Potential of Bacterial Inoculants to Mitigate Soil Compaction Effects on Gossypium hirsutum Growth
Abstract
1. Introduction
2. Material and Methods
2.1. Experiment Planning and Installation
2.2. Substrate Used and Normal Proctor Test
2.3. Obtaining Microbial Accessions and Bacterial Production and Inoculation
2.4. Assessments of Vegetative Growth Promotion
2.4.1. Determination of the Nutritional Content of Plants
2.4.2. Microbial Density in Plant Tissues and Rhizosphere
2.5. Statistical Analysis
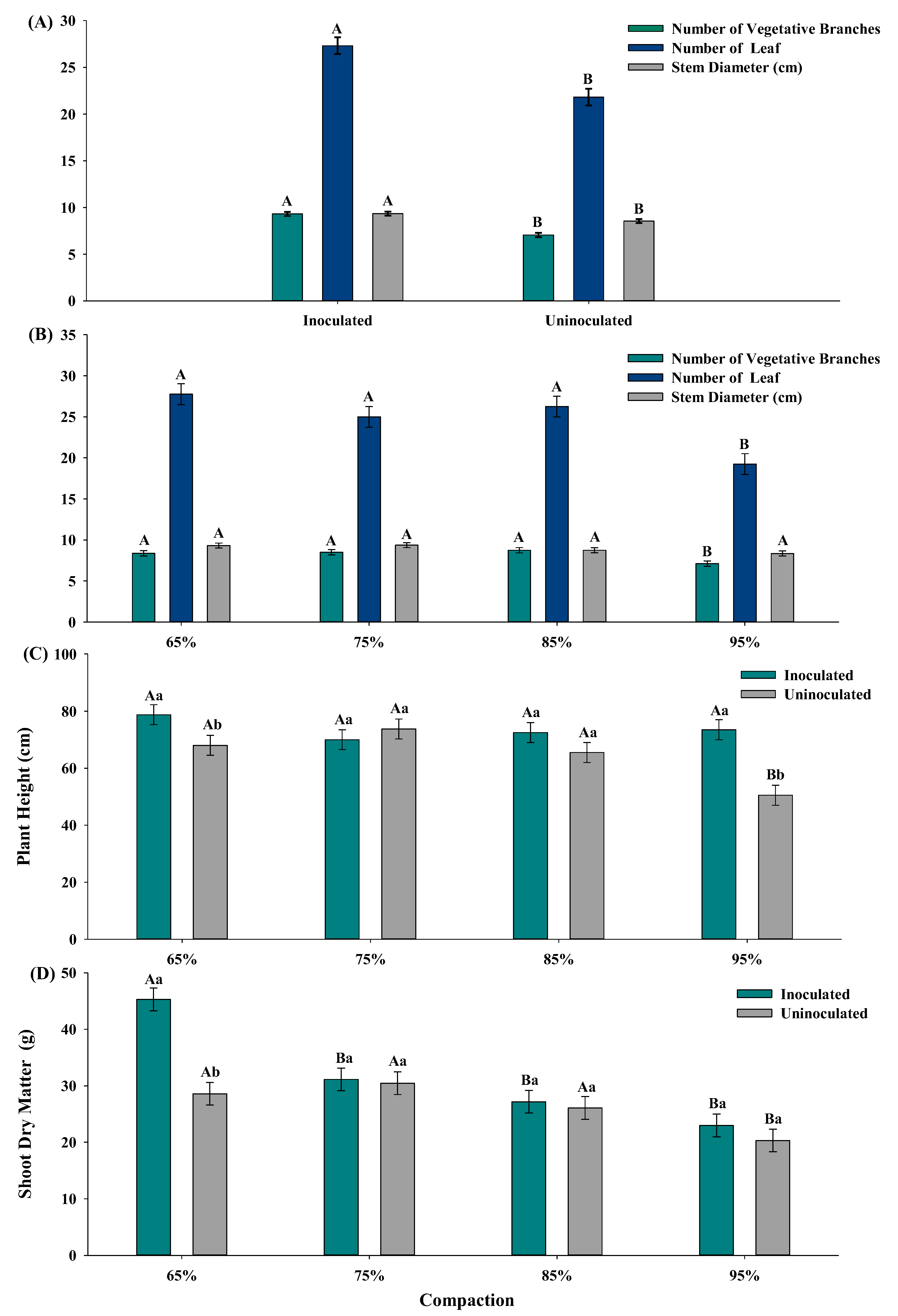
3. Result
3.1. Initial Growth of Cotton Plant (Gossypium hirsutum L.)
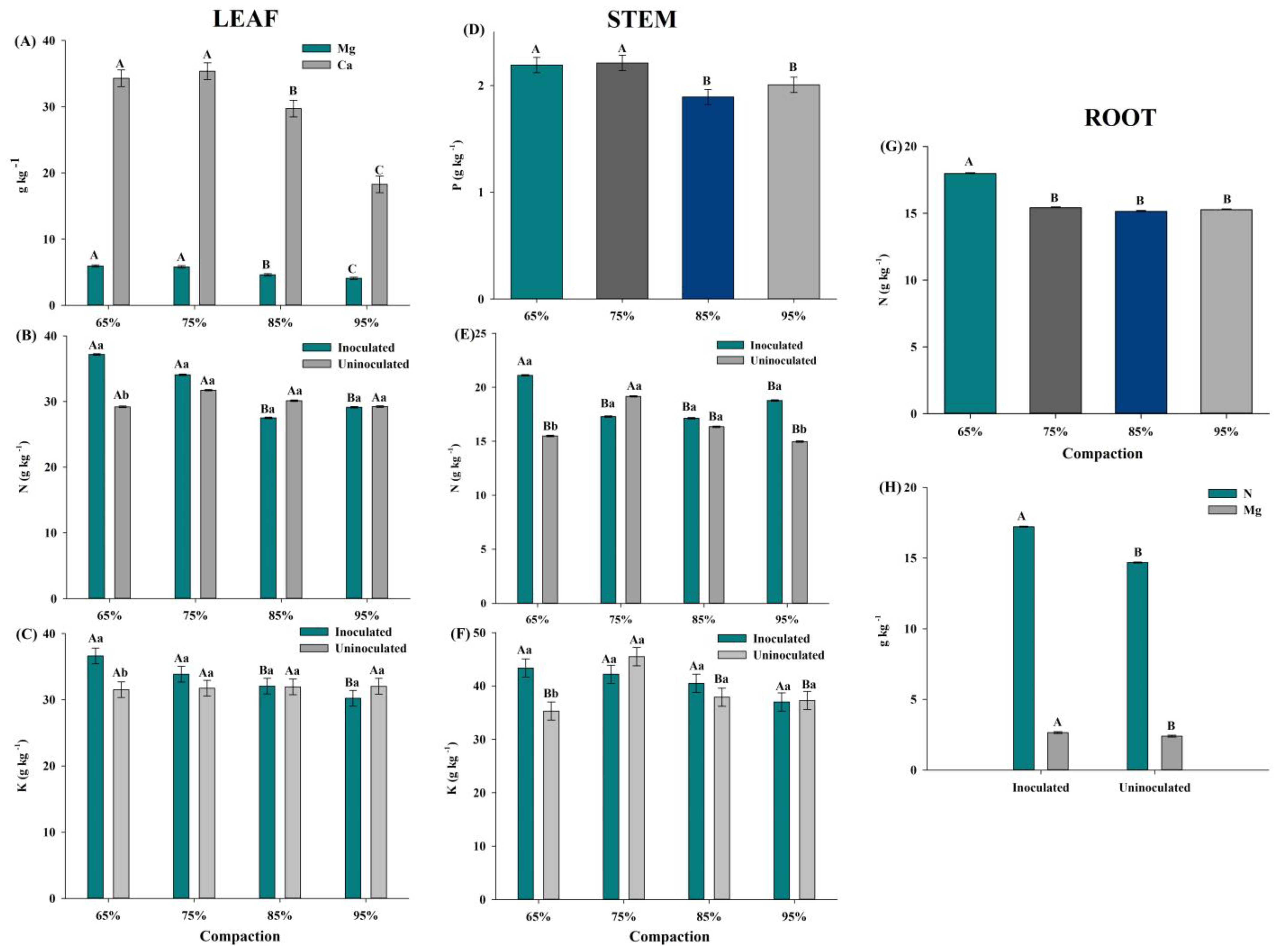
3.2. Macronutrient Contents in Plant Tissues
3.2.1. Leaf
3.2.2. Stem
3.2.3. Roots
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meyer, L.A.; Dew, T. Cotton and Wool Outlook: December 2021; U.S. Department of Agriculture, Economic Research Service: Washington, DC, USA, 2021. [Google Scholar]
- Cordeiro, C.F.S.; Rodrigues, D.R.; Echer, F.R. Cover crops and controlled-release urea decrease need for mineral nitrogen fertilizer for cotton in sandy soil. Field Crops Res. 2022, 276, 108387. [Google Scholar] [CrossRef]
- Goreux, L.; Macrae, J. Reforming the Cotton Sector in Sub-Saharan Africa; Africa Region Working Paper Series No. 47; World Bank: Washington, DC, USA, 2003. [Google Scholar]
- U.S. Department of Agriculture (USDA). Production, Supply and Distribution (PSD); U.S. Department of Agriculture: Washington, DC, USA, 2021. Available online: https://apps.fas.usda.gov/psdonline/app/index.html#/app/downloads (accessed on 9 June 2025).
