Genus Echium L.: Phytochemical Characterization and Bioactivity Evaluation for Drug Discovery
Abstract
1. Introduction
Aims and Methodology
2. Phytochemical Characterization
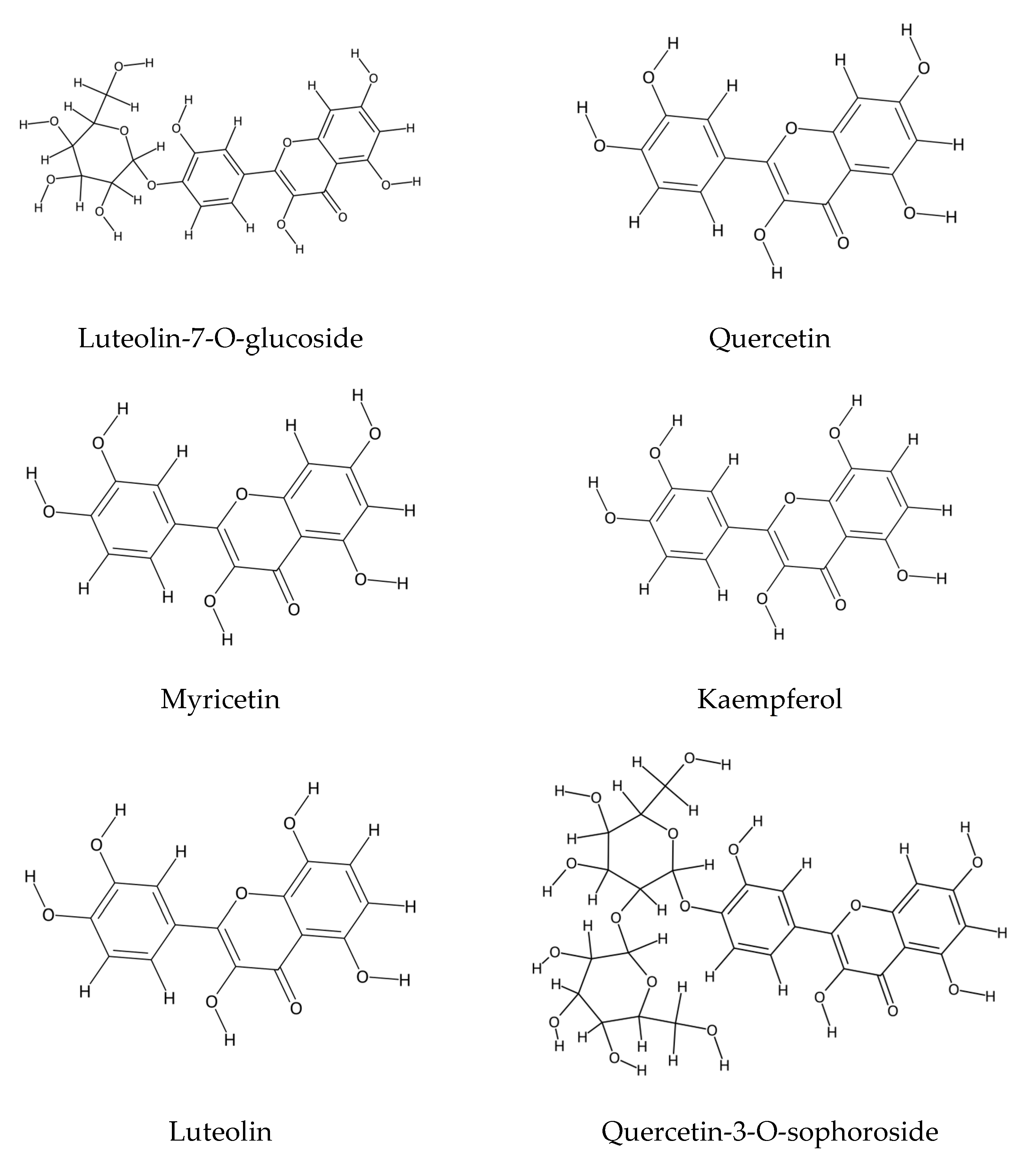
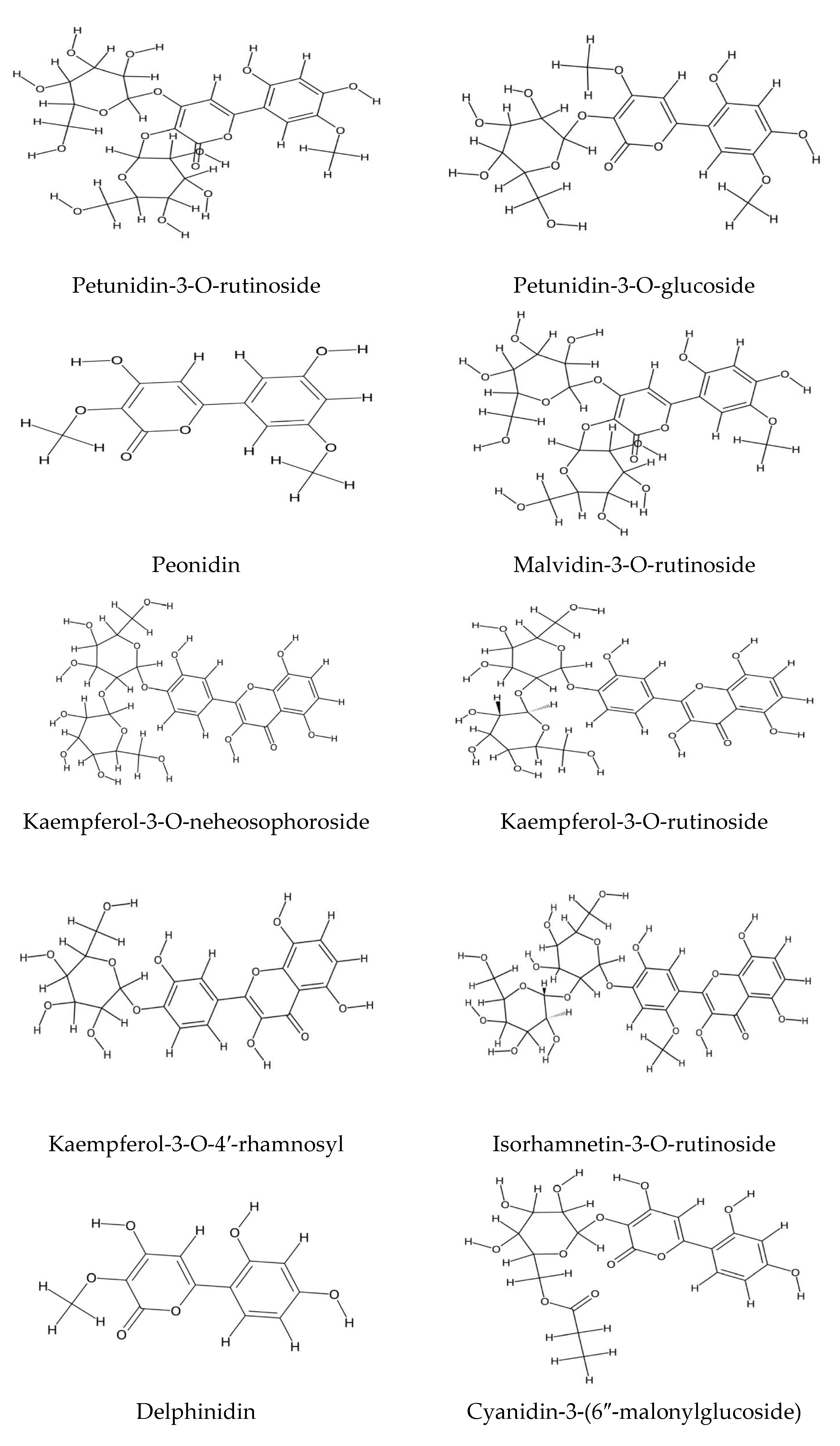
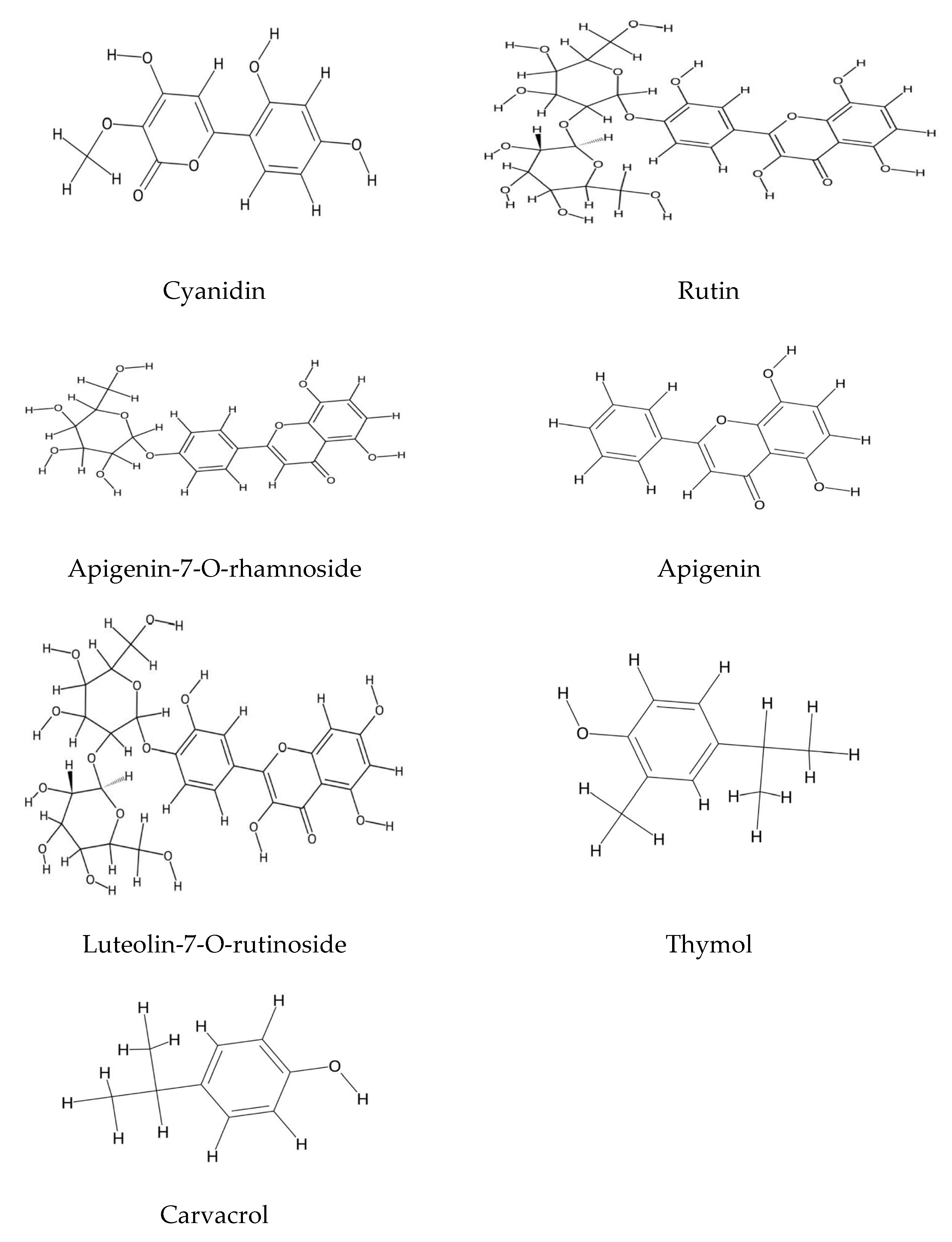
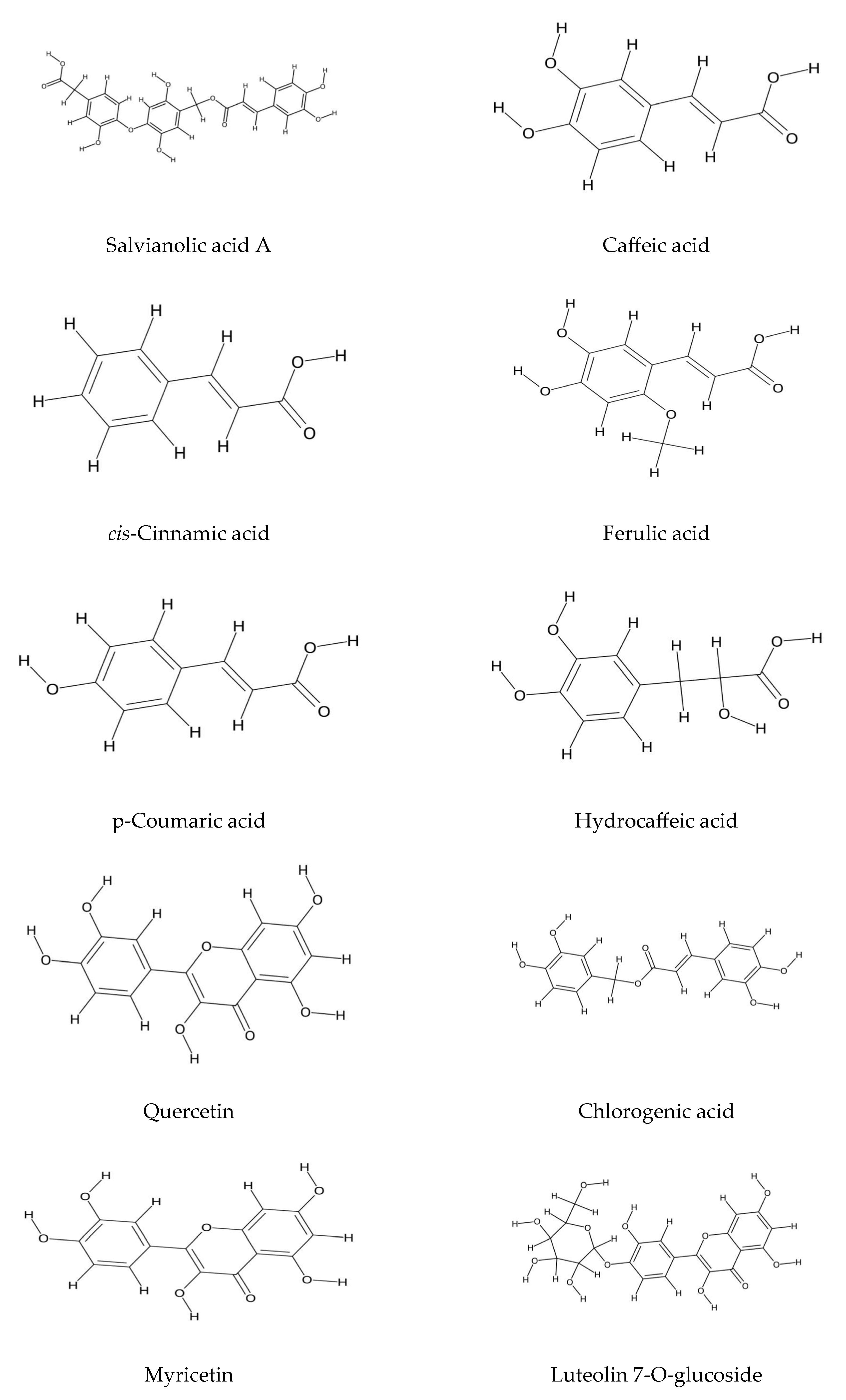
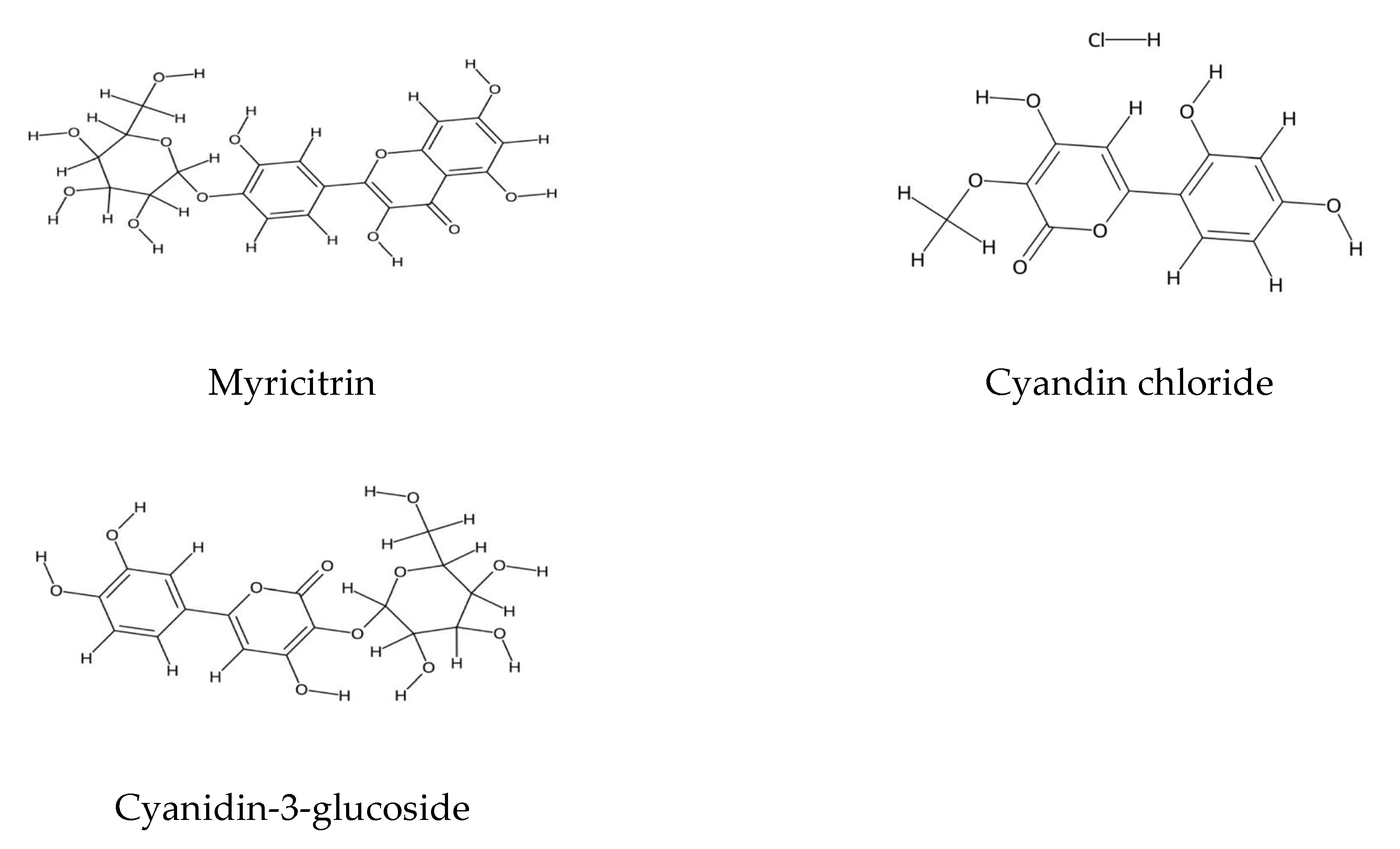
2.1. Polyphenolic Compound Extraction and Phenolic Profiles in Echium Species: Key Findings and Analytical Insights
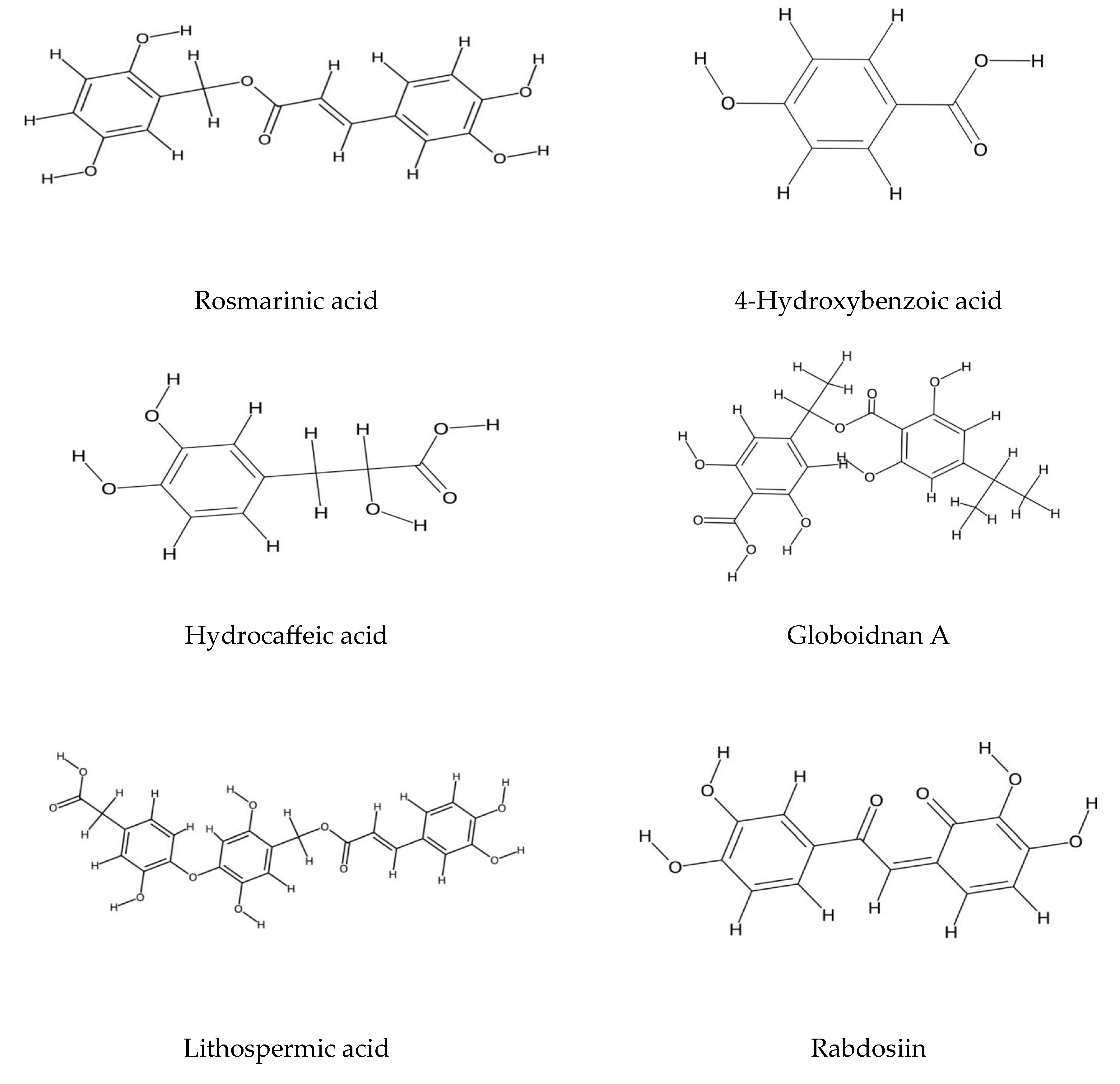
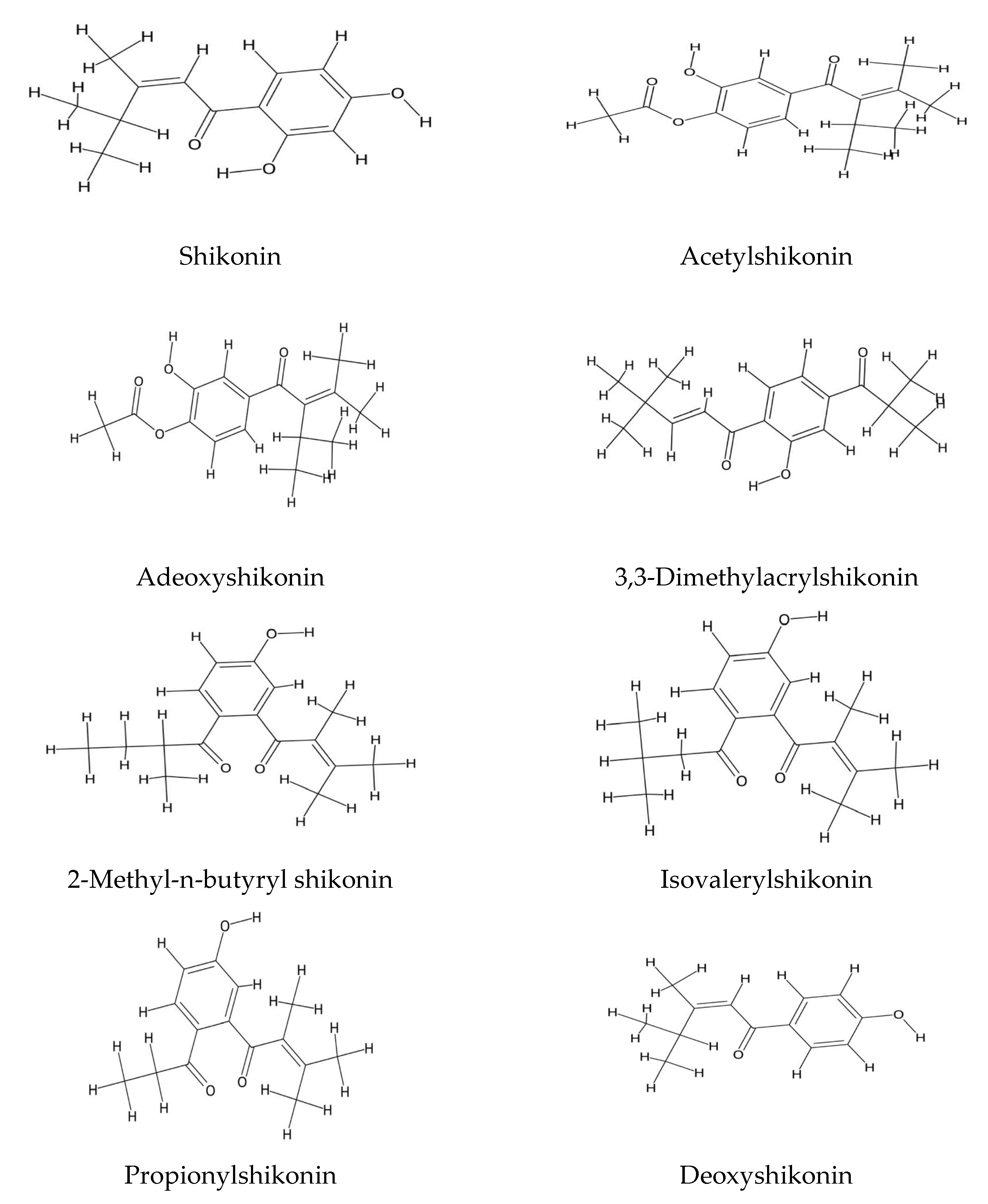
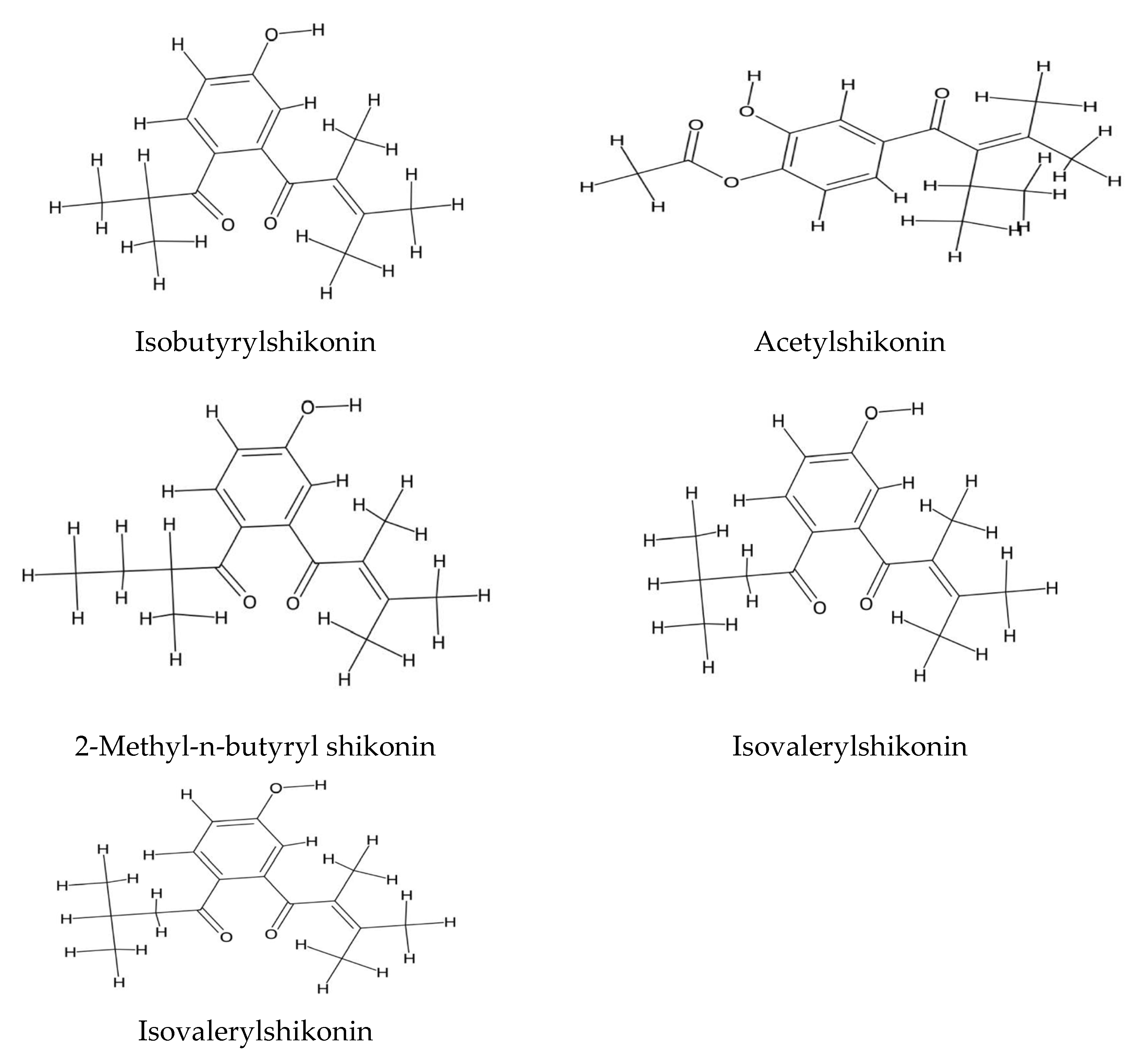
2.2. Echium Roots: Focus on Alkanin and Shikonin Compounds
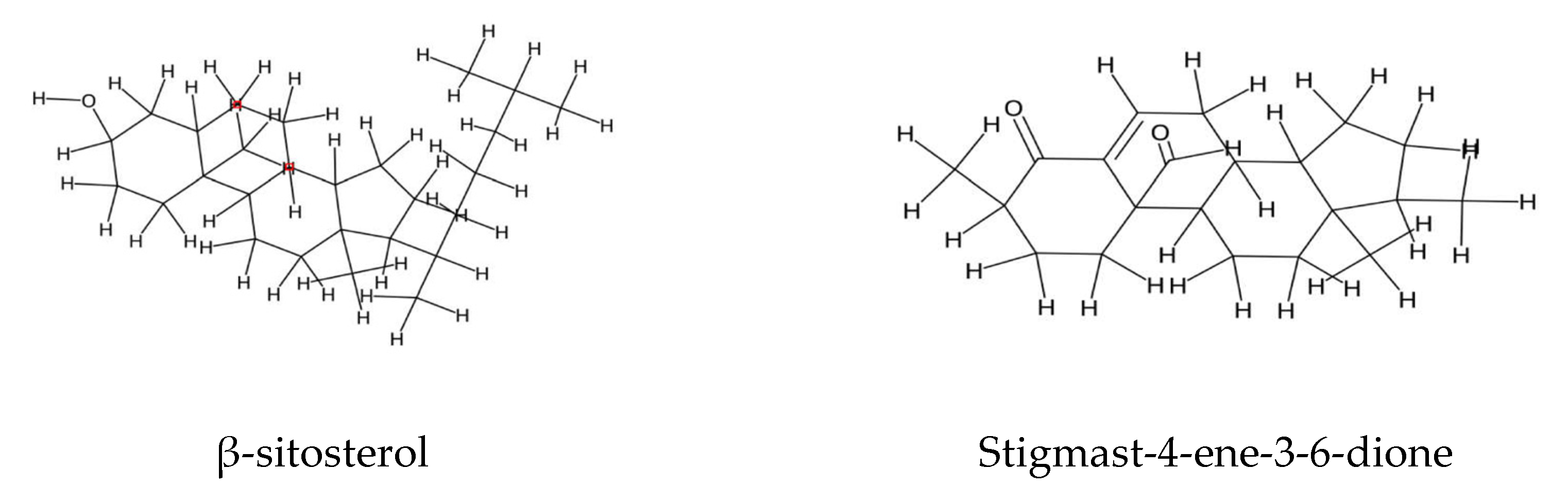
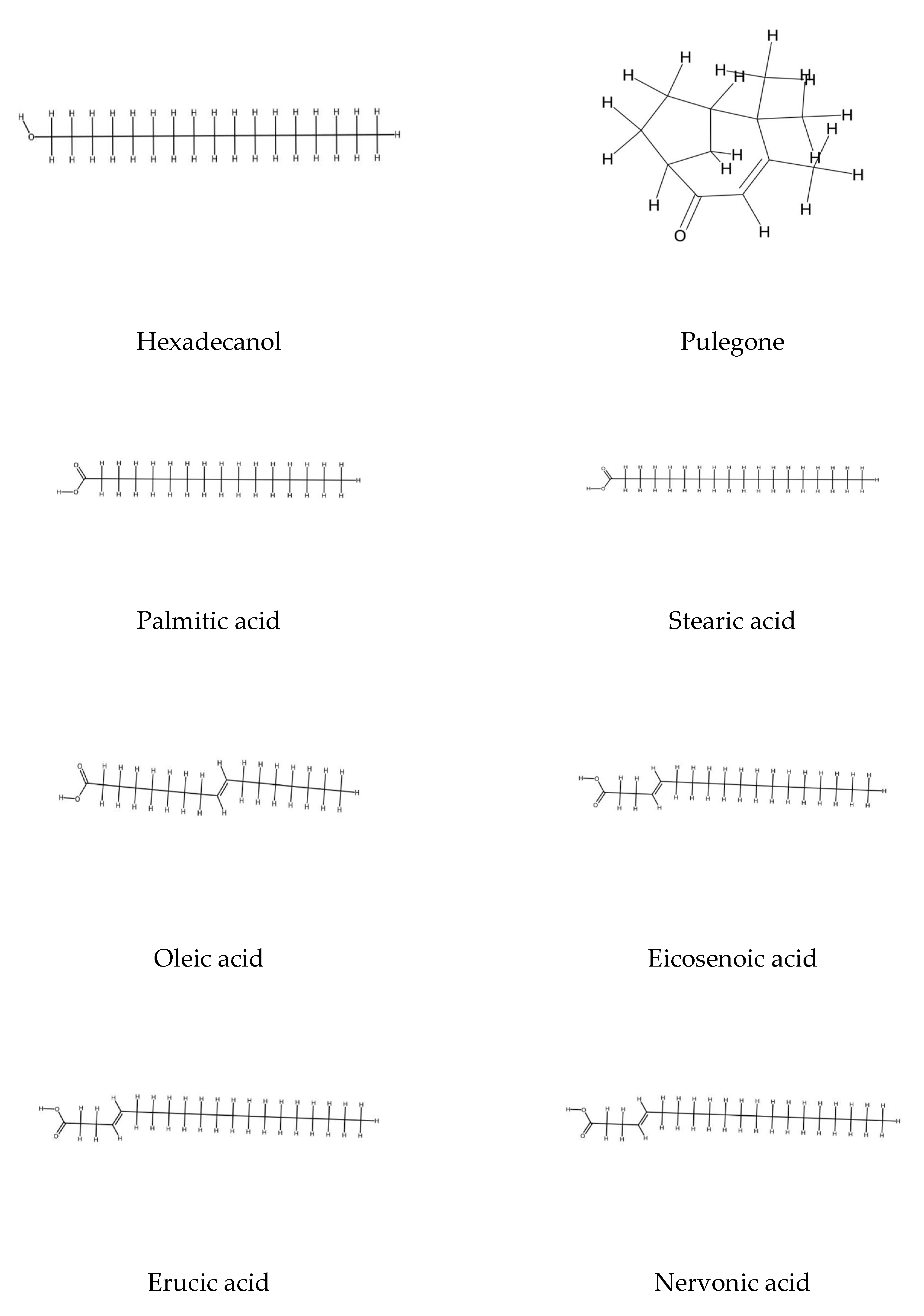
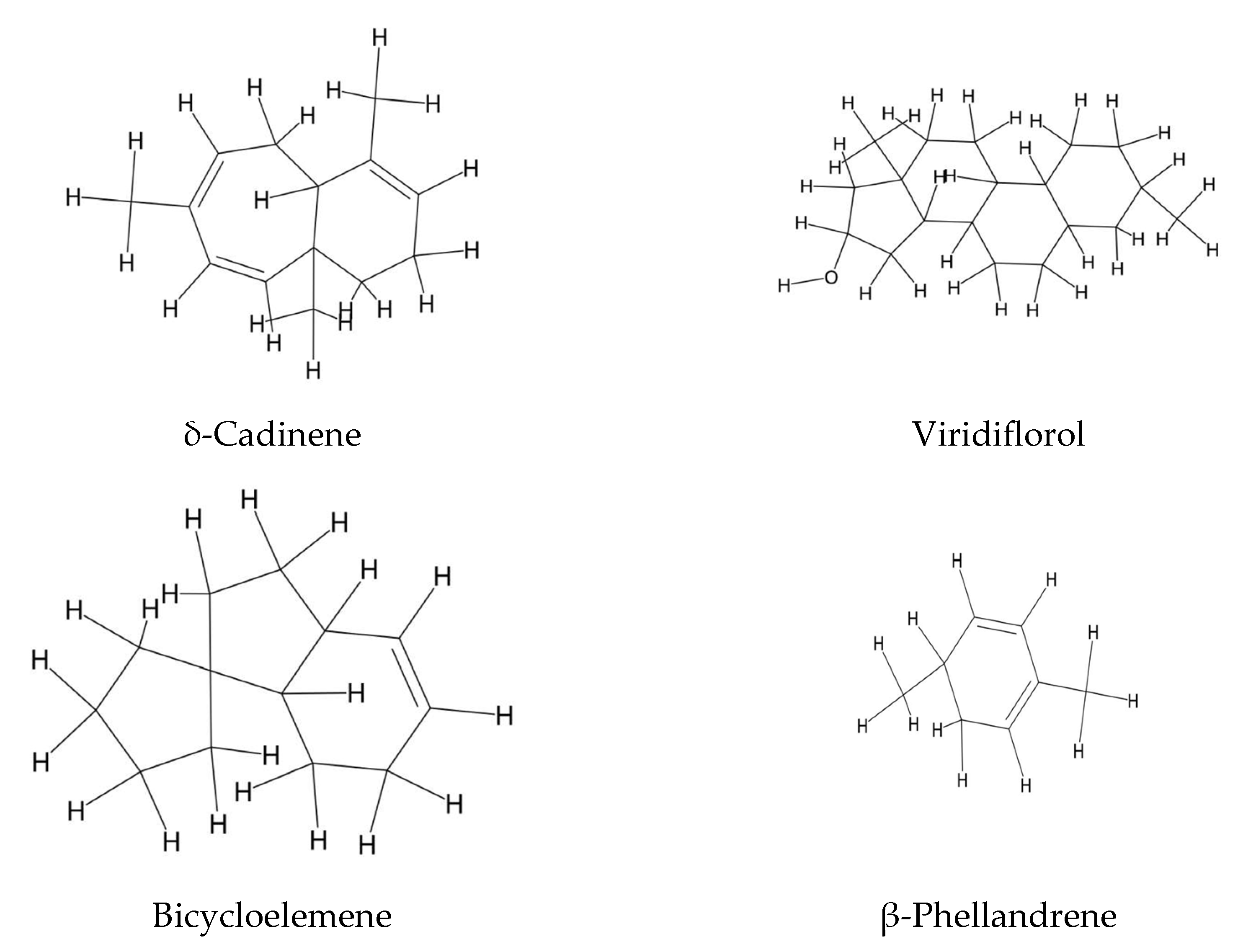
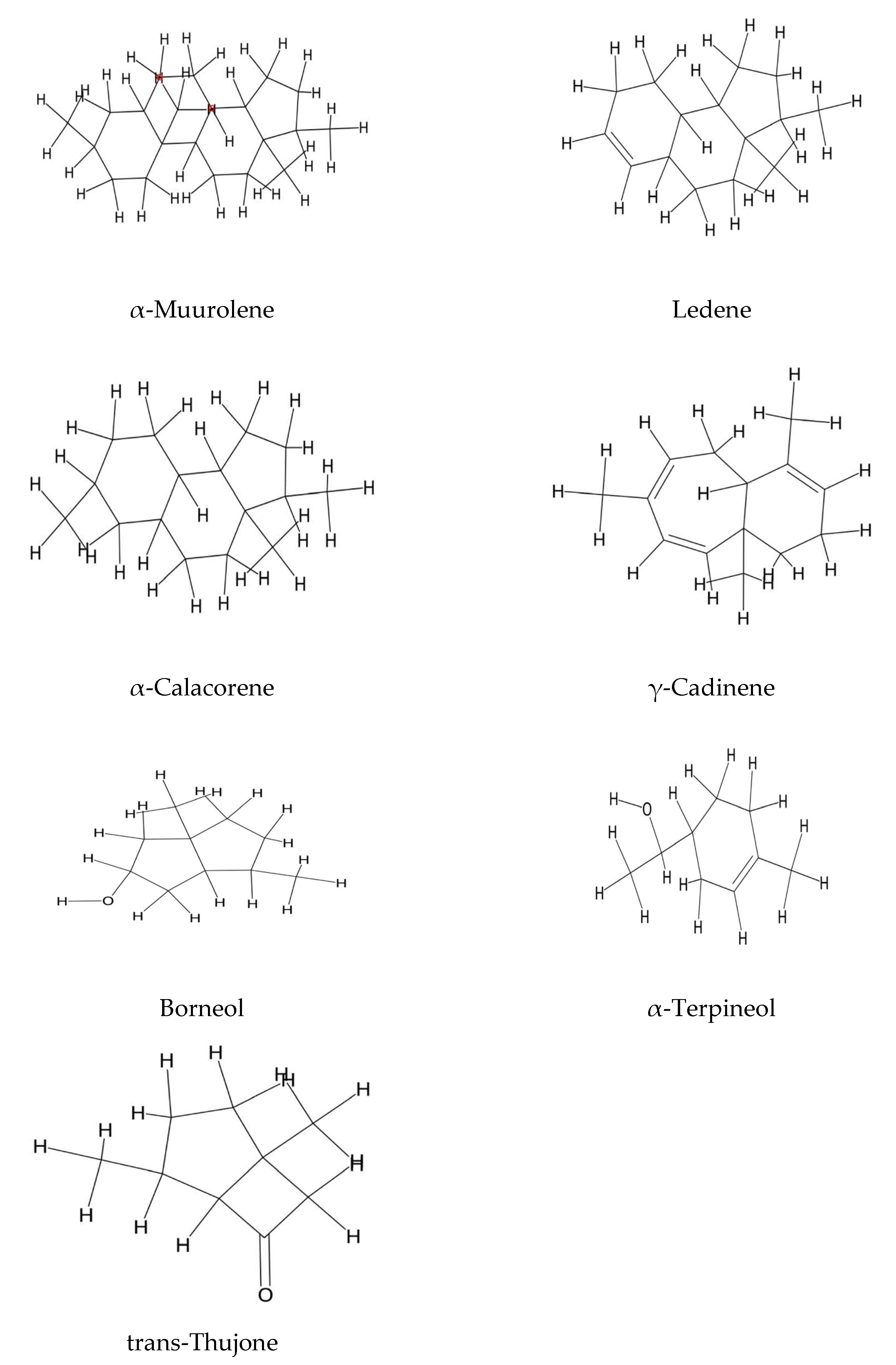
2.3. Steroids and Polyunsaturated Fatty Acids
2.4. Pyrrolizidine Alkaloids in Echium Species: Toxicity, Challenges and Mitigation-Strategies
3. General Applications, Medicinal Uses, and Pharmacological Studies of Echium spp.
| Species | Medicinal Uses | Part of Plant | Extraction Techniques | Country |
|---|---|---|---|---|
| Echium italicum L. | Allergic disease | Hairy roots | ND1 | ND1 [101] |
| Echium italicum L. | Wounds, anti-inflammatory | Leaf, root | Poultice | Turkey [18] |
| Echium vulgare L. and E. russicum J.F. Gmellin | Vulnerary | Root | Poultice | Turkey [18] |
| Echium vulgare L. | Wound healing, ulcer, bruising, pulled muscles, ligaments and sprains | Root | Ethanol and chloroform | Turkey [110] |
| Echium vulgare L. | Blood purification, heal wounds, tonic, diaphoretic, diuretic, astringent, treatment of snakebite | Leaves and flowering stems | Methanol and ethanol | East Serbia [80] |
| Echium italicum L. (PORUN—ADN 3851) BORAGINACEAE. | Depurative, diaphoretic, diuretic, emollient for healing respiratory infections | Aerial part | Decoction | Italy [111] |
| Echium amoenum, Fisch. And May. Ahvazi 637 (IMPH) | Demulcent, anti-inflammatory and analgesic, especially for common cold, pneumonia, anxiolytic, sedative, and other psychiatric symptoms, including obsession | Flower | Brewed | Iran [6] |
| Echium amoenum (F.M.) | Infectious diseases and for controlling fever | Flower | Aqueous extraction | Iran [37] |
| Echium vulgare L. | Antitussive, aphrodisiac, demulcent, diaphoretic, diuretic, pectoral, vulnerary, efficacious in the treatment of snake bites, for cleaning up the blood and healing wounds. | Leaves and flowering stems | ND1 | Serbia [21] |
| Echium Italicum L. | Diuretic, sweet, and sedative | aerial parts | Decoction | Turkey [14] |
| Echium italicum L., Echium vulgare L. and Echium angustifolium Miller | Wound healing | Root and Aerial parts | Hydroalcoholic extract | Turkey [10] |
| Echium plantagineum | Diuretic and diaphoretic, | Aerial part | Decoction | Turkey [19] |
| Echium Arabicum. R. Mill | Antiplasmodial and antitrypanosomal activity | Aerial parts | Methanolic extract | Saudi Arabia [116] |
| Echium flavum Desf | Antiseptics and wound healing for ulcers and herpe | Root | Mashed or fried in olive oil | Spain [117] |
| Echium vulgare L. | Diuretic | Leaves | Aqueous EtOH, through maceration | Italy [118] |
| Echium amoenum Fisch. & C.A.Mey. | Cold and flu | Flower | ND1 | Iran [119] |
| Echium amoenum | Reduce anxiety | Flower | Aqueous EtOH | Iran [120] |
| Echium amoenum Fisch. et May. | Improve depression | Flower | Aqueous extract | Iran [30] |
| Echium amoenum | Alleviate PMS symptoms | Aqueous extract | Iran [87] | |
| Echium italicum L. | Sedative, Burns, Rheumatism, Uterus infection | Flower, root | Infusion, Cataplasm, Decoction | Iran [121] |
| Echium amoenum Fisch. & C.A. | Sore throat, nerve system relaxant, digestive | Therophytes, Aerial parts | Infusion, Decoction | Iran [3] |
| Echium amoenum Fisch and May. Ahvazi 637 (IMPH) | Sedative, exhilarating, diuretic, analgesic, antioxidant, anxiolytic, diaphoretic | Flowers | Infusion | Iran [5,6,12,13,122] |
| Echium hipernopicum | Dietetic | Seed oil | Essential oil | Cape Verde [115] |
| Echium stenosiphon | Cough syrup | ND | Essential oil | Cape Verde [115] |
| Echium vulcanorum | Dietetic | Seed oil | Essential oil | Cape Verde [115] |
| Echium vulgare L. | As a revitalizing, anti-inflammatory, cough-relieving, asthma-relieving and phlegm-resolving agent, dry colds, biliary diseases, depression, heart and brain defciency syndromes, palpitations, insomnia | Flower | ND1 | China [123] |
4. Bioactivities of the Echium Genus
4.1. Antimicrobial Activity
| Species | Virus/Bacteria | Part of Plant | Extract | Assay | Country |
|---|---|---|---|---|---|
| Echium amenum L. | herpes simplex virus type I (HSV-1, KOS strain) and Hep-2 | Flower | Aqueous | Cytopathic effect inhibition assay (CPE) | Iran [36] |
| Echium amenum L. | Staphylococcus aureus (ATCC 25913), Listeria monocytogenes (ATCC 19117), Escherichia coli (ATCC 8739), Yersinia enterocolitica (ATCC 9610), and Salmonella typhimurium strain (ATCC 14028) | Flower | Aqueous and ethanolic | Disk diffusion method, and MIC1 | Iran [124] |
| Echium amoenum Fisch. & C.A.Mey. | methicillin-resistant Staphylococcus aureus (MRSA), Staphylococcus epidermidis, Pseudomonas aeruginosa, Candida albicans, and Aspergillus niger | Seed | Chloroform and methanol | MIC | Iran [125] |
| Echium amoenum Fisch. & C.A.Mey. | Staphylococcus aureus 8327 | Flower | Aqueous | Agar-well diffusion method and MIC | Iran [37] |
| Echium amoenum Fisch. & C.A.Mey. | Acinetobacter baumannii | Flower | Methanolic | disk diffusion method | Iran [126] |
| Echium arenarium (Guss) | Bacillus cereus ATCC 14579, Listeria monocytogenes ATCC 19115, Staphylococcus aureus ATCC 25923, methicillin-resistant Staphylococcus aureus (MRSA), Enterrococcus faecalis ATCC 29212, Escherichia coli ATCC 35214, Pseudomonas aeruginosa ATCC 27853, and Klebsiella pneumonia CIP 104727 | Aerial part | Water, cyclohexane, dichloromethane and ethyl acetate | Disk diffusion method and MIC | Tunisia [45] |
