The Impact of Cd Pollution on Arbuscular Mycorrhizal Fungal Communities in Paddy Fields
Abstract
1. Introduction
2. Results
2.1. Soil Physicochemical Properties
2.2. Analysis of AMF Community Structure and Diversity
2.3. AMF Community Alpha Diversity Index
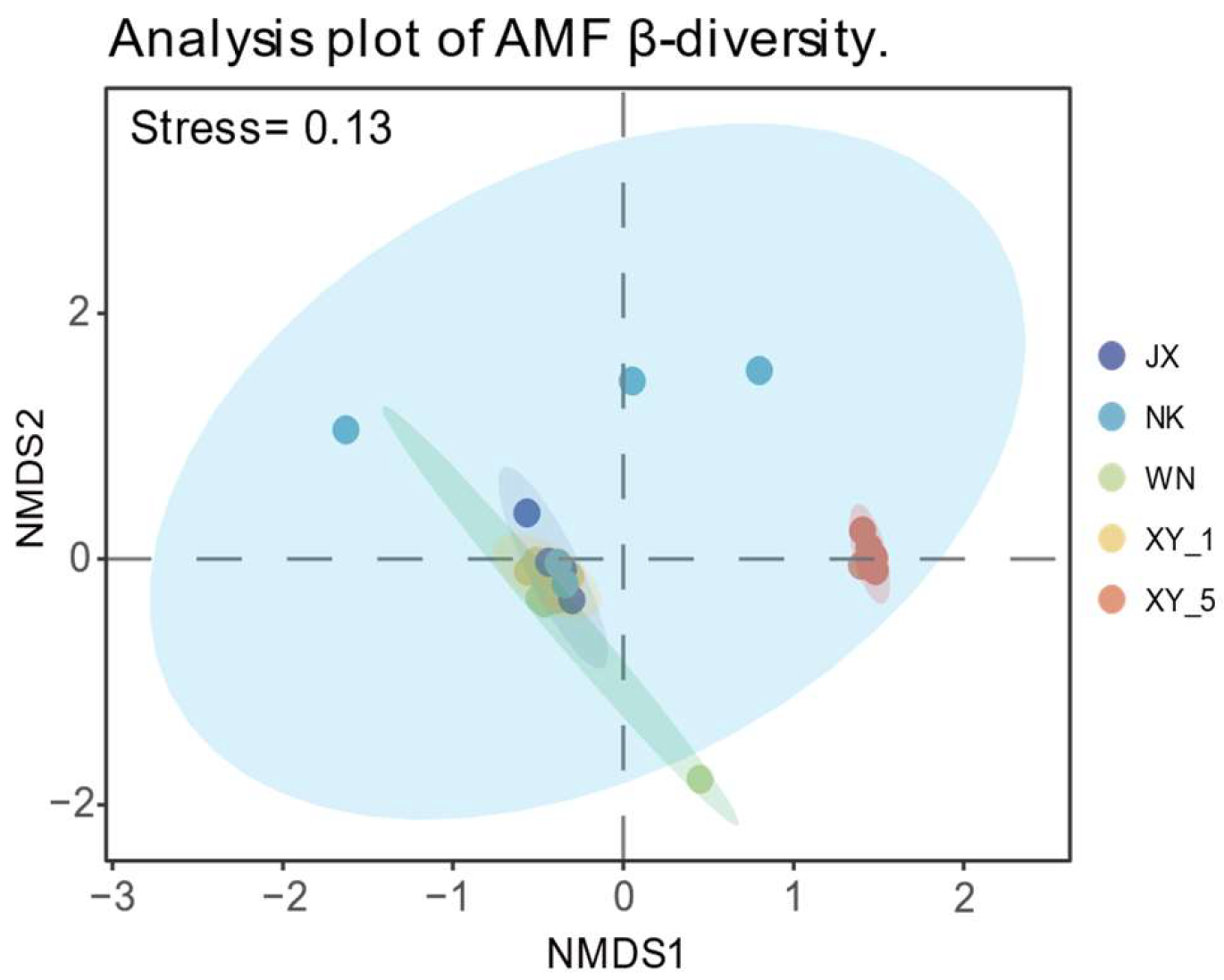
2.4. AMF Community β-Diversity Index
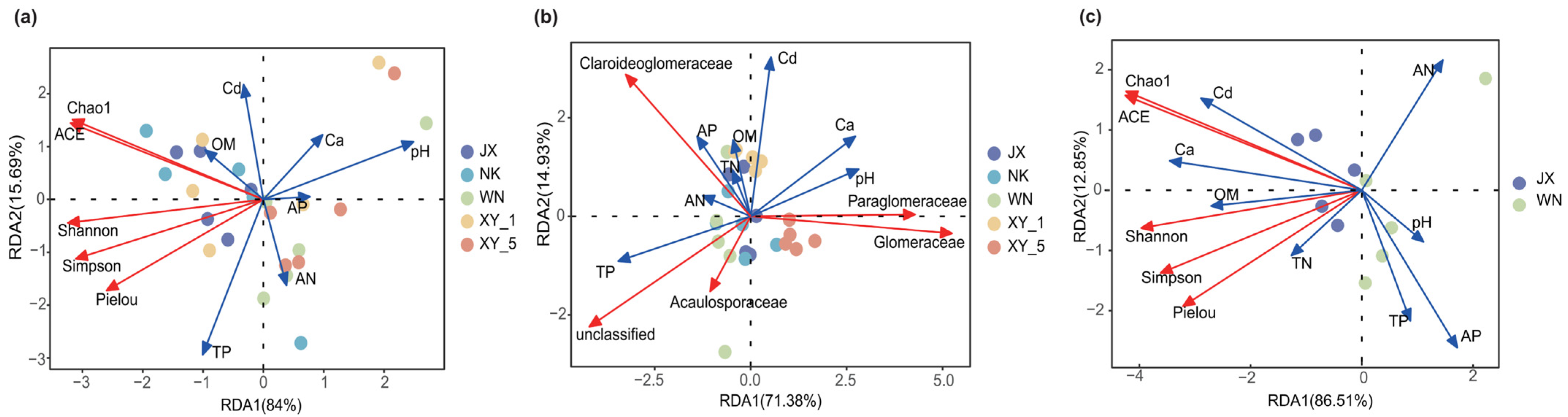
2.5. Environmental Factors and AMF Communities
3. Discussion
3.1. Dominant AMF Families in Cd-Contaminated Paddy Fields
3.2. Cd-Induced Changes in AMF Community Structure
3.3. Regulatory Mechanisms of Multiple Environmental Factors on AMF Communities
4. Materials and Methods
4.1. Study Area
4.2. Collection of Soil Samples
4.3. Determination of Soil Physicochemical Parameters
4.4. Determination of AMF Community Structure
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, W.; Chen, S.; Liu, J.; Chen, L.; Song, N.; Li, N.; Liu, B. Variation of Cd Concentration in Various Rice Cultivars and Derivation of Cadmium Toxicity Thresholds for Paddy Soil by Species-Sensitivity Distribution. J. Integr. Agric. 2015, 14, 1845–1854. [Google Scholar] [CrossRef]
- Yuan, X.; Xue, N.; Han, Z. A Meta-Analysis of Heavy Metals Pollution in Farmland and Urban Soils in China over the Past 20 Years. J. Environ. Sci. 2021, 101, 217–226. [Google Scholar] [CrossRef]
- Shahabivand, S.; Maivan, H.Z.; Goltapeh, E.M.; Sharifi, M.; Aliloo, A.A. The Effects of Root Endophyte and Arbuscular Mycorrhizal Fungi on Growth and Cadmium Accumulation in Wheat Under Cadmium Toxicity. Plant Physiol. Biochem. 2012, 60, 53–58. [Google Scholar] [CrossRef] [PubMed]
- McBride, M.B.; Shayler, H.A.; Spliethoff, H.M.; Mitchell, R.G.; Marquez-Bravo, L.G.; Ferenz, G.S.; Russell-Anelli, J.M.; Casey, L.; Bachman, S. Concentrations of Lead, Cadmium and Barium in Urban Garden-Grown Vegetables: The Impact of Soil Variables. Environ. Pollut. 2014, 194, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Uraguchi, S.; Fujiwara, T. Cadmium Transport and Tolerance in Rice: Perspectives for Reducing Grain Cadmium Accumulation. Rice 2012, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, Y.; Mao, W.; Sui, H.; Yong, L.; Yang, D.; Jiang, D.; Zhang, L.; Gong, Y. Dietary Cadmium Exposure Assessment Among the Chinese Population. PLoS ONE 2017, 12, e0177978. [Google Scholar] [CrossRef]
- Hu, Y.; Ge, Y.; Zhang, C.; Ju, T.; Cheng, W. Cadmium Toxicity and Translocation in Rice Seedlings Are Reduced by Hydrogen Peroxide Pretreatment. Plant Growth Regul. 2009, 59, 51–61. [Google Scholar] [CrossRef]
- Tsukahara, T.; Ezaki, T.; Moriguchi, J.; Furuki, K.; Shimbo, S.; Matsuda-Inoguchi, N.; Ikeda, M. Rice as the Most Influential Source of Cadmium Intake Among General Japanese Population. Sci. Total Environ. 2003, 305, 41–51. [Google Scholar] [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of Heavy Metal(Loid)s Contaminated Soils—To Mobilize or to Immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef]
- Chen, X.W.; Wu, L.; Luo, N.; Mo, C.H.; Wong, M.H.; Li, H. Arbuscular Mycorrhizal Fungi and the Associated Bacterial Community Influence the Uptake of Cadmium in Rice. Geoderma 2019, 337, 749–757. [Google Scholar] [CrossRef]
- Wu, F.Y.; Bi, Y.L.; Leung, H.M.; Ye, Z.H.; Lin, X.G.; Wong, M.H. Accumulation of As, Pb, Zn, Cd and Cu and Arbuscular Mycorrhizal Status in Populations of Cynodon Dactylon Grown on Metal-Contaminated Soils. Appl. Soil Ecol. 2010, 44, 213–218. [Google Scholar] [CrossRef]
- Mi, Y.; Bai, X.; Li, X.; Zhou, M.; Liu, X.; Wang, F.; Su, H.; Chen, H.; Wei, Y. Soil Mercury Pollution Changes Soil Arbuscular Mycorrhizal Fungal Community Composition. J. Fungi 2023, 9, 395. [Google Scholar] [CrossRef] [PubMed]
- Neeratanaphan, L.; Khamma, S.; Benchawattananon, R.; Ruchuwararak, P.; Appamaraka, S.; Intamat, S. Heavy Metal Accumulation in Rice (Oryza sativa) near Electronic Waste Dumps and Related Human Health Risk Assessment. Hum. Ecol. Risk Assess. Int. J. 2017, 23, 1086–1098. [Google Scholar] [CrossRef]
- Wu, Z.; McGrouther, K.; Huang, J.; Wu, P.; Wu, W.; Wang, H. Decomposition and the Contribution of Glomalin-Related Soil Protein (GRSP) in Heavy Metal Sequestration: Field Experiment. Soil Biol. Biochem. 2014, 68, 283–290. [Google Scholar] [CrossRef]
- Xu, Y.; Lambers, H.; Feng, J.; Tu, Y.; Peng, Z.; Huang, J. The Role of Arbuscular Mycorrhizal Fungi in Micronutrient Homeostasis and Cadmium Uptake and Transfer in Rice Under Different Flooding Intensities. Ecotoxicol. Environ. Saf. 2024, 284, 116978. [Google Scholar] [CrossRef]
- Ting, Z.; Li, W.; Jixian, Y.; Fang, M. Causal Analysis between Rice Growth and Cadmium Accumulation and Transfer Under Arbuscular Mycorrhizal Inoculation. Rice Sci. 2024, 31, 226–236. [Google Scholar] [CrossRef]
- Del Val, C.; Barea, J.M.; Azcón-Aguilar, C. Diversity of Arbuscular Mycorrhizal Fungus Populations in Heavy-Metal-Contaminated Soils. Appl. Environ. Microbiol. 1999, 65, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shi, Y.; Xia, W.; Wang, X.; Xin, Z.; Liao, Y.; Sun, X. Soil Amendments Altered Arbuscular Mycorrhizal Fungal Communities in Cadmium-Contaminated Vegetable Fields. Front. Microbiol. 2024, 15, 1470137. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W. Arbuscular Mycorrhizal Symbiosis of Viola Baoshanensis at Baoshan Pb/Zn Mine in China. J. Environ. Eng. Landscape Manag. 2024, 32, 143–151. [Google Scholar] [CrossRef]
- GB 15618-2018; Environmental Quality Standards for Soil—Risk Control Standards for Soil Pollution of Agricultural Land (Trial). Ministry of Ecology and Environment, State Administration for Market Regulation: Beijing, China, 2018.
