Adaptability of Foxtail Millet Varieties Based on Photosynthetic Performance and Agronomic Traits
Abstract
1. Introduction
2. Results
2.1. Comparison of Physiological and Ecological Parameters of Different Foxtail Millet Varieties
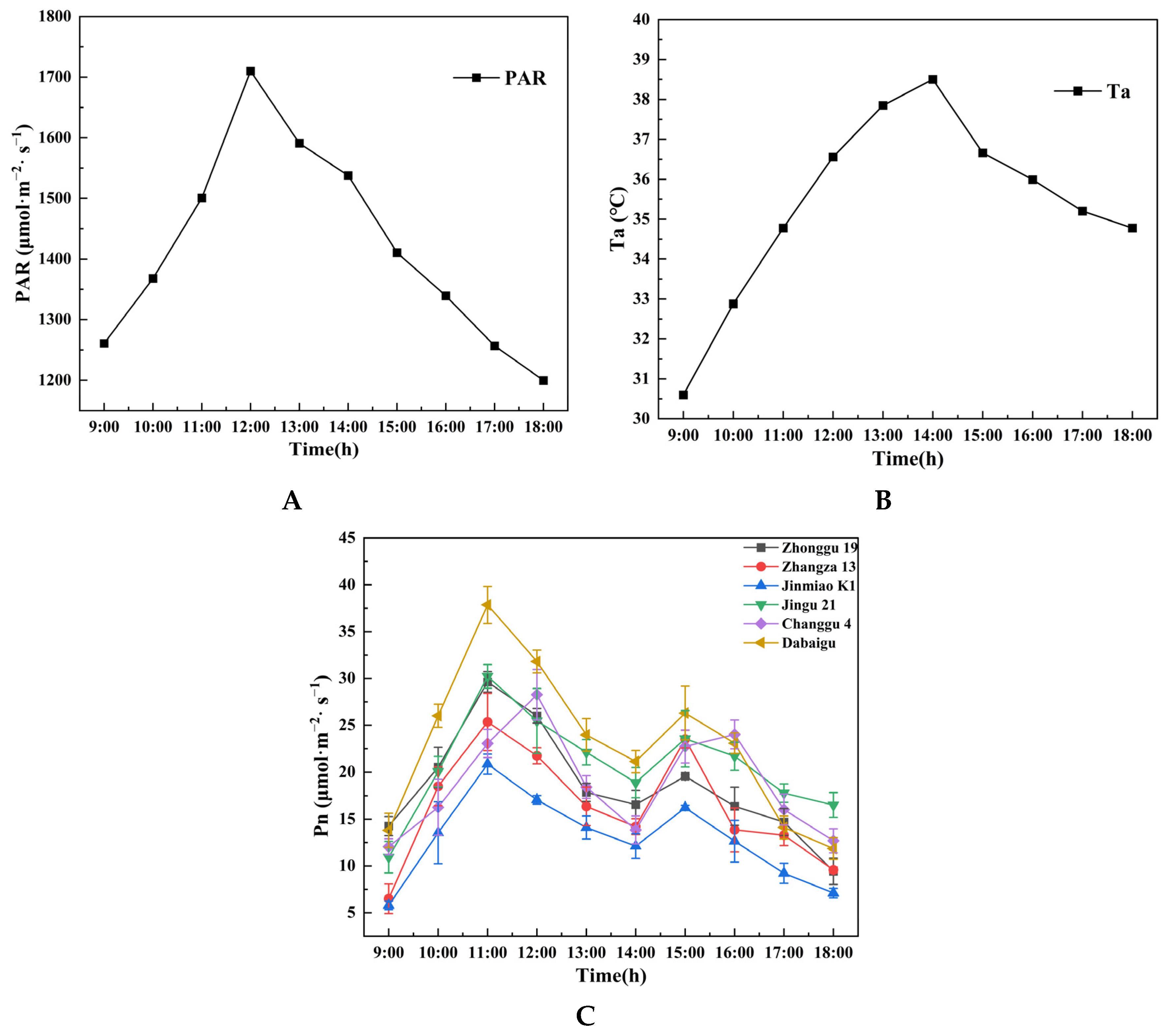
2.1.1. Comparison of Diurnal Variation in Net Photosynthetic Rate
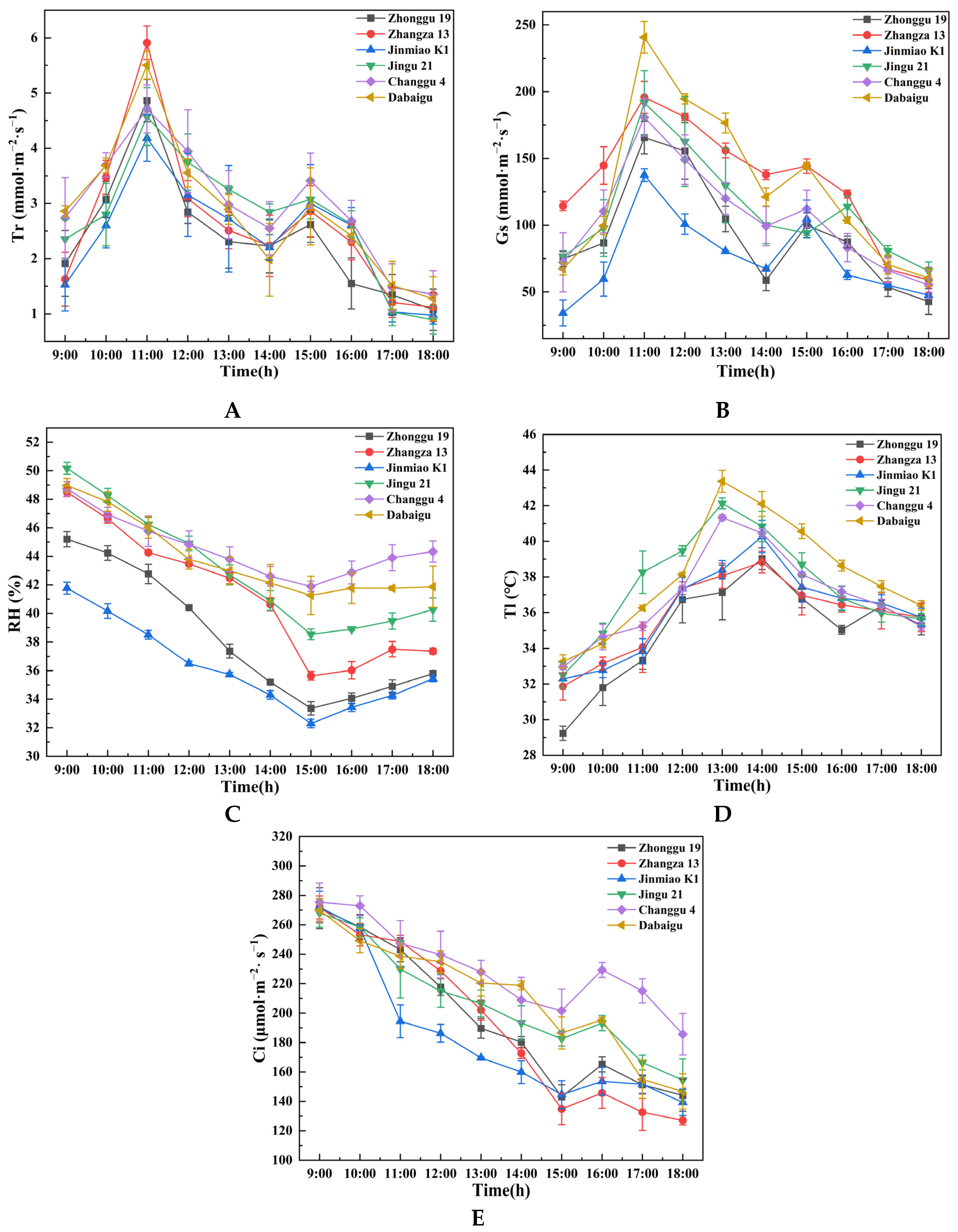
2.1.2. Comparison of Additional Parameters
2.1.3. Comparison of Leaf Water Use Efficiency
2.1.4. Comparison of Mean Diurnal Variation in Ecophysiological Parameters
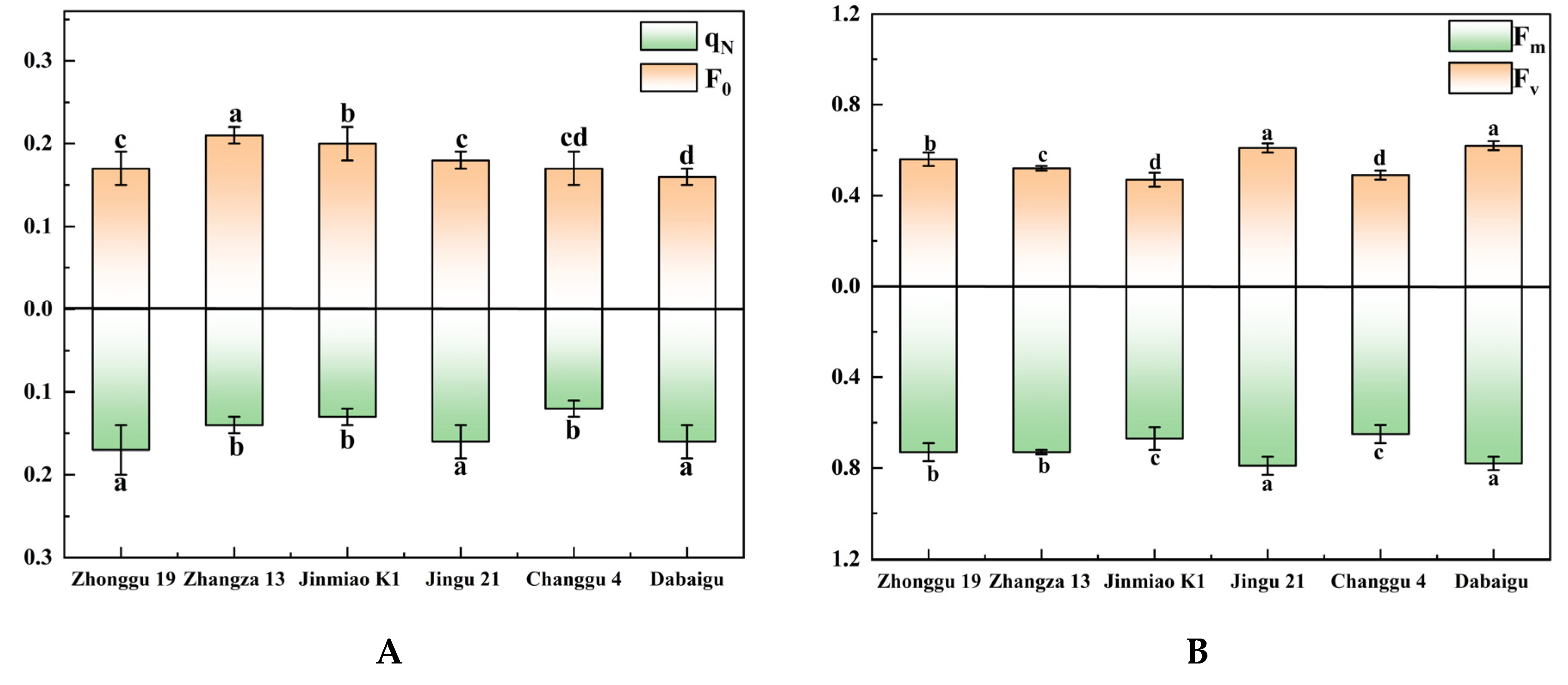
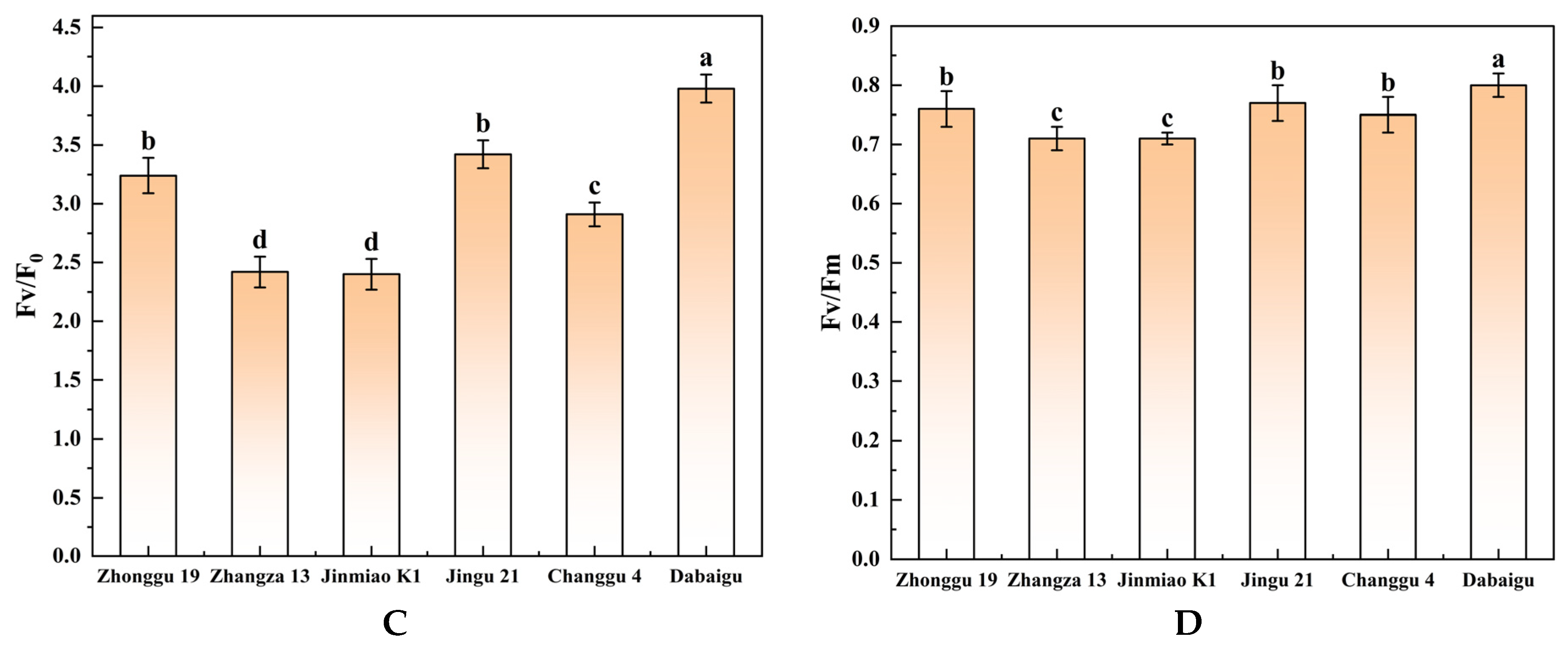
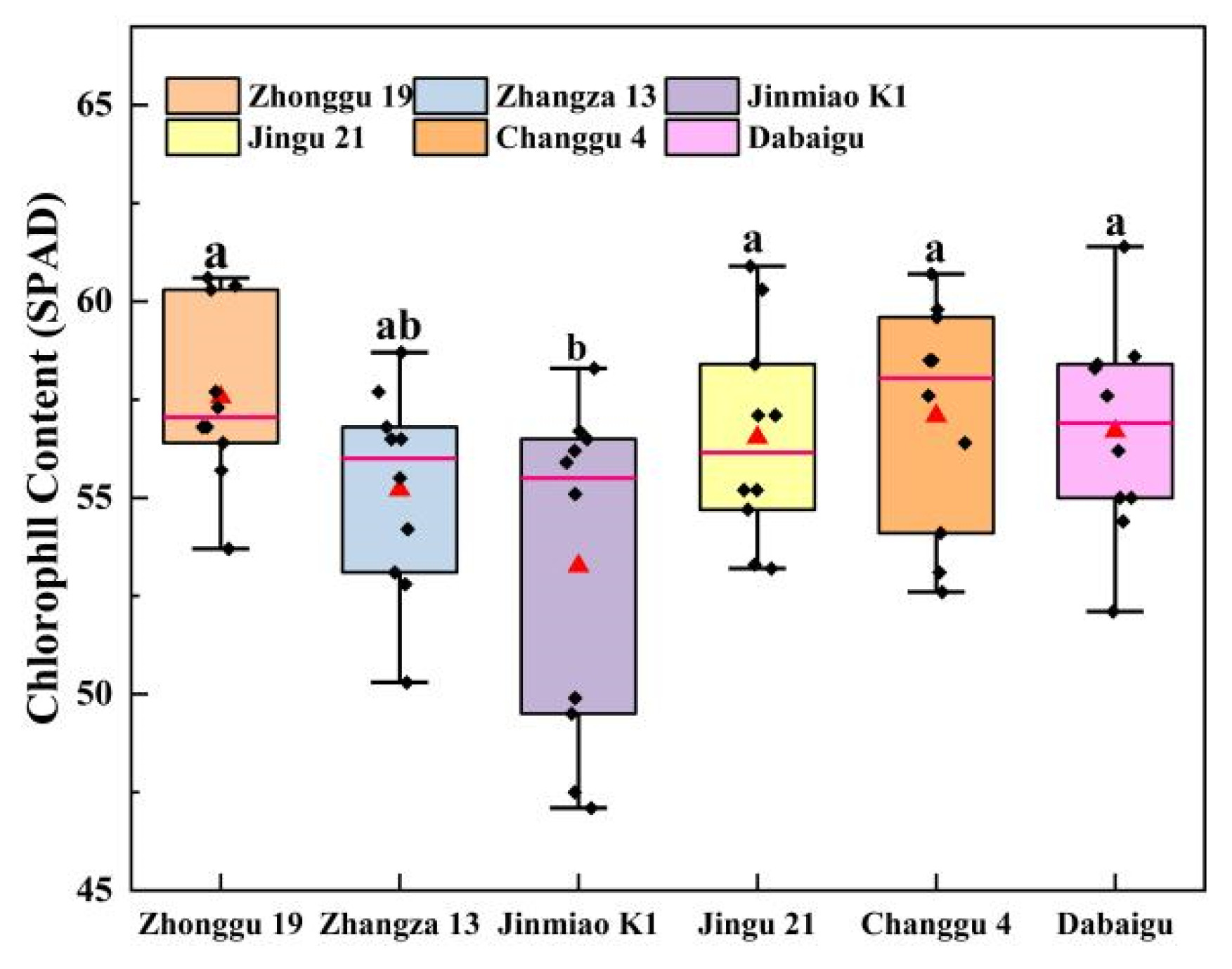
2.2. Comparison of Chlorophyll Fluorescence Kinetics and Chlorophyll Content
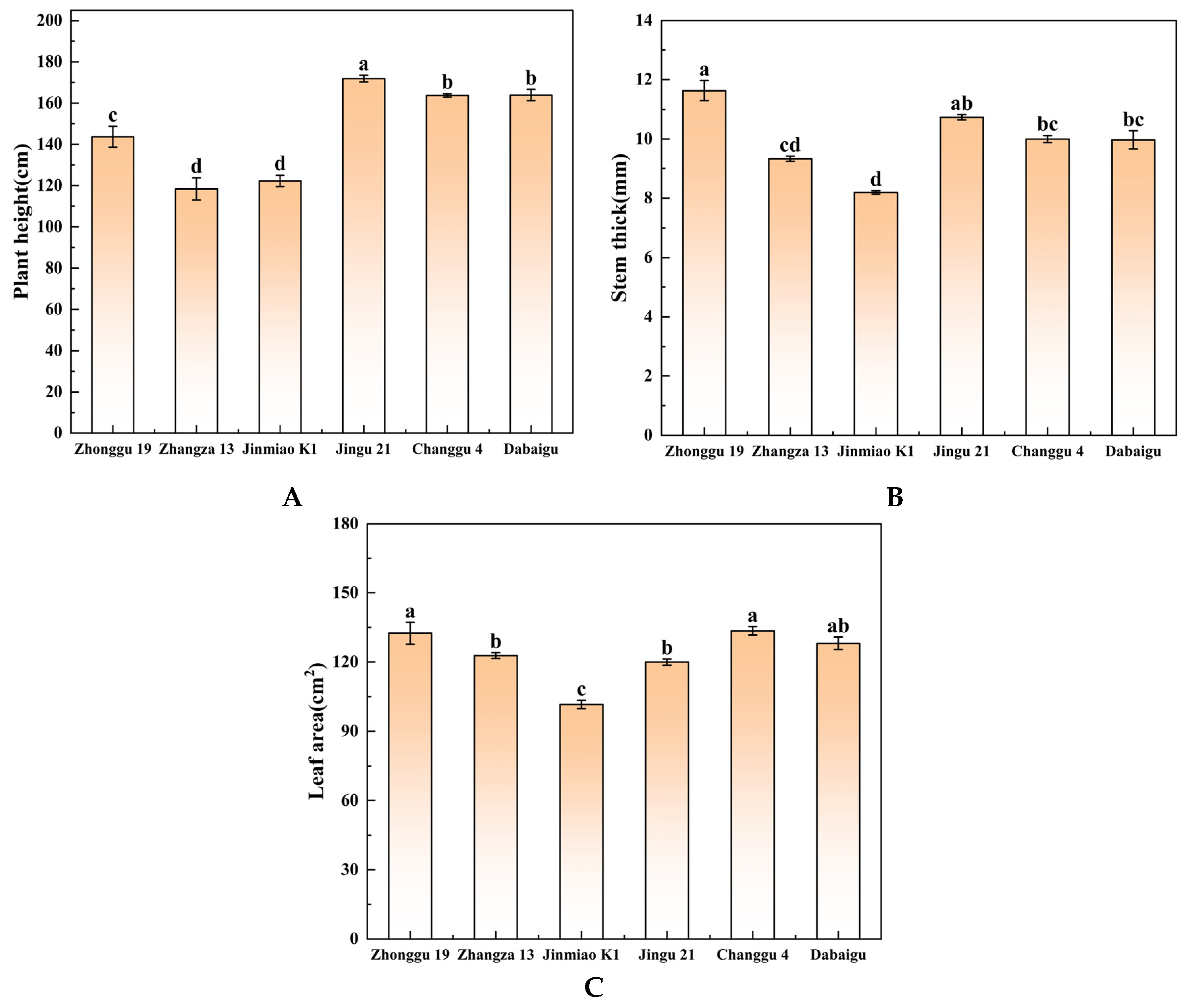
2.3. Comparison of Agronomic Traits
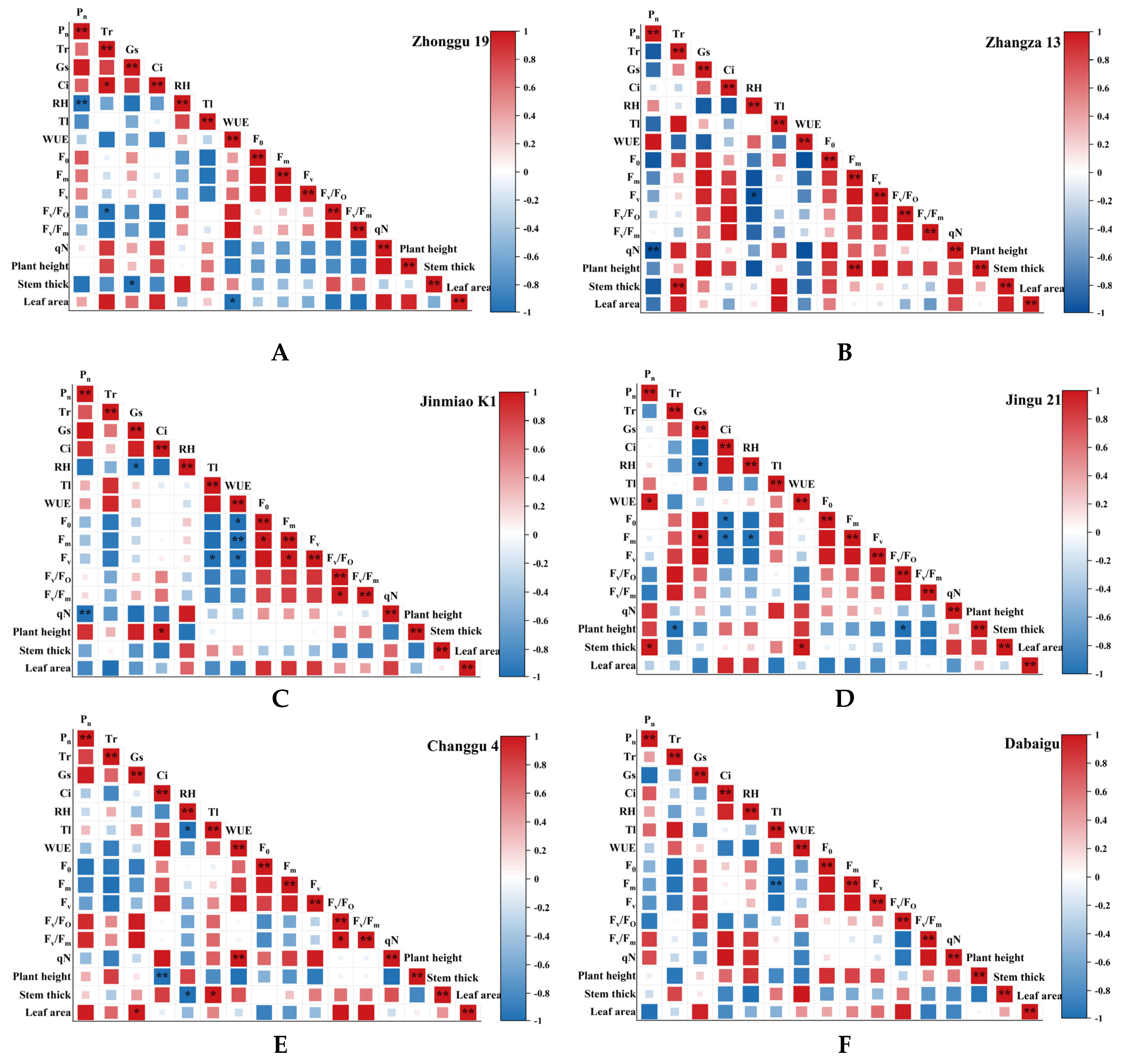
2.4. Correlation Analysis
3. Discussion
3.1. Diurnal Variation of Photosynthesis and Cultivar Specificity
3.2. Coordination Between Stomatal Behavior and Water Use
3.3. Synergistic Effects of Morphological Traits and Photosynthetic Performance
3.4. Linkage Between Chlorophyll Fluorescence Kinetics and Photoprotective Mechanisms
3.5. Environmental Adaptation
4. Materials and Methods
4.1. Test Materials
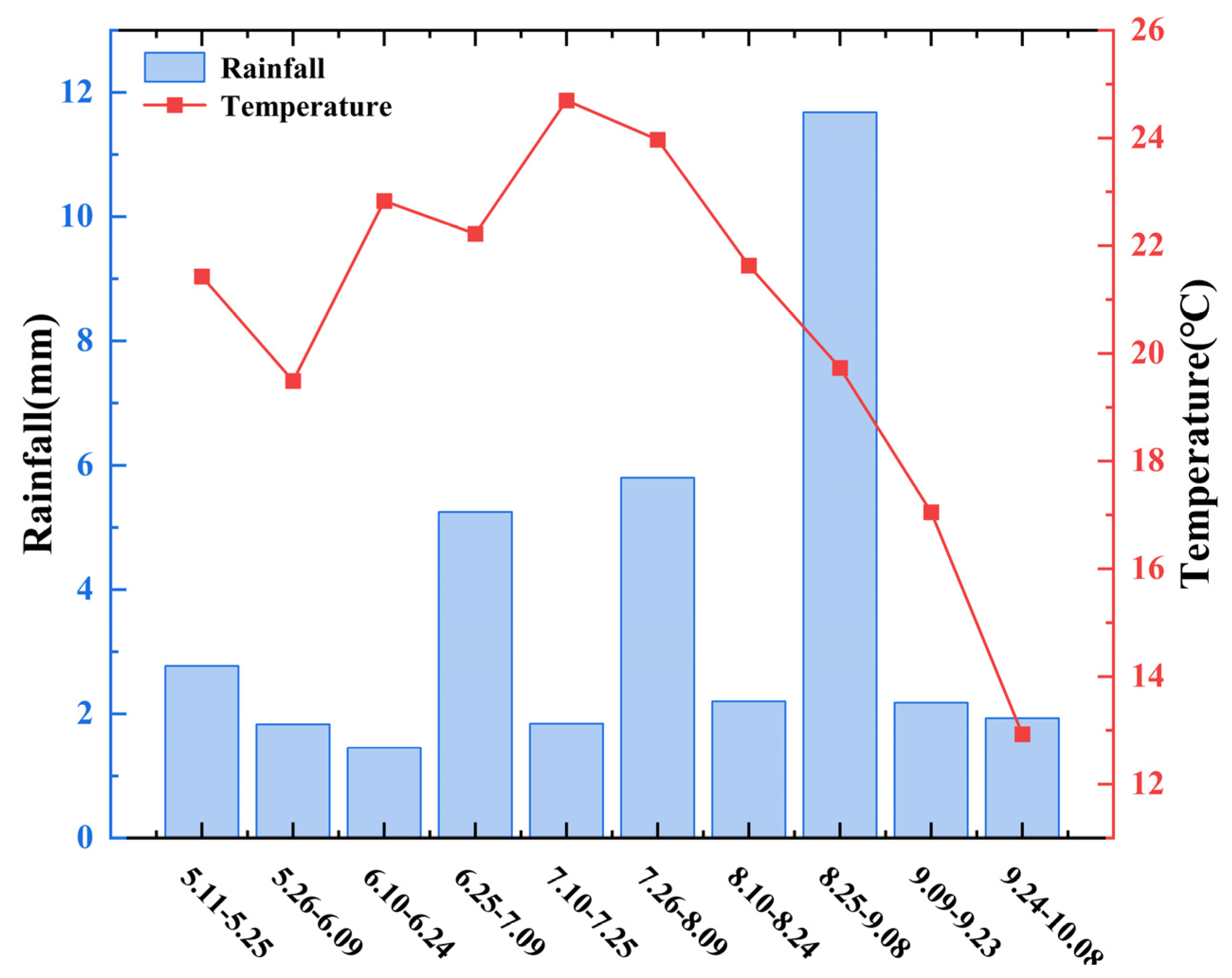
4.2. Experimental Design
4.3. Determination Indicators and Methods
4.3.1. Determination of Agronomic Traits
4.3.2. Determination of Leaf Photosynthetic Data
4.3.3. Determination of Chlorophyll Fluorescence Data
Fv/Fm = (Fm − F0)/Fm
Fv/F0 = (Fm − F0)/F0
4.3.4. Determination of SPAD in Leaves
4.4. Data Processing
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, G.; Shi, S.; Wang, C.; Niu, Z.; Chai, Y.; Zhi, H.; Diao, X. Molecular diversity and population structure of Chinese green foxtail [Setaria viridis (L.) Beauv.] revealed by microsatellite analysis. J. Exp. Bot. 2013, 64, 3645–3656. [Google Scholar] [CrossRef]
- Doust, A.N.; Kellogg, E.A.; Devos, K.M.; Bennetzen, J.L. Foxtail millet: A sequence-driven grass model system. Plant Physiol. 2009, 149, 137–141. [Google Scholar] [CrossRef]
- Chen, H.; Ge, W. Identification, Molecular Characteristics, and Evolution of GRF Gene Family in Foxtail Millet (Setaria italica L.). Front. Genet. 2022, 12, 727674. [Google Scholar] [CrossRef]
- Lata, C.; Gupta, S.; Prasad, M. Foxtail millet: A model crop for genetic and genomic studies in bioenergy grasses. Crit. Rev. Biotechnol. 2013, 33, 328–343. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, N.; Yan, J.; Zhang, S. Effects of soft rock and biochar applications on millet (Setaria italica L.) crop performance in sandy soil. Agronomy 2020, 10, 669. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Yang, Y.; Xu, Y.; Fan, X.; Guo, Z.; Han, Y.; Lin, X. Physiological Mechanisms and Core Genes in Response to Saline-Alkali Stress in Foxtail Millet (Setaria italica L.). Biomolecules 2025, 15, 859. [Google Scholar] [CrossRef]
- Luo, W.; Tang, Y.; Li, S.; Zhang, L.; Liu, Y.; Zhang, R.; Diao, X.; Yu, J. The m6A reader SiYTH1 enhances drought tolerance by affecting the messenger RNA stability of genes related to stomatal closure and reactive oxygen species scavenging in Setaria italica. J. Integr. Plant Biol. 2023, 65, 2569–2586. [Google Scholar] [CrossRef]
- He, Q.; Tang, S.; Zhi, H.; Chen, J.; Zhang, J.; Liang, H.; Alam, O.; Li, H.; Zhang, H.; Xing, L.; et al. A graph-based genome and pan-genome variation of the model plant Setaria. Nat. Genet. 2023, 55, 1232–1242. [Google Scholar] [CrossRef]
