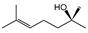
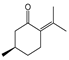
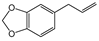
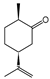
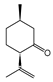
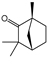
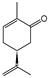
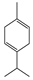
Abstract
Moniliophthora roreri, the causal agent of moniliasis, severely affects cacao production in Latin America, and sustainable control alternatives remain limited. This study aimed to evaluate the antifungal potential of essential oils (EOs) from Piper species and selected volatile compounds against M. roreri. A total of 34 EOs obtained by steam distillation were assessed for mycelial growth inhibition under fumigation conditions. The most active EOs (≥60% inhibition) were chemically characterized by GC-MS, and their median inhibitory concentrations (IC50) were determined. Additionally, 40 structurally diverse volatile compounds were selected and evaluated for their antifungal activity to identify the main contributors and explore structure–activity relationships. Most of the active EOs exhibited a high proportion of phenylpropanoids and oxygenated monoterpenoids, with IC50 values ranging from 0.58 to 184.27 µL·L−1; the most active were those from P. holtonii and P. aduncum. Among the 28 most active compounds, IC50 values ranged from 0.48 to 109.81 µL·L−1; the most potent were myristicin and dillapiole. The most potent molecules were phenylpropanoids bearing methoxy and methylenedioxy groups, followed by oxygenated monoterpenoids and long-chain ketones. This is the first report of antifungal activity against M. roreri for most of the evaluated EOs and all tested compounds, highlighting the potential of the Piper genus as a source of natural alternatives for sustainable disease management in cacao cultivation.
1. Introduction
Moniliophthora roreri (Cif.) H.C. Evans (Marasmiaceae) is a highly aggressive Basidiomycete fungus and the causal agent of frosty pod rot (FPR), one of the most devastating diseases affecting cacao (Theobroma cacao L.) in Latin America [1,2,3]. This pathogen primarily infects the fruit, leading to total or partial pod rot, with symptoms including premature ripening, hypertrophy, deformities, subepidermal oily spots, brown lesions covered by a cream-colored mycelial mat, wilting, and fruit necrosis, depending on developmental stage and environmental conditions. [1,4]. Native to Colombia, M. roreri has spread to major cacao-producing regions across Central and South America [3,5,6]. Yield losses caused by this pathogen can reach up to 90% under warm, humid climates with annual rainfall between 1200 and 4000 mm, making it one of the main drivers of crop abandonment among smallholder farmers [1,4,7].
Control strategies have focused on quarantine measures and cultural practices, often supplemented by the application of synthetic fungicides [4,8,9]. However, the limited efficacy of some commercial products, combined with environmental and health concerns and the emergence of resistant fungal strains, underscores the need for sustainable and safe alternatives [10,11,12]. Given this scenario, it becomes imperative to explore sustainable and safe alternatives for the control of M. roreri that can reduce dependence on synthetic products and mitigate their associated adverse effects. In this context, plants represent a promising source of bioactive compounds for crop protection [13,14,15].
Essential oils (EOs) are complex mixtures of volatile secondary metabolites derived from plants, which have shown promising activity against agricultural pests and pathogens [6,16,17,18,19,20]. In general, essential oils have been reported to exert multiple antifungal effects, including (1) disruption of membrane function and structure through the denaturation of membrane proteins; (2) inhibition of cellular respiration and alteration in membrane permeability at low concentrations; (3) severe membrane damage, homeostatic imbalance, and cell death at higher concentrations; (4) precipitation of cellular proteins and inhibition of key enzymes involved in energy production and biosynthesis of structural components; (5) increased membrane permeability leading to leakage of vital intracellular constituents; and (6) inhibition of mycotoxin production [21,22,23,24,25]. Despite this potential, research on the use of EOs against M. roreri remains limited, often restricted to preliminary screenings, with little exploration of their major constituents or mechanisms of action [26,27,28,29,30].
Among plant genera of interest, Piper (Piperaceae) has received growing attention due to its chemical diversity and the antifungal properties of many of its species [2,10,31,32,33]. Prior studies have reported antifungal activity of P. aduncum and P. auritum EOs against M. roreri [26,34,35]. Additionally, ethanolic extracts of P. peltatum have demonstrated significant antifungal effects, and a broader screening of ethanolic extracts from eleven Piper species identified P. asperiusculum, P. grande, P. statarium, P. artanthe, and P. nigrum as particularly effective, with IC50 values for mycelial growth inhibition ranging from 692 ppm to below 125 ppm [36,37]. Studies on P. pesaresanum and P. ceanothifolium revealed the antifungal properties of their extracts and some chemical constituents against this phytopathogen and allowed for the establishment of preliminary structure–activity relationships. These investigations highlighted the inhibitory activity of alkylphenols, chalcones, and prenylated derivatives of benzoic acid [2,10]. This study evaluates the antifungal potential of 34 Piper essential oils and 40 structurally diverse volatile compounds against M. roreri, characterizes their chemical profiles, and explores structure–activity relationships to support the development of natural vapor-phase control strategies.
2. Results and Discussion
2.1. Screening of Antifungal Activity of Piper EOs Against M. roreri
This study assessed the mycelial growth inhibition (MGI) of M. roreri by 34 EOs obtained from Piper species, using a concentration of 183 µL/L of air, evaluated through a vapor-phase diffusion assay specifically chosen due to the volatile and hydrophobic nature of essential oil and their constituents, which limits their effectiveness in conventional agar-based methods such as dilution or disc diffusion. These traditional techniques rely on diffusion through aqueous media, where volatile substances may not dissolve efficiently and tend to evaporate during incubation, reducing their contact with the fungal target. In contrast, the vapor-phase diffusion assay enables a controlled vapor-phase exposure, allowing for accurate assessment of antifungal activity by calculating the concentration of EO in the headspace (µL/L of air), which more closely reflects real conditions of application for volatile agents. The results revealed that 20 of these EOs inhibited more than 60% of the radial fungal growth (Figure 1). Among these, the EOs from P. holtoni, P. aduncum, P. asperiusculum, P. tenue, P. auritum, and P. statarium stood out for their strong antifungal activity, each exhibiting inhibition rates above 99%. Additionally, EOs from P. tuberculatum, P. divortans, P. marginatum, and P. albomaculatum showed inhibition rates exceeding 90%. In contrast, moderate antifungal activity (60–89%) was observed for EOs from P. elmetanum, P. lanceifolium, P. eriopodon, P. aequale, and P. haugtii, while others, such as those from P. imperiale, P. pertomentellum, P. grande, P. arborium, Piper sp., and P. marequitense, exhibited considerably lower efficacy. Interestingly, essential oils from P. rusticum and P. tomas-albertoi promoted mycelial growth instead of inhibiting it. Similar stimulatory effects have been reported in the literature, where certain essential oils or their volatile constituents enhance sporulation or growth in fungi under sublethal chemical stress. For example, Hountondji, 2006 [38], showed that citrus essential oils increased sporulation in Phaeoramularia angolensis, while other studies suggest that some volatiles may interfere with fungal regulatory pathways, triggering adaptive or compensatory responses [39,40,41].
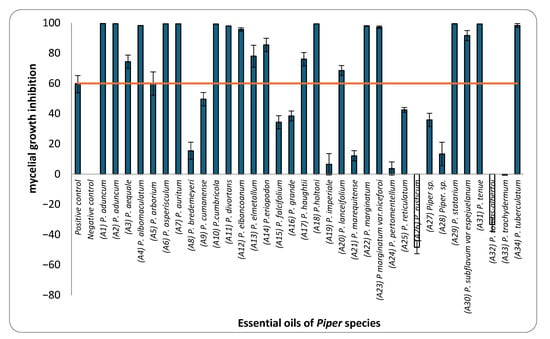
Figure 1.
Mycelial growth inhibition (MGI) of M. roreri by essential oils from Piper species at a concentration of 183 µL/L of air.
Among the 34 EOs tested, only those from P. aduncum, P. cumanense, and P. auritum had been previously reported to inhibit M. roreri. In earlier studies, EO from P. aduncum, extracted from shoots, leaves, and inflorescences, significantly inhibited fungal growth through both contact and fumigation, particularly at volumes of 15 and 30 µL [34]. Similarly, P. cumanense demonstrated complete inhibition in contact assays at concentrations near 52 mg/mL [35]. For P. auritum, a partial reduction in fungal growth was reported, although the specific concentrations used were not provided [26]. Although research on the antifungal activity of Piper EOs against M. roreri remains limited, prior studies have documented their efficacy against other cacao-associated phytopathogens. For instance, EOs from P. chaba, P. callosum, P. enckea, P. divaricatum, P. marginatum, P. marginatum var. anisatum, and P. dilatatum have shown activity against Fusarium solani, Phytophthora palmivora and P. capsici, pathogens that share ecological niches with M. roreri [42,43,44]. Within this context, the present findings significantly expand the existing knowledge base by providing the first report of vapor-phase diffusion activity against M. roreri for 31 of the 34 EOs evaluated.