- Ma, S.; Li, T.; Zhang, B.; Zhai, L.; Liu, X.; Zhang, J. Unveiling the Influence of Seawater Intrusion and Vegetation Type on Coastal Arbuscular Mycorrhizal Fungal Communities in China. Land Degrad. Dev. 2024, 35, 4935–4947. [Google Scholar] [CrossRef]
- Van Geel, M.; Ceustermans, A.; Van Hemelrijck, W.; Lievens, B.; Honnay, O. Decrease in Diversity and Changes in Community Composition of Arbuscular Mycorrhizal Fungi in Roots of Apple Trees with Increasing Orchard Management Intensity Across a Regional Scale. Mol. Ecol. 2015, 24, 941–952. [Google Scholar] [CrossRef]
- Dai, M.; Bainard, L.D.; Hamel, C.; Gan, Y.; Lynch, D. Impact of Land Use on Arbuscular Mycorrhizal Fungal Communities in Rural Canada. Appl. Environ. Microbiol. 2013, 79, 6719–6729. [Google Scholar] [CrossRef]
- Dai, M.; Hamel, C.; Bainard, L.D.; Arnaud, M.S.; Grant, C.A.; Lupwayi, N.Z.; Malhi, S.S.; Lemke, R. Negative and Positive Contributions of Arbuscular Mycorrhizal Fungal Taxa to Wheat Production and Nutrient Uptake Efficiency in Organic and Conventional Systems in the Canadian Prairie. Soil Biol. Biochem. 2014, 74, 156–166. [Google Scholar] [CrossRef]
- Colombo, R.P.; Benavidez, M.E.; Fernandez Bidondo, L.; Silvani, V.A.; Bompadre, M.J.; Statello, M.; Scorza, M.V.; Scotti, A.; Godeas, A.M. Arbuscular Mycorrhizal Fungi in Heavy Metal Highly Polluted Soil in the Riachuelo River Basin. Rev. Argent. Microbiol. 2020, 52, 145–149. [Google Scholar] [CrossRef]
- Cornejo, P.; Meier, S.; Borie, G.; Rillig, M.C.; Borie, F. Glomalin-Related Soil Protein in a Mediterranean Ecosystem Affected by a Copper Smelter and Its Contribution to Cu and Zn Sequestration. Sci. Total Environ. 2008, 406, 154–160. [Google Scholar] [CrossRef]
- Hao, B.; Zhang, Z.; Bao, Z.; Hao, L.; Diao, F.; Li, F.Y.; Guo, W. Claroideoglomus Etunicatum Affects the Structural and Functional Genes of the Rhizosphere Microbial Community to Help Maize Resist Cd and La Stresses. Environ. Pollut. 2022, 307, 119559. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.E.D.; Boon, E.; St-Arnaud, M.; Hijri, M. Molecular Biodiversity of Arbuscular Mycorrhizal Fungi in Trace Metal-Polluted Soils. Mol. Ecol. 2011, 20, 3469–3483. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhao, F.; Wang, M.; Qi, K.; Wu, J.; Zhang, S. Soil Chemical Properties and Geographical Distance Exerted Effects on Arbuscular Mycorrhizal Fungal Community Composition in Pear Orchards in Jiangsu Province, China. Appl. Soil Ecol. 2019, 142, 18–24. [Google Scholar] [CrossRef]
- Parvin, S.; Van Geel, M.; Yeasmin, T.; Lievens, B.; Honnay, O. Variation in Arbuscular Mycorrhizal Fungal Communities Associated with Lowland Rice (Oryza sativa) Along a Gradient of Soil Salinity and Arsenic Contamination in Bangladesh. Sci. Total Environ. 2019, 686, 546–554. [Google Scholar] [CrossRef]
