu, F., Chang, J., Li, M., Wang, S., Wang, C., & Zhan, H. (2025). Silicon Accumulation and Photosynthetic Capacity of Dendrocalamus brandisii in Response to Sodium Silicate Foliar Application Across Vegetative Phenological Stages. Plants, 14(17), 2624. https://doi.org/10.3390/plants14172624