- Tang, S.; Zhao, Z.; Liu, X.; Sui, Y.; Zhang, D.; Zhi, H.; Gao, Y.; Zhang, H.; Zhang, L.; Wang, Y.; et al. An E2-E3 pair contributes to seed size control in grain crops. Nat. Commun. 2013, 14, 3091. [Google Scholar] [CrossRef]
- Xu, B.; Gao, X.; Dong, K.; Li, X.; Yang, P.; Yang, T.; Feng, B. Grain protein content comparison and proteomic analysis of foxtail millet (Setaria italica L.) seed response to different drought stress levels. Acta Physiol. Plant. 2020, 42, 20. [Google Scholar] [CrossRef]
- Langham, D.G. The effect of light on growth habit of plants. Am. J. Bot. 1941, 28, 951–956. [Google Scholar] [CrossRef]
- von Caemmerer, S.; Ghannoum, O.; Furbank, R.T. C4 photosynthesis: 50 years of discovery and innovation. J. Exp. Bot. 2017, 68, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Kromdijk, J.; Głowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 2016, 354, 857–861. [Google Scholar] [CrossRef]
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-regulated plant growth and development. Curr. Top. Dev. Biol. 2010, 91, 29–66. [Google Scholar] [PubMed]
- Nasar, J.; Wang, G.-Y.; Ahmad, S.; Muhammad, I.; Zeeshan, M.; Gitari, H.; Adnan, M.; Fahad, S.; Bin Khalid, M.H.; Zhou, X.-B.; et al. Nitrogen fertilization coupled with iron foliar application improves the photosynthetic characteristics, photosynthetic nitrogen use efficiency, and the related enzymes of maize crops under different planting patterns. Front. Plant Sci. 2022, 13, 988055. [Google Scholar] [CrossRef]
- Leakey, A.D.B.; Uribelarrea, M.; Ainsworth, E.A.; Naidu, S.L.; Rogers, A.; Ort, D.R.; Long, S.P. Photosynthesis, productivity, and yield of maize are not affected by open-air elevation of CO2 concentration in the absence of drought. Plant Physiol. 2006, 140, 779–790. [Google Scholar] [CrossRef]
- Fryer, M.J.; Oxborough, K.; Martin, B.; Ort, D.R.; Baker, N.R. Factors associated with depression of photosynthetic quantum efficiency in maize at low growth temperature. Plant Physiol. 1995, 108, 761–767. [Google Scholar] [CrossRef]
- Zhang, Q.; Tian, S.; Chen, G.; Tang, Q.; Zhang, Y.; Fleming, A.J.; Zhu, X.; Wang, P. Regulatory NADH dehydrogenase-like complex optimizes C4 photosynthetic carbon flow and cellular redox in maize. New Phytol. 2024, 241, 82–101. [Google Scholar] [CrossRef]