- Parvin, S.; Van Geel, M.; Ali, M.M.; Yeasmin, T.; Lievens, B.; Honnay, O. A Comparison of the Arbuscular Mycorrhizal Fungal Communities Among Bangladeshi Modern High Yielding and Traditional Rice Varieties. Plant Soil 2021, 462, 109–124. [Google Scholar] [CrossRef]
- Lin, L.; Chen, Y.; Qu, L.; Zhang, Y.; Ma, K. Cd Heavy Metal and Plants, Rather than Soil Nutrient Conditions, Affect Soil Arbuscular Mycorrhizal Fungal Diversity in Green Spaces during Urbanization. Sci. Total Environ. 2020, 726, 138594. [Google Scholar] [CrossRef]
- Faggioli, V.; Menoyo, E.; Geml, J.; Kemppainen, M.; Pardo, A.; Salazar, M.J.; Becerra, A.G. Soil Lead Pollution Modifies the Structure of Arbuscular Mycorrhizal Fungal Communities. Mycorrhiza 2019, 29, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Gai, J.P.; Fan, J.Q.; Zhang, S.B.; Mi, N.N.; Christie, P.; Li, X.L.; Feng, G. Direct Effects of Soil Cadmium on the Growth and Activity of Arbuscular Mycorrhizal Fungi. Rhizosphere 2018, 7, 43–48. [Google Scholar] [CrossRef]
- Xu, P.; Tu, X.; An, Z.; Mi, W.; Wan, D.; Bi, Y.; Song, G. Cadmium-Induced Physiological Responses, Biosorption and Bioaccumulation in Scenedesmus Obliquus. Toxics 2024, 12, 262. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Kim, C.-G.; Subramanian, P.; Kim, K.-Y.; Selvakumar, G.; Sa, T.-M. Arbuscular Mycorrhizal Fungi Community Structure, Abundance and Species Richness Changes in Soil by Different Levels of Heavy Metal and Metalloid Concentration. PLoS ONE 2015, 10, e0128784. [Google Scholar] [CrossRef]
- He, X.; Nara, K. Element Biofortification: Can Mycorrhizas Potentially Offer a More Effective and Sustainable Pathway to Curb Human Malnutrition? Trends Plant Sci. 2007, 12, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Hempel, S.; Wubet, T.; Schäfer, T.; Savaghebi, G.; Jouzani, G.S.; Nekouei, M.K.; Buscot, F. Molecular Diversity of Arbuscular Mycorrhizal Fungi in Relation to Soil Chemical Properties and Heavy Metal Contamination. Environ. Pollut. 2010, 158, 2757–2765. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, B.; Ohtomo, R. Mycorrhizal Effects on Growth, P Uptake and Cd Tolerance of the Host Plant Vary among Different AM Fungal Species. Soil Sci. Plant Nutr. 2015, 61, 359–368. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, X.; Kang, Y.; Xie, C.; An, X.; Dong, C.; Xu, Y.; Shen, Q. Arbuscular Mycorrhizal Fungal Communities in the Rhizospheric Soil of Litchi and Mango Orchards as Affected by Geographic Distance, Soil Properties and Manure Input. Appl. Soil Ecol. 2020, 152, 103593–103605. [Google Scholar] [CrossRef]