| Echium italicum L. | Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Salmonella typhi, Pseudomonas aeruginosa, Aspergilus niger and Candida albicans | Flowering aerial parts | Hydrodistillation | Disk diffusion method and MIC | Iran [84] |
| Echium italicum L. | Bacillus megaterium, Escherichia. coli, Staphylococcus aureus, Candida albicans, and Klebsiella pneumonia | Flower, stems, and leaves | Methanol and hexane | Agar-well diffusion method and MIC | Turkey [128] |
| Echium serbicum L. | Bacillus mycoides ATCC 6462, Bacillus subtilis ATCC 6633, Enterococcus faecalis ATCC 29212, Micrococcus luteus ATCC 1024, Micrococcus lysodeikticus ATCC 4698, Staphylococcus aureus ATCC 25923, Staphylococcus epidermidis ATCC 14990, Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 13883, Pseudomonas aeruginosa ATCC 19429 | Flowers, leaves, stems, and roots | Methanol | MIC | [127] |
| Echium italicum L. | Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Salmonella typhi, Pseudomonas aeruginosa, Aspergilus niger and Candida albicans | Aerial part | Essential oil | Disk diffusion method | Iran [84] |
| Echium amoenum Fisch. & C.A.Mey. | Staphylococcus aureus | Flower | Aqueous | Agar-well diffusion method | Iran [35] |
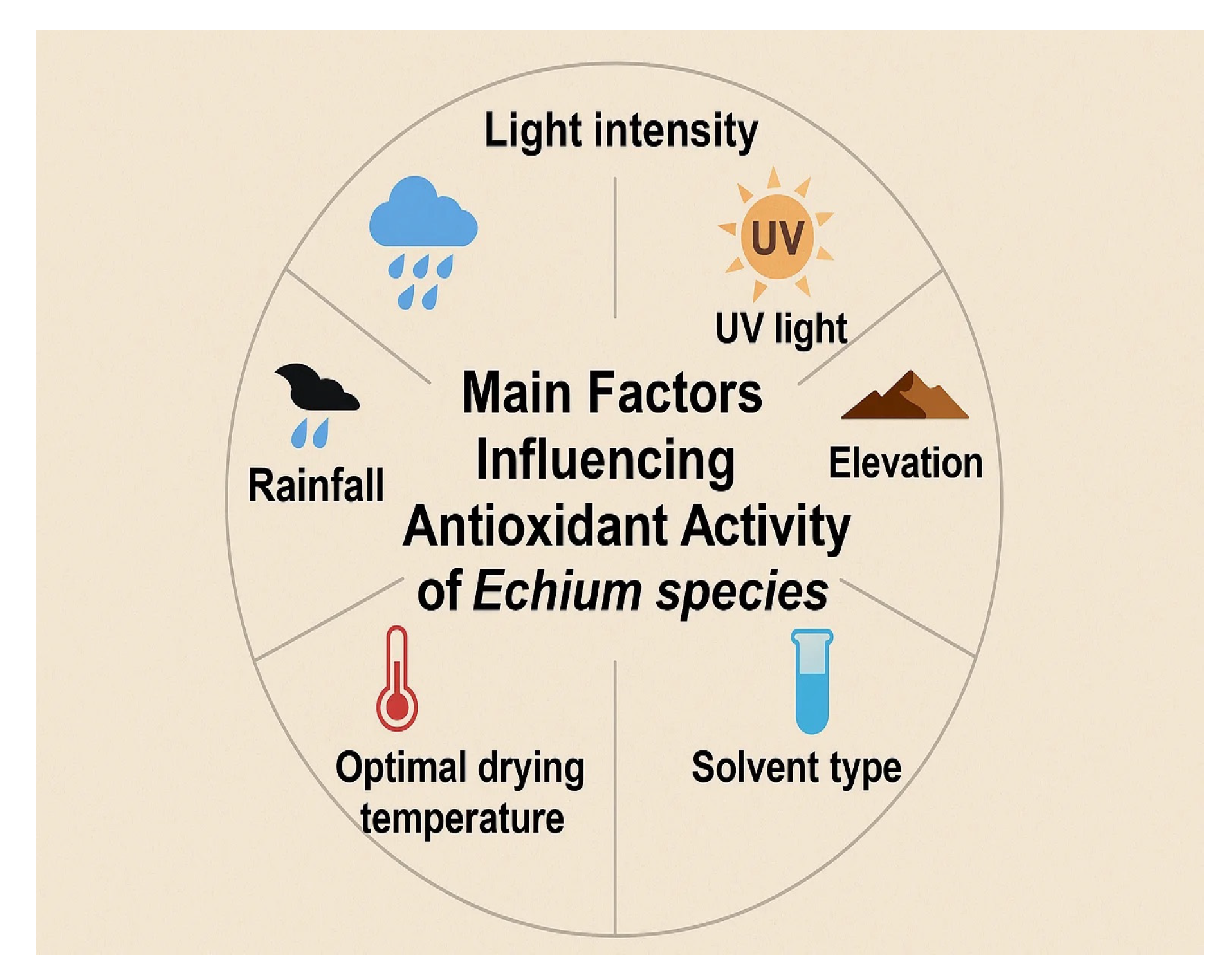
4.2. Antioxidant Activity
| Scientific Name | Part of Plant | Extract | Type of Study | Reference |
|---|---|---|---|---|
| Echium Fisch. & C.A. Mey. | Petals | Dichloromethane | Ferric Reducing Antioxidant Power Assay (FRAP) | [23] |
| Echium amoenum | Petals | Ethanol, methanol, acetone, ethanol 80% and water extracts | 1,1 Diphenyl 2-Picryl Hydrazyl (DPPH) | [129] |
| Echium arenarium (Guss) | Aerial part | Ethyl acetate extracts cyclohexane, dichloromethane, and water | 1DPPH; β-carotene bleaching assay | [45] |
| Echium amaenum Fisch & C.A. Mey | Flowers | Decoction | blood total antioxidant capacity (TAC), lipid peroxidation (LPO) and total thiol (SH) molecules | [12] |
| Echium amoenum | Flower | Ethanol, water, methanol and a choline chloride and glycerol (CHGLY) | DPPH and FRAP | [53] |
| Echium amoenum Fisch & C.A. Mey | Petals | Ultrasonic extract, Polyphenol fraction, and Alkaloid fraction | DPPH | [143] |
| Echium pycnanthum Pomel | Roots | Hydromethanolic | DPPH, b-carotene bleaching test, 1,1-diphenyl-2-picrylhydrazyl, 2,2-azino-bis-3-ethyl benzthiazoline-6-sulfonic acid (ABTS), Chelating effect on ferrous ions, Iron reducing power | [145] |
| Echium sericeum (Vahl) | Aerial parts | Chloroform, ethyl acetate, and n-butanol, the ethanolic extract | DPPH | [11] |
| Echium amoenum Fisch & C.A, Echium italicum L. | Leaf, stem, and seed | Aqueous EtOH | DPPH and FRAP | [149] |
| Echium amoenum Fisch & C.A | Petal | Methanol and 70% acetone | DPPH | [131] |
| Echium italicum L. | Root | n-Hexane | DPPH | [134] |
| Echium amoenum | Flower | Decoction, infusion, methanolic, and hydroalcoholic extract | DPPH, FRAP, ABST | [59] |
| Echium vulgare L. and, Echium rubrum L. | Aerial parts | Methanol and ethanol | total antioxidant capacity, DPPH free-radical scavenging, the inhibitory activity toward lipid peroxidation, Fe3+- reducing power, Fe2+- chelating ability, and hydroxyl radical scavenging activity | [21] |
| Echium italicum L. | Flowers, stems, and leaves | Methanol and hexane | DPPH | [128] |
| Echium italicum L | Aerial parts | Chloroform, ethyl acetate, ethanol, acetone, and petroleum ether extracts | DPPH, total phenolic content, flavonoid content, inhibitory activity against lipid peroxidation, and hydroxyl radical scavenging activity | [133] |
| Echium serbicum L. | Flowers, leaves, stems, and roots | Methanol | DPPH, ABTS, reducing power activity, Inhibition of lipid peroxidation | [127] |
| Echium humile Desf | Aerial part | Hexane, dichloromethane, ethyl acetate, methanol, and aqueous | DPPH, ABTS, FRAP, and TAC | [151] |
| Echium vulgare L. and Echium italicum L. | Flower | Chloroform, ethyl acetate, ethanol, acetone, petroleum | Determination of inhibition of lipid peroxidation by ammonium thiocyanate | [152] |
4.3. Cytotoxicity Activity
| Taxon | Extract | Biological Activity | Assay | Part of Plant | Ref. |
|---|---|---|---|---|---|
| Echium italicum L. | Methanol and hexane | HepG2, MCf-7 | MTT | Seed | [154] |
| Echium vulgare L and Echium italicum L. | Chloroform, ethyl acetate, ethanol, acetone, and petroleum | Hep2, RD, L2OB | MTT | Flower | [152] |
| Echium amoenum | Hexane, dichloromethane, and ethyl acetate | J774.1A macrophage cell line | MTT | Flower | [155] |
| Echium serbicum L. | Methanol | HCT-116, SW-480, MDAMB-231 and MRC-5 | MTT | Flowers, leaves, stems, and roots | [127] |
| Echium plantagineum L. | Methanol | AGS and MRC-5 | MTT | Pollen | [41] |
| Echium arenarium Guss | Hydromethanolic | U266 | MTT | Root and aerial parts | [45] |
| Echium angustifolium Mill | n-Hexane (nonpolar) | HCT116 and HEPG2 | SRB | Aerial part | [158] |
| Echium creticum | Aqueous, ethyl acetate extracts, and methanol | MCF-7 | MTT | Leaves and stem | [156] |
| Echium italicum L. | Methanol and hexane | HepG2 and MCF 7 | MTT | Flowers, stems, and leaves | [128] |
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ziberna, L.; Tramer, F.; Moze, S.; Vrhovsek, U.; Mattivi, F.; Passamonti, S. Transport and bioactivity of cyanidin 3-glucoside into the vascular endothelium. Free Radic. Biol. Med. 2012, 52, 1750–1759. [Google Scholar] [CrossRef]
- Sheydaei, P.; Duarte, A.P. The genus Tripleurospermum Sch. Bip. (Asteraceae): A comprehensive review of its ethnobotanical utilizations, pharmacology, phytochemistry, and toxicity. Life 2023, 13, 1323. [Google Scholar] [CrossRef]
- Mirdeilami, S.Z.; Barani, H.; Mazandarani, M.; Heshmati, G.A. Ethnopharmacological survey of medicinal plants in Maraveh Tappeh region, north of Iran. Iran. J. Plant Physiol. 2011, 2, 327–338. [Google Scholar]
- Ministry of Health and Medical Education Deputy Ministry for Food and Drug. Iranian Herbal Pharmacopoeia; Ministry of Health and Medical Education Deputy Ministry for Food and Drug: Tehran, Iran, 2002; Volume 108, pp. 110–111. [Google Scholar]
- Heidari, M.R.; Azad, E.M.; Mehrabani, M. Evaluation of the analgesic effect of Echium amoenum Fisch & CA Mey. extract in mice: Possible mechanism involved. J. Ethnopharmacol. 2006, 103, 345–349. [Google Scholar] [CrossRef]
- Ahvazi, M.; Khalighi-Sigaroodi, F.; Charkhchiyan, M.M.; Mojab, F.; Mozaffarian, V.-A.; Zakeri, H. Introduction of medicinal plants species with the most traditional usage in Alamut region. Iran. J. Pharm. Res. IJPR 2012, 11, 185. [Google Scholar]
- Böhle, U.-R.; Hilger, H.H.; Martin, W.F. Island colonization and evolution of the insular woody habit in Echium L. (Boraginaceae). Proc. Natl. Acad. Sci. USA 1996, 93, 11740–11745. [Google Scholar] [CrossRef] [PubMed]
- Retief, E.; Van Wyk, A. The genus Echium (Boraginaceae) in southern Africa. Bothalia 1998, 28, 167–177. [Google Scholar] [CrossRef]
- Nabli, M.A. La Mise à Jour de la Flore de la Tunisie: Programme Flore Et Végétation Tunisiennes; Laboratoire de Botanique Fondamentale et Appliquée, Faculté des Sciences de Tunis, Université de Tunis El Manar: Tunis, Tunisia, 2015. [Google Scholar]
- Eruygur, N.; Yılmaz, G.; Kutsal, O.; Yücel, G.; Üstün, O. Bioassay-guided isolation of wound healing active compounds from Echium species growing in Turkey. J. Ethnopharmacol. 2016, 185, 370–376. [Google Scholar] [CrossRef]
- Radwan, H.; Shams, K.; Mohamed, W.; Ismail, I. Phytochemical and biological investigation of Echium sericeum (Vahl) growing in Egypt. Planta Med. 2007, 73, P_334. [Google Scholar] [CrossRef]
- Ranjbar, A.; Khorami, S.; Safarabadi, M.; Shahmoradi, A.; Malekirad, A.A.; Vakilian, K.; Mandegary, A.; Abdollahi, M. Antioxidant activity of Iranian Echium amoenum Fisch & CA Mey flower decoction in humans: A cross-sectional before/after clinical trial. Evid.-Based Complement. Altern. Med. 2006, 3, 469–473. [Google Scholar]
- Shafaghi, B.; Naderi, N.; Tahmasb, L.; Kamalinezhad, M. Anxiolytic effect of Echium amoenum L. in mice. Iran. J. Pharm. Res. 2002, 1, 37–41. [Google Scholar]
- Cakilcioglu, U.; Turkoglu, I. An ethnobotanical survey of medicinal plants in Sivrice (Elazığ-Turkey). J. Ethnopharmacol. 2010, 132, 165–175. [Google Scholar] [CrossRef]
- Tabata, M.; Sezik, E.; Honda, G.; Yeşilada, E.; Fukui, H.; Goto, K.; Ikeshiro, Y. Traditional medicine in Turkey III. Folk medicine in East Anatolia, van and Bitlis provinces. Int. J. Pharm. 1994, 32, 3–12. [Google Scholar] [CrossRef]
- Fujita, T.; Sezik, E.; Tabata, M.; Yeşilada, E.; Honda, G.; Takeda, Y.; Tanaka, T.; Takaishi, Y. Traditional medicine in Turkey VII. Folk medicine in middle and west Black Sea regions. Econ. Bot. 1995, 49, 406–422. [Google Scholar] [CrossRef]
- Simsek, I.; Aytekin, F.; Yesilada, E.; Yildirimli, Ş. An Ethnobotanical survey of the beypazari, ayas, and G¸ d¸ l district towns of Ankara Province (Turkey). Econ. Bot. 2004, 58, 705–720. [Google Scholar]
- Altundag, E.; Ozturk, M. Ethnomedicinal studies on the plant resources of east Anatolia, Turkey. Procedia-Soc. Behav. Sci. 2011, 19, 756–777. [Google Scholar] [CrossRef]
- Ünsal, Ç.; Vural, H.; Sariyar, G.; Özbek, B.; Ötük, G. Traditional medicine in Bilecik province (Turkey) and antimicrobial activities of selected species. Turk. J. Pharm. Sci. 2010, 7, 139. [Google Scholar]
- Sezik, E.; Yesilada, E.; Tabata, M.; Honda, G.; Takaishi, Y.; Fujita, T.; Tanaka, T.; Takeda, Y. Traditional medicine in Turkey VIII. Folk medicine in East Anatolia; Erzurum, Erzincan, Agn, Kars, Iğdır Provinces. Econ. Bot. 1997, 51, 195–211. [Google Scholar] [CrossRef]
- Nićiforović, N.; Mihailović, V.; Mašković, P.; Solujić, S.; Stojković, A.; Muratspahić, D.P. Antioxidant activity of selected plant species; potential new sources of natural antioxidants. Food Chem. Toxicol. 2010, 48, 3125–3130. [Google Scholar] [CrossRef]
- Ferreira, I.C.; Aires, E.; Barreira, J.C.; Estevinho, L.M. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Safaeian, L.; Javanmard, S.H.; Ghanadian, M.; Seifabadi, S. Cytoprotective and antioxidant effects of Echium amoenum anthocyanin-rich extract in human endothelial cells (HUVECs). Avicenna J. Phytomed. 2015, 5, 157. [Google Scholar] [PubMed]
- Tahmouzi, S. Extraction, antioxidant and antilisterial activities of polysaccharides from the flower of viper’s bugloss. Int. J. Biol. Macromol. 2014, 69, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Abed, A.; Minaiyan, M.; Ghannadi, A.; Mahzouni, P.; Babavalian, M.R. Effect of Echium amoenum Fisch. et Mey a traditional Iranian herbal remedy in an experimental model of acute pancreatitis. Int. Sch. Res. Not. 2012, 2012, 141548. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Ioele, G.; Statti, G.; Marrelli, M.; Ragno, G.; Menichini, F. Antiproliferative activity against human tumor cell lines and toxicity test on Mediterranean dietary plants. Food Chem. Toxicol. 2008, 46, 3325–3332. [Google Scholar] [CrossRef]
- Uysal, H.; Kızılet, H.; Ayar, A.; Taheri, A. The use of endemic Iranian plant, Echium amoenum, against the ethyl methanesulfonate and the recovery of mutagenic effects. Toxicol. Ind. Health 2015, 31, 44–51. [Google Scholar] [CrossRef]
- Wassel, G.; El-Menshawi, B.; Saeed, A.; Mahran, G.; El-Merzabani, M. Screening of selected plants for pyrrolizidine alkaloids and antitumor activity. Die Pharm. 1987, 42, 709. [Google Scholar]
- Moalem, S.A.; Hosseinzadeh, H.; Ghoncheh, F. Evaluation of antidepressant effects of aerial parts of Echium vulgare on mice. Pharmazie 2007, 42, 709. [Google Scholar]
- Sayyah, M.; Sayyah, M.; Kamalinejad, M. A preliminary randomized double blind clinical trial on the efficacy of aqueous extract of Echium amoenum in the treatment of mild to moderate major depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 30, 166–169. [Google Scholar] [CrossRef]
- Sayyah, M.; Boostani, H.; Pakseresht, S.; Malaieri, A. Efficacy of aqueous extract of Echium amoenum in treatment of obsessive–compulsive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 1513–1516. [Google Scholar] [CrossRef]
- Gholamzadeh, S.; Zare, S.; Ilkhanipoor, M. Evaluation of the anxiolytic effect of Echium amoenum petals extract, during chronic treatment in rat. Res. Pharm. Sci. 2009, 2, 91–95. [Google Scholar]