- Alaoui, A.; Lipiec, J.; Gerke, H.H. A review of the changes in the soil pore system due to soil deformation: A hydrodynamic perspective. Soil Tillage Res. 2011, 115, 1–15. [Google Scholar] [CrossRef]
- Anghinoni, G.; Anghinoni, F.B.G.; Tormena, C.A.; Braccini, A.L.; de Carvalho Mendes, I.; Zancanaro, L.; Lal, R. Conservation agriculture strengthens sustainability of Brazilian grain production and food security. Land Use Policy 2021, 108, 105591. [Google Scholar] [CrossRef]
- Hamza, M.A.; Anderson, W.K. Soil compaction in cropping systems: A review of the nature, causes and possible solutions. Soil Tillage Res. 2005, 82, 121–145. [Google Scholar] [CrossRef]
- McKenzie, B.M.; Tisdall, J.M.; Vance, W.H. Soil physical quality. In Encyclopedia of Agrophysics; Glinski, J., Horabik, Lipiec, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 757–761. [Google Scholar] [CrossRef]
- Jamali, H.; Nachimuthu, G.; Palmer, B.; Hodgson, D.; Hundt, A.; Nunn, C.; Braunack, M. Soil compaction in a new light: Know the cost of doing nothing–A cotton case study. Soil Tillage Res. 2021, 213, 105158. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.A.; Marriel, I.E.; Gomes, E.A.; Mattos, B.B.; Santos, F.C.; Oliveira, M.C.; Alves, V.M.C. Methodology for the Application of Phosphorus Solubilizing Microorganisms in Seeds for Better Use of This Nutrient by Plants; Embrapa: Brasília, Brazil, 2013; 29p, Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/95211/1/bol-88.pdf (accessed on 9 June 2025).
- Lopes, R.; Tsui, S.; Gonçalves, P.J.; de Queiroz, M.V. A look into a multifunctional toolbox: Endophytic Bacillus species provide broad and underexploited benefits for plants. World J. Microbiol. Biotechnol. 2018, 34, 94. [Google Scholar] [CrossRef]
- Verma, S.K.; Kingsley, K.; Bergen, M.; English, C.; Elmore, M.; Kharwar, R.N.; White, J.F. Bacterial endophytes from rice cut grass (Leersia oryzoides L.) increase growth, promote root gravitropic response, stimulate root hair formation, and protect rice seedlings from disease. Plant Soil 2018, 422, 223–238. [Google Scholar] [CrossRef]
- Kasana, R.C.; Pandey, C.B. Exiguobacterium: An overview of a versatile genus with potential in industry and agriculture. Crit. Rev. Biotechnol. 2018, 38, 141–156. [Google Scholar] [CrossRef]
- Eid, A.M.; Fouda, A.; Abdel-Rahman, M.A.; Salem, S.S.; Elsaied, A.; Oelmüller, R.; Hijri, M.; Bhowmik, A.; Elkelish, A.; Hassan, S.E.-D. Harnessing bacterial endophytes for promotion of plant growth and biotechnological applications: An overview. Plants 2021, 10, 935. [Google Scholar] [CrossRef]
- Guimarães, P.T.G.; Guimarães, P.T.G.; Ribeiro, A.C.; Alvarez, V.H.; Garcia, A.W.R.; Prezotti, L.C.; Viana, A.S.; Miguel, A.E.; Malavolta, E.; Corrêa, J.B.; et al. Coffee. In Recommendations for the Use of Correctives and Fertilizers in Minas Gerais: 5th Approach; Ribeiro, A.C., Gontijo, P.T., Alvarez, V.H., Eds.; Soil Fertility Commission of the State of Minas Gerais: Viçosa, Brazil, 1999; pp. 289–302. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). World Reference Base for Soil Resources; FAO: Rome, Italy, 2006. [Google Scholar]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Cunha, T.J.F.; Oliveira, J.B. Brazilian Soil Classification System, 5th ed.; Embrapa: Seropédica, Brazil, 2018; Available online: http://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/1094001 (accessed on 9 June 2025).
- Stancati, G.; Nogueira, J.B.; Vilar, O.M. Laboratory Tests in Soil Mechanics. 1992. Available online: https://repositorio.usp.br/item/000847718 (accessed on 9 June 2025).