- Soares, C.R.F.S.; Siqueira, J.O. Mycorrhiza and Phosphate Protection of Tropical Grass Species against Heavy Metal Toxicity in Multi-Contaminated Soil. Biol. Fertil. Soils 2008, 44, 833–841. [Google Scholar] [CrossRef]
- Liu, X.; Feng, Z.; Zhou, Y.; Zhu, H.; Yao, Q. Plant Species-Dependent Effects of Liming and Plant Residue Incorporation on Soil Bacterial Community and Activity in an Acidic Orchard Soil. Appl. Sci. 2020, 10, 5681. [Google Scholar] [CrossRef]
- Davison, J.; Moora, M.; Semchenko, M.; Adenan, S.B.; Ahmed, T.; Akhmetzhanova, A.A.; Alatalo, J.M.; Al-Quraishy, S.; Andriyanova, E.; Anslan, S.; et al. Temperature and pH Define the Realised Niche Space of Arbuscular Mycorrhizal Fungi. New Phytol. 2021, 231, 763–776. [Google Scholar] [CrossRef]
- Zarei, M.; Saleh-Rastin, N.; Jouzani, G.S.; Savaghebi, G.; Buscot, F. Arbuscular Mycorrhizal Abundance in Contaminated Soils around a Zinc and Lead Deposit. Eur. J. Soil Biol. 2008, 44, 381–391. [Google Scholar] [CrossRef]
- Chen, Y.X.; Lin, Q.; Luo, Y.M.; He, Y.F.; Zhen, S.J.; Yu, Y.L.; Tian, G.M.; Wong, M.H. The Role of Citric Acid on the Phytoremediation of Heavy Metal Contaminated Soil. Chemosphere 2003, 50, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, K.; Wang, W.; Ren, G.; Khan, A.; Feng, Y.; Yang, G. Changes in Soil Enzymes, Soil Properties, and Maize Crop Productivity Under Wheat Straw Mulching in Guanzhong, China. Soil Tillage Res. 2018, 182, 94–102. [Google Scholar] [CrossRef]
- Li, Q.; Peng, X.; Yang, H.; Wang, H.; Shu, Y. Deficiency of Multidrug and Toxin Extrusion 1 Enhances Renal Accumulation of Paraquat and Deteriorates Kidney Injury in Mice. Mol. Pharm. 2011, 8, 2476–2483. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, W.; Liu, Y.; Yun, W.; Luo, B.; Chai, R.; Zhang, C.; Xiang, X.; Su, X. Changes in Phosphorus Mobilization and Community Assembly of Bacterial and Fungal Communities in Rice Rhizosphere under Phosphate Deficiency. Front. Microbiol. 2022, 13, 953340. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Young, J.P.W. Improved PCR Primers for the Detection and Identification of Arbuscular Mycorrhizal Fungi: PCR Primers for Arbuscular Mycorrhizal Fungi. FEMS Microbiol. Ecol. 2008, 65, 339–349. [Google Scholar] [CrossRef]
- Sato, K.; Suyama, Y.; Saito, M.; Sugawara, K. A New Primer for Discrimination of Arbuscular Mycorrhizal Fungi with Polymerase Chain Reaction-Denature Gradient Gel Electrophoresis. Grassl. Sci. 2005, 51, 179–181. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Öpik, M.; Vanatoa, A.; Vanatoa, E.; Moora, M.; Davison, J.; Kalwij, J.M.; Reier, Ü.; Zobel, M. The Online Database Maarj AM Reveals Global and Ecosystemic Distribution Patterns in Arbuscular Mycorrhizal Fungi (Glomeromycota). New Phytol. 2010, 188, 223–241. [Google Scholar] [CrossRef] [PubMed]


| Treatment | OM (g/kg) | AP (mg/kg) | TN (g/kg) | AN (mg/kg) | TP (mg/kg) | Ca (mg/kg) | Cd (mg/kg) | pH |
|---|---|---|---|---|---|---|---|---|
| JX (27.960° N, 116.834° E) | 40.98 + 6.26 ab | 15.44 + 5.80 b | 2.53 + 0.33 a | 0.03 + 0.01 a | 560.0 + 157.65 ab | 808.20 + 179.29 b | 0.67 + 0.17 b | 5.02 + 0.08 c |
| NK (25.718° N, 114.747° E) | 26.48 + 3.66 d | 14.61 + 13.45 b | 1.71 + 0.18 c | 0.02 + 0.01 a | 589.2 + 136.40 ab | 1168.4 + 1030.08 b | 0.36 + 0.07 c | 5.39 + 0.65 c |
| WN (28.772° N, 117.078° E) | 35.98 + 2.80 bc | 57.10 + 29.56 a | 2.52 + 0.20 a | 0.04 + 0.02 a | 707.00 + 128.34 a | 532.40 + 109.62 b | 0.39 + 0.05 c | 5.14 + 0.13 c |
| XY_1 (27.794° N, 114.994° E) | 43.52 + 2.35 a | 56.82 + 10.69 a | 2.66 + 0.19 a | 0.03 + 0.00 a | 518.40 + 19.49 bc | 2254.00 + 114.59 a | 1.36 + 0.40 a | 5.97 + 0.22 b |
| XY_5 (27.789° N, 115.000° E) | 31.44 + 3.38 cd | 16.90 + 5.79 b | 2.17 + 0.25 b | 0.03 + 0.01 a | 399.40 + 60.58 c | 2954.00 + 686.75 a | 0.56 + 0.02 bc | 6.86 + 0.23 a |
| Treatment | Chao1 Index | ACE Index | Simpson Index | Shannon Index |
|---|---|---|---|---|
| JX | 31.90 ± 9.75 a | 32.09 ± 9.52 a | 0.69 ± 0.07 a | 2.24 ± 0.34 a |
| NK | 31.19 ± 19.35 a | 32.03 ± 19.64 a | 0.65 ± 0.15 ab | 2.19 ± 0.73 a |
| WN | 11.50 ± 4.80 b | 12.55 ± 6.02 b | 0.47 ± 0.24 ab | 1.25 ± 0.61 b |
| XY_1 | 25.67 ± 11.95 ab | 26.07 ± 12.10 ab | 0.53 ± 0.28 ab | 1.79 ± 0.97 ab |
| XY_5 | 12.00 ± 3.54 b | 12.44 ± 3.62 b | 0.38 ± 0.20 b | 1.12 ± 0.52 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, W.; Liao, Y.; Chen, X.; Li, L.; Shi, Y.; Liu, Y.; Zhang, J.; Fu, J. The Impact of Cd Pollution on Arbuscular Mycorrhizal Fungal Communities in Paddy Fields. Plants 2025, 14, 2501. https://doi.org/10.3390/plants14162501
Xia W, Liao Y, Chen X, Li L, Shi Y, Liu Y, Zhang J, Fu J. The Impact of Cd Pollution on Arbuscular Mycorrhizal Fungal Communities in Paddy Fields. Plants. 2025; 14(16):2501. https://doi.org/10.3390/plants14162501
Chicago/Turabian StyleXia, Wangbiao, Yingchun Liao, Xinyi Chen, Liang Li, Yanning Shi, Yaxin Liu, Jingmin Zhang, and Jiankang Fu. 2025. "The Impact of Cd Pollution on Arbuscular Mycorrhizal Fungal Communities in Paddy Fields" Plants 14, no. 16: 2501. https://doi.org/10.3390/plants14162501
APA StyleXia, W., Liao, Y., Chen, X., Li, L., Shi, Y., Liu, Y., Zhang, J., & Fu, J. (2025). The Impact of Cd Pollution on Arbuscular Mycorrhizal Fungal Communities in Paddy Fields. Plants, 14(16), 2501. https://doi.org/10.3390/plants14162501