- Hosseinzadeh, H.; Shahandeh, S.; Shahsavand, S. Anxiolytic and hypnotic effects of aqueous and ethanolic extracts of aerial parts of Echium italicum L. in mice. Jundishapur J. Nat. Pharm. Prod. 2012, 7, 71. [Google Scholar] [CrossRef]
- Gholamzadeh, S.; Zare, S.; Ilkhanipoor, M. Anxiolytic effect of Echium amoenum during different treatment courses. Res. J. Biol. Sci. 2008, 3, 176–178. [Google Scholar]
- Abolhassani, M. Antiviral activity of borage (Echium amoenum). Arch. Med. Sci. 2010, 6, 366–369. [Google Scholar] [CrossRef]
- Farahani, M.; Branch, Q.; Azad, I. Antiviral effect assay of aqueous extract of Echium amoenum-L against HSV-1. Zahedan J. Res. Med. Sci. 2013, 15, 46–48. [Google Scholar]
- Abolhassani, M. Antibacterial effect of borage (Echium amoenum) on Staphylococcus aureus. Braz. J. Infect. Dis. 2004, 8, 382–385. [Google Scholar] [CrossRef]
- Mohsin, R.; Choudhary, M.I. Medicinal plants with anticonvulsant activities. Stud. Nat. Prod. Chem. 2000, 22, 507–553. [Google Scholar]
- Bainbridge, M.L.; Lock, A.L.; Kraft, J. Lipid-encapsulated echium oil (Echium plantagineum) increases the content of stearidonic acid in plasma lipid fractions and milk fat of dairy cows. J. Agric. Food Chem. 2015, 63, 4827–4835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Boudyguina, E.; Wilson, M.D.; Gebre, A.K.; Parks, J.S. Echium oil reduces plasma lipids and hepatic lipogenic gene expression in apoB100-only LDL receptor knockout mice. J. Nutr. Biochem. 2008, 19, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.; Fernandes, F.; Valentão, P.; Pereira, D.M.; Andrade, P.B. Echium plantagineum L. honey: Search of pyrrolizidine alkaloids and polyphenols, anti-inflammatory potential and cytotoxicity. Food Chem. 2020, 328, 127169. [Google Scholar] [CrossRef]
- Moreira, R.; Pereira, D.M.; Valentão, P.; Andrade, P.B. Pyrrolizidine alkaloids: Chemistry, pharmacology, toxicology and food safety. Int. J. Mol. Sci. 2018, 19, 1668. [Google Scholar] [CrossRef]
- Boppré, M.; Colegate, S.M.; Edgar, J.A. Pyrrolizidine alkaloids of Echium vulgare honey found in pure pollen. J. Agric. Food Chem. 2005, 53, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Kefi, S.; Essid, R.; Mkadmini, K.; Kefi, A.; Haddada, F.M.; Tabbene, O.; Limam, F. Phytochemical investigation and biological activities of Echium arenarium (Guss) extracts. Microb. Pathog. 2018, 118, 202–210. [Google Scholar] [CrossRef]
- Di Paola-Naranjo, R.D.; Sánchez-Sánchez, J.; González-Paramás, A.M.a.; Rivas-Gonzalo, J.C. Liquid chromatographic–mass spectrometric analysis of anthocyanin composition of dark blue bee pollen from Echium plantagineum. J. Chromatogr. A 2004, 1054, 205–210. [Google Scholar] [CrossRef]
- Moita, E.; Gil-Izquierdo, A.; Sousa, C.; Ferreres, F.; Silva, L.R.; Valentao, P.; Dominguez-Perles, R.; Baenas, N.; Andrade, P.B. Integrated analysis of COX-2 and iNOS derived inflammatory mediators in LPS-stimulated RAW macrophages pre-exposed to Echium plantagineum L. bee pollen extract. PLoS ONE 2013, 8, e59131. [Google Scholar] [CrossRef]
- Ferreres, F.; Pereira, D.M.; Valentão, P.; Andrade, P.B. First report of non-coloured flavonoids in Echium plantagineum bee pollen: Differentiation of isomers by liquid chromatography/ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 801–806. [Google Scholar] [CrossRef]
- de Almeida Pontes, P.V.; Shiwaku, I.A.; Maximo, G.J.; Batista, E.A.C. Choline chloride-based deep eutectic solvents as potential solvent for extraction of phenolic compounds from olive leaves: Extraction optimization and solvent characterization. Food Chem. 2021, 352, 129346. [Google Scholar] [CrossRef]
- Cui, Z.; Djocki, A.V.E.; Yao, J.; Wu, Q.; Zhang, D.; Nan, S.; Gao, J.; Li, C. COSMO-SAC-supported evaluation of natural deep eutectic solvents for the extraction of tea polyphenols and process optimization. J. Mol. Liq. 2021, 328, 115406. [Google Scholar] [CrossRef]
- da Silva, D.T.; Pauletto, R.; da Silva Cavalheiro, S.; Bochi, V.C.; Rodrigues, E.; Weber, J.; da Silva, C.d.B.; Morisso, F.D.P.; Barcia, M.T.; Emanuelli, T. Natural deep eutectic solvents as a biocompatible tool for the extraction of blueberry anthocyanins. J. Food Compost. Anal. 2020, 89, 103470. [Google Scholar] [CrossRef]
- Zannou, O.; Koca, I.; Aldawoud, T.M.; Galanakis, C.M. Recovery and stabilization of anthocyanins and phenolic antioxidants of roselle (Hibiscus sabdariffa L.) with hydrophilic deep eutectic solvents. Molecules 2020, 25, 3715. [Google Scholar] [CrossRef] [PubMed]
- Zannou, O.; Pashazadeh, H.; Ghellam, M.; Ibrahim, S.A.; Koca, I. Extraction of anthocyanins from borage (Echium amoenum) flowers using choline chloride and a glycerol-based, deep eutectic solvent: Optimization, antioxidant activity, and in vitro bioavailability. Molecules 2021, 27, 134. [Google Scholar] [CrossRef] [PubMed]
- Morteza-Semnani, K.; Saeedi, M. Essential oil composition of Echium amoenum Fisch. & CA Mey. J. Essent. Oil Bear. Plants 2005, 8, 61–64. [Google Scholar] [CrossRef]
- Mehrabani, M.; Ghassemi, N.; Sajjadi, E.; Ghanadi, A.; Shams, A.M. Main phenolic compound of petals of Echium amoenum Fisch. and CA Mey., a famous medicinal plant of Iran. DARU J. Pharm. Sci. 2005, 13, 65–69. [Google Scholar]
- Olennikov, D.; Daironas, Z.V.; Zilfikarov, I. Shikonin and rosmarinic-acid derivatives from Echium russicum roots. Chem. Nat. Compd. 2017, 53, 953–955. [Google Scholar] [CrossRef]
- Dresler, S.; Kubrak, T.; Bogucka-Kocka, A.; Szymczak, G. Determination of shikonin and rosmarinic acid in Echium vulgare L. and Echium russicum JF Gmel. by capillary electrophoresis. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 698–701. [Google Scholar] [CrossRef]
- Dresler, S.; Rutkowska, E.; Bednarek, W.; Stanisławski, G.; Kubrak, T.; Bogucka-Kocka, A.; Wójcik, M. Selected secondary metabolites in Echium vulgare L. populations from nonmetalliferous and metalliferous areas. Phytochemistry 2017, 133, 4–14. [Google Scholar] [CrossRef]
- Asghari, B.; Mafakheri, S.; Zarrabi, M.; Erdem, S.; Orhan, I.; Bahadori, M. Therapeutic target enzymes inhibitory potential, antioxidant activity, and rosmarinic acid content of Echium amoenum. S. Afr. J. Bot. 2019, 120, 191–197. [Google Scholar] [CrossRef]
- Jamnani, M.J.; Holmelid, B.; Vedeler, A.; Parsian, H.H.; Andersen, H.L.; Fossen, T. Natural Products from Leaves of the Ancient Iranian Medicinal Plant Echium amoenum Fisch. & CA Mey. Molecules 2023, 28, 385. [Google Scholar] [PubMed]
- Jin, J.; Holland, D.C.; Carroll, A.R.; Zunk, M. Echiumin E, an aryl dihydronaphthalene lignan from the Australian invasive plant Paterson’s Curse (Echium plantagineum). J. Nat. Prod. 2022, 85, 2474–2479. [Google Scholar] [CrossRef] [PubMed]
- Abd Haghighi, M.J.; Azadbakht, M.; Habibi, E.; Vahedi, L.; Hosseinimehr, S.J. Phytochemical profile, antioxidant, and wound healing activities of Echium amoenum (Boraginaceae). Nat. Prod. Res. 2024, 27, 1–7. [Google Scholar] [CrossRef]
- Papageorgiou, V.P.; Assimopoulou, A.N.; Couladouros, E.A.; Hepworth, D.; Nicolaou, K. The chemistry and biology of alkannin, shikonin, and related naphthazarin natural products. Angew. Chem. Int. Ed. 1999, 38, 270–301. [Google Scholar] [CrossRef]
- Kheiri, A.; Amini, S.; Javidan, A.N.; Saghafi, M.M.; Khorasani, G. The effects of Alkanna tinctoria Tausch on split-thickness skin graft donor site management: A randomized, blinded placebo-controlled trial. BMC Complement. Altern. Med. 2017, 17, 253. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Ohno, A.; Abe, K.; Saito, Y.; Satake, M. Comparative study on accelerative effect of Koushikon and Nanshikon and their constituents on proliferation of granulation tissue in rats. Jpn. J. Pharmacol. 1993, 61, 162. [Google Scholar] [CrossRef]
- Akkol, E.K.; Koca, U.; Peşin, I.; Yılmazer, D.; Toker, G.; Yeşilada, E. Exploring the wound healing activity of Arnebia densiflora (Nordm.) Ledeb. by in vivo models. J. Ethnopharmacol. 2009, 124, 137–141. [Google Scholar] [CrossRef]
- Sidhu, G.S.; Singh, A.K.; Banaudha, K.K.; Gaddipati, J.P.; Patnaik, G.K.; Maheshwari, R.K. Arnebin-1 accelerates normal and hydrocortisone-induced impaired wound healing. J. Invest. Dermatol. 1999, 113, 773–781. [Google Scholar] [CrossRef][Green Version]
- Weston, L.A.; Ryan, P.R.; Watt, M. Mechanisms for cellular transport and release of allelochemicals from plant roots into the rhizosphere. J. Exp. Bot. 2012, 63, 3445–3454. [Google Scholar] [CrossRef] [PubMed]
- Weston, L.; Weston, P.; McCully, M. Production of bioactive napthoquinones by roots of Paterson’s Curse (Echium plantagineum L.): Implications for invasion success? In Proceedings of 23rd Asian-Pacific Weed Science Society Conference (APWSS): Weed Management in a Changing World, Cairns, Australia, 26–29 September 2011; pp. 576–581. [Google Scholar]
- Weston, P.A.; Weston, L.A.; Hildebrand, S. Metabolic profiling in Echium plantagineum: Presence of bioactive pyrrolizidine alkaloids and napthoquinones from accessions across southeastern Australia. Phytochem. Rev. 2013, 12, 831–837. [Google Scholar] [CrossRef]
- Albreht, A.; Vovk, I.; Simonovska, B.; Srbinoska, M. Identification of shikonin and its ester derivatives from the roots of Echium italicum L. J. Chromatogr. A 2009, 1216, 3156–3162. [Google Scholar] [CrossRef]
- Assimopoulou, A.; Karapanagiotis, I.; Vasiliou, A.; Kokkini, S.; Papageorgiou, V. Analysis of alkannin derivatives from Alkanna species by high-performance liquid chromatography/photodiode array/mass spectrometry. Biomed. Chromatogr. 2006, 20, 1359–1374. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jiang, Z.; Leung, K.S.-Y.; Zhao, Z. Simultaneous determination of naphthoquinone derivatives in Boraginaceous herbs by high-performance liquid chromatography. Anal. Chim. Acta 2006, 577, 26–31. [Google Scholar] [CrossRef]
- Gara, R.K.; Srivastava, V.K.; Duggal, S.; Bagga, J.K.; Bhatt, M.; Sanyal, S.; Mishra, D.P. Shikonin selectively induces apoptosis in human prostate cancer cells through the endoplasmic reticulum stress and mitochondrial apoptotic pathway. J. Biomed. Sci. 2015, 22, 26. [Google Scholar] [CrossRef]
- Trivedi, R.; Müller, G.A.; Rathore, M.S.; Mishra, D.P.; Dihazi, H. Anti-leukemic activity of shikonin: Role of ERP57 in shikonin induced apoptosis in acute myeloid leukemia. Cell. Physiol. Biochem. 2016, 39, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Wu, L.-F.; Jiang, H.-L. Reviews on natural quinones and their bioactivities of medicinal Zicao. Nat. Prod. Res. Dev. 2020, 32, 694–707. [Google Scholar]
- Binder, R.G.; Benson, M.E.; Flath, R.A. Eight 1, 4-naphthoquinones from Juglans. Phytochemistry 1989, 28, 2799–2801. [Google Scholar] [CrossRef]
- Brigham, L.A.; Michaels, P.J.; Flores, H.E. Cell-specific production and antimicrobial activity of naphthoquinones in roots of Lithospermum erythrorhizon. Plant Physiol. 1999, 119, 417–428. [Google Scholar] [CrossRef]
- Babula, P.; Adam, V.; Havel, L.; Kizek, R. Noteworthy secondary metabolites naphthoquinones-their occurrence, pharmacological properties and analysis. Curr. Pharm. Anal. 2009, 5, 47–68. [Google Scholar] [CrossRef]
- Duke, J.A. A Field Guide to Medicinal Plants: Eastern and Central North America; Houghton Mifflin: Boston, MA, USA, 1990; Volume 40. [Google Scholar]
- Butler, M.S. The role of natural product chemistry in drug discovery. J. Nat. Prod. 2004, 67, 2141–2153. [Google Scholar] [CrossRef]
- Paniagua-Pérez, R.; Madrigal-Bujaidar, E.; Reyes-Cadena, S.; Alvarez-González, I.; Sánchez-Chapul, L.; Pérez-Gallaga, J.; Hernández, N.; Flores-Mondragón, G.; Velasco, O. Cell protection induced by beta-sitosterol: Inhibition of genotoxic damage, stimulation of lymphocyte production, and determination of its antioxidant capacity. Arch. Toxicol. 2008, 82, 615–622. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, A.K.; Dobhal, M.; Sharma, M.; Gupta, R. Antidiabetic and antioxidant potential of β-sitosterol in streptozotocin-induced experimental hyperglycemia. J. Diabetes 2011, 3, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Morteza-Semnani, K.; Saeedi, M.; Akbarzadeh, M. Chemical composition and antimicrobial activity of essential oil of Echium italicum L. J. Essent. Oil Bear. Plants 2009, 12, 557–561. [Google Scholar] [CrossRef]
- Ghassemi, N.; Sajadi, S.; Ghannadi, A.; Shams, A.M.; Mehrabani, M. Volatile constituents of a medicinal plant of Iran, Echium amoenum Fisch. and CA Mey. DARU J. Pharm. Sci. 2003, 11, 32–33. [Google Scholar]
- Ghoreishi, S.M.; Bataghva, E. Supercritical extraction of essential oil from Echium amoenum seed: Experimental, modeling and genetic algorithm parameter estimation. Korean J. Chem. Eng. 2014, 31, 1632–1640. [Google Scholar] [CrossRef]
- Abbaszadeh, F.; Taghizadeh, M.; Akbari, H.; Farzin, N.; Mahlouji, M.; Memarzadeh, M.R.; Sharifi, N. The effect of an oral tablet containing a combination of extracts from Vitex agnus-castus, Echium amoenum, and Chamomile plus vitamin B6 on premenstrual syndrome: A randomized clinical trial. Basic Clin. Biochem. Nutr. 2024, 1, 30–38. [Google Scholar]
- Farajdokht, F.; Vosoughi, A.; Ziaee, M.; Araj-Khodaei, M.; Mahmoudi, J.; Sadigh-Eteghad, S. The role of hippocampal GABAA receptors on anxiolytic effects of Echium amoenum extract in a mice model of restraint stress. Mol. Biol. Rep. 2020, 47, 6487–6496. [Google Scholar] [CrossRef]
- Azizi, H.; Ghafari, S.; Ghods, R.; Shojaii, A.; Salmanian, M.; Ghafarzadeh, J. A review study on pharmacological activities, chemical constituents, and traditional uses of Echium amoenum. Pharmacogn Rev. 2018, 12, 208–213. [Google Scholar]
- Molyneux, R.; Gardner, D.; Colegate, S.; Edgar, J. Pyrrolizidine alkaloid toxicity in livestock: A paradigm for human poisoning? Food Addit. Contam. Part A 2011, 28, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Chain, E.P.o.C.i.t.F. Scientific opinion on pyrrolizidine alkaloids in food and feed. EFSA J. 2011, 9, 2406. [Google Scholar] [CrossRef]
- Chmit, M.S.; Horn, G.; Dübecke, A.; Beuerle, T. Pyrrolizidine alkaloids in the food chain: Is horizontal transfer of natural products of relevance? Foods 2021, 10, 1827. [Google Scholar] [CrossRef] [PubMed]
- Rather, I.A.; Koh, W.Y.; Paek, W.K.; Lim, J. The sources of chemical contaminants in food and their health implications. Front. Pharmacol. 2017, 8, 830. [Google Scholar] [CrossRef]
- Dusemund, B.; Nowak, N.; Sommerfeld, C.; Lindtner, O.; Schäfer, B.; Lampen, A. Risk assessment of pyrrolizidine alkaloids in food of plant and animal origin. Food Chem. Toxicol. 2018, 115, 63–72. [Google Scholar] [CrossRef]
- Casado, N.; Morante-Zarcero, S.; Sierra, I. The concerning food safety issue of pyrrolizidine alkaloids: An overview. Trends Food Sci. Technol. 2022, 120, 123–139. [Google Scholar] [CrossRef]
- Agency, E.M. Public Statement on the Use of Herbal Medicinal Products Containing Toxic, Unsaturated Pyrroliz-Idine Alkaloids (PAs); European Medicines Agency: London, UK, 2014. [Google Scholar]
- Bundesverband der Pharmazeutischen Industrie. Code of Practice to Prevent and Reduce Pyrrolizidine Alkaloid Contaminations of Medicinal Products of Plant Origin. Available online: https://media.journals.elsevier.com/content/files/cop-revision-20090245.pdf (accessed on 14 July 2025).
- Dittrich, H.; Hösel, K.; Sievers, H.; Klier, B.; Waimer, F.; Heuberger, H.; Plescher, A.; Armbrüster, N.; Steinhoff, B. Code of practice zur Vermeidung und Verringerung von Kontaminationen pflanzlicher Arzneimittel mit Pyrrolizidinalkaloiden. Pharm. Ind. 2016, 78, 836–845. [Google Scholar]
- Colegate, S.M.; Edgar, J.A.; Knill, A.M.; Lee, S.T. Solid-phase extraction and HPLC-MS profiling of pyrrolizidine alkaloids and their N-oxides: A case study of Echium plantagineum. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2005, 16, 108–119. [Google Scholar] [CrossRef] [PubMed]
- El-Shazly, A.; Sarg, T.; Ateya, A.; Abdel Aziz, A.; El-Dahmy, S.; Witte, L.; Wink, M. Pyrrolizidine alkaloids from Echium setosum and Echium vulgare. J. Nat. Prod. 1996, 59, 310–313. [Google Scholar] [CrossRef]
- Tepe, M. Antihistaminic Activity of Shikonin from Biotechnologically Grown Echium italicum L. In Biotechnology of Medicinal Plants with Antiallergy Properties: Research Trends and Prospects; Springer: Berlin/Heidelberg, Germany, 2024; pp. 219–234. [Google Scholar]
- Guo, C.; He, J.; Song, X.; Tan, L.; Wang, M.; Jiang, P.; Li, Y.; Cao, Z.; Peng, C. Pharmacological properties and derivatives of shikonin—A review in recent years. Pharmacol. Res. 2019, 149, 104463. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Sharma, A.; Nayik, G.A.; Cooper, R.; Bhardwaj, G.; Sohal, H.S.; Mutreja, V.; Kaur, R.; Areche, F.O.; AlOudat, M. Review of shikonin and derivatives: Isolation, chemistry, biosynthesis, pharmacology and toxicology. Front. Pharmacol. 2022, 13, 905755. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, C.; Zhou, F.; Zhang, Y. Shikonin potentiates therapeutic efficacy of oxaliplatin through reactive oxygen species-mediated intrinsic apoptosis and endoplasmic reticulum stress in oxaliplatin-resistant colorectal cancer cells. Drug Dev. Res. 2023, 84, 537–550. [Google Scholar] [CrossRef]
- Zhang, N.; Peng, F.; Wang, Y.; Yang, L.; Wu, F.; Wang, X.; Ye, C.; Han, B.; He, G. Shikonin induces colorectal carcinoma cells apoptosis and autophagy by targeting galectin-1/JNK signaling axis. Int. J. Biol. Sci. 2020, 16, 147. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Li, H.; Guo, X.; Guo, D.; Yang, Y.; Wang, X.; Zhang, C.; Shan, Z.; Xia, X. Antibiofilm activity of shikonin against Listeria monocytogenes and inhibition of key virulence factors. Food Control 2021, 120, 107558. [Google Scholar] [CrossRef]
- Kourounakis, A.P.; Assimopoulou, A.N.; Papageorgiou, V.P.; Gavalas, A.; Kourounakis, P.N. Alkannin and Shikonin: Effect on free radical processes and on inflammation-a preliminary pharmacochemical investigation. Arch. Pharm. Int. J. Pharm. Med. Chem. 2002, 335, 262–266. [Google Scholar] [CrossRef]
- Tepe, M.; Abadan, Ş.; Saglam, M.F.; Süzerer, V.; Erçin, P.B.; Atilla, D.; Baykal, E.E.; Şeker, M.G.; Yağcı, T.; Çiftçi, Y.Ö. In vitro mass production, chemical modification, and cytotoxicity of shikonin derivatives on breast cancer cells. Ind. Crops Prod. 2023, 192, 116087. [Google Scholar] [CrossRef]
- Roy, S.; Kim, H.-J.; Rhim, J.-W. Effect of blended colorants of anthocyanin and shikonin on carboxymethyl cellulose/agar-based smart packaging film. Int. J. Biol. Macromol. 2021, 183, 305–315. [Google Scholar] [CrossRef]
- Eruygur, N. A simple isocratic high-perfomance liquid chromatography method for the simultaneous determination of shikonin derivatives in some Echium species growing wild in Turkey. Turk. J. Pharm. Sci. 2018, 15, 38. [Google Scholar]
- De Natale, A.; Pollio, A. Plants species in the folk medicine of Montecorvino Rovella (inland Campania, Italy). J. Ethnopharmacol. 2007, 109, 295–303. [Google Scholar] [CrossRef]
- Araj-khodaei, M.; Nouri, M.; Farajdokht, F.; Torbati, M.; Kuchaksaray, F.R.; Hamedyazdan, S.; Sadigh-Eteghad, S. A Close Look at Echium amoenum Processing, Neuroactive Components, and Effects on Neuropsychiatric Disorders. Galen Med. J. 2019, 8, e1559. [Google Scholar] [CrossRef]
- Gomes, N.G.; Campos, M.G.; Órfão, J.M.; Ribeiro, C.A. Plants with neurobiological activity as potential targets for drug discovery. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 1372–1389. [Google Scholar] [CrossRef] [PubMed]
- Quintans-Júnior, L.; Moreira, J.C.; Pasquali, M.A.; Rabie, S.M.; Pires, A.S.; Schröder, R.; Rabelo, T.K.; Santos, J.P.; Lima, P.S.; Cavalcanti, S.C. Antinociceptive activity and redox profile of the monoterpenes (+)-camphene, p-cymene, and geranyl acetate in experimental models. Int. Sch. Res. Not. 2013, 2013, 459530. [Google Scholar] [CrossRef]
- Lima, I.S.M. Medicinal and Aromatic Plants of Cape Verde: Characterization of Volatile Metabolites of Endemic and Native Species; Universidade de Coimbra: Coimbra, Portugal, 2013. [Google Scholar]
- Abdel-Sattar, E.; Harraz, F.M.; Al-Ansari, S.M.; El-Mekkawy, S.; Ichino, C.; Kiyohara, H.; Otoguro, K.; Omura, S.; Yamada, H. Antiplasmodial and antitrypanosomal activity of plants from the Kingdom of Saudi Arabia. J. Nat. Med. 2009, 63, 232–239. [Google Scholar] [CrossRef]
- González-Tejero, M.; Molero-Mesa, J.; Casares-Porcel, M.; Lirola, M.M. New contributions to the ethnopharmacology of Spain. J. Ethnopharmacol. 1995, 45, 157–165. [Google Scholar] [CrossRef]
- Conforti, F.; Sosa, S.; Marrelli, M.; Menichini, F.; Statti, G.A.; Uzunov, D.; Tubaro, A.; Menichini, F. The protective ability of Mediterranean dietary plants against the oxidative damage: The role of radical oxygen species in inflammation and the polyphenol, flavonoid and sterol contents. Food Chem. 2009, 112, 587–594. [Google Scholar] [CrossRef]
- Kakhki, M.V.; Koushki, E.; Khalilzadeh, S.; Pouya, M.M. Green Synthesis and Characterization of Silver Nanoparticles Using Echium amoenum Fisch. & C. A. Mey. Extract: Colorimetric Assay and Nonlinear Optical Absorption. Plasmonics 2024, 19, 2483–2492. [Google Scholar]
- Rabbani, M.; Sajjadi, S.; Vaseghi, G.; Jafarian, A. Anxiolytic effects of Echium amoenum on the elevated plus-maze model of anxiety in mice. Fitoterapia 2004, 75, 457–464. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Sadeghi, Z.; Hosseini, S.V.; Bussmann, R.W. Ethnopharmacological study of medicinal plants in Sarvabad, Kurdistan province, Iran. J. Ethnopharmacol. 2022, 288, 114985. [Google Scholar] [CrossRef]
- Pharmacopoeia, I.H. Ministry of Health and Medical Education. Tehran Food Drug Deputy 2002, 1, 51–52. [Google Scholar]
- Wang, W.; Jin, J.; Xu, H.; Shi, Y.; Boersch, M.; Yin, Y. Comparative analysis of the main medicinal substances and applications of Echium vulgare L. and Echium plantagineum L.: A review. J. Ethnopharmacol. 2022, 285, 114894. [Google Scholar] [CrossRef]
- Shariatifar, N.; Fathabad, A.E.; Madihi, S. Antibacterial activity of aqueous and ethanolic extracts of Echium amoenum on food-borne pathogens. J. Food Saf. Hyg. 2016, 2, 63–66. [Google Scholar]
- Hamedi, J.; Vatani, M. Antibacterial and antifungal effects of evening primrose “Oenothera biennis L.” and Borage “Echium amoenum Fisch. & CA Mey. oils. Nova Biol. Reper. 2015, 2, 199–206. [Google Scholar]
- Sabour, M.; Vala, M.H.; Motamed, S.M.; Eiji, M. Evaluation of the antibacterial effect of Echium amoenum Fisch. et Mey. against multidrug resistant Acinetobacter baumannii strains isolated from burn wound infection. Nov. Biomed. 2015, 3, 38–42. [Google Scholar]
- Vukic, M.D.; Vukovic, N.L.; Djelic, G.T.; Obradovic, A.; Kacaniova, M.M.; Markovic, S.; Popović, S.; Baskić, D. Phytochemical analysis, antioxidant, antibacterial and cytotoxic activity of different plant organs of Eryngium serbicum L. Ind. Crops Prod. 2018, 115, 88–97. [Google Scholar] [CrossRef]
- Atessahin, D.A.; Dalkilic, S.; Dalkilic, L.K.; Bayindir, D.; Cetinkaya, E. Evaluation of cytotoxic, antimicrobial, and antioxidant activi-ties of Echium italicum L. in MCF-7 and HepG2 cell lines. Int. J. Plant Based Pharm. 2025, 5, 25–32. [Google Scholar] [CrossRef]
- Adel Pilerood, S.; Prakash, J. Evaluation of nutritional composition and antioxidant activity of Borage (Echium amoenum) and Valerian (Valerian officinalis). J. Food Sci. Technol. 2014, 51, 845–854. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteu methods. Food Chem. 2006, 99, 835–841. [Google Scholar] [CrossRef]
- Nadi, F. Bioactive compound retention in Echium amoenum Fisch. & CA Mey. petals: Effect of fluidized bed drying conditions. Int. J. Food Prop. 2017, 20, 2249–2260. [Google Scholar]
- Uysal, İ.; Mohammed, F.S.; Şabik, A.E.; Kına, E.; Sevindik, M. Antioxidant and Oxidant status of medicinal plant Echium italicum collected from different regions. Turk. J. Agric.-Food Sci. Technol. 2021, 9, 1902–1904. [Google Scholar] [CrossRef]
- Boškovic, I.; Đukić, D.; Mašković, P.; Mandić, L. Phytochemical composition and biological activity of Echium italicum L. plant extracts. Bulg. Chem. Commun. 2017, 49, 836–845. [Google Scholar]
- Gheisary, B.; Fattahi, M.; Alipour, H. Enhancing extraction of shikonin and phenolic antioxidants from Echium italicum L. using ultrasound and response surface methodology: Optimizing temperature, time, and liquid–solid ratio. Biomass Convers. Biorefinery 2025, 15, 15619–15630. [Google Scholar] [CrossRef]
- Velioglu, Y.; Mazza, G.; Gao, L.; Oomah, B. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Lugasi, A.; Hóvári, J. Antioxidant properties of commercial alcoholic and nonalcoholic beverages. Food/Nahrung 2003, 47, 79–86. [Google Scholar] [CrossRef]
- Frankel, E.N.; Huang, S.-W.; Aeschbach, R.; Prior, E. Antioxidant activity of a rosemary extract and its constituents, carnosic acid, carnosol, and rosmarinic acid, in bulk oil and oil-in-water emulsion. J. Agric. Food Chem. 1996, 44, 131–135. [Google Scholar] [CrossRef]
- Fuhrman, B.; Volkova, N.; Rosenblat, M.; Aviram, M. Lycopene synergistically inhibits LDL oxidation in combination with vitamin E, glabridin, rosmarinic acid, carnosic acid, or garlic. Antioxid. Redox Signal. 2000, 2, 491–506. [Google Scholar] [CrossRef]
- Kimura, Y.; Okuda, H.; Okuda, T.; Hatano, T.; Arichi, S. Studies on the activities of tannins and related compounds, X. Effects of caffeetannins and related compounds on arachidonate metabolism in human polymorphonuclear leukocytes. J. Nat. Prod. 1987, 50, 392–399. [Google Scholar] [CrossRef]
- Englberger, W.; Hadding, U.; Etschenberg, E.; Graf, E.; Leyck, S.; Winkelmann, J.; Parnham, M. Rosmarinic acid: A new inhibitor of complement C3-convertase with anti-inflammatory activity. Int. J. Immunopharmacol. 1988, 10, 729–737. [Google Scholar] [CrossRef]
- Ito, H.; Miyazaki, T.; Ono, M.; Sakurai, H. Antiallergic activities of rabdosiin and its related compounds: Chemical and biochemical evaluations. Biorg. Med. Chem. 1998, 6, 1051–1056. [Google Scholar] [CrossRef]
- Bekhradnia, S.; Ebrahimzadeh, M.A. Antioxidant activity of Echium amoenum. Rev. Chim. J. 2016, 67, 223–226. [Google Scholar]
- Bourgou, S.; Ksouri, R.; Bellila, A.; Skandrani, I.; Falleh, H.; Marzouk, B. Phenolic composition and biological activities of Tunisian Nigella sativa L. shoots and roots. C. R. Biol. 2008, 331, 48–55. [Google Scholar] [CrossRef]
- Chaouche, T.M.; Haddouchi, F.; Ksouri, R.; Atik-Bekkara, F. Evaluation of antioxidant activity of hydromethanolic extracts of some medicinal species from South Algeria. J. Chin. Med. Assoc. 2014, 77, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Oloumi, H.; Hassibi, N. Study the correlation between some climate parameters and the content of phenolic compounds in roots of Glycyrrhiza glabra. J. Med. Plant Res. 2011, 5, 6011–6016. [Google Scholar]
- Ghasemi, K.; Ghasemi, Y.; Ehteshamnia, A.; Nabavi, S.M.; Nabavi, S.F.; Ebrahimzadeh, M.A.; Pourmorad, F. Influence of environmental factors on antioxidant activity, phenol and flavonoids contents of walnut (Juglans regia L.) green husks. J. Med. Plants Res. 2011, 5, 1128–1133. [Google Scholar]
- Jovančević, M.; Balijagić, J.; Menković, N.; Šavikin, K.; Zdunić, G.; Janković, T.; Dekić-Ivanković, M. Analysis of phenolic compounds in wild populations of bilberry (Vaccinium myrtillus L.) from Montenegro. J. Med. Plants Res. 2011, 5, 910–914. [Google Scholar]
- Abbaszadeh, S.; Rajabian, T.; Taghizadeh, M. Antioxidant activity, phenolic and flavonoid contents of Echium species from different geographical locations of Iran. J. Med. Plants By-Prod. 2013, 2, 23–31. [Google Scholar]
- Saifullah, M.; McCullum, R.; McCluskey, A.; Vuong, Q. Effects of different drying methods on extractable phenolic compounds and antioxidant properties from lemon myrtle dried leaves. Heliyon 2019, 5, e03044. [Google Scholar] [CrossRef]
- Aouadi, K.; Hajlaoui, H.; Arraouadi, S.; Ghannay, S.; Snoussi, M.; Kadri, A. HPLC/MS phytochemical profiling with antioxidant activities of Echium humile Desf. extracts: ADMET prediction and computational study targeting human peroxiredoxin 5 receptor. Agronomy 2021, 11, 2165. [Google Scholar] [CrossRef]
- Boskovic, I.; Dukić, D.; Mandic, L.; Mašković, P.; Govedarica-Lucic, A. Antioxidant and cytotoxic potential of selected plant species of the boraginaceae family. Agric. For. 2021, 67, 53–61. [Google Scholar] [CrossRef]
- Jin, J.; Boersch, M.; Nagarajan, A.; Davey, A.K.; Zunk, M. Antioxidant properties and reported ethnomedicinal use of the genus Echium (Boraginaceae). Antioxidants 2020, 9, 722. [Google Scholar] [CrossRef]
- Ateşşahin, D.A.; Dalkılıç, L.K.; Özeren, Y.; Dalkılıç, S.; Çakmak, K.; Çiçek, T.A. Investigation of Cytotoxic, Antimicrobial and Antioxidant Activity of Echium vulgare L. Seed. Int. J. Nat. Life Sci. 2023, 7, 129–135. [Google Scholar] [CrossRef]
- Naseri, N.; Kalantar, K.; Amirghofran, Z. Anti-inflammatory activity of Echium amoenum extract on macrophages mediated by inhibition of inflammatory mediators and cytokines expression. Res. Pharm. Sci. 2018, 13, 73–81. [Google Scholar] [CrossRef]
- Rammal, H.; Farhan, H.; Mohsen, H.; Hijazi, A.; Kobeissy, A.; Daher, A.; Badran, B. Antioxidant, cytotoxic properties and phytochemical screening of two Lebanese medicinal plants. Int. Res. J. Pharm 2013, 4, 132–136. [Google Scholar]
- Wink, M.; Ashour, M.L.; El-Readi, M.Z. Secondary metabolites from plants inhibiting ABC transporters and reversing resistance of cancer cells and microbes to cytotoxic and antimicrobial agents. Front. Microbiol. 2012, 3, 130. [Google Scholar] [CrossRef] [PubMed]
- El-Tantawy, H.M.; Hassan, A.R.; Taha, H.E. Anticancer mechanism of the non-polar extract from Echium angustifolium Mill. aerial parts in relation to its chemical content. Egypt. J. Chem. 2022, 65, 17–26. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheydaei, P.; Amaral, M.E.; Duarte, A.P. Genus Echium L.: Phytochemical Characterization and Bioactivity Evaluation for Drug Discovery. Plants 2025, 14, 2548. https://doi.org/10.3390/plants14162548
Sheydaei P, Amaral ME, Duarte AP. Genus Echium L.: Phytochemical Characterization and Bioactivity Evaluation for Drug Discovery. Plants. 2025; 14(16):2548. https://doi.org/10.3390/plants14162548
Chicago/Turabian StyleSheydaei, Parvaneh, Maria Emília Amaral, and Ana Paula Duarte. 2025. "Genus Echium L.: Phytochemical Characterization and Bioactivity Evaluation for Drug Discovery" Plants 14, no. 16: 2548. https://doi.org/10.3390/plants14162548
APA StyleSheydaei, P., Amaral, M. E., & Duarte, A. P. (2025). Genus Echium L.: Phytochemical Characterization and Bioactivity Evaluation for Drug Discovery. Plants, 14(16), 2548. https://doi.org/10.3390/plants14162548