Based on these results, EOs exhibiting ≥ 60% inhibition were selected for dose–response assays to determine their IC50 values. This quantitative approach enabled a comparative ranking of the fungicidal potency of the selected EOs and contributed to a more comprehensive understanding of their activity against the pathogen. Table 1 presents the IC50 values in ascending order, from the most to the least active EOs. The IC50 values ranged from 0.58 to 184.27 µL L−1 of air. The one-way analysis of variance (ANOVA) performed on the IC50 values of the EOs revealed significant differences (F = 3844.77; p < 0.001). Tukey’s post hoc multiple comparison test allowed for the grouping of the EOs into 13 homogeneous subsets, designated with the letters A to M in the corresponding table. Group A included the most active EOs, with IC50 values ranging from 0.58 to 2.05 µL L−1, while group M comprised the least active EO, with an IC50 of 184.27 µL L−1. Notably, the EOs from P. holtoni (A18), P. aduncum (A1 and A2), and P. statarium (A29) exhibited IC50 values below 3 µL L−1, classifying them as highly active. In contrast, the EOs from P. albomaculatum, P. elmetanum, and P. lanceifolium had IC50 values above 100 µL L−1, indicating limited efficacy.

Table 1.
Inhibitory concentrations (IC50) of EOs from bioactive Piper species against M. roreri.
2.2. Chemical Characterization of EOs with Potential Activity Against M. roreri
The chemical composition of the 20 bioactive EOs was analyzed using gas chromatography–mass spectrometry (GC-MS) with orthogonal polarity columns. This approach allowed for the identification of 122 compounds, which together accounted for more than 90% of the total composition of the analyzed oils. Table 2 summarizes the major constituents of each EO (defined as those exceeding 3% relative area). Detailed compositions obtained with both DB-5MS and HP-INNOWax columns are provided in Table S3.

Table 2.
Major volatile components (% area) identified by GC-MS in bioactive EOs from Piper species.
Figure 2 illustrates the relative distribution of the main chemical classes identified in the EOs, including hydrocarbon and oxygenated sesquiterpenoids, hydrocarbon and oxygenated monoterpenoids, and, to a lesser extent, phenylpropanoids. The abundance of these chemical groups varied considerably among the different Piper species. EOs from P. subflavum, P. asperiusculum, and P. aequale were characterized by a predominance of monoterpenes such as α- and β-pinene, limonene, and piperitone. In contrast, P. huantlii, P. eriopodon, P. lanceolifolium, and P. tuberculatum exhibited higher levels of sesquiterpenes, with β-caryophyllene, germacrene D, and α-copaene among the most abundant. Phenylpropanoids were the dominant class in the EOs of P. aduncum, P. marginatum, P. holtonii, and P. auritum, where major constituents included estragole, dillapiole, apiole, and safrole. Although no definitive trend was established linking a specific chemical class to stronger antifungal effects, EOs with high phenylpropanoid content tended to show enhanced activity against M. roreri. These oils also demonstrated substantial structural diversity, with major components such as dillapiole, myristicin, elemicin, apiole, and safrole, which have been previously associated with antifungal activity and are recognized as typical metabolites in various Piperaceae species [26,45,46,47,48].
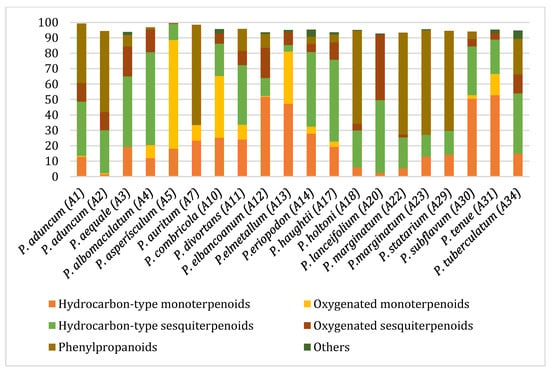
Figure 2.
Distribution of major chemical classes in EOs from Piper species with antifungal activity against M. roreri.
From a chemical perspective, previous studies on Piper species have demonstrated that the volatile fractions from aerial parts are predominantly composed of monoterpenoids and sesquiterpenoids, with occasional reports of diterpenes and phenylpropanoids [49]. The chemical profiles observed in this study are largely consistent with the existing literature, though notable interspecific differences were detected. These differences may result from environmental and geographic factors, as well as variations in extraction techniques and analytical methodologies [49,50,51].
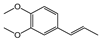
For instance, the EO from Piper aduncum analyzed in this study exhibited high levels of myristicin and dillapiole, followed by germacrene D and caryophyllene. These results contrast significantly with previously reported profiles, which describe a marked presence of caryophyllene oxide (37.0%), piperitone (23.7%), and viridiflorol (14.5%), as dominant components, in addition to a diverse mixture of monoterpenes and sesquiterpenes such as linalool, camphene, and germacrene D [52]. In another reported chemotype of P. aduncum, dillapiole (23.3%) was again predominant, followed by apiole (7.3%), myristicin (5.6%), and viridiflorol (4.1%), supporting the dominance of phenylpropanoids in this species. Nevertheless, other studies have reported substantially higher dillapiole content (73.7%) and only trace levels of the remaining compounds, suggesting that the observed variation in phenolic composition may be attributed to factors such as phenological stage, genetic divergence among populations, or differences in extraction methodologies [49,53,54,55].
The EO of P. aequale was primarily composed of E-nerolidol, germacrene B, β-caryophyllene and safrole, reflecting a combination of oxygenated sesquiterpenoids and phenylpropanoids. This composition diverges from previous reports, which highlight α-pinene (12.6%), δ-elemene (19.0%), and cubebol (7.2%) as dominant components, along with limonene (7.9%) and β-pinene (3.3%) as non-major constituents [56]. The EO of P. auritum was rich in safrole and camphor, partially consistent with the literature that reports even higher safrole content (93.2%) and detectable myristicin (4.3%) [57]. In P. asperiusculum, piperitone was the major constituent, contrasting with prior studies of fresh leaves in which dillapiole (48.5%) and myristicin (11.5%) predominated [58], suggesting that sample condition and plant origin may influence chemical profiles.
In P. eriopodon, the EO featured a balanced mixture of β-caryophyllene, α-pinene, β-pinene, α-copaene, germacrene B and apiole, while the presence of α-copaene and β-caryophyllene was consistent with research data [59]. The EO from P. huaghtii was characterized by germacrene D, β-caryophyllene and α-copaene, together with cadina-1,4-diene and apiole, in contrast to prior reports where dillapiole (48.2%) was dominant, and β-caryophyllene and piperitone were present at lower levels [60]. In P. holtonii EO, the main compounds were apiole, dillapiole and germacrene D; although the dillapiole content was lower than the 64.4% previously described, the consistent presence of germacrene D supports its chemotaxonomic relevance [60].
The EO of P. lanceolifolium was dominated by β-caryophyllene, farnesol and eudesmol. In contrast, earlier studies reported safrole (48.3%) as the primary compound, which was not detected in our samples [61]. P. marginatum exhibited a profile dominated by estragole and E-anethole, whereas previous research identified γ-asarone (64.5%) as the major metabolite [51]. In P. subflavum, high levels of α-pinene and β-pinene were found, accompanied by germacrene D and β-caryophyllene. This monoterpene-rich profile was largely consistent with previous reports, with minor differences such as the presence of apiole (3.4%) [59]. The EO of P. tuberculatum was characterized by β-caryophyllene, germacrene D and E-nerolidol. Although β-caryophyllene was also dominant in earlier studies (40.2%), such discrepancies may be attributed to differences in the plant organ or environmental conditions [49].
Several Piper EOs have demonstrated significant antifungal activity against phytopathogenic fungi of agricultural importance. For instance, P. auritum has shown strong inhibition against Fusarium species, with inhibition rates of 75.32% against F. oxysporum f. sp. comiteca, 86.57% against F. oxysporum f. sp. tequilana, and 63.36% against F. solani f. sp. comiteca [62,63]. Similarly, P. holtonii EO has been shown to inhibit the mycelial growth of Colletotrichum acutatum, C. gloeosporioides, and Botryodiplodia theobromae at concentrations of 400 µg/mL [64]. In vitro studies on P. aduncum reported inhibition rates of up to 94% against F. solani and 91% against Phytophthora sp. [26]. To the best of our knowledge, this work provides the first chemical characterization of the EOs from eight species: P. albomaculatum, P. cumbricola, P. divortans, P. elbanoanum, P. elmetanum, P. marginatum var. niceforoi, P. statarium, and P. tenue. These new profiles expand the phytochemical knowledge of the genus Piper and support its potential in the development of botanical fungicides.
2.3. Antifungal Potential of Selected Chemical Constituents Against M. roreri
To identify individual compounds potentially responsible for the vapor-phase activity observed in the most effective essential oils (EOs), a set of 40 representative substances was selected. This set included 25 compounds detected in the analyzed EOs and 15 additional molecules with relevant structural features for exploring structure–activity relationships. Among the selected compounds were 23 monoterpenoids, 13 phenylpropanoids, 2 sesquiterpenoids, and 2 aliphatic ketones, labeled from C1 to C40 (Table S2). Fumigation-based mycelial growth inhibition (MGI) assays were conducted at a maximum concentration of 122 µL·L−1 of air, revealing substantial variability in antifungal responses, with inhibition values ranging from −46.65% to 100%. A total of 28 compounds inhibited more than 60% of M. roreri growth (Figure 3). The most active compounds belonged primarily to the classes of phenylpropanoids, oxygenated monoterpenoids, and aliphatic ketones. Among the compounds that achieved over 90% inhibition, all shared the presence of oxygenated groups such as alcohols, ketones, methylenedioxy moieties, or conjugated double bonds inside chains. This study represents the first report of vapor-phase diffusion antifungal activity against M. roreri for all tested compounds.
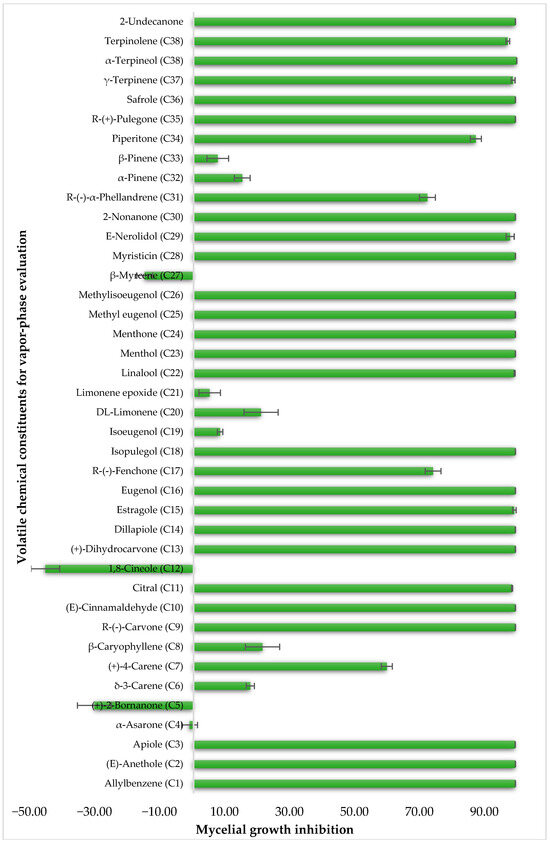
Figure 3.
Growth inhibition of M. roreri by selected volatile compounds in vapor exposure assays.
For the 28 promising compounds, median inhibitory concentration (IC50) values were determined, and the results are presented in Table 3. A broad range of antifungal potency was observed, with IC50 values ranging from 0.48 to 109.8 µL·L−1 of air. The one-way analysis of variance (ANOVA) performed on the IC50 values of the bioactive compounds revealed significant differences among the evaluated compounds (F = 3844.77; p < 0.001). Tukey’s post hoc multiple comparison test allowed for the grouping of the compounds into 13 homogeneous subsets, designated with the letters A to K in the corresponding table. Group A included the most active compounds, with IC50 values of less than 1 µL L−1, while group K comprised the least active compounds, with an IC50 greater than 100 µL L−1. The most active compounds were predominantly phenylpropanoids and oxygenated monoterpenoids, characterized by functional groups such as methylenedioxy-substituted aromatic rings, methoxy groups, α,β-unsaturated aldehydes, or tertiary alcohols. Myristicin and dillapiole were particularly notable for their high efficacy, with IC50 values below 1 µL·L−1, suggesting a key role for these structural motifs in inhibiting mycelial growth. In contrast, the compounds with the lowest activity were mainly hydrocarbon monoterpenes such as γ-terpinene and α-phellandrene.

Table 3.
IC50 values of volatile bioactive compounds against M. roreri.
Although none of the evaluated compounds had been previously reported as active against M. roreri, some have been studied in other fungal models. Myristicin, for example, has demonstrated potent antifungal activity in nutmeg essential oil, with inhibition rates exceeding 80% against Fusarium oxysporum, Aspergillus flavus, and A. ochraceus at concentrations between 0.1% and 0.3% [65,66]. It has also been shown to enhance the toxicity of other compounds, such as xanthotoxin, through inhibition of fungal microsomal enzymes [67]. Dillapiole has likewise shown strong antifungal effects, including inhibition of basidiospore germination in Crinipellis perniciosa at concentrations as low as 0.6–1.0 ppm [45], and selective inhibition of aflatoxin G1 production in Aspergillus parasiticus (IC50 = 0.15 μM) [68]. Structurally related compounds such as apiole and myristicin have also shown antifungal activity, with reported IC50 values of 0.24 and 3.5 μM, respectively.
With respect to oxygenated monoterpenoids, previous studies have documented antifungal activity for compounds such as citral, linalool, eugenol, methyl eugenol, 1,8-cineole, and α-phellandrene, although these have typically been tested at higher concentrations than those used in the present study [69]. Most Piper species are known to produce essential oils with a high proportion of phenylpropanoids. In this context, the results reported here support the hypothesis that these compounds contribute substantially to the antifungal activity observed in the vapor phase against M. roreri. EOs rich in oxygenated components and/or phenylpropanoids may therefore be considered promising candidates for the development of biocontrol agents applicable to agricultural systems.
Based on the IC50 values obtained for the phenylpropanoids evaluated, preliminary structure–activity relationships can be proposed in relation to their antifungal activity against M. roreri. The data indicate that the presence, number, and position of functional groups on the aromatic ring and the side chain significantly influence inhibitory potency. The most active compounds were myristicin (C29, IC50 = 0.48 µL·L−1) and dillapiole (C14, IC50 = 0.56 µL·L−1), both bearing a methylenedioxy-substituted aromatic ring and methoxy groups in ortho and para positions. In contrast, apiole (C3, IC50 = 3.66 µL·L−1), which also possesses a methylenedioxy group, exhibited approximately sevenfold-lower activity. This difference highlights the importance of the methoxylation pattern, suggesting that the specific positioning of methoxy substituents adjacent to the methylenedioxy group, as observed in apiole, may be less favorable for antifungal efficacy.
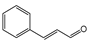
The relevance of methoxy groups is further supported by comparison with safrole (C36, IC50 = 15.04 µL·L−1), which lacks methoxy substituents on the aromatic ring and showed significantly lower activity. In fact, its IC50 value was 4 to 30 times higher than that of C3, C14, and C29, reinforcing the idea that methoxy substitution may play a key role in enhancing biological activity. In addition to aromatic substitution, the position of the double bond in the side chain may also contribute to antifungal performance, although to a lesser extent. Comparisons among compounds such as C26 and C27, or C15 and C2 reveal that those with a terminal double bond in the propyl side chain (C15 and C26) were slightly more active than those with conjugation extending into the aromatic ring (C2 and C27). However, the differences in activity between these pairs did not exceed 1.36-fold, indicating that substituents on the aromatic ring exert a stronger influence on activity than modifications to the side chain.
The number of methoxy groups on the ring also appears to play a significant role. Methyl eugenol (C26) and methyl isoeugenol (C27), each containing two methoxy groups, were approximately ten times more active than estragole (C15) and anethole (C2), each of which contains only one. Conversely, α-asarone (C4), with three methoxy groups, exhibited no antifungal activity under the conditions tested. These results suggest that while the introduction of methoxy groups can enhance activity, excessive substitution may lead to a loss of efficacy. Substitution of phenolic hydroxyl groups with methoxy groups was also associated with increased activity. For example, methyl eugenol (C26, IC50 = 4.84 µL·L−1) was significantly more active than eugenol (C16, IC50 = 85.42 µL·L−1), representing an 18-fold improvement. A similar trend was observed for isoeugenol and methyl isoeugenol, with only the methylated compound exhibiting inhibition greater than 60% under the experimental conditions. These results suggest that hydroxyl methylation may enhance antifungal activity by altering physicochemical properties such as stability, lipophilicity, and membrane permeability.
Allylbenzene (C1), which lacks oxygenated substituents, displayed lower activity than most oxygenated phenylpropanoids. However, it was more effective than eugenol, isoeugenol, and α-asarone, indicating that the presence of hydroxyl or multiple methoxy groups does not necessarily confer improved efficacy. These findings emphasize that both the presence and the spatial arrangement of functional groups on the aromatic ring are critical for biological performance. Methoxy and methylenedioxy substituents, when appropriately positioned, appear to be particularly favorable for enhancing the antifungal activity of phenylpropanoids against M. roreri.
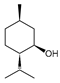
Among cyclic oxygenated monoterpenes, higher antifungal activity was observed when the hydroxyl group was attached to the tertiary carbon of the isopropyl moiety rather than directly to the ring. This trend was evident in the comparison of compounds C38, C18, and C24, with C38 (α-terpineol) showing the greatest activity. The presence of an exocyclic double bond in the side chain also appeared to have a positive influence. Isopulegol (C18), which contains such a feature, was approximately twice as active as menthol (C24), its saturated analog. For cyclic monoterpene ketones, the presence of an α,β-unsaturated carbonyl system did not correlate with increased activity. Dihydrocarvone (C13), which contains a non-conjugated double bond, was more effective than carvone (C9), piperitone (C34), and pulegone (C35). In addition, menthone (C25), lacking double bonds in the ring, was the least active of this group. These results suggest that non-conjugated unsaturations in the carbocyclic skeleton may contribute to antifungal potency.
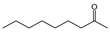
In the case of acyclic aliphatic ketones, carbon chain length had a notable effect on activity. 2-undecanone (C40), with a longer hydrocarbon chain, was approximately five times more active than 2-nonanone (C31). A similar trend was observed among acyclic alcohols: nerolidol (C30), a sesquiterpenic alcohol with a longer side chain, showed three-fold greater activity than linalool (C23), a monoterpenic counterpart. These observations suggest that, in oxygenated acyclic structures, the length of the hydrocarbon chain may enhance the inhibitory effect of volatile compounds against the pathogen.
Finally, the strong antifungal activity of phenylpropanoids against M. roreri underscores their potential as effective biocontrol agents. However, compounds such as safrole, myristicin, and apiole, as well as essential oils rich in phenylpropanoids, have been linked to potential phytotoxic effects in some plant species [70,71,72]. These may include impacts on germination, root growth, or other physiological functions, depending on dose and plant sensitivity. Further research is needed to assess their safety and compatibility with cacao under greenhouse and field conditions to support their use in sustainable disease management.
3. Materials and Methods
3.1. General Experimental Procedures
GC–MS analyses were performed using a GC 2010 Plus gas chromatograph coupled to a GCMS-TQ 8040 triple quadrupole mass spectrometer (Shimadzu®, Kyoto, Japan). The instrument was operated in electron impact mode (70 eV, 100 µA) in full scan acquisition (scan rate: 4.57 s−1), covering a mass range of m/z 40–400. The ion source (trap) and transfer line temperatures were both set at 280 °C. Essential oils (EOs) were analyzed using two capillary columns with orthogonal polarities: a DB-5MS column ((5%)-phenyl-methylpolysiloxane, 60 m × 0.25 mm i.d., 0.25 µm film thickness) and an HP-INNOWax column (polyethylene glycol, 60 m × 0.25 mm i.d., 0.25 µm film) (Agilent Technologies, Santa Clara, CA, USA). Linear retention indices (LRIs) were calculated based on a standard solution of n-alkanes (C7–C40, 1000 ppm; Sigma-Aldrich®, St. Louis, MO, USA).
Isolation and purification of natural constituents from the EOs were carried out by flash chromatography (FC) using SiliaFlash® P60 silica gel (particle size 25–40 µm; SiliCycle®, Quebec, QC, Canada). Fractions were monitored and analyzed by thin-layer chromatography (TLC) on SiliaPlate™ aluminum sheets coated with silica gel P60 F254 (5–20 µm; SiliCycle®), using UV light at 254 and 365 nm and iodine vapor for visualization. Solvent removal was conducted using a rotary evaporator (Hei-VAP, Heidolph Instruments GmbH & Co. KG, Schwabach, Germany). All solvents used in chromatographic procedures were of technical grade, commercially acquired, and distilled and dried prior to use. Other reagents were used as received without further purification. Structural characterization of the isolated compounds was performed by 1H-NMR and 13C-NMR (APT) spectroscopy. NMR spectra were recorded at 400 MHz for 1H and 100 MHz for 13C using a Bruker Advance AC-400 spectrometer (Bruker®, Hamburg, Germany) in CDCl3 at 25 °C. Chemical shifts (δ) are reported in parts per million (ppm) and coupling constants (J) in Hertz (Hz).
3.2. Screening of Antifungal Activity of Piper EOs Against M. roreri
3.2.1. Plant Material
The samples of 34 plant species of Piper genus were randomly collected during different field trips in the departments of Cundinamarca and Boyacá (Colombia). A specimen of each sample gathered was sent to an herbarium (Herbario Nacional Colombiano or Herbario Universidad de Antioquia) for taxonomic determination (Table S1). The collection of plant species was carried out under the contract of access to genetic resources and derived products No. 121 (22 January 2016), with OTROSI No. 21 celebrated between Ministerio de Medio Ambiente y Desarrollo Sostenible and Universidad Nacional de Colombia and under the amnesty framework established in Article 6 of Law 1955 of 2019.
3.2.2. EOs Extraction
Fresh aerial parts of the Piper species were subjected to steam distillation for approximately 2 h. The EOs were collected by condensation using a Clevenger-type apparatus, separated by decantation, dried over anhydrous sodium sulfate, and stored at 4 °C in refrigeration until analysis.
3.2.3. Fungal Strain
The M. roreri strain used in this study was maintained under controlled conditions at 24 ± 1 °C in the dark and preserved in the fungal collection. Its identity was confirmed through morphological and molecular analyses. Macroscopic and microscopic observations were conducted on colonies grown on potato dextrose agar (PDA) incubated at 25 °C for four weeks. Colony characteristics and reproductive structures were recorded weekly, and microscopic features stained with lactophenol blue were consistent with reference descriptions for M. roreri [73,74]. For molecular identification, DNA was extracted from the isolate and the internal transcribed spacer (ITS) region of the ribosomal DNA was amplified using primers ITS4 and ITS5. PCR products were verified on 1% agarose gels and sequenced by AGROSAVIA (Corporación Colombiana de Investigación Agropecuaria, Bogotá-Colombia) using the Sanger method. Sequences were edited with BioEdit (v7.1.9) and compared to GenBank entries using the BLASTn algorithm, showing ≥99% identity and an E-value of 0.0 with M. roreri reference sequences.
3.2.4. In Vitro Antifungal Evaluation of the EOs Using a Vapor-Phase Diffusion Assay Against M. roreri
The antifungal activity of the essential oils (EOs) was evaluated using an in vitro vapor-phase diffusion assay, a fumigation-type method based on confined vapor exposure, adapted from methods previously reported in the literature [75,76,77,78,79,80]. This approach is particularly suitable for assessing the efficacy of volatile, hydrophobic compounds such as EOs in sealed environments, where conventional contact-based methods are less effective.
The assay was conducted in sterile 90 mm diameter × 15 mm height glass Petri dishes containing potato dextrose agar (PDA) as the culture medium. At the center of each plate, 2 µL of a conidial suspension of M. roreri (1 × 106 conidia/mL) was deposited. Surrounding the inoculation site, three sterile Whatman® No. 1 filter paper discs (5 mm in diameter) were affixed equidistantly at approximately 2 cm. A volume of 5 µL of EO was applied to each disc, totaling 15 µL per plate. The Petri dishes were immediately sealed with Parafilm® to prevent vapor loss and to maintain a confined atmosphere. The vapor-phase concentration was expressed in µL/L of air and was calculated based on the total applied volume (15 µL) and the internal headspace of the Petri dish (~82 mL). Under these conditions, the exposure concentration for the initial screening was approximately 183 µL/L of air. Plates were incubated at 24 ± 1 °C in the dark. Fungal growth was monitored daily, and the experiment was terminated once the radial growth in the negative control (EO-free) plates reached approximately 60% of the total plate diameter (~50 mm). This typically occurred between 5 and 7 days post-inoculation. Digital images of each plate were taken, and radial growth was measured using ImageJ version 1.53t. A positive control with Mancozeb® 80 WP (Vecol S.A, Bogotá-Colombia) was included to confirm fungal inhibition (1000 µg/mL). All treatments were performed using three biological replicates, each consisting of four technical replicates. Mycelial growth inhibition (MGI) was calculated using the following formula:
where MGIc represents the mycelial growth in the control and MGIt corresponds to the growth under treatment.
EOs that showed ≥60% inhibition under screening conditions were considered bioactive and selected for further testing. For these, dose–response assays were performed using volumes ranging from 0.5 µL to 20 µL per dish, corresponding to vapor-phase concentrations between 0.58 and 243 µL/L of air. The median inhibitory concentration (IC50) for each EO or pure compound was determined from dose–response data by nonlinear regression analysis (probit model), using IBM SPSS Statistics 28. All results are reported as mean IC50 ± standard deviation (SD).
3.2.5. Statistical Analysis
A one-way analysis of variance (ANOVA) was performed using the IC50 values as the response variable. Once significant overall differences were detected (p < 0.05), a multiple comparison procedure was applied using Tukey’s HSD post hoc test, with the sample size adjusted using the harmonic mean of the groups (n = 4.0).
3.3. Chemical Characterization of EOs with Potential Activity Against M. roreri
3.3.1. Sample Preparation
For each sample, 25 μL of EO was diluted with n-hexane to a final volume of 1.0 mL. Similarly, a standard hydrocarbon mixture, (C7–C40) (Sigma-Aldrich, Saint Louis, MO, USA), was prepared by diluting 25 μL of the stock solution in n-hexane to a final volume of 1.0 mL.
3.3.2. GC–MS Analysis
The chromatographic analyses were performed using two columns. The first was a DB-5MS column, with a 1 μL injection volume and a 20:1 split ratio, at an injection temperature of 280 °C. Helium (99.9995%) was used as the carrier gas at a linear velocity of 25.5 cm/s and a constant flow rate of 1 mL/min. The temperature program started at 40 °C (2 min hold), and then increased to 123 °C at 4 °C/min (2 min hold), followed by a ramp to 160 °C at 4 °C/min (5 min hold), then 220 °C at 5 °C/min (8 min hold), and finally to 280 °C at 5 °C/min (4 min hold), with a total runtime of 75 min. The second analysis used a HP-INNOWax column under the same injection conditions. The temperature program began at 45 °C (4 min hold), ramped to 120 °C at 3 °C/min (2 min hold), and then increased to 250 °C at 4 °C/min (8 min hold), with a total analysis time of 71.5 min.
3.3.3. Tentative Identification of EO Constituents
Chemical compounds were tentatively identified by comparing their mass spectra and linear retention indices (LRIs) with those from the NIST 14.L, Wiley 8.1, and Pherobase databases, along with values reported by Adams [58,81,82]. The LRI values were calculated using a series of n-alkanes run under the same chromatographic conditions as the EO samples [81].
3.4. In Vitro Antifungal Evaluation of Selected Chemical Constituents Using a Vapor-Phase Diffusion Assay Against M. roreri
3.4.1. Chemicals
The plant-derived volatile compounds (VCs) evaluated in this study were selected based on two criteria: (i) their presence in essential oils that showed antifungal activity against M. roreri, and (ii) their structural similarity to those constituents, allowing for a structure–activity relationship (SAR) analysis. Of the 40 compounds tested, 35 were commercially sourced, as detailed in Supplementary Materials’ Table S2. Estragole, apiole, dillapiole and myristicin had been previously isolated from the essential oils of Artemisia dracunculus, P. holtonii, P. aduncum, and P. asperiusculum, and were available in the laboratory (Table S2) [83,84,85]. Piperitone was isolated via flash chromatography from the EOs of P. asperiusculum and was characterized by NMR and GC-MS (Tables S4 and S5, Figures S2–S4).
3.4.2. In Vitro Antifungal Evaluation of the VCs Using a Vapor-Phase Diffusion Assay
The antifungal activity of the volatile compounds (VCs) was evaluated using the same vapor-phase methodology described for the EOs in Section 3.2.4, using a maximum concentration of 122 μL/L air. Compounds that exhibited ≥ 60% mycelial growth inhibition were considered active and were further evaluated to determine their median inhibitory concentration (IC50). IC50 values were estimated by nonlinear regression analysis using a concentration range between 0.48 and 122 μL/L air. All assays were conducted with four replicates, and standard deviations were calculated. The data are presented as mean IC50 ± standard deviation (SD).
3.4.3. Statistical Analysis
A one-way analysis of variance (ANOVA) was performed using the IC50 values as the response variable. Once significant overall differences were detected (p < 0.05), a multiple comparison procedure was applied using Tukey’s HSD post hoc test, with the sample size adjusted using the harmonic mean of the groups (n = 4.0).
4. Conclusions
This study provides new evidence of the antifungal effectiveness of Piper-derived essential oils and volatile compounds in the vapor phase against M. roreri. For the majority of the tested essential oils and all individual constituents, this constitutes the first report of activity against this phytopathogen. Notably, several oils showed high efficacy, particularly those rich in phenylpropanoids and oxygenated compounds. The EOs of Piper holtonii and Piper aduncum were the most active, and among the individual compounds, myristicin and dillapiole showed the highest antifungal activity. The preliminary structure–activity relationship (SAR) analysis revealed structural features associated with antifungal potency. In phenylpropanoids, increased activity was linked to the presence of methoxy substituents and methylenedioxy groups on the aromatic ring, as well as to the methylation of phenolic hydroxyl groups. Among cyclic monoterpenoids, the most active compounds were those containing oxygenated functionalities such as alcohols and ketones, particularly when the structure also included non-conjugated double bonds. In acyclic compounds, including aliphatic ketones and alcohols, a longer hydrocarbon chain was associated with greater antifungal efficacy. Overall, these findings highlight the potential of Piper species as a valuable source of antifungal volatiles and support their use in the development of natural products for the sustainable control of M. roreri in cacao cultivation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14162514/s1. Table S1: Collection information of the 34 Piper species evaluated; Table S2: Selected volatile chemical constituents for fumigant evaluation against M. roreri; Table S3: Chemical compositions of the EOs of 20 essential oils from Piper species with potential fungicidal activity; Figure S1: Table of results of the Tukey test EOs; Table S4: Spectroscopic data and NMR spectra of compound C34 [86]; Figure S2: NMR 1H spectrum C34 (CDCl3, 400 MHz); Figure S3: NMR APT spectrum (CDCl3, 100 MHz); Figure S4: Chromatographic profile (TIC) from GC-MS analysis (DB-5MS) of piperitone isolated from Piper asperisculum; Table S5: Chemical composition of piperitone isolated from Piper asperisculum (DB-5MS); Figure S5: Table of results of the compound Tukey test.
Author Contributions
Conceptualization, O.J.P.-L. and J.A.P.-R.; methodology, N.V.D.-B. and J.A.P.-R.; formal analysis, N.V.D.-B., O.J.P.-L. and J.A.P.-R.; study investigation, N.V.D.-B. and J.A.P.-R.; data curation and interpretation, N.V.D.-B., O.J.P.-L. and J.A.P.-R.; original draft preparation, N.V.D.-B. and J.A.P.-R.; supervision, O.J.P.-L.; funding acquisition, O.J.P.-L. and J.A.P.-R. All authors contributed to the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research and the APC were funded by Ministerio de Ciencia Tecnología e Innovación de Colombia and Universidad Nacional de Colombia, grant number 232-2023, approved under Call No. 935 of 2023.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within this article and the Supplementary Materials.
Acknowledgments
The authors express their sincere gratitude to the research groups for their valuable collaboration in this research: QUIPRONAB and BIOMOLUN, belonging to the Universidad Nacional de Colombia and GIFUJ, belonging to the Pontificia Universidad Javeriana. The authors also acknowledge the contract of access to genetic resources and derived products No. 121 (22 January 2016), with OTROSI No. 21 celebrated between Ministerio de Medio Ambiente y Desarrollo Sostenible and Universidad Nacional de Colombia and for the amnesty established in article 6° of the law of 1955 of 2019.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ANOVA | A one-way analysis of variance |
| APT | Attached Proton Test |
| CDCl3 | Deuterated chloroform |
| 13C-NMR | Carbon Nuclear Magnetic Resonance |
| d | Doublet |
| DCM | Dichloromethane |
| EI | Electron impact |
| EO | Essential oil |
| EOs | Essential oils |
| eV | Electron volt |
| ExpLRI | Experimental Linear Retention Index |
| FC | Flash chromatography |
| g | Grams |
| CG | Gas chromatography |
| GC–MS | Gas chromatography with mass spectrometry |
| GCMS-TQ | Gas chromatography–mass spectrometry with triple quadrupole |
| Hz | Hertz |
| 1H-NMR | Proton Nuclear Magnetic Resonance |
| IC50 | Median inhibitory concentration |
| i.d | Internal diameter |
| J | Coupling constant |
| L | Liter |
| m | Multiplet |
| m | Meters |
| mg | Milligrams |
| MGI | Mycelial growth inhibition |
| MHz | Megahertz |
| min | Minutes |
| mL | Milliliter |
| mm | Millimeter |
| m/z | Mass/charge |
| nm | Nanometers |
| NMR | Nuclear Magnetic Resonance |
| p | p value |
| ppm | Parts per million |
| q | Quartet |
| rt | Retention time |
| Ref | Reference |
| REF LRI | Reference linear retention index |
| s | Singlet |
| SD | Standard deviation |
| UV | Ultraviolet |
| VCs | Volatile compounds |
| °C | Degree Celsius |
| δ | Chemical shift |
| δC | Carbon shift |
| δH | Hydrogen shift |
| µL | Microliter |
| µm | Micrometer |
References
- Compañía Nacional de Chocolate S.A.S. La Moniliasis Del Cacao: Daños, Síntomas, Epidemiología y Manejo. Available online: https://www.agrosavia.co/media/11540/69317.pdf (accessed on 26 June 2025).
- Mahecha, Y.S.; Patiño, O.J.; Prieto, J.A. Chemical Constituents and Antifungal Properties of Piper ceanothifolium Kunth Against Phytopathogens Associated with Cocoa Crops. Plants 2025, 14, 934. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, F.; Amaya, D.; Pinto, C.M.; Villavicencio, M.; Sosa Del Castillo, D.; Pérez, S. Multiple Introductions of Moniliophthora roreri from the Amazon to the Pacific Region in Ecuador and Shared High Azoxystrobin Sensitivity. Agronomy 2022, 12, 1119. [Google Scholar] [CrossRef]
- Perez, L. Moniliophthora roreri H.C. Evans et al. and Moniliophthora perniciosa (Stahel) Aime: Impact, Symptoms, Diagnosis, Epidemiology and Management. Rev. Prot. Veg. 2018, 33, 1–13. [Google Scholar]
- Contreras, L.Y.S. Identificación Molecular de Aislamientos de Moniliophthora roreri En Huertos de Cacao de Norte de Santander, Colombia. Acta Agron. 2016, 65, 51–57. [Google Scholar] [CrossRef]
- Yu, J. Chemical Composition of Essential Oils and Their Potential Applications in Postharvest Storage of Cereal Grains. Molecules 2025, 30, 683. [Google Scholar] [CrossRef]
- Plasencia-Vázquez, A.H.; Vilchez-Ponce, C.R.; Ferrer-Sánchez, Y.; Veloz-Portillo, C.E.; Plasencia-Vázquez, A.H.; Vilchez-Ponce, C.R.; Ferrer-Sánchez, Y.; Veloz-Portillo, C.E. Efecto Del Cambio Climático Sobre La Distribución Potencial Del Hongo Moniliophthora roreri y El Cultivo de Cacao (Theobroma cacao) En Ecuador Continental. Terra Latinoam. 2022, 40, 1–14.e1151. [Google Scholar] [CrossRef]
- Tirado, P.; Lopera, A.; Ríos, L.A. Estrategias de Control de Moniliophthora roreri y Moniliophthora perniciosa En Theobroma cacao L.: Revisión Sistemática. Cienc. Tecnol. Agropecu. 2016, 17, 417–430. [Google Scholar] [CrossRef]
- Gómez, H.; Lopez, M.; Garrido, E.; Solís, J.; Zamarripa, A.; Avendaño, C.; Mendoza, A. La Moniliasis (Moniliophthora roreri Cif & Par) Del Cacao: Búsqueda de Estrategias Del Manejo. Available online: https://www.researchgate.net/publication/257066495_LA_MONILIASIS_Moniliophthora_roreri_Cif_Par_DEL_CACAO_BUSQUEDA_DE_ESTRATEGIAS_DE_MANEJO (accessed on 31 May 2025).
- Chitiva, L.C.; Ladino, C.; Cuca, L.E.; Prieto, J.A.; Patiño, O.J. Antifungal Activity of Chemical Constituents from Piper pesaresanum C. DC. and Derivatives against Phytopathogen Fungi of Cocoa. Molecules 2021, 26, 3256. [Google Scholar] [CrossRef]
- Fungicide Resistence Action Committee Fungal Control Agents Sorted by Cross-Resistance Pattern and Mode of Action (Including Coding for FRAC Groups on Product Labels). Available online: https://cpb-us-w2.wpmucdn.com/u.osu.edu/dist/b/28945/files/2020/02/frac-code-list-2020-final.pdf (accessed on 26 June 2025).
- Kumar, S.; Singh, V.; Chakdar, H.; Choudhary, P. Harmful Effects of Fungicides: Current Status. Int. J. Agric. Environ. Biotechnol. 2018, 11, 1011–1019. [Google Scholar]
- Deresa, E.M.; Diriba, T.F. Phytochemicals as Alternative Fungicides for Controlling Plant Diseases: A Comprehensive Review of Their Efficacy, Commercial Representatives, Advantages, Challenges for Adoption, and Possible Solutions. Heliyon 2023, 9, e13810. [Google Scholar] [CrossRef]
- Butu, M.; Rodino, S.; Butu, A. Biopesticide Formulations—Current Challenges and Future Perspectives. In Biopesticides: Volume 2: Advances in Bio-Inoculants; Elsevier: Amsterdam, The Netherlands, 2022; pp. 19–29. [Google Scholar] [CrossRef]
- Zhang, P.; Duan, C.B.; Jin, B.; Ali, A.S.; Han, X.; Zhang, H.; Zhang, M.Z.; Zhang, W.H.; Gu, Y.C. Recent Advances in the Natural Products-Based Lead Discovery for New Agrochemicals. Adv. Agrochem. 2023, 2, 324–339. [Google Scholar] [CrossRef]
- Li, H.; Qiao, S.; Zhang, S. Essential Oils in Grain Storage: A Comprehensive Review of Insecticidal and Antimicrobial Constituents, Mechanisms, and Applications for Grain Security. J. Stored Prod. Res. 2025, 111, 102537. [Google Scholar] [CrossRef]
- Gupta, I.; Singh, R.; Muthusamy, S.; Sharma, M.; Grewal, K.; Singh, H.P.; Batish, D.R. Plant Essential Oils as Biopesticides: Applications, Mechanisms, Innovations, and Constraints. Plants 2023, 12, 2916. [Google Scholar] [CrossRef] [PubMed]
- Ayllón-Gutiérrez, R.; Díaz-Rubio, L.; Montaño-Soto, M.; Haro-Vázquez, M.d.P.; Córdova-Guerrero, I. Applications of Plant Essential Oils in Pest Control and Their Encapsulation for Controlled Release: A Review. Agriculture 2024, 14, 1766. [Google Scholar] [CrossRef]
- Jyotsna, B.; Patil, S.; Prakash, Y.S.; Rathnagiri, P.; Kavi Kishor, P.B.; Jalaja, N. Essential Oils from Plant Resources as Potent Insecticides and Repellents: Current Status and Future Perspectives. Biocatal. Agric. Biotechnol. 2024, 61, 103395. [Google Scholar] [CrossRef]
- Petrović, E.; Vrandečić, K.; Ćosić, J.; Siber, T.; Godena, S. Antifungal Efficacy of Essential Oils and Their Predominant Components Against Olive Fungal Pathogens. Agriculture 2025, 15, 340. [Google Scholar] [CrossRef]
- Başer, K.H.; Buchbauer, G. Handbook of Essential Oils: Science, Technology, and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 1–1095. [Google Scholar] [CrossRef]
- Alam, A.; Tripathi, A.; Sharma, V.; Sharma, N. Essential Oils: A Novel Consumer and Eco-Friendly Approach to Combat Postharvest Phytopathogens. J. Adv. Biol. Biotechnol. 2017, 11, 1–16. [Google Scholar] [CrossRef]
- Jiménez, M.F.; Carrasco, H.; Olea, A.; Silva, E. Natural Compounds: A Sustainable Alternative to the Phytopathogens Control. J. Chil. Chem. Soc. 2019, 64, 4459–4465. [Google Scholar] [CrossRef]
- Sil, A.; Pramanik, K.; Samantaray, P.; Mondal, M.F.; Yadav, V. Essential Oils: A Boon towards Eco-Friendly Management of Phytopathogenic Fungi. J. Entomol. Zool. Stud. 2020, 8, 1884–1891. [Google Scholar]
- Seepe, H.A.; Nxumalo, W.; Amoo, S.O. Natural Products from Medicinal Plants against Phytopathogenic Fusarium Species: Current Research Endeavours, Challenges and Prospects. Molecules 2021, 26, 6539. [Google Scholar] [CrossRef]
- Scalvenzi, L.; Yaguache-Camacho, B.; Cabrera-Martínez, P.; Guerrini, A. Actividad Antifúngica in Vitro de Aceites Esenciales de Ocotea quixos (Lam.) Kosterm. Y Piper aduncum L. Bioagro 2016, 28, 39–46. [Google Scholar]
- Gómez-López, A.; Martínez-Bolaños, L.; Ortiz-Gil, G.; Martínez-Bolaños, M.; Avendaño-Arrazate, C.H.; Hernández-Meneses, E. Bioaceites Esenciales Inhiben a Moniliophthora roreri (Cif. y Par.) Evans et al., Causante de La Moniliasis En El Cultivo Del Cacao. Acta Agríc. Pecu. 2020, 6, E0061016. [Google Scholar] [CrossRef]
- Ramos, P.; Rodríguez, W.; Castrillón, B.; Ramos, F.; Suárez, J. Potential Use of Siparuna Guianensis Essential Oil for the Control of Moniliophthora roreri in Cacao. Acta Agron. 2022, 71, 178–185. [Google Scholar] [CrossRef]
- Lozada, B.S.; Herrera, L.V.; Perea, J.A.; Stashenko, E.; Escobar, P. Efecto in Vitro de Aceites Esenciales de Tres Especies de Lippia Sobre Moniliophthora roreri (Cif. y Par.) Evans et al., Agente Causante de La Moniliasis Del Cacao (Theobroma cacao L.). Acta Agron. 2012, 61, 102–110. [Google Scholar]
- Pansera, M.R.; Silvestre, W.P.; Gonzatti, F.; Pauletti, G.F.; Sartori, V.C. Chemical Composition and Antifungal Activity of the Essential Oils from Native Species of the ‘Campos de Cima Da Serra’ Region, South Brazil. J. Essent. Oil Res. 2021, 33, 488–501. [Google Scholar] [CrossRef]
- da Silva, H.A.; Yamaguchi, L.F.; Young, M.C.M.; Ramos, C.S.; Amorim, A.M.A.; Kato, M.J.; Batista, R. Antifungal Piperamides from Piper mollicomum Kunth (Piperaceae). Eclet. Quim. 2021, 43, 33–38. [Google Scholar] [CrossRef]
- Xu, W.-H.; Li, X.-C. Antifungal Compounds from Piper Species. Curr. Bioact. Compd. 2011, 7, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.; Mesia, L.R.; Ceferino, H.; Mesia, W.R.; Andrés, M.F.; Díaz, C.E.; Gonzalez, A. Antifungal and Herbicidal Potential of Piper Essential Oils from the Peruvian Amazonia. Plants 2022, 11, 1793. [Google Scholar] [CrossRef] [PubMed]
- Huaman, C.J.; Cabezas, O.E. Matico Oil (Piper aduncum) in the Control of Moniliophthora roreri Causal Agent of Cocoa Moniliasis. Peruv. Agric. Res. 2019, 1, 53–57. [Google Scholar] [CrossRef]
- Sendoya, P. Evaluación de la Actividad Antimicrobiana in Vitro de Aceites Esenciales de Piper cumanense Kunth Frente a Moniliophthora rorero (Cif.) H.C. Evans, Stalpers, Samson & Benny. Bachelor’s Thesis, Universidad de Ibagué, Ibagué, Colombia, 2023. [Google Scholar]
- Martinez, A.P.G.; Ortíz, S.P.S.; Rodríguez, L.C.G.; Álvarez, L.N.C.; Beltrán, G.M.P.; Motta, D.M.; Moyano, E.M. Potential Use of Piper peltatum Essential Oil for the Control of Moniliophthora roreri in Cacao Plants Cultivated in the Colombian Amazon Region. Res. Sq. 2025; preprint. [Google Scholar] [CrossRef]
- Sanabria, N. Evaluaciónde La Actividad Antifúngica de Extractos Del Género Piper Contra Moniliophthora perniciosa, Agente Causal de Escoba de Bruja En El Cacao. Bachelor’s Thesis, Pontificia Universidad Javeriana, Bogotá, Colombia, 2019. [Google Scholar]
- Hountondji, F.C.C.; Hanna, R.; Sabelis, M.W. Does Methyl Salicylate, a Component of Herbivore-Induced Plant Odour, Promote Sporulation of the Mite-Pathogenic Fungus Neozygites tanajoae? Exp. Appl. Acarol. 2006, 39, 63–74. [Google Scholar] [CrossRef]
- Simas, D.; de Amorim, S.; Goulart, F.; Alviano, C.; Alviano, D.; da Silva, A. Citrus Species Essential Oils and Their Components Can Inhibit or Stimulate Fungal Growth in Fruit. Ind. Crops Prod. 2017, 98, 108–115. [Google Scholar] [CrossRef]
- Kuate, J.; Foko, J.; Ndindeng, S.; Jazet, P.; Fouré, E.; Damesse, F.; Bella, M.; Ducelier, D. Effect of Essential Oils from Citrus Varieties on in Vitro Growth and Sporulation of Phaeoramularia Angolensis Causing Citrus Leaf and Fruit Spot Disease. Eur. J. Plant Pathol. 2006, 114, 151–161. [Google Scholar] [CrossRef]
- Kadoglidou, K.; Lagopodi, A.; Karamanoli, K.; Vokou, D.; Bardas, G.A.; Menexes, G.; Constantinidou, H.I.A. Inhibitory and Stimulatory Effects of Essential Oils and Individual Monoterpenoids on Growth and Sporulation of Four Soil-Borne Fungal Isolates of Aspergillus Terreus, Fusarium oxysporum, Penicillium Expansum, and Verticillium Dahliae. Eur. J. Plant Pathol. 2011, 130, 297–309. [Google Scholar] [CrossRef]
- Silva, J.K.R.D.; Silva, J.R.A.; Nascimento, S.B.; Luz, S.F.M.D.; Meireles, E.N.; Alves, C.N.; Ramos, A.R.; Maia, J.G.S. Antifungal Activity and Computational Study of Constituents from Piper Divaricatum Essential Oil against Fusarium Infection in Black Pepper. Molecules 2014, 19, 17926–17942. [Google Scholar] [CrossRef] [PubMed]
- Mariana, D.; Silva, M.H.; Bastos, C.N. Atividade Antifúngica de Óleos Essenciais de Espécies de Piper Sobre Crinipellis perniciosa, Phytophthora palmivora e Phytophthora capsici. Fitopatol. Bras. 2007, 32, 143–145. [Google Scholar] [CrossRef]
- Rahman, A.; Al-Reza, S.M.; Kang, S.C. Antifungal Activity of Essential Oil and Extracts of Piper Chaba Hunter against Phytopathogenic Fungi. JAOCS J. Am. Oil Chem. Soc. 2011, 88, 573–579. [Google Scholar] [CrossRef]
- De Almeida, R.R.P.; Souto, R.N.P.; Bastos, C.N.; Da Silva, M.H.L.; Maia, J.G.S. Chemical Variation in Piper aduncum and Biological Properties of Its Dillapiole-Rich Essential Oil. Chem. Biodivers. 2009, 6, 1427–1434. [Google Scholar] [CrossRef]
- Potzernheim, M.C.L.; Bizzo, H.R.; Silva, J.P.; Vieira, R.F. Chemical Characterization of Essential Oil Constituents of Four Populations of Piper aduncum L. from Distrito Federal, Brazil. Biochem. Syst. Ecol. 2012, 42, 25–31. [Google Scholar] [CrossRef]
- François, T.; Dongmo, J.; Michel, P.; Lambert, S.M.; Ndifor, F.; Nangue, W.; Vyry, A.; Zollo, A.; Henri, P.; Chantal, M. Comparative Essential Oils Composition and Insecticidal Effect of Different Tissues of Piper capense L., Piper guineense Schum. et Thonn., Piper nigrum L. and Piper umbellatum L. Grown in Cameroon. Afr. J. Biotechnol. 2009, 8, 424–431. [Google Scholar]
- Salazar, L.C.; Ortiz-Reyes, A.; Rosero, D.M.; Lobo-Echeverri, T. Dillapiole in Piper holtonii as an Inhibitor of the Symbiotic Fungus Leucoagaricus gongylophorus of Leaf-Cutting Ants. J. Chem. Ecol. 2020, 46, 668–674. [Google Scholar] [CrossRef]
- Debonsi Navickiene, H.M.; de Morandim, A.A.; Alécio, A.C.; Regasini, L.O.; Cristina Bergamo, D.B.; Telascrea, M.; Cavalheiro, A.J.; Lopes, M.N.; da Bolzani Maysa Furlan, V.S.; Young, M.C.M.; et al. Composition and Antifungal Activity of Essential Oils from Piper aduncum, Piper arboreum and Piper tuberculatum. Quim. Nova 2006, 29, 467–470. [Google Scholar] [CrossRef]
- Celis, A.; Mendoza, C.; Pachón, M.; Cardona, J.; Delgado, W.; Cuca, L. Plant Extracts Used as Biocontrol with Emphasis on Piperaceae Family. A Review. Agron. Colomb. 2007, 26, 97–106. [Google Scholar]
- De Lima, C.H.M.; Camara, C.A.G.; Monteiro, V.B.; Santos, M.F.; Pontes, W.J.T.; Moraes, M.M.; Rodrigues, L.V.B. Bioactivity of Piper aduncum and Piper marginatum Essential Oils on Planococcus citri (RISSO, 1813) (Hemiptera: Pseudococcidae). Int. J. Trop. Insect Sci. 2024, 44, 2539–2548. [Google Scholar] [CrossRef]
- Monzote, L.; Scull, R.; Cos, P.; Setzer, W. Essential Oil from Piper aduncum: Chemical Analysis, Antimicrobial Assessment, and Literature Review. Medicines 2017, 4, 49. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.C.; Fernandes, M. Organ- and Season-Dependent Variation in the Essential Oil Composition of Salvia officinalis L. Cultivated at Two Different Sites. J. Agric. Food Chem. 2001, 49, 2908–2916. [Google Scholar] [CrossRef]
- Masotti, V.; Juteau, F.; Bessière, J.M.; Viano, J. Seasonal and Phenological Variations of the Essential Oil from the Narrow Endemic Species Artemisia Molinieri and Its Biological Activities. J. Agric. Food Chem. 2003, 51, 7115–7121. [Google Scholar] [CrossRef]
- Guerrini, A.; Sacchetti, G.; Rossi, D.; Paganetto, G.; Muzzoli, M.; Andreotti, E.; Tognolini, M.; Maldonado, M.E.; Bruni, R. Bioactivities of Piper aduncum L. and Piper obliquum Ruiz & Pavon (Piperaceae) Essential Oils from Eastern Ecuador. Environ. Toxicol. Pharmacol. 2009, 27, 39–48. [Google Scholar] [CrossRef]
- Kelly Da Silva, J.K.R.; Pinto, L.C.; Burbano, R.M.R.; Montenegro, R.C.; Andrade, E.H.A.; Maia, J.G.S. Composition and Cytotoxic and Antioxidant Activities of the Oil of Piper aequale Vahl. Lipids Health Dis. 2016, 15, 174. [Google Scholar] [CrossRef]
- Caballero, K.; Olivero, J.; Pino, N.; Stashenko, E. Chemical Composition and Bioactivity of Piper auritum and P. multiplinervium Essential Oils against the Red Flour Beetle, Tribolium castaneum (Herbst). Plantas Med. Aromát. 2014, 13, 10–19. [Google Scholar]
- Prieto, J.A.; Patiño, W.R.; Patiño, O.J. Chemical Composition, Insecticidal and Repellent Activities of Essential Oils from Piper asperiusculum and Piper pertomentellum against Red flour Weevil. Rec. Nat. Prod. 2025, 19, 169–181. [Google Scholar] [CrossRef]
- Velandia, S.A.; Quintero, E.; Stashenko, E.E.; Ocazionez, R.E. Antiproliferative Activity of Essential Oils from Colombian Plants. Acta Biol. Colomb. 2018, 23, 189–198. [Google Scholar] [CrossRef]
- Muñoz-Acevedo, A.; González, M.C.; Alonso, J.E.; Flórez, K.C. The Repellent Capacity against Sitophilus zeamais (Coleoptera: Curculionidae) and In Vitro Inhibition of the Acetylcholinesterase Enzyme of 11 Essential Oils from Six Plants of the Caribbean Region of Colombia. Molecules 2024, 29, 1753. [Google Scholar] [CrossRef] [PubMed]
- Valarezo, E.; Benítez, L.; Palacio, C.; Aguilar, S.; Armijos, C.; Calva, J.; Ramírez, J. Volatile and Non-Volatile Metabolite Study of Endemic Ecuadorian Specie Piper lanceifolium Kunth. J. Essent. Oil Res. 2020, 33, 182–188. [Google Scholar] [CrossRef]
- Chacón, C.; Miranda-Granados, J.; Ruiz-Lau, N.; Lagunas-Rivera, S.; Ruíz-Valdiviezo, V.M.; Gutiérrez-Miceli, F.A. Actividad Antifúngica de Extractos de Hierba Santa (Piper auritum) y Jarilla (Baccharis glutinosa) Contra fusarium spp. Agrociencia 2020, 54, 531–538. [Google Scholar] [CrossRef]
- Duarte, Y.; Pino Pérez, O.; Martínez, B. Efecto de Cuatro Aceites Esenciales Sobre fusarium spp. Rev. Prot. Veg. 2013, 28, 232–235. [Google Scholar]
- Pineda, R.; Vizcaíno, S.; García, C.M.; Gil, J.H.; Durango, D.L. Chemical Composition and Antifungal Activity of Piper auritum Kunth and Piper holtonii C. DC. against Phytopatogenic fungi. Chil. J. Agric. Res. 2012, 72, 507–515. [Google Scholar] [CrossRef]
- Valente, V.M.M.; Jham, G.N.; Jardim, C.M.; Dhingra, O.D.; Ghiviriga, I. Major Antifungals in Nutmeg Essential Oil against Aspergillus flavus and A. Ochraceus. J. Food Res. 2014, 4, 51. [Google Scholar] [CrossRef]
- Fernando, A.; Senevirathne, W. Raw Material from Nutmeg Myristica Fragrans) as Effective Fungicide against Fusarium oxysporum and the Oleoresin Profile of Nutmeg. J. Appl. Life Sci. Int. 2019, 22, 1–10. [Google Scholar] [CrossRef][Green Version]
- Berenbaum, M.; Neal, J.J. Synergism between Myristicin and Xanthotoxin, a Naturally Cooccurring Plant Toxicant. J. Chem. Ecol. 1985, 11, 1349–1358. [Google Scholar] [CrossRef]
- Razzaghi-Abyaneh, M.; Yoshinari, T.; Shams-Ghahfarokhi, M.; Rezaee, M.B.; Nagasawa, H.; Sakuda, S. Dillapiol and Apiol as Specific Inhibitors of the Biosynthesis of Aflatoxin G1 in Aspergillus parasiticus. Biosci. Biotechnol. Biochem. 2007, 71, 2329–2332. [Google Scholar] [CrossRef] [PubMed]
- Boulogne, I.; Petit, P.; Ozier-Lafontaine, H.; Desfontaines, L.; Loranger-Merciris, G. Insecticidal and Antifungal Chemicals Produced by Plants: A Review. Environ. Chem. Lett. 2012, 10, 325–347. [Google Scholar] [CrossRef]
- Herrera, O.; Saleh, A.; Mahmood, A.; Khalaf, M.; Calva, J.; Loyola, E.; Tataje, F.; Chávez, H.; Almeida-Galindo, J.S.; Chavez-Espinoza, J.H.; et al. The Essential Oil of Petroselinum crispum (Mill) Fuss Seeds from Peru: Phytotoxic Activity and In Silico Evaluation on the Target Enzyme of the Glyphosate Herbicide. Plants 2023, 12, 2288. [Google Scholar] [CrossRef] [PubMed]
- Andrés, M.F.; Rossa, G.E.; Cassel, E.; Vargas, R.M.F.; Santana, O.; Díaz, C.E.; González, A. Biocidal Effects of Piper hispidinervum (Piperaceae) Essential Oil and Synergism among Its Main Components. Food Chem. Toxicol. 2017, 109, 1086–1092. [Google Scholar] [CrossRef]
- Jop, B.; Wawrzyńczak, K.; Polaszek, K.; Synowiec, A. Analysis of the Sensitivity of Spring Wheat and White Mustard Seedlings to the Essential Oil of Parsley Seeds. Biol. Life Sci. Forum 2021, 3, 12. [Google Scholar] [CrossRef]
- Bailey, B.; Evans, H.; Phillips, W.; Ali, S.; Meinhardt, L. Moniliophthora roreri, Causal Agent of Cacao Frosty Pod Rot. Mol. Plant Pathol. 2018, 19, 1580–1594. [Google Scholar] [CrossRef]
- Phillips, W.; Aime, M.; Wilkinson, M. Biodiversity and Biogeography of the Cacao (Theobroma cacao) Pathogen Moniliophthora roreri in Tropical America. Plant Pathol. 2007, 56, 911–922. [Google Scholar] [CrossRef]
- Massoud, M.A.; Sayed, A.; Saad, A.K.; Soliman, E.; Yasseen El-Moghazy, A. Antifungal Activity of Some Essential Oils Applied as Fumigants against Two Stored Grains Fungi. J. Adv. Agric. Res. 2012, 17, 296–306. [Google Scholar]
- Hlebová, M.; Foltinová, D.; Vešelényiová, D.; Medo, J.; Šramková, Z.; Tančinová, D.; Mrkvová, M.; Hleba, L. The Vapor Phase of Selected Essential Oils and Their Antifungal Activity In Vitro and In Situ against Penicillium commune, a Common Contaminant of Cheese. Foods 2022, 11, 3517. [Google Scholar] [CrossRef]
- Prieto, J.A.; Patiño, O.J.; Delgado, W.A.; Moreno, J.P.; Cuca, L.E. Chemical Composition, Insecticidal and Antifungical Activities of Fruit Essential Oils of Three Colombian Zanthoxylum Species. Chil. J. Agric. Res. 2011, 71, 73–82. [Google Scholar] [CrossRef]
- Lin, H.J.; Lin, Y.L.; Huang, B.B.; Lin, Y.T.; Li, H.K.; Lu, W.J.; Lin, T.C.; Tsui, Y.C.; Lin, H.T.V. Solid- and Vapour-Phase Antifungal Activities of Six Essential Oils and Their Applications in Postharvest Fungal Control of Peach (Prunus persica L. Batsch). LWT 2022, 156, 113031. [Google Scholar] [CrossRef]
- Tančinová, D.; Mašková, Z.; Mendelová, A.; Foltinová, D.; Barboráková, Z.; Medo, J. Antifungal Activities of Essential Oils in Vapor Phase against Botrytis Cinerea and Their Potential to Control Postharvest Strawberry Gray Mold. Foods 2022, 11, 2945. [Google Scholar] [CrossRef]
- Mrvová, M.; Medo, J.; Lakatošová, J.; Barboráková, Z.; Golian, M.; Mašková, Z.; Tančinová, D. Vapor-Phase Essential Oils as Antifungal Agents against Penicillium olsonii Causing Postharvest Cherry Tomato Rot. Foods 2024, 13, 3202. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation, Ed.; Carol Stream: Carol Stream, IL, USA, 2012; ISBN 978-1-932633-21-4. [Google Scholar]
- Pherobase Pherobase The Pherobase: Database of Pheromones and Semiochemicals|The World Largest Database of Behavioural Modifying Chemicals. Available online: https://www.pherobase.com/ (accessed on 26 June 2025).
- Ripoll-Aristizábal, D.C.; Patiño-Ladino, O.J.; Prieto-Rodríguez, J.A. Essential oils and phenylpropanoids from Piper: Bioactivity and enzyme inhibition in Sitophilus zeamais and Tribolium castaneum. J. Stored Prod. Res. 2025, 114, 102714. [Google Scholar] [CrossRef]
- Ripoll, D. Insecticidal Potential of Essential Oils from Piper Species with High Phenylpropanoid Content for the Control of Sitophilus zeamais and Tribolium castaneum; National University of Colombia: Bogotá, Colombia, 2021; Available online: https://repositorio.unal.edu.co/handle/unal/87732 (accessed on 23 May 2025).
- Nagles Galeano, L. Study of the Insecticidal Action of Chemical Constituents Present in Essential Oils and Their Effect on Detoxifying Enzymes and Motor Function for Sitophilus zeamais; National University of Colombia: Bogotá, Colombia, 2021; Available online: https://repositorio.unal.edu.co/handle/unal/80633 (accessed on 23 May 2025).
- Telci, I.; Demirtas, I.; Bayram, E.; Arabaci, O.; Kacar, O. Environmental variation on aroma components of pulegone/piperitone rich spearmint (Mentha spicata L.). Ind. Crops Prod. 2010, 32, 588–592. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).