- Nogueira, J.B. Soil Mechanics: Laboratory Tests; São Carlos School of Engineering, University of São Paulo: São Paulo, Brazil, 1995; 248p. [Google Scholar]
- Associação Brasileira de Normas Técnicas—ABNT. Departamento Nacional de Estradas de Rodagem. DNER-M162/94: Soil: Compaction Test; ABNT: Rio de Janeiro, Brazil, 1994; 10p. [Google Scholar]
- Associação Brasileira de Normas Técnicas—ABNT. Departamento Nacional de Estradas de Rodagem. NBR 7182: Soil: Compaction Test; ABNT: Rio de Janeiro, Brazil, 1986; 10p. [Google Scholar]
- Blake, G.R.; Hartge, K.H. Bulk density. In Methods of Soil Analysis: Part 1. Physical and Mineralogical Methods, 2nd ed.; Klute, A., Ed.; Soil Science Society of America: Madison, WI, USA, 1986; Volume 5, pp. 363–375. [Google Scholar] [CrossRef]
- Vargas, M. Introduction to Soil Mechanics; McGraw-Hill: Columbus, OH, USA; University of São Paulo Press: São Paulo, Brazil, 1977. [Google Scholar]
- Ramires, R.V. Endophytic Microorganisms in Eucalyptus. Ph.D. Thesis, Federal University of the Jequitinhonha and Mucuri Valleys, Diamantina, Brazil, 2021. [Google Scholar]
- Bertani, G. Studies on lysogenesis I: The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951, 62, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Döbereiner, J.; Baldani, V.L.D.; Baldani, J.I. How to Isolate and Identify Diazotrophic Bacteria from Non-Leguminous Plants; Embrapa-SPI: Brasilia, Brazil, 1995; 60p. [Google Scholar]
- Silva, M.F.D.; Oliveira, P.J.D.; Xavier, G.R.; Rumjanek, N.G.; Reis, V.M. Inoculants containing polymers and endophytic bacteria for the sugarcane crop. Pesqui. Agropecuária Bras. 2009, 44, 1437–1443. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Assessment of the Nutritional Status of Plants: Principles and Applications, 2nd ed.; Potafós: São Paulo, Brazil, 1997; 201p. [Google Scholar]
- Meneghetti, A.M. Manual of Sampling Procedures and Chemical Analysis of Plants, Soil and Fertilizers; EDUTFPR: Curitiba, Brazil, 2018. [Google Scholar]
- Oliveira, A.M.; de Abreu, C.M.; Grazziotti, P.H.; de Andrade, G.F.P.; Gomes, J.V.; Avelino, N.R.; Menezes, J.F.S.; Barroso, G.M.; dos Santos, J.B.; da Costa, M.R. Production of seedlings of Corymbia citriodora inoculated with endophytic bacteria. Forests 2024, 15, 905. [Google Scholar] [CrossRef]
- Thomas, P.; Sekhar, A.C.; Mujawar, M.M. Nonrecovery of varying proportions of viable bacteria during spread plating governed by the extent of spreader usage and proposal for an alternate spotting-spreading approach to maximize the CFU. J. Appl. Microbiol. 2012, 113, 339–350. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 9 June 2025).
- Bhagat, N.; Raghav, M.; Dubey, S.; Bedi, N. Bacterial exopolysaccharides: Insight into their role in plant abiotic stress tolerance. J. Microbiol. Biotechnol. 2021, 31, 1045–1059. [Google Scholar] [CrossRef]
- Zhang, F.; Hou, Y.; Rengel, Z.; Mauchline, T.H.; Shen, J.; Zhang, F.; Jin, K. Root exudation of organic acid anions and recruitment of beneficial actinobacteria facilitate phosphorus uptake by maize in compacted silt loam soil. Soil Biol. Biochem. 2023, 184, 109074. [Google Scholar] [CrossRef]
- de Oliveira, A.M.; da Costa, M.R.; Grazziotti, P.H.; de Abreu, C.M.; Bispo, N.D.S.; Roa, J.P.B.; Silva, D.M.; Miranda, J.M. Brazilian scenario of inoculant production: A look at patents. Rev. Bras. Ciência Solo 2022, 46, e0210081. [Google Scholar] [CrossRef]
- Delegan, Y.; Kocharovskaya, Y.; Bogun, A.; Sizova, A.; Solomentsev, V.; Iminova, L.; Lyakhovchenko, N.; Zinovieva, A.; Goyanov, M.; Solyanikova, I. Characterization and genomic analysis of Exiguobacterium alkaliphilum B-3531D, an efficient crude oil degrading strain. Biotechnol. Rep. 2021, 32, e00678. [Google Scholar] [CrossRef]
- Chen, W.; Jin, M.; Ferré, T.; Liu, Y.; Huang, J.; Xian, Y. Soil conditions affect cotton root distribution and cotton yield under mulched drip irrigation. Field Crops Res. 2020, 249, 107743. [Google Scholar] [CrossRef]
- Adeniji, A.; Huang, J.; Li, S.; Lu, X.; Guo, R. Hot viewpoint on how soil texture, soil nutrient availability, and root exudates interact to shape microbial dynamics and plant health. Plant Soil 2024, 498, 1–22. [Google Scholar] [CrossRef]
- Nakhforoosh, A.; Nagel, K.A.; Fiorani, F.; Bodner, G. Deep soil exploration vs. topsoil exploitation: Distinctive rooting strategies between wheat landraces and wild relatives. Plant Soil 2021, 459, 397–421. [Google Scholar] [CrossRef] [PubMed]
- St Aime, R.; Rhodes, G.; Jones, M.; Campbell, B.T.; Narayanan, S. Evaluation of root traits and water use efficiency of different cotton genotypes in the presence or absence of a soil-hardpan. Crop J. 2021, 9, 945–953. [Google Scholar] [CrossRef]
- Falkoski Filho, J.; Batista, I.; Rosolem, C.A. Sensitivity of cotton cultivars to soil compaction. Semin. Ciências Agrárias 2013, 34 (Suppl. S1), 3645–3653. [Google Scholar] [CrossRef]
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; Hafeez Laghari, A.; Mustafa Bhabhan, G.; Hussain Talpur, K.; Bhutto, T.A.; Wahocho, S.A.; Lashari, A.A. Role of nitrogen for plant growth and development: A review. Adv. Environ. Biol. 2016, 10, 209–219. [Google Scholar]
- Ahmad, A.; Aslam, Z.; Javed, T.; Hussain, S.; Raza, A.; Shabbir, R.; Mora-Poblete, F.; Saeed, T.; Zulfiqar, F.; Ali, M.M.; et al. Screening of wheat (Triticum aestivum L.) genotypes for drought tolerance through agronomic and physiological response. Agronomy 2022, 12, 287. [Google Scholar] [CrossRef]
- Staut, L.A.; Kurihara, C.H. Liming and fertilization. In Cotton: Production Technology; EMBRAPA Agriculture West: Brasília, Brazil; EMBRAPA Cotton: Campina Grande, Brazil, 2001; pp. 103–123. Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/241150/1/14233.pdf (accessed on 9 June 2025).
- Munir, N.; Hanif, M.; Abideen, Z.; Sohail, M.; El-Keblawy, A.; Radicetti, E.; Mancinelli, R.; Haider, G. Mechanisms and strategies of plant microbiome interactions to mitigate abiotic stresses. Agronomy 2022, 12, 2069. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, F.H.V.; da Conceição, C.A.; Batista, A.C.; de Andrade, G.F.P.; de Abreu, C.M.; Grazziotti, P.H.; da Silva, R.S. Potential of Bacterial Inoculants to Mitigate Soil Compaction Effects on Gossypium hirsutum Growth. Plants 2025, 14, 1844. https://doi.org/10.3390/plants14121844
Araújo FHV, da Conceição CA, Batista AC, de Andrade GFP, de Abreu CM, Grazziotti PH, da Silva RS. Potential of Bacterial Inoculants to Mitigate Soil Compaction Effects on Gossypium hirsutum Growth. Plants. 2025; 14(12):1844. https://doi.org/10.3390/plants14121844
Chicago/Turabian StyleAraújo, Fausto Henrique Viera, Crislaine Alves da Conceição, Adriene Caldeira Batista, Gabriel Faria Parreiras de Andrade, Caique Menezes de Abreu, Paulo Henrique Grazziotti, and Ricardo Siqueira da Silva. 2025. "Potential of Bacterial Inoculants to Mitigate Soil Compaction Effects on Gossypium hirsutum Growth" Plants 14, no. 12: 1844. https://doi.org/10.3390/plants14121844
APA StyleAraújo, F. H. V., da Conceição, C. A., Batista, A. C., de Andrade, G. F. P., de Abreu, C. M., Grazziotti, P. H., & da Silva, R. S. (2025). Potential of Bacterial Inoculants to Mitigate Soil Compaction Effects on Gossypium hirsutum Growth. Plants, 14(12), 1844. https://doi.org/10.3390/plants14121844