- Pengelly, J.J.L.; Sirault, X.R.R.; Tazoe, Y.; Evans, J.R.; Furbank, R.T.; von Caemmerer, S. Growth of the C4 dicot Flaveria bidentis: Photosynthetic acclimation to low light through shifts in leaf anatomy and biochemistry. J. Exp. Bot. 2010, 61, 4109–4122. [Google Scholar] [CrossRef]
- Langdale, J.A. C4 cycles: Past, present, and future research on C4 photosynthesis. Plant Cell 2011, 23, 3879–3892. [Google Scholar] [CrossRef]
- Borba, A.R.; Reyna-Llorens, I.; Dickinson, P.J.; Steed, G.; Gouveia, P.; Górska, A.M.; Gomes, C.; Kromdijk, J.; Webb, A.A.R.; Saibo, N.J.M.; et al. Compartmentation of photosynthesis gene expression in C4 maize depends on time of day. Plant Physiol. 2023, 193, 2306–2320. [Google Scholar] [CrossRef]
- Li, X.; Wang, P.; Li, J.; Wei, S.; Yan, Y.; Yang, J.; Zhao, M.; Langdale, J.A.; Zhou, W. Maize GOLDEN2-LIKE genes enhance biomass and grain yields in rice by improving photosynthesis and reducing photoinhibition. Commun. Biol. 2020, 3, 151. [Google Scholar] [CrossRef]
- Harrison, E.L.; Arce Cubas, L.; Gray, J.E.; Hepworth, C. The influence of stomatal morphology and distribution on photosynthetic gas exchange. Plant J. 2020, 101, 768–779. [Google Scholar] [CrossRef]
- Morgan, J.M. Osmoregulation and Water Stress in Higher Plants. Annu. Rev. Plant Biol. 2003, 35, 299–319. [Google Scholar] [CrossRef]
- Allen, L.H.; Kakani, V.G.; Vu, J.C.V.; Boote, K.J. Elevated CO2 increases water use efficiency by sustaining photosynthesis of water-limited maize and sorghum. J. Plant Physiol. 2011, 168, 1909–1918. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Jiang, Q.; Roche, D.; Monaco, T.A.; Durham, S. Gas exchange, chlorophyll fluorescence parameters and carbon isotope discrimination of 14 barley genetic lines in response to salinity. Field Crops Res. 2006, 96, 269–278. [Google Scholar] [CrossRef]
- Frani, M.; Mazur, M.; Volenik, M.; Brkić, J.; Imšić, D. Effect of plant density on agronomic traits and photosynthetic performance in the maize IBM population. Poljoprivreda 2015, 21, 36–40. [Google Scholar] [CrossRef]
- Csajbók, J.; Kutasy, E.; Pepó, P. Photosynthetic and agronomic traits of winter barley (Hordeum vulgare L.) varieties. Agronomy 2020, 10, 1999. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Araus, J.L.; Sánchez, C.; Cabrera-Bosquet, L. Is heterosis in maize mediated through better water use? New Phytol. 2010, 187, 392–406. [Google Scholar] [CrossRef]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef]
- Srivastava, A.C.; Khanna, Y.P.; Meena, R.C.; Pal, M.; Sengupta, U.K. Diurnal changes in photosynthesis, sugars, and nitrogen of wheat and mungbean grown under elevated CO2 concentration. Photosynthetica 2002, 40, 221–225. [Google Scholar] [CrossRef]
- Hurry, V.M.; Krol, M.; Öquist, G.; Huner, N.P. Effect of long-term photoinhibition on growth and photosynthesis of cold-hardened spring and winter wheat. Planta 1992, 188, 369–375. [Google Scholar] [CrossRef]
- Bashir, N.; Athar, H.-u.-R.; Kalaji, H.M.; Wróbel, J.; Mahmood, S.; Zafar, Z.U.; Ashraf, M. Is photoprotection of PSII one of the key mechanisms for drought tolerance in maize? Int. J. Mol. Sci. 2021, 22, 13490. [Google Scholar] [CrossRef]
- Murchie, E.H.; Niyogi, K.K. Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol. 2011, 155, 86–92. [Google Scholar] [CrossRef]
- Yang, Q.Y.; Zhang, Y.W.; Liu, N.Y.; Sun, H.; Zhang, S.B.; Timm, S.; Huang, W. Variation in photosynthetic efficiency among maize cultivars and its implications for breeding strategy. J. Exp. Bot. 2025, in press. [Google Scholar] [CrossRef]
- Qu, M.; Essemine, J.; Xu, J.; Ablat, G.; Perveen, S.; Wang, H.; Chen, K.; Zhao, Y.; Chen, G.; Chu, C.; et al. