Recent Advances and Developments in Bacterial Endophyte Identification and Application: A 20-Year Landscape Review
Abstract
1. Introduction
2. Diversity and Distribution of Bacterial Endophytes
3. Biological Roles, Benefits, and Prospective Application of Bacterial Endophytes
3.1. Biocontrol Role
3.2. Nanoparticle Biosynthesizer Role
3.3. Plant Growth-Promotion Role
3.4. Phytoremediation Role
4. Isolation Techniques
5. Identification and Characterization of Bacterial Endophytes
5.1. Culture-Dependent Techniques
5.2. Culture-Independent Techniques
Meta-Omics Approaches
- Metagenomics
- b.
- Metatranscriptomics
- c.
- Metaproteomics
- d.
- Metabolomics
6. Literature Search Criteria
6.1. Review Strategy
6.2. Inclusion and Exclusion Criteria
7. Conclusions and Future Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABA | abscisic acid |
| ACC | 1-aminocyclopropane-1-carboxylate |
| AgNPs | silver nanoparticles |
| BLAST | basic local alignment search tool |
| CE-MS | capillary electrophoresis-mass spectrometry |
| DNA | deoxyribonucleic acid |
| GC-MS | gas chromatography-mass spectrometry |
| GNPS | global natural products social molecular networking |
| IAA | indole-3-acetic acid |
| IMG/M | integrated microbial genomes and microbiomes |
| ISR | induce systemic resistance |
| JA | jasmonic acid |
| KEGG | Kyoto encyclopedia of genes and genomes |
| LB | Luria–Bertani |
| LC-MS | liquid chromatography–mass spectrometry |
| MG-RAST | metagenome rapid annotation using subsystems technology |
| mRNA | messenger RNA |
| miRNAs | micro-RNAs |
| NCBI | the national center for biotechnology information |
| NMR | nuclear magnetic resonance spectroscopy |
| NPs | nanoparticles |
| OQDS | olive quick decline syndrome |
| PCR | polymerase chain reaction |
| PGPB | plant growth-promoting bacteria |
| PICRUSt | ribosomal database project |
| PRISMA | preferred reporting items for systematic reviews and meta-analyses |
| QIIME | quantitative insights into microbial ecology |
| RT-PCR | reverse transcription polymerase chain reaction |
| RNA | ribonucleic acid |
| rRNA | ribosomal RNA |
| RDP | ribosomal database project |
| tRNA | transfer RNA |
| SEM | scanning electron microscopy |
| TEM | transmission electron microscopy |
| UniProt | universal protein resource |
References
- Araújo, W.L.; Marcon, J.; Maccheroni, W.; van Elsas, J.D.; van Vuurde, J.W.L.; Azevedo, J.L. Diversity of Endophytic Bacterial Populations and Their Interaction with Xylella fastidiosa in Citrus Plants. Appl. Environ. Microbiol. 2002, 68, 4906–4914. [Google Scholar] [CrossRef]
- Nair, D.N.; Padmavathy, S. Impact of Endophytic Microorganisms on Plants, Environment and Humans. Sci. World J. 2014, 2014, 250693. [Google Scholar] [CrossRef]
- Gupta, K.; Dubey, N.K.; Singh, S.P.; Kheni, J.K.; Gupta, S.; Varshney, A. Plant Growth-Promoting Rhizobacteria (PGPR): Current and Future Prospects for Crop Improvement. In Current Trends in Microbial Biotechnology for Sustainable Agriculture; Yadav, A.N., Singh, J., Singh, C., Yadav, N., Eds.; Springer: Singapore, 2021; pp. 203–226. ISBN 978-981-15-6949-4. [Google Scholar]
- Chebotar, V.K.; Malfanova, N.V.; Shcherbakov, A.V.; Ahtemova, G.A.; Borisov, A.Y.; Lugtenberg, B.; Tikhonovich, I.A. Endophytic Bacteria in Microbial Preparations That Improve Plant Development (Review). Appl. Biochem. Microbiol. 2015, 51, 271–277. [Google Scholar] [CrossRef]
- Ou, T.; Xu, W.; Wang, F.; Strobel, G.; Zhou, Z.; Xiang, Z.; Liu, J.; Xie, J. A Microbiome Study Reveals Seasonal Variation in Endophytic Bacteria Among Different Mulberry Cultivars. Comput. Struct. Biotechnol. J. 2019, 17, 1091–1100. [Google Scholar] [CrossRef]
- Bamisile, B.S.; Akutse, K.S.; Siddiqui, J.A.; Xu, Y. Model Application of Entomopathogenic fungi as Alternatives to Chemical Pesticides: Prospects, Challenges, and Insights for Next-Generation Sustainable Agriculture. Front. Plant Sci. 2021, 12, 741804. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd_Allah, E.F.; Hashem, A. Phytohormones and Beneficial Microbes: Essential Components for Plants to Balance Stress and Fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef]
- Kabiraj, A.; Biswas, R.; Halder, U.; Bandopadhyay, R. Bacterial Arsenic Metabolism and Its Role in Arsenic Bioremediation. Curr. Microbiol. 2022, 79, 131. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, C.; Kaur, G.; Kaur, J.; Rath, S.K.; Dwibedi, V. From Microscopy to Omics: A Comprehensive Review of Tools and Techniques in Studying Endophytic Adaptation Under Abiotic and Biotic Stress. J. Plant Growth Regul. 2024. [Google Scholar] [CrossRef]
- Reinhold-Hurek, B.; Hurek, T. Living Inside Plants: Bacterial Endophytes. Curr. Opin. Plant Biol. 2011, 14, 435–443. [Google Scholar] [CrossRef]
- Resti, Z.; Liswarni, Y.; Martinius, M. Endophytic Bacterial Consortia as Biological Control of Bacterial Leaf Blight and Plant Growth Promoter of Rice (Oryza sativa L.). J. Appl. Agric. Sci. Technol. 2020, 4, 134–145. [Google Scholar] [CrossRef]
- Mundt, J.O.; Hinkle, N.F. Bacteria Within Ovules and Seeds. Appl. Environ. Microbiol. 1976, 32, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Bhore, S.; Christina, A.; Christapher, V. Endophytic Bacteria as a Source of Novel Antibiotics: An Overview. Pharmacogn. Rev. 2013, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Pal, T.; Yadav, N.; Singh, V.K.; Tripathi, V.; Choudhary, K.K.; Shukla, A.K.; Sunita, K.; Kumar, A.; Bontempi, E.; et al. Current Scenario and Future Prospects of Endophytic Microbes: Promising Candidates for Abiotic and Biotic Stress Management for Agricultural and Environmental Sustainability. Microb. Ecol. 2023, 86, 1455–1486. [Google Scholar] [CrossRef]
- Neelam Geat, D.S.; Rajawat, M.V.S.; Prasanna, R.; Rajeev Kaushik, A.K.S. Isolation and Characterization of Plant Growth Promoting Endophytic Diazotrophic Bacteria from Wheat Genotypes and Their Influence on Plant Growth Promotion. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1533–1540. [Google Scholar] [CrossRef][Green Version]
- Musa, Z.; Ma, J.; Egamberdieva, D.; Abdelshafy Mohamad, O.A.; Abaydulla, G.; Liu, Y.; Li, W.-J.; Li, L. Diversity and Antimicrobial Potential of Cultivable Endophytic Actinobacteria Associated with the Medicinal Plant Thymus Roseus. Front. Microbiol. 2020, 11, 191. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant Growth-Promoting Bacteria in the Rhizo- and Endosphere of Plants: Their Role, Colonization, Mechanisms Involved and Prospects for Utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Lopez-Echartea, E.; Strejcek, M.; Mukherjee, S.; Uhlik, O.; Yrjälä, K. Bacterial Succession in Oil-Contaminated Soil Under Phytoremediation with Poplars. Chemosphere 2020, 243, 125242. [Google Scholar] [CrossRef]
- Suman, A.; Yadav, A.N.; Verma, P. Endophytic Microbes in Crops: Diversity and Beneficial Impact for Sustainable Agriculture. In Microbial Inoculants in Sustainable Agricultural Productivity: Vol. 1: Research Perspectives; Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer: New Delhi, India, 2016; pp. 117–143. ISBN 978-81-322-2647-5. [Google Scholar]
- Wu, J.; Zhao, N.; Li, X.; Zhang, P.; Li, T.; Lu, Y. Nitrogen-Mediated Distinct Rhizosphere Soil Microbes Contribute to Sorghum bicolor (L.) Moench and Solanum nigrum L. for Phytoremediation of Cadmium-Polluted Soil. Plant Soil 2024, 495, 723–740. [Google Scholar] [CrossRef]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant Beneficial Endophytic Bacteria: Mechanisms, Diversity, Host Range and Genetic Determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhai, Y.; Cao, L.; Tan, H.; Zhang, R. Endophytic Bacterial and Fungal Microbiota in Sprouts, Roots and Stems of Rice (Oryza sativa L.). Microbiol. Res. 2016, 188–189, 1–8. [Google Scholar] [CrossRef]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; del Rio, T.G.; et al. Defining the Core Arabidopsis Thaliana Root Microbiome. Nature 2012, 488, 86–90. [Google Scholar] [CrossRef]
- Shen, S.Y.; Fulthorpe, R. Seasonal Variation of Bacterial Endophytes in Urban Trees. Front. Microbiol. 2015, 6, 427. [Google Scholar] [CrossRef]
- Al-Hawamdeh, F.; Ayad, J.Y.; Alananbeh, K.M.; Akash, M.W. Bacterial Endophytes and Their Contributions to Alleviating Drought and Salinity Stresses in Wheat: A Systematic Review of Physiological Mechanisms. Agriculture 2024, 14, 769. [Google Scholar] [CrossRef]
- Romero, F.M.; Marina, M.; Pieckenstain, F.L. The Communities of Tomato (Solanum lycopersicum L.) Leaf Endophytic Bacteria, Analyzed by 16S-Ribosomal RNA Gene Pyrosequencing. FEMS Microbiol. Lett. 2014, 351, 187–194. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, Y.; Lai, X. Antagonistic Endophytic Bacteria Associated with Nodules of Soybean (Glycine max L.) and Plant Growth-Promoting Properties. Braz. J. Microbiol. 2018, 49, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.M.; Salim, S.S.; Hassan, S.E.-D.; Ismail, M.A.; Fouda, A. Role of Endophytes in Plant Health and Abiotic Stress Management. In Microbiome in Plant Health and Disease: Challenges and Opportunities; Kumar, V., Prasad, R., Kumar, M., Choudhary, D.K., Eds.; Springer: Singapore, 2019; pp. 119–144. ISBN 978-981-13-8495-0. [Google Scholar]
- Luo, W.; Wang, D.; Qi, H.; Wang, T.; Liu, Z.; Tian, L.; Dai, X.; Chen, J.; Mijiti, M. Identification of Antagonistic Bacterium Strain KRS022 and Its Inhibition Effect on Verticillium dahliae. Sci. Agric. Sin. 2023, 56, 649–664. [Google Scholar] [CrossRef]
- Rabasco-Vílchez, L.; Bolívar, A.; Morcillo-Martín, R.; Pérez-Rodríguez, F. Exploring the Microbiota of Tomato and Strawberry Plants as Sources of Bio-Protective Cultures for Fruits and Vegetables Preservation. Future Foods 2024, 9, 100344. [Google Scholar] [CrossRef]
- Karabulut, F.; Parray, J.A.; Shafi, N.; Ikram, M. Chapter 8—Endophytes: Role in Maintaining Plant Health Under Stress Conditions. In Plant Endophytes and Secondary Metabolites; Egamberdieva, D., Parray, J.A., Davranov, K., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 105–132. ISBN 978-0-443-13365-7. [Google Scholar]
- Wu, T.; Xu, J.; Liu, J.; Guo, W.-H.; Li, X.-B.; Xia, J.-B.; Xie, W.-J.; Yao, Z.-G.; Zhang, Y.-M.; Wang, R.-Q. Characterization and Initial Application of Endophytic Bacillus safensis Strain ZY16 for Improving Phytoremediation of Oil-Contaminated Saline Soils. Front. Microbiol. 2019, 10, 991. [Google Scholar] [CrossRef]
- Lata, R.; Chowdhury, S.; Gond, S.K.; White, J.F., Jr. Induction of Abiotic Stress Tolerance in Plants by Endophytic Microbes. Lett. Appl. Microbiol. 2018, 66, 268–276. [Google Scholar] [CrossRef]
- Kaur, M.; Karnwal, A. Screening of Endophytic Bacteria from Stress-Tolerating Plants for Abiotic Stress Tolerance and Plant Growth-Promoting Properties: Identification of Potential Strains for Bioremediation and Crop Enhancement. J. Agric. Food Res. 2023, 14, 100723. [Google Scholar] [CrossRef]
- Gange, A.C.; Gadhave, K.R. Plant Growth-Promoting Rhizobacteria Promote Plant Size Inequality. Sci. Rep. 2018, 8, 13828. [Google Scholar] [CrossRef]
- Compant, S.; Cambon, M.C.; Vacher, C.; Mitter, B.; Samad, A.; Sessitsch, A. The Plant Endosphere World—Bacterial Life Within Plants. Environ. Microbiol. 2021, 23, 1812–1829. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Carrozza, G.P.; Vettori, L.; Felici, C.; Cinelli, F.; Toffanin, A. Plant Beneficial Microbes and Their Application in Plant Biotechnology. Innov. Biotechnol. 2012, 57–72. [Google Scholar]
- Harish, S.; Kavino, M.; Kumar, N.; Balasubramanian, P.; Samiyappan, R. Induction of Defense-Related Proteins by Mixtures of Plant Growth Promoting Endophytic Bacteria Against Banana bunchy Top Virus. Biol. Control 2009, 51, 16–25. [Google Scholar] [CrossRef]
- Glick, B.R. Introduction to Plant Growth-Promoting Bacteria. In Beneficial Plant-Bacterial Interactions; Glick, B.R., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–37. ISBN 978-3-030-44368-9. [Google Scholar]
- Narayanan, Z.; Glick, B.R. Secondary Metabolites Produced by Plant Growth-Promoting Bacterial Endophytes. Microorganisms 2022, 10, 2008. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Fan, X.; Cai, X.; Hu, F. The Inhibitory Mechanisms by Mixtures of Two Endophytic Bacterial Strains Isolated from Ginkgo biloba Against Pepper Phytophthora Blight. Biol. Control 2015, 85, 59–67. [Google Scholar] [CrossRef]
- Ma, L.; Cao, Y.H.; Cheng, M.H.; Huang, Y.; Mo, M.H.; Wang, Y.; Yang, J.Z.; Yang, F.X. Phylogenetic Diversity of Bacterial Endophytes of Panax notoginseng with Antagonistic Characteristics Towards Pathogens of Root-Rot Disease Complex. Antonie Van Leeuwenhoek 2013, 103, 299–312. [Google Scholar] [CrossRef]
- Jayakumar, A.; Krishna, A.; Mohan, M.; Nair, I.C.; Radhakrishnan, E.K. Plant Growth Enhancement, Disease Resistance, and Elemental Modulatory Effects of Plant Probiotic Endophytic Bacillus sp. Fcl1. Probiotics Antimicrob. Proteins 2019, 11, 526–534. [Google Scholar] [CrossRef]
- Rakotoniriana, E.F.; Rafamantanana, M.; Randriamampionona, D.; Rabemanantsoa, C.; Urveg-Ratsimamanga, S.; El Jaziri, M.; Munaut, F.; Corbisier, A.-M.; Quetin-Leclercq, J.; Declerck, S. Study In Vitro of the Impact of Endophytic Bacteria Isolated from Centella asiatica on the Disease Incidence Caused by the Hemibiotrophic Fungus Colletotrichum higginsianum. Antonie Van Leeuwenhoek 2013, 103, 121–133. [Google Scholar] [CrossRef]
- He, R.-L.; Wang, G.-P.; Liu, X.-H.; Zhang, C.-L.; Lin, F.-C. Antagonistic Bioactivity of an Endophytic Bacterium Isolated from Epimedium brevicornu Maxim. Afr. J. Biotechnol. 2009, 8, 191–195. [Google Scholar]
- Hong, C.E.; Jo, S.H.; Jo, I.-H.; Park, J.M. Diversity and Antifungal Activity of Endophytic Bacteria Associated with Panax ginseng Seedlings. Plant Biotechnol. Rep. 2018, 12, 409–418. [Google Scholar] [CrossRef]
- Karthikeyan, B.; Joe, M.M.; Islam, M.d.R.; Sa, T. ACC Deaminase Containing Diazotrophic Endophytic Bacteria Ameliorate Salt Stress in Catharanthus roseus Through Reduced Ethylene Levels and Induction of Antioxidative Defense Systems. Symbiosis 2012, 56, 77–86. [Google Scholar] [CrossRef]
- Xu, J.-Y.; Han, Y.-H.; Chen, Y.; Zhu, L.-J.; Ma, L.Q. Arsenic Transformation and Plant Growth Promotion Characteristics of As-Resistant Endophytic Bacteria from As-Hyperaccumulator Pteris vittata. Chemosphere 2016, 144, 1233–1240. [Google Scholar] [CrossRef]
- Khaksar, G.; Siswanto, D.; Treesubsuntorn, C.; Thiravetyan, P. Euphorbia milii-Endophytic Bacteria Interactions Affect Hormonal Levels of the Native Host Differently Under Various Airborne Pollutants. Mol. Plant-Microbe Interact. 2016, 29, 663–673. [Google Scholar] [CrossRef]
- Qin, S.; Feng, W.-W.; Zhang, Y.-J.; Wang, T.-T.; Xiong, Y.-W.; Xing, K. Diversity of Bacterial Microbiota of Coastal Halophyte Limonium sinense and Amelioration of Salinity Stress Damage by Symbiotic Plant Growth-Promoting Actinobacterium Glutamicibacter halophytocola KLBMP 5180. Appl. Environ. Microbiol. 2018, 84, e01533-18. [Google Scholar] [CrossRef]
- Govarthanan, M.; Mythili, R.; Selvankumar, T.; Kamala-Kannan, S.; Rajasekar, A.; Chang, Y.-C. Bioremediation of Heavy Metals Using an Endophytic Bacterium Paenibacillus sp. RM Isolated from the Roots of Tridax Procumbens. 3 Biotech 2016, 6, 242. [Google Scholar] [CrossRef]
- Zhu, X.; Jin, L.; Sun, K.; Li, S.; Ling, W.; Li, X. Potential of Endophytic Bacterium Paenibacillus sp. PHE-3 Isolated from Plantago asiatica L. for Reduction of PAH Contamination in Plant Tissues. Int. J. Environ. Res. Public Health 2016, 13, 633. [Google Scholar] [CrossRef]
- Aswathy, A.J.; Jasim, B.; Jyothis, M.; Radhakrishnan, E.K. Identification of Two Strains of Paenibacillus sp. as Indole 3 Acetic Acid-Producing Rhizome-Associated Endophytic Bacteria from Curcuma longa. 3 Biotech 2013, 3, 219–224. [Google Scholar] [CrossRef]
- Hassan, S.E.-D. Plant Growth-Promoting Activities for Bacterial and Fungal Endophytes Isolated from Medicinal Plant of Teucrium polium L. J. Adv. Res. 2017, 8, 687–695. [Google Scholar] [CrossRef]
- Ibrahim, E.; Fouad, H.; Zhang, M.; Zhang, Y.; Qiu, W.; Yan, C.; Li, B.; Mo, J.; Chen, J. Biosynthesis of Silver Nanoparticles Using Endophytic Bacteria and Their Role in Inhibition of Rice Pathogenic Bacteria and Plant Growth Promotion. RSC Adv. 2019, 9, 29293–29299. [Google Scholar] [CrossRef]
- Vendan, R.T.; Yu, Y.J.; Lee, S.H.; Rhee, Y.H. Diversity of Endophytic Bacteria in Ginseng and Their Potential for Plant Growth Promotion. J. Microbiol. 2010, 48, 559–565. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, Y.; Lai, X.-H.; Shan, C.; Deng, Z.; Ji, Y. Screening and Characterization of Endophytic Bacillus and Paenibacillus Strains from Medicinal Plant Lonicera japonica for Use as Potential Plant Growth Promoters. Braz. J. Microbiol. 2015, 46, 977–989. [Google Scholar] [CrossRef]
- Devi, K.A.; Pandey, P.; Sharma, G.D. Plant Growth-Promoting Endophyte Serratia marcescens AL2-16 Enhances the Growth of Achyranthes aspera L., a Medicinal Plant. HAYATI J. Biosci. 2016, 23, 173–180. [Google Scholar] [CrossRef]
- Pereira, S.I.A.; Monteiro, C.; Vega, A.L.; Castro, P.M.L. Endophytic Culturable Bacteria Colonizing Lavandula dentata L. Plants: Isolation, Characterization and Evaluation of Their Plant Growth-Promoting Activities. Ecol. Eng. 2016, 87, 91–97. [Google Scholar] [CrossRef]
- Yin, D.D.; Wang, Y.L.; Yang, M.; Yin, D.K.; Wang, G.K.; Xu, F. Analysis of Chuanxiong Rhizoma Substrate on Production of Ligustrazine in Endophytic Bacillus subtilis by Ultra High Performance Liquid Chromatography with Quadrupole Time-of-Flight Mass Spectrometry. J. Sep. Sci. 2019, 42, 3067–3076. [Google Scholar] [CrossRef]
- Fu, Y. Biotransformation of Ginsenoside Rb1 to Gyp-XVII and Minor Ginsenoside Rg3 by Endophytic Bacterium Flavobacterium sp. GE 32 Isolated from Panax ginseng. Lett. Appl. Microbiol. 2019, 68, 134–141. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Q.; Zang, P.; Li, X.; Ji, Q.; He, Z.; Zhao, Y.; Yang, H.; Zhao, X.; Zhang, L. An Endophytic Bacterium Isolated from Panax ginseng C.A. Meyer Enhances Growth, Reduces Morbidity, and Stimulates Ginsenoside Biosynthesis. Phytochem. Lett. 2015, 11, 132–138. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Li, X.; Zhao, D.; Deng-Wang, M.-Y.; Dai, C.-C. Reactive Oxygen Species and Hormone Signaling Cascades in Endophytic Bacterium Induced Essential Oil Accumulation in Atractylodes lancea. Planta 2016, 244, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-R.; Yuan, J.; Liu, L.-H.; Zhang, W.; Chen, F.; Dai, C.-C. Endophytic Pseudomonas fluorescens Induced Sesquiterpenoid Accumulation Mediated by Gibberellic Acid and Jasmonic Acid in Atractylodes macrocephala Koidz Plantlets. Plant Cell Tissue Organ Cult. PCTOC 2019, 138, 445–457. [Google Scholar] [CrossRef]
- Li, J.; Zhao, G.Z.; Varma, A.; Qin, S.; Xiong, Z.; Huang, H.Y.; Zhu, W.Y.; Zhao, L.X.; Xu, L.H.; Zhang, S.; et al. An Endophytic Pseudonocardia Species Induces the Production of Artemisinin in Artemisia annua. PLoS ONE 2012, 7, e51410. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Behrendt, U. Exploiting the Biocontrol Potential of Plant-Associated Pseudomonads—A Step Towards Pesticide-Free Agriculture? Biol. Control 2021, 155, 104538. [Google Scholar] [CrossRef]
- Djaya, L.; Hersanti; Istifadah, N.; Hartati, S.; Joni, I.M. In Vitro Study of Plant Growth Promoting Rhizobacteria (PGPR) and Endophytic Bacteria Antagonistic to Ralstonia Solanacearum Formulated with Graphite and Silica Nano Particles as a Biocontrol Delivery System (BDS). Biocatal. Agric. Biotechnol. 2019, 19, 101153. [Google Scholar] [CrossRef]
- Zheng, T.; Liu, L.; Nie, Q.; Hsiang, T.; Sun, Z.; Zhou, Y. Isolation, Identification and Biocontrol Mechanisms of Endophytic Bacterium D61-A from Fraxinus hupehensis against Rhizoctonia solani. Biol. Control 2021, 158, 104621. [Google Scholar] [CrossRef]
- Arora, N.K.; Mishra, J. Next Generation Microbe-Based Bioinoculants for Sustainable Agriculture and Food Security. Environ. Sustain. 2024, 7, 1–4. [Google Scholar] [CrossRef]
- Laskar, I.H.; Vandana, U.K.; Das, N.; Pandey, P.; Mazumder, P.B. Role of Microbial Bio-Inoculants in Sustainable Agriculture. In Microbial Biotechnology for Sustainable Agriculture Volume 2; Arora, N.K., Bouizgarne, B., Eds.; Springer Nature: Singapore, 2024; pp. 1–28. ISBN 978-981-97-2355-3. [Google Scholar]
- Rabiey, M.; Hailey, L.E.; Roy, S.R.; Grenz, K.; Al-Zadjali, M.A.S.; Barrett, G.A.; Jackson, R.W. Endophytes vs. Tree Pathogens and Pests: Can They Be Used as Biological Control Agents to Improve Tree Health? Eur. J. Plant Pathol. 2019, 155, 711–729. [Google Scholar] [CrossRef]
- Mamarasulov, B.; Davranov, K.; Umruzaqov, A.; Ercisli, S.; Ali Alharbi, S.; Ansari, M.J.; Krivosudská, E.; Datta, R.; Jabborova, D. Evaluation of the Antimicrobial and Antifungal Activity of Endophytic Bacterial Crude Extracts from Medicinal Plant Ajuga turkestanica (Rgl.) Brig (Lamiaceae). J. King Saud Univ. Sci. 2023, 35, 102644. [Google Scholar] [CrossRef]
- Spantidos, T.-N.; Douka, D.; Katinakis, P.; Venieraki, A. Genomic Insights into Plant Growth Promotion and Biocontrol of Bacillus velezensis Amfr20, an Olive Tree Endophyte. Horticulturae 2025, 11, 384. [Google Scholar] [CrossRef]
- Zicca, S.; De Bellis, P.; Masiello, M.; Saponari, M.; Saldarelli, P.; Boscia, D.; Sisto, A. Antagonistic Activity of Olive Endophytic Bacteria and of Bacillus spp. Strains against Xylella fastidiosa. Microbiol. Res. 2020, 236, 126467. [Google Scholar] [CrossRef]
- Marian, M.; Ohno, T.; Suzuki, H.; Kitamura, H.; Kuroda, K.; Shimizu, M. A Novel Strain of Endophytic Streptomyces for the Biocontrol of Strawberry Anthracnose Caused by Glomerella cingulata. Microbiol. Res. 2020, 234, 126428. [Google Scholar] [CrossRef]
- Dowarah, B.; Agarwal, H.; Krishnatreya, D.B.; Sharma, P.L.; Kalita, N.; Agarwala, N. Evaluation of Seed Associated Endophytic Bacteria from Tolerant Chilli cv. Firingi jolokia for Their Biocontrol Potential against Bacterial Wilt Disease. Microbiol. Res. 2021, 248, 126751. [Google Scholar] [CrossRef]
- Figueiredo, J.E.F.; Diniz, G.d.F.D.; Marins, M.S.; Silva, F.C.; Ribeiro, V.P.; Lanza, F.E.; Oliveira-Paiva, C.A.d.; Cruz-Magalhães, V. Bacillus velezensis CNPMS-22 as Biocontrol Agent of Pathogenic Fungi and Plant Growth Promoter. Front. Microbiol. 2025, 16, 1522136. [Google Scholar] [CrossRef]
- Cui, L.; Yang, C.; Wei, L.; Li, T.; Chen, X. Isolation and Identification of an Endophytic Bacteria Bacillus velezensis 8-4 Exhibiting Biocontrol Activity Against Potato Scab. Biol. Control 2020, 141, 104156. [Google Scholar] [CrossRef]
- Verma, M.L.; Kumar, A.; Chintagunta, A.D.; Samudrala, P.J.K.; Bardin, M.; Lichtfouse, E. Microbial Production of Biopesticides for Sustainable Agriculture. Sustainability 2024, 16, 7496. [Google Scholar] [CrossRef]
- Mei, C.; Amaradasa, B.S.; Chretien, R.L.; Liu, D.; Snead, G.; Samtani, J.B.; Lowman, S. A Potential Application of Endophytic Bacteria in Strawberry Production. Horticulturae 2021, 7, 504. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Karishma, S.; Vo, D.-V.N.; Jeevanantham, S.; Yaashikaa, P.R.; George, C.S. A Review on Biosynthesis of Metal Nanoparticles and Its Environmental Applications. Chemosphere 2021, 264, 128580. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Ahmad, W.; Kalra, D. Green Synthesis, Characterization and Anti Microbial Activities of ZnO Nanoparticles Using Euphorbia Hirta Leaf Extract. J. King Saud Univ. Sci. 2020, 32, 2358–2364. [Google Scholar] [CrossRef]
- Rahman, S.; Rahman, L.; Khalil, A.T.; Ali, N.; Zia, D.; Ali, M.; Shinwari, Z.K. Endophyte-Mediated Synthesis of Silver Nanoparticles and Their Biological Applications. Appl. Microbiol. Biotechnol. 2019, 103, 2551–2569. [Google Scholar] [CrossRef]
- Nero, B.F. Phytoremediation of Petroleum Hydrocarbon-Contaminated Soils with Two Plant Species: Jatropha curcas and Vetiveria zizanioides at Ghana Manganese Company Ltd. Int. J. Phytoremediation 2021, 23, 171–180. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Abdo, A.M.; El-Gamal, M.S. Antimicrobial, Antioxidant and Larvicidal Activities of Spherical Silver Nanoparticles Synthesized by Endophytic streptomyces spp. Biol. Trace Elem. Res. 2020, 195, 707–724. [Google Scholar] [CrossRef]
- Baker, S.; Satish, S. Biosynthesis of Gold Nanoparticles by Pseudomonas Veronii AS41G Inhabiting Annona squamosa L. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2015, 150, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Iqbal, A.; Ashraf, M.A. Bacterial-Mediated Synthesis of Silver Nanoparticles and Their Significant Effect Against Pathogens. Environ. Sci. Pollut. Res. 2020, 27, 37347–37356. [Google Scholar] [CrossRef]
- Hassan, S.E.-D.; Salem, S.S.; Fouda, A.; Awad, M.A.; El-Gamal, M.S.; Abdo, A.M. New Approach for Antimicrobial Activity and Bio-Control of Various Pathogens by Biosynthesized Copper Nanoparticles Using Endophytic Actinomycetes. J. Radiat. Res. Appl. Sci. 2018, 11, 262–270. [Google Scholar] [CrossRef]
- Hassan, S.E.-D.; Fouda, A.; Radwan, A.A.; Salem, S.S.; Barghoth, M.G.; Awad, M.A.; Abdo, A.M.; El-Gamal, M.S. Endophytic Actinomycetes streptomyces spp. Mediated Biosynthesis of Copper Oxide Nanoparticles as a Promising Tool for Biotechnological Applications. JBIC J. Biol. Inorg. Chem. 2019, 24, 377–393. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Mortimer, P.E.; Xu, J.; Ebenso, E.E.; Babalola, O.O. Biosynthesis of Nanoparticles Using Endophytes: A Novel Approach for Enhancing Plant Growth and Sustainable Agriculture. Sustainability 2022, 14, 10839. [Google Scholar] [CrossRef]
- Bogas, A.C.; Henrique Rodrigues, S.; Gonçalves, M.O.; De Assis, M.; Longo, E.; Paiva De Sousa, C. Endophytic Microorganisms From the Tropics as Biofactories for the Synthesis of Metal-Based Nanoparticles: Healthcare Applications. Front. Nanotechnol. 2022, 4, 823236. [Google Scholar] [CrossRef]
- Tripathy, P.; Sethi, S.; Panchal, D.; Prakash, O.; Sharma, A.; Mondal, R.B.; Pal, S. Chapter 13—Biogenic Synthesis of Nanoparticles by Amalgamating Microbial Endophytes: Potential Environmental Applications and Future Perspectives. In Microbial Endophytes and Plant Growth; Solanki, M.K., Yadav, M.K., Singh, B.P., Gupta, V.K., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 215–231. ISBN 978-0-323-90620-3. [Google Scholar]
- Long, H.H.; Schmidt, D.D.; Baldwin, I.T. Native Bacterial Endophytes Promote Host Growth in a Species-Specific Manner; Phytohormone Manipulations Do Not Result in Common Growth Responses. PLoS ONE 2008, 3, e2702. [Google Scholar] [CrossRef]
- Goswami, S.K.; Kashyap, A.S.; Kumar, R.; Gujjar, R.S.; Singh, A.; Manzar, N. Harnessing Rhizospheric Microbes for Eco-Friendly and Sustainable Crop Production in Saline Environments. Curr. Microbiol. 2023, 81, 14. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, R.; Bhutani, N.; Bhardwaj, A.; Suneja, P. Functional Diversity of Cultivable Endophytes from Cicer Arietinum and Pisum Sativum: Bioprospecting Their Plant Growth Potential. Biocatal. Agric. Biotechnol. 2019, 20, 101229. [Google Scholar] [CrossRef]
- Aleem, A.; Sidik, N.J.; Wan, W.R.; Razak, A.; Yunus, N.M. Secretion of Root Exudates in Response to Biotic and Abiotic Environment. Sarhad J. Agric. 2024, 40, 760–773. [Google Scholar] [CrossRef]
- Duhan, P.; Bansal, P.; Rani, S. Isolation, Identification and Characterization of Endophytic Bacteria from Medicinal Plant Tinospora Cordifolia. S. Afr. J. Bot. 2020, 134, 43–49. [Google Scholar] [CrossRef]
- Khalaf, E.M.; Raizada, M.N. Taxonomic and Functional Diversity of Cultured Seed Associated Microbes of the Cucurbit Family. BMC Microbiol. 2016, 16, 131. [Google Scholar] [CrossRef]
- Saad, D.M.; El- Demerdash, M.E.; Abdellatif, Y.M.; Hassan, E.A.E.-T. Isolation and Identification of Bacteria Producing Indole from Rhizospheric Plant. Arab Univ. J. Agric. Sci. 2024, 32, 199–203. [Google Scholar] [CrossRef]
- Frank, A.; Saldierna Guzmán, J.; Shay, J. Transmission of Bacterial Endophytes. Microorganisms 2017, 5, 70. [Google Scholar] [CrossRef]
- Vyas, P.; Singh, B. Plant Growth-Promoting Endophytes from Stevia rebaudiana Identified to Possess Bio-Control Potential against Maize Sheath Blight Pathogen Rhizoctonia solani. Eur. J. Plant Pathol. 2024, 168, 571–591. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.d.C.; Flores, A.; Rojas-Sánchez, B.; Urtis-Flores, C.A.; Morales-Cedeño, L.R.; Valencia-Marin, M.F.; Chávez-Avila, S.; Rojas-Solis, D.; Santoyo, G. Plant Growth-Promoting Bacteria as Bioinoculants: Attributes and Challenges for Sustainable Crop Improvement. Agronomy 2021, 11, 1167. [Google Scholar] [CrossRef]
- Kaur, G.; Patel, A.; Dwibedi, V.; Rath, S.K. Harnessing the Action Mechanisms of Microbial Endophytes for Enhancing Plant Performance and Stress Tolerance: Current Understanding and Future Perspectives. Arch. Microbiol. 2023, 205, 303. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Nisar, M.; Ali, H.; Hazrat, A.; Hayat, K.; Keerio, A.A.; Ihsan, M.; Laiq, M.; Ullah, S.; Fahad, S.; et al. Drought Tolerance Improvement in Plants: An Endophytic Bacterial Approach. Appl. Microbiol. Biotechnol. 2019, 103, 7385–7397. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant Growth-Promoting Bacterial Endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.-S.; Patra, J.K. Endophytes: A Treasure House of Bioactive Compounds of Medicinal Importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kumar, A.; Singh, R.; Pandey, K.D. Endophytic Bacteria: A New Source of Bioactive Compounds. 3 Biotech 2017, 7, 315. [Google Scholar] [CrossRef]
- Panigrahi, S.; Rath, C.C. In Vitro Characterization of Antimicrobial Activity of an Endophytic Bacterium Enterobacter cloacae (MG001451) Isolated from Ocimum sanctum. S. Afr. J. Bot. 2021, 143, 90–96. [Google Scholar] [CrossRef]
- Raimi, A.; Adeleke, R. Bioprospecting of Endophytic Microorganisms for Bioactive Compounds of Therapeutic Importance. Arch. Microbiol. 2021, 203, 1917–1942. [Google Scholar] [CrossRef]
- Tlou, M.; Ndou, B.; Mabona, N.; Khwathisi, A.; Ateba, C.; Madala, N.; Serepa-Dlamini, M.H. Next Generation Sequencing-Aided Screening, Isolation, Molecular Identification, and Antimicrobial Potential for Bacterial Endophytes from the Medicinal Plant, Elephantorrhiza elephantina. Front. Microbiol. 2024, 15, 1383854. [Google Scholar] [CrossRef] [PubMed]
- Chandwani, S.; Amaresan, N. Role of ACC Deaminase Producing Bacteria for Abiotic Stress Management and Sustainable Agriculture Production. Environ. Sci. Pollut. Res. 2022, 29, 22843–22859. [Google Scholar] [CrossRef]
- Gamalero, E.; Lingua, G.; Glick, B.R. Ethylene, ACC, and the Plant Growth-Promoting Enzyme ACC Deaminase. Biology 2023, 12, 1043. [Google Scholar] [CrossRef]
- Morales-Cedeño, L.R.; Orozco-Mosqueda, M.d.C.; Loeza-Lara, P.D.; Parra-Cota, F.I.; de los Santos-Villalobos, S.; Santoyo, G. Plant Growth-Promoting Bacterial Endophytes as Biocontrol Agents of Pre- and Post-Harvest Diseases: Fundamentals, Methods of Application and Future Perspectives. Microbiol. Res. 2021, 242, 126612. [Google Scholar] [CrossRef]
- Wahab, A.; Batool, F.; Abdi, G.; Muhammad, M.; Ullah, S.; Zaman, W. Role of Plant Growth-Promoting Rhizobacteria in Sustainable Agriculture: Addressing Environmental and Biological Challenges. J. Plant Physiol. 2025, 307, 154455. [Google Scholar] [CrossRef]
- Ratnaningsih, H.R.; Noviana, Z.; Dewi, T.K.; Loekito, S.; Wiyono, S.; Gafur, A.; Antonius, S. IAA and ACC Deaminase Producing-Bacteria Isolated from the Rhizosphere of Pineapple Plants Grown under Different Abiotic and Biotic Stresses. Heliyon 2023, 9, e16306. [Google Scholar] [CrossRef] [PubMed]
- Saikia, J.; Sarma, R.K.; Dhandia, R.; Yadav, A.; Bharali, R.; Gupta, V.K.; Saikia, R. Alleviation of Drought Stress in Pulse Crops with ACC Deaminase Producing Rhizobacteria Isolated from Acidic Soil of Northeast India. Sci. Rep. 2018, 8, 3560. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.T.; McComb, J.A.; van der Moezel, P.G.; Bennett, I.J.; Kabay, E.D. Comparisons of Selected and Cloned Plantlets against Seedlings for Rehabilitation of Saline and Waterlogged Discharge Zones in Australian Agricultural Catchments. Aust. For. 1994, 57, 69–75. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. A Halotolerant Bacterium Bacillus licheniformis HSW-16 Augments Induced Systemic Tolerance to Salt Stress in Wheat Plant (Triticum aestivum). Front. Plant Sci. 2016, 7, 1890. [Google Scholar] [CrossRef]
- Durairaj, K.; Velmurugan, P.; Park, J.-H.; Chang, W.-S.; Park, Y.-J.; Senthilkumar, P.; Choi, K.-M.; Lee, J.-H.; Oh, B.-T. Potential for Plant Biocontrol Activity of Isolated Pseudomonas aeruginosa and Bacillus stratosphericus Strains against Bacterial Pathogens Acting Through Both Induced Plant Resistance and Direct Antagonism. FEMS Microbiol. Lett. 2017, 364, fnx225. [Google Scholar] [CrossRef] [PubMed]
- Stępniewska, Z.; Kuźniar, A. Endophytic Microorganisms—Promising Applications in Bioremediation of Greenhouse Gases. Appl. Microbiol. Biotechnol. 2013, 97, 9589–9596. [Google Scholar] [CrossRef]
- Liu, L.; Quan, S.; Li, L.; Lei, G.; Li, S.; Gong, T.; Zhang, Z.; Hu, Y.; Yang, W. Endophytic Bacteria Improve Bio- and Phytoremediation of Heavy Metals. Microorganisms 2024, 12, 2137. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Glick, B.R. The Role of Bacteria in Phytoremediation. In Applied Bioengineering; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 327–353. ISBN 978-3-527-80059-9. [Google Scholar]
- He, W.; Mallavarapu, M.; Chun-Ya, W.; Suresh, R.S.; Dai, C.-C. Endophyte-Assisted Phytoremediation: Mechanisms and Current Application Strategies for Soil Mixed Pollutants. Crit. Rev. Biotechnol. 2020, 40, 31–45. [Google Scholar] [CrossRef]
- Datta, S.; Singh, S.; Kumar, V.; Dhanjal, D.S.; Sidhu, G.K.; Amin, D.S.; Kumar, S.; Singh, J.; Singh, J. Endophytic Bacteria in Xenobiotic Degradation. In Microbial Endophytes; Kumar, A., Singh, V.K., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 125–156. ISBN 978-0-12-818734-0. [Google Scholar]
- Ren, Z.; Cheng, R.; Chen, P.; Xue, Y.; Xu, H.; Yin, Y.; Huang, G.; Zhang, W.; Zhang, L. Plant-Associated Microbe System in Treatment of Heavy Metals–Contaminated Soil: Mechanisms and Applications. Water. Air. Soil Pollut. 2023, 234, 39. [Google Scholar] [CrossRef]
- Afzal, M.; Khan, Q.M.; Sessitsch, A. Endophytic Bacteria: Prospects and Applications for the Phytoremediation of Organic Pollutants. Chemosphere 2014, 117, 232–242. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Alimov, J.; Shurigin, V.; Alaylar, B.; Wirth, S.; Bellingrath-Kimura, S.D. Diversity and Plant Growth-Promoting Ability of Endophytic, Halotolerant Bacteria Associated with Tetragonia tetragonioides (Pall.) Kuntze. Plants 2022, 11, 49. [Google Scholar] [CrossRef]
- Zhang, Y.; He, L.; Chen, Z.; Zhang, W.; Wang, Q.; Qian, M.; Sheng, X. Characterization of Lead-Resistant and ACC Deaminase-Producing Endophytic Bacteria and Their Potential in Promoting Lead Accumulation of Rape. J. Hazard. Mater. 2011, 186, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, H.; Xiong, Y.; Zhang, L.; Cui, J.; Li, G.; Du, C.; Wen, K. Remediation Mechanism of High Concentrations of Multiple Heavy Metals in Contaminated Soil by Sedum alfredii and Native Microorganisms. J. Environ. Sci. 2025, 147, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Luo, S.; Li, X.; Wan, Y.; Chen, J.; Liu, C. Interaction of Cd-Hyperaccumulator Solanum nigrum L. and Functional Endophyte pseudomonas sp. Lk9 on Soil Heavy Metals Uptake. Soil Biol. Biochem. 2014, 68, 300–308. [Google Scholar] [CrossRef]
- Mamphogoro, T.P.; Babalola, O.O.; Aiyegoro, O.A. Sustainable Management Strategies for Bacterial Wilt of Sweet Peppers (Capsicum annuum) and Other Solanaceous Crops. J. Appl. Microbiol. 2020, 129, 496–508. [Google Scholar] [CrossRef]
- Azmi, N.S.A. Phytomicrobiome Engineering for Crop Growth and Resilience. In Plant Microbiome Engineering; Dhanasekaranand, D., Narayanan, A.S., Eds.; Springer: New York, NY, USA, 2025; pp. 485–492. ISBN 978-1-0716-4180-4. [Google Scholar]
- Compant, S.; Mitter, B.; Colli-Mull, J.G.; Gangl, H.; Sessitsch, A. Endophytes of Grapevine Flowers, Berries, and Seeds: Identification of Cultivable Bacteria, Comparison with Other Plant Parts, and Visualization of Niches of Colonization. Microb. Ecol. 2011, 62, 188–197. [Google Scholar] [CrossRef]
- Papik, J.; Folkmanova, M.; Polivkova-Majorova, M.; Suman, J.; Uhlik, O. The Invisible Life inside Plants: Deciphering the Riddles of Endophytic Bacterial Diversity. Biotechnol. Adv. 2020, 44, 107614. [Google Scholar] [CrossRef]
- Mahlangu, S.G.; Serepa-Dlamini, M.H. First Report of Bacterial Endophytes from the Leaves of Pellaea calomelanos in South Africa. S. Afr. J. Sci. 2018, 114, 1–9. [Google Scholar] [CrossRef]
- Ogofure, A.G.; Green, E. Bioactivity and Metabolic Profiling of Crude Extracts from Endophytic Bacteria Linked to Solanum mauritianum Scope: Discovery of Antibacterial and Anticancer Properties. Heliyon 2025, 11, e40525. [Google Scholar] [CrossRef]
- Jacob, J.; Krishnan, G.V.; Thankappan, D.; Amma, D.K.B.N.S. Endophytic Bacterial Strains Induced Systemic Resistance in Agriculturally Important Crop Plants. In Microbial Endophytes; Kumar, A., Radhakrishnan, E.K., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 75–105. ISBN 978-0-12-819654-0. [Google Scholar]
- Sonowal, S.; Ahmed, R.; Chikkaputtaiah, C.; Basar, E.; Velmurugan, N. A Comprehensive Characterization of Culturable Endophytic Bacteria of Paris polyphylla and Their Potential Role in Microalgal Growth in Co-Culture. Appl. Soil Ecol. 2022, 174, 104410. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Biocontrol of Plant Diseases by Bacillus spp. Physiol. Mol. Plant Pathol. 2023, 126, 102048. [Google Scholar] [CrossRef]
- Hallmann, J.; Quadt-Hallmann, A.; Mahaffee, W.F.; Kloepper, J.W. Bacterial Endophytes in Agricultural Crops. Can. J. Microbiol. 1997, 43, 895–914. [Google Scholar] [CrossRef]
- Eevers, N.; Gielen, M.; Sánchez-López, A.; Jaspers, S.; White, J.C.; Vangronsveld, J.; Weyens, N. Optimization of Isolation and Cultivation of Bacterial Endophytes Through Addition of Plant Extract to Nutrient Media. Microb. Biotechnol. 2015, 8, 707–715. [Google Scholar] [CrossRef]
- Makuwa, S.C.; Serepa-Dlamini, M.H. The Antibacterial Activity of Crude Extracts of Secondary Metabolites from Bacterial Endophytes Associated with Dicoma anomala. Int. J. Microbiol. 2021, 2021, 8812043. [Google Scholar] [CrossRef]
- Anwar, N.; Jiang, Y.; Ma, W.; Yao, Y.; Li, J.; Ababaikeli, G.; Li, G.; Ma, T. Culturable Bacteria Diversity in Stem Liquid and Resina from Populus euphratica and Screening of Plant Growth-Promoting Bacteria. BMC Microbiol. 2022, 22, 322. [Google Scholar] [CrossRef]
- Singh, M.; Naskar, A.; Rupashree, A.; Rajput, M.; Singh, V.K. Analysis of Endophytic Microbes Harboring in Medicinal Plants of Himalayan Region with Their Medicinal Properties. Biocatal. Agric. Biotechnol. 2023, 53, 102857. [Google Scholar] [CrossRef]
- Adeleke, R.A.; Mashiane, A.R.; Bezuidenhout, C.C.; Chirima, G. Community Composition and Functions of Endophytic Bacteria of Bt Maize. S. Afr. J. Sci. 2018, 114, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, M.G.; Burragoni, S.; Amrutha, S.; Muthupandi, M.; Parveen, A.B.M.; Sivakumar, V.; Ulaganathan, K. Diversity of Bacterial Endophyte in Eucalyptus Clones and Their Implications in Water Stress Tolerance. Microbiol. Res. 2020, 241, 126579. [Google Scholar] [CrossRef] [PubMed]
- Abbamondi, G.R.; Tommonaro, G.; Weyens, N.; Thijs, S.; Sillen, W.; Gkorezis, P.; Iodice, C.; de Melo Rangel, W.; Nicolaus, B.; Vangronsveld, J. Plant Growth-Promoting Effects of Rhizospheric and Endophytic Bacteria Associated with Different Tomato Cultivars and New Tomato Hybrids. Chem. Biol. Technol. Agric. 2016, 3, 1. [Google Scholar] [CrossRef]
- Abiraami, T.V.; Singh, S.; Nain, L. Soil Metaproteomics as a Tool for Monitoring Functional Microbial Communities: Promises and Challenges. Rev. Environ. Sci. Biotechnol. 2020, 19, 73–102. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O. Meta-Omics of Endophytic Microbes in Agricultural Biotechnology. Biocatal. Agric. Biotechnol. 2022, 42, 102332. [Google Scholar] [CrossRef]
- Taheri, E.; Tarighi, S.; Taheri, P. An Endophytic Bacterium with Biocontrol Activity Against Important Wheat Pathogens. Biol. Control 2023, 183, 105243. [Google Scholar] [CrossRef]
- Pei, C.; Mi, C.; Sun, L.; Liu, W.; Li, O.; Hu, X. Diversity of Endophytic Bacteria of Dendrobium officinale Based on Culture-Dependent and Culture-Independent Methods. Biotechnol. Biotechnol. Equip. 2017, 31, 112–119. [Google Scholar] [CrossRef]
- Zhao, C.; Onyino, J.; Gao, X. Current Advances in the Functional Diversity and Mechanisms Underlying Endophyte–Plant Interactions. Microorganisms 2024, 12, 779. [Google Scholar] [CrossRef] [PubMed]
- War Nongkhlaw, F.; Joshi, S. Microscopic Study on Colonization and Antimicrobial Property of Endophytic Bacteria Associated with Ethnomedicinal Plants of Meghalaya. J. Microsc. Ultrastruct. 2017, 5, 132. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.R.A.; Yin, Q.; Oliveira, R.S.; Silva, E.F.; Novo, L.A.B. Plant Growth-Promoting Bacteria in Phytoremediation of Metal-Polluted Soils: Current Knowledge and Future Directions. Sci. Total Environ. 2022, 838, 156435. [Google Scholar] [CrossRef]
- Khanna, A.; Raj, K.; Kumar, P.; Wati, L. Antagonistic and Growth-Promoting Potential of Multifarious Bacterial Endophytes against Fusarium Wilt of Chickpea. Egypt. J. Biol. Pest Control 2022, 32, 17. [Google Scholar] [CrossRef]
- Kandel, S.L.; Firrincieli, A.; Joubert, P.M.; Okubara, P.A.; Leston, N.D.; McGeorge, K.M.; Mugnozza, G.S.; Harfouche, A.; Kim, S.-H.; Doty, S.L. An In Vitro Study of Bio-Control and Plant Growth Promotion Potential of Salicaceae Endophytes. Front. Microbiol. 2017, 8, 386. [Google Scholar] [CrossRef]
- Valdez-Nuñez, R.A.; Ríos-Ruiz, W.F.; Ormeño-Orrillo, E.; Torres-Chávez, E.E.; Torres-Delgado, J. Genetic Characterization of Rice Endophytic Bacteria (Oryza sativa L.) with Antimicrobial Activity Against Burkholderia glumae. Rev. Argent. Microbiol. 2020, 52, 315–327. [Google Scholar] [CrossRef]
- Kandasamy, G.D.; Kathirvel, P. Insights into Bacterial Endophytic Diversity and Isolation with a Focus on Their Potential Applications—A Review. Microbiol. Res. 2023, 266, 127256. [Google Scholar] [CrossRef]
- Ali, M.; Ali, Q.; Sohail, M.A.; Ashraf, M.F.; Saleem, M.H.; Hussain, S.; Zhou, L. Diversity and Taxonomic Distribution of Endophytic Bacterial Community in the Rice Plant and Its Prospective. Int. J. Mol. Sci. 2021, 22, 10165. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, C.; Oliveira, R.S.; Freitas, H.; Luo, Y. Bioaugmentation with Endophytic Bacterium E6S Homologous to Achromobacter piechaudii Enhances Metal Rhizoaccumulation in Host Sedum plumbizincicola. Front. Plant Sci. 2016, 7, 75. [Google Scholar] [CrossRef]
- de Moura, G.G.D.; de Barros, A.V.; Machado, F.; Martins, A.D.; da Silva, C.M.; Durango, L.G.C.; Forim, M.; Alves, E.; Pasqual, M.; Doria, J. Endophytic Bacteria from Strawberry Plants Control Gray Mold in Fruits via Production of Antifungal Compounds against Botrytis cinerea L. Microbiol. Res. 2021, 251, 126793. [Google Scholar] [CrossRef]
- Beukes, C.W.; Palmer, M.; Manyaka, P.; Chan, W.Y.; Avontuur, J.R.; van Zyl, E.; Huntemann, M.; Clum, A.; Pillay, M.; Palaniappan, K.; et al. Genome Data Provides High Support for Generic Boundaries in Burkholderia Sensu Lato. Front. Microbiol. 2017, 8, 1154. [Google Scholar] [CrossRef]
- Marokane-Radebe, C.; Raimi, A.; Amoo, S.; Adeleke, R. Metabolomic Profiling and 16 S rRNA Metabarcoding of Endophytes of Two Aloe Species Revealed Diverse Metabolites. AMB Express 2024, 14, 122. [Google Scholar] [CrossRef]
- Li, F.; Neves, A.L.A.; Ghoshal, B.; Guan, L.L. Symposium Review: Mining Metagenomic and Metatranscriptomic Data for Clues about Microbial Metabolic Functions in Ruminants1. J. Dairy Sci. 2018, 101, 5605–5618. [Google Scholar] [CrossRef] [PubMed]
- Wijayawardene, N.N.; Bahram, M.; Sánchez-Castro, I.; Dai, D.-Q.; Ariyawansa, K.G.S.U.; Jayalal, U.; Suwannarach, N.; Tedersoo, L. Current Insight into Culture-Dependent and Culture-Independent Methods in Discovering Ascomycetous Taxa. J. Fungi 2021, 7, 703. [Google Scholar] [CrossRef]
- Ambikapathy, V.; Babu, S.; Anbukumaran, A.; Shijila Rani, A.S. Screening of Endophytes for Biosurfactant Production. In Endophytic Microbes: Isolation, Identification, and Bioactive Potentials; Springer: New York, NY, USA, 2023; pp. 209–212. [Google Scholar]
- Johnston-Monje, D.; Lundberg, D.S.; Lazarovits, G.; Reis, V.M.; Raizada, M.N. Bacterial Populations in Juvenile Maize Rhizospheres Originate from Both Seed and Soil. Plant Soil 2016, 405, 337–355. [Google Scholar] [CrossRef]
- Kumar, P.; Rani, S.; Sarita, S.; Dang, A.; Suneja, P. Detection of Endophytes by Electron Microscope. In Endophytic Microbes: Isolation, Identification, and Bioactive Potentials; Sankaranarayanan, A., Amaresan, N., Dwivedi, M.K., Eds.; Springer: New York, NY, USA, 2023; pp. 71–76. ISBN 978-1-0716-2827-0. [Google Scholar]
- de França Bettencourt, G.M.; Degenhardt, J.; dos Santos, G.D.; Vicente, V.A.; Soccol, C.R. Metagenomic Analyses, Isolation and Characterization of Endophytic Bacteria Associated with Eucalyptus urophylla BRS07-01 In Vitro Plants. World J. Microbiol. Biotechnol. 2021, 37, 164. [Google Scholar] [CrossRef]
- Deb, L.; Dutta, P.; Tombisana Devi, R.K.; Thakuria, D.; Majumder, D. Endophytic Beauveria Bassiana Can Protect the Rice Plant from Sheath Blight of Rice Caused by Rhizoctonia solani and Enhance Plant Growth Parameters. Arch. Microbiol. 2022, 204, 587. [Google Scholar] [CrossRef]
- Morare, R.; Ubomba-Jaswa, E.; Serepa-Dlamini, M. Isolation and Identification of Bacterial Endophytes from Crinum macowanii Baker. Afr. J. Biotechnol. 2018, 17, 1040–1047. [Google Scholar] [CrossRef]
- Jana, S.K.; Islam, M.M.; Hore, S.; Mandal, S. Rice Seed Endophytes Transmit into the Plant Seedling, Promote Plant Growth and Inhibit Fungal Phytopathogens. Plant Growth Regul. 2023, 99, 373–388. [Google Scholar] [CrossRef]
- Agtuca, B.J.; Stopka, S.A.; Tuleski, T.R.; do Amaral, F.P.; Evans, S.; Liu, Y.; Xu, D.; Monteiro, R.A.; Koppenaal, D.W.; Paša-Tolić, L.; et al. In-Situ Metabolomic Analysis of Setaria viridis Roots Colonized by Beneficial Endophytic Bacteria. Mol. Plant-Microbe Interact. 2020, 33, 272–283. [Google Scholar] [CrossRef]
- Sipriyadi; Masrukhin; Wibowo, R.H.; Darwis, W.; Yudha, S.; Purnaningsih, I.; Siboro, R. Potential Antimicrobe Producer of Endophytic Bacteria from Yellow Root Plant (Arcangelisia flava (L.)) Originated from Enggano Island. Int. J. Microbiol. 2022, 2022, 6435202. [Google Scholar] [CrossRef] [PubMed]
- Bikel, S.; Valdez-Lara, A.; Cornejo-Granados, F.; Rico, K.; Canizales-Quinteros, S.; Soberón, X.; Del Pozo-Yauner, L.; Ochoa-Leyva, A. Combining Metagenomics, Metatranscriptomics and Viromics to Explore Novel Microbial Interactions: Towards a Systems-Level Understanding of Human Microbiome. Comput. Struct. Biotechnol. J. 2015, 13, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Bhatt, A. Microbial Endophytes: Emerging Trends and Biotechnological Applications. Curr. Microbiol. 2023, 80, 249. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; Ver Loren van Themaat, E.; Ahmadinejad, N.; Assenza, F.; Rauf, P.; Huettel, B.; Reinhardt, R.; Schmelzer, E.; et al. Revealing Structure and Assembly Cues for Arabidopsis Root-Inhabiting Bacterial Microbiota. Nature 2012, 488, 91–95. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–Microbiome Interactions: From Community Assembly to Plant Health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Molefe, R.R.; Amoo, A.E.; Babalola, O.O. Metagenomic Insights into the Bacterial Community Structure and Functional Potentials in the Rhizosphere Soil of Maize Plants. J. Plant Interact. 2021, 16, 258–269. [Google Scholar] [CrossRef]
- Maropola, M.K.A.; Ramond, J.-B.; Trindade, M. Impact of Metagenomic DNA Extraction Procedures on the Identifiable Endophytic Bacterial Diversity in Sorghum bicolor (L. Moench). J. Microbiol. Methods 2015, 112, 104–117. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Ayangbenro, A.S.; Babalola, O.O. Metagenomic Profiling of the Community Structure, Diversity, and Nutrient Pathways of Bacterial Endophytes in Maize Plant. Antonie Van Leeuwenhoek 2020, 113, 1559–1571. [Google Scholar] [CrossRef]
- Liljeqvist, M.; Ossandon, F.J.; González, C.; Rajan, S.; Stell, A.; Valdes, J.; Holmes, D.S.; Dopson, M. Metagenomic Analysis Reveals Adaptations to a Cold-Adapted Lifestyle in a Low-Temperature Acid Mine Drainage Stream. FEMS Microbiol. Ecol. 2015, 91, fiv011. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.E.; Kim, J.U.; Lee, J.W.; Bang, K.H.; Jo, I.H. Metagenomic Analysis of Bacterial Endophyte Community Structure and Functions in Panax ginseng at Different Ages. 3 Biotech 2019, 9, 300. [Google Scholar] [CrossRef]
- Alawiye, T.T.; Babalola, O.O. Metagenomic Insight into the Community Structure and Functional Genes in the Sunflower Rhizosphere Microbiome. Agriculture 2021, 11, 167. [Google Scholar] [CrossRef]
- Funnicelli, M.I.G.; de Carvalho, L.A.L.; Teheran-Sierra, L.G.; Dibelli, S.C.; de Macedo Lemos, E.G.; Pinheiro, D.G. Unveiling Genomic Features Linked to Traits of Plant Growth-Promoting Bacterial Communities from Sugarcane. Sci. Total Environ. 2024, 947, 174577. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Sharma, T.; Dhar, M.K. “Omics” Tools for Better Understanding the Plant–Endophyte Interactions. Front. Plant Sci. 2016, 7, 955. [Google Scholar] [CrossRef]
- Gómez-Godínez, L.J.; Fernandez-Valverde, S.L.; Martinez Romero, J.C.; Martínez-Romero, E. Metatranscriptomics and Nitrogen Fixation from the Rhizoplane of Maize Plantlets Inoculated with a Group of PGPRs. Syst. Appl. Microbiol. 2019, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wei, Y.; Zhao, Z.; Li, J.; Li, H.; Yang, P.; Tian, S.; Ryder, M.; Toh, R.; Yang, H.; et al. Microbial Communities along the Soil-Root Continuum Are Determined by Root Anatomical Boundaries, Soil Properties, and Root Exudation. Soil Biol. Biochem. 2022, 171, 108721. [Google Scholar] [CrossRef]
- Lima, J.C.d.; Loss-Morais, G.; Margis, R. MicroRNAs Play Critical Roles during Plant Development and in Response to Abiotic Stresses. Genet. Mol. Biol. 2012, 35, 1069–1077. [Google Scholar] [CrossRef]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic Stress Responses and Microbe-Mediated Mitigation in Plants: The Omics Strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [CrossRef]
- Yongpisanphop, J.; Babel, S.; Kurisu, F.; Kruatrachue, M.; Pokethitiyook, P. Isolation and Characterization of Pb-Resistant Plant Growth Promoting Endophytic Bacteria and Their Role in Pb Accumulation by Fast-Growing Trees. Environ. Technol. 2020, 41, 3598–3606. [Google Scholar] [CrossRef]
- Ma, Y. Abiotic Stress Responses and Microbe-Mediated Mitigation in Plants. Agronomy 2023, 13, 1844. [Google Scholar] [CrossRef]
- Dubey, A.; Saiyam, D.; Kumar, A.; Hashem, A.; Abd_Allah, E.F.; Khan, M.L. Bacterial Root Endophytes: Characterization of Their Competence and Plant Growth Promotion in Soybean (Glycine max (L.) Merr.) under Drought Stress. Int. J. Environ. Res. Public Health 2021, 18, 931. [Google Scholar] [CrossRef]
- Moreira, L.M.; Soares, M.R.; Facincani, A.P.; Ferreira, C.B.; Ferreira, R.M.; Ferro, M.I.T.; Gozzo, F.C.; Felestrino, É.B.; Assis, R.A.B.; Garcia, C.C.M.; et al. Proteomics-Based Identification of Differentially Abundant Proteins Reveals Adaptation Mechanisms of Xanthomonas citri subsp. Citri during Citrus Sinensis Infection. BMC Microbiol. 2017, 17, 155. [Google Scholar] [CrossRef]
- Kleiner, M. Metaproteomics: Much More than Measuring Gene Expression in Microbial Communities. mSystems 2019, 4, 10–1128. [Google Scholar] [CrossRef]
- Suárez-Acevedo, S.; Chaves-Bedoya, G.; Guariz-Pinheiro, D.; Cristina-Lopes, A.; Mari-Murata, M.; Hirochi-Herai, R.; Aparecido-Ferro, J.; Rodas-Mendoza, E. Comparative Transcriptional Analyzes of Xanthomonas citri subsp. Citri Reveal Mechanisms of Adaptation and Bacterial Virulence in the Early Stage of Citrus Canker Disease. Eur. J. Plant Pathol. 2022, 163, 557–572. [Google Scholar] [CrossRef]
- Peng, J.; Zhou, X.; Rensing, C.; Liesack, W.; Zhu, Y.-G. Soil Microbial Ecology Through the Lens of Metatranscriptomics. Soil Ecol. Lett. 2023, 6, 230217. [Google Scholar] [CrossRef]
- Pan, H.; Wattiez, R.; Gillan, D. Soil Metaproteomics for Microbial Community Profiling: Methodologies and Challenges. Curr. Microbiol. 2024, 81, 257. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Okubo, T.; Kubota, K.; Kasahara, Y.; Tsurumaru, H.; Anda, M.; Ikeda, S.; Minamisawa, K. Metaproteomic Identification of Diazotrophic Methanotrophs and Their Localization in Root Tissues of Field-Grown Rice Plants. Appl. Environ. Microbiol. 2014, 80, 5043–5052. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Pandove, G. Understanding the Beneficial Interaction of Plant Growth Promoting Rhizobacteria and Endophytic Bacteria for Sustainable Agriculture: A Bio-Revolution Approach. J. Plant Nutr. 2023, 46, 3569–3597. [Google Scholar] [CrossRef]
- Pan Chongleand Banfield, J.F. Quantitative Metaproteomics: Functional Insights into Microbial Communities. In Environmental Microbiology: Methods and Protocols; Paulsen, I.T., Holmes, A.J., Eds.; Humana Press: Totowa, NJ, USA, 2014; pp. 231–240. ISBN 978-1-62703-712-9. [Google Scholar]
- Lohmann, P.; Schäpe, S.S.; Haange, S.-B.; Oliphant, K.; Allen-Vercoe, E.; Jehmlich, N.; Bergen, M.V. Function Is What Counts: How Microbial Community Complexity Affects Species, Proteome and Pathway Coverage in Metaproteomics. Expert Rev. Proteom. 2020, 17, 163–173. [Google Scholar] [CrossRef]
- Muth, T.; Renard, B.Y.; Martens, L. Metaproteomic Data Analysis at a Glance: Advances in Computational Microbial Community Proteomics. Expert Rev. Proteom. 2016, 13, 757–769. [Google Scholar] [CrossRef]
- Dobrzyński, J.; Jakubowska, Z.; Kulkova, I.; Kowalczyk, P.; Kramkowski, K. Biocontrol of Fungal Phytopathogens by Bacillus pumilus. Front. Microbiol. 2023, 14, 1194606. [Google Scholar] [CrossRef]
- Lankadurai, B.P.; Nagato, E.G.; Simpson, M.J. Environmental Metabolomics: An Emerging Approach to Study Organism Responses to Environmental Stressors. Environ. Rev. 2013, 21, 180–205. [Google Scholar] [CrossRef]
- Fan, W.; Xiao, Y.; Dong, J.; Xing, J.; Tang, F.; Shi, F. Variety-Driven Rhizosphere Microbiome Bestows Differential Salt Tolerance to Alfalfa for Coping with Salinity Stress. Front. Plant Sci. 2023, 14, 1324333. [Google Scholar] [CrossRef] [PubMed]
- Spina, R.; Saliba, S.; Dupire, F.; Ptak, A.; Hehn, A.; Piutti, S.; Poinsignon, S.; Leclerc, S.; Bouguet-Bonnet, S.; Laurain-Mattar, D. Molecular Identification of Endophytic Bacteria in Leucojum Aestivum In Vitro Culture, NMR-Based Metabolomics Study and LC-MS Analysis Leading to Potential Amaryllidaceae Alkaloid Production. Int. J. Mol. Sci. 2021, 22, 1773. [Google Scholar] [CrossRef] [PubMed]
- Kohl, K.D. Ecological and Evolutionary Mechanisms Underlying Patterns of Phylosymbiosis in Host-Associated Microbial Communities. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190251. [Google Scholar] [CrossRef] [PubMed]
- Brader, G.; Compant, S.; Mitter, B.; Trognitz, F.; Sessitsch, A. Metabolic Potential of Endophytic Bacteria. Curr. Opin. Biotechnol. 2014, 27, 30–37. [Google Scholar] [CrossRef]
- Araldi-Brondolo, S.J.; Spraker, J.; Shaffer, J.P.; Woytenko, E.H.; Baltrus, D.A.; Gallery, R.E.; Arnold, A.E. Bacterial Endosymbionts: Master Modulators of Fungal Phenotypes. Microbiol. Spectr. 2017, 5, 10–1128. [Google Scholar] [CrossRef]
- Sharma, M.; Sudheer, S.; Usmani, Z.; Rani, R.; Gupta, P. Deciphering the Omics of Plant-Microbe Interaction: Perspectives and New Insights. Curr. Genom. 2020, 21, 343–362. [Google Scholar] [CrossRef]
- Chowdhury, T.; Shishir, M.d.A.; Fakruddin, M.d. Endophytic Bacteria Are Potential Source of Medicinal Plant Therapeutics and Bioactive Compound Synthesis. Aust. Herb. Insight 2024, 7, 1–12. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Meyer, F.; Paarmann, D.; D’Souza, M.; Olson, R.; Glass, E.; Kubal, M.; Paczian, T.; Rodriguez, A.; Stevens, R.; Wilke, A.; et al. The Metagenomics RAST Server—A Public Resource for the Automatic Phylogenetic and Functional Analysis of Metagenomes. BMC Bioinform. 2008, 9, 386. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive Functional Profiling of Microbial Communities Using 16S rRNA Marker Gene Sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Sato, Y.; Kawashima, M. KEGG Mapping Tools for Uncovering Hidden Features in Biological Data. Protein Sci. 2022, 31, 47–53. [Google Scholar] [CrossRef]
- Consortium, T.U. UniProt: The Universal Protein Knowledgebase in 2025. Nucleic Acids Res. 2024, 53, D609–D617. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Caspi, R.; Billington, R.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Midford, P.E.; Ong, W.K.; Paley, S.; Subhraveti, P.; Karp, P.D. The MetaCyc Database of Metabolic Pathways and Enzymes—A 2019 Update. Nucleic Acids Res. 2020, 48, D445–D453. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Cole, J.R.; Wang, Q.; Fish, J.A.; Chai, B.; McGarrell, D.M.; Sun, Y.; Brown, C.T.; Porras-Alfaro, A.; Kuske, C.R.; Tiedje, J.M. Ribosomal Database Project: Data and Tools for High Throughput rRNA Analysis. Nucleic Acids Res. 2014, 42, D633–D642. [Google Scholar] [CrossRef]
- Chen, I.-M.A.; Chu, K.; Palaniappan, K.; Ratner, A.; Huang, J.; Huntemann, M.; Hajek, P.; Ritter, S.; Varghese, N.; Seshadri, R.; et al. The IMG/M Data Management and Analysis System v.6.0: New Tools and Advanced Capabilities. Nucleic Acids Res. 2021, 49, D751–D763. [Google Scholar] [CrossRef] [PubMed]
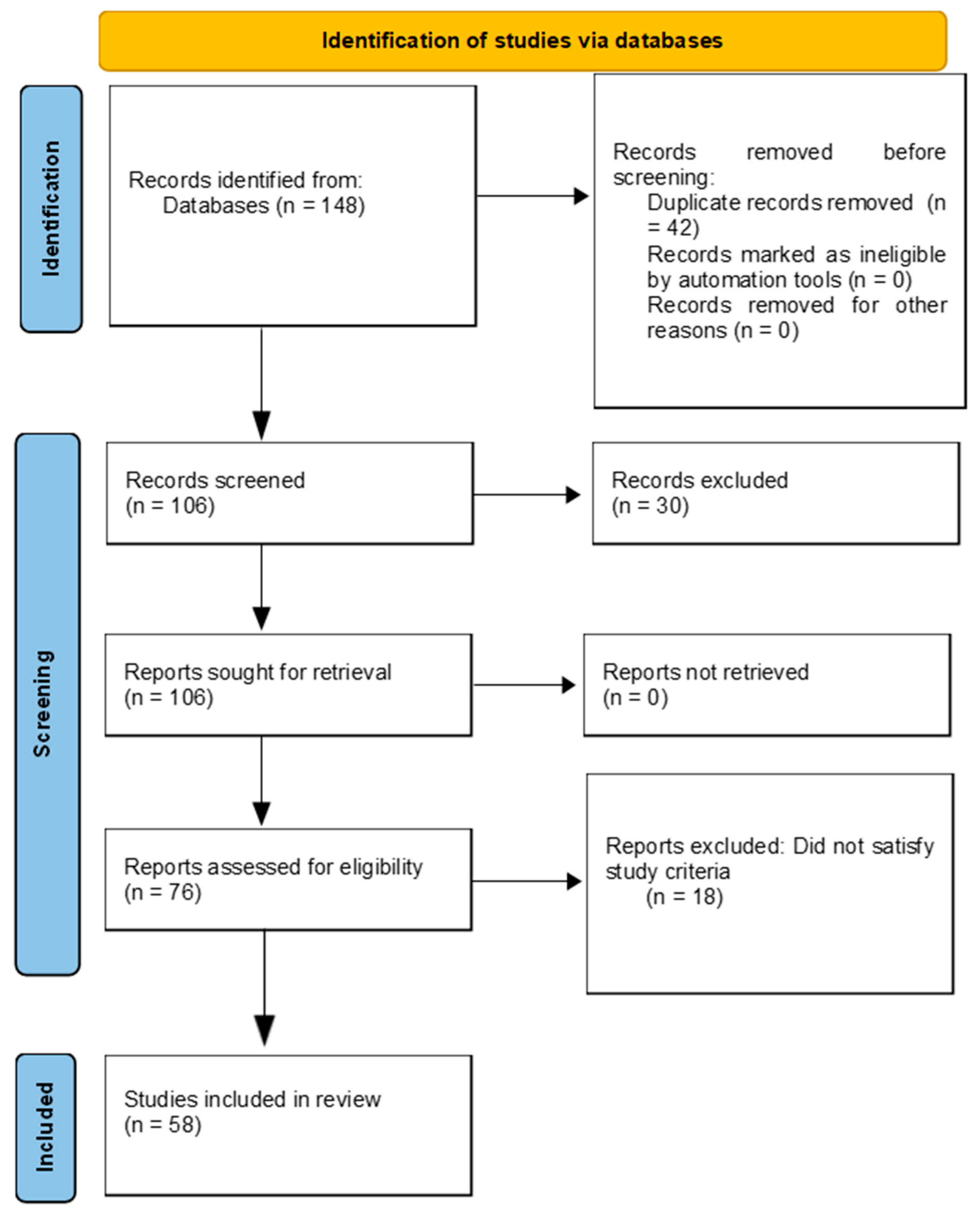
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]

| Role of Beneficial Bacteria | Bacterial Endophyte | Host Plant | References |
|---|---|---|---|
| Enhance plant resistance to phytopathogens | Bacillus amyloliquefaciens | Ginkgo biloba and Panax notoginseng | [41,42] |
| Bacillus sp. | Curcuma longa | [43] | |
| Cohnella sp., Paenibacillus sp., and Pantoea sp. | Centella asiatica | [44] | |
| Phyllobacterium myrsinacearum | Epimedium brevicornu | [45] | |
| Stenotrophomonas maltophilia, and Bacillus sp. | Panax ginseng | [46] | |
| Improved plant abiotic stress tolerance | Achromobacter xylosoxidans | Catharanthus roseus | [47] |
| Agrobacterium spp., and Bacillus spp. | Pteris vittata | [48] | |
| Citrobacter putida | Euphorbia milii | [49] | |
| Glutamicibacter halophytocola | Limonium sinense | [50] | |
| Paenibacillus sp. | Plantago asiatica and Tridax procumbens | [51,52] | |
| Plant growth promotion | Bacillus and Paenibacillus spp. | Curcuma longa | [53] |
| Bacillus cereus and Bacillus subtilis | Teucrium polium | [54] | |
| Bacillus siamensis | Coriandrum sativum | [55] | |
| Micrococcus luteus and Lysinibacillus fusiformis | Panax ginseng | [56] | |
| Paenibacillus and Bacillus spp. | Lonicera japonica | [57] | |
| Serratia marcescens | Achyranthes aspera | [58] | |
| Variovorax sp. | Lavandula dentata | [59] | |
| Promotion of plant metabolite accumulation | Bacillus subtilis | Ligusticum chuanxiong | [60] |
| Burkholderia sp. and Paenibacillus polymyxa | Panax ginseng | [61,62] | |
| Pseudomonas fluorescens | Atractylodes lancea and Atractylodes macrocephala | [63,64] | |
| Pseudonocardia sp. | Artemisia annua | [65] |
| Techniques | Criteria |
|---|---|
| Culture-dependent |
|
| Culture-independent |
|
|
| Data Platform | Type | Omics Approach | Function | Reference |
|---|---|---|---|---|
| QIIME2 | Tool | Metagenomics | Analysis of amplicon sequencing data for microbial community profiling | [215] |
| MG-RAST | Tool | Metagenomics, Metatranscriptomics | Automated annotation and analysis of metagenomic and metatranscriptomic data | [216] |
| PICRUSt | Tool | Functional inference | Predicts functional potential of microbial communities from 16S rRNA data | [217] |
| KEGG | Database | All (Genomics, Transcriptomics, Proteomics, Metabolomics) | Maps genes, proteins, and metabolites to known metabolic pathways | [218] |
| UniProt | Database | Proteomics | Repository of protein sequences and functional annotation | [219] |
| GNPS | Database | Metabolomics | Annotates and visualizes microbial secondary metabolites; excellent for environmental and microbial metabolomics | [220] |
| MetaCyc | Database | Metabolomics | Curated database of microbial metabolic pathways and enzymes | [221] |
| SILVA | Database | Metagenomics | Curated rRNA gene sequence database for taxonomic classification | [222] |
| Greengenes | Database | Metagenomics | 16S rRNA gene sequence database for bacterial taxonomy | [223] |
| RDP | Database | Metagenomics | Ribosomal Database Project for rRNA gene taxonomy and analysis | [224] |
| IMG/M | Tool and Database | Metagenomics | Integrated microbial genome/metagenome comparative analysis system | [225] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mametja, N.M.; Ramadwa, T.E.; Managa, M.; Masebe, T.M. Recent Advances and Developments in Bacterial Endophyte Identification and Application: A 20-Year Landscape Review. Plants 2025, 14, 2506. https://doi.org/10.3390/plants14162506
Mametja NM, Ramadwa TE, Managa M, Masebe TM. Recent Advances and Developments in Bacterial Endophyte Identification and Application: A 20-Year Landscape Review. Plants. 2025; 14(16):2506. https://doi.org/10.3390/plants14162506
Chicago/Turabian StyleMametja, Neo M., Thanyani E. Ramadwa, Muthumuni Managa, and Tracy M. Masebe. 2025. "Recent Advances and Developments in Bacterial Endophyte Identification and Application: A 20-Year Landscape Review" Plants 14, no. 16: 2506. https://doi.org/10.3390/plants14162506
APA StyleMametja, N. M., Ramadwa, T. E., Managa, M., & Masebe, T. M. (2025). Recent Advances and Developments in Bacterial Endophyte Identification and Application: A 20-Year Landscape Review. Plants, 14(16), 2506. https://doi.org/10.3390/plants14162506






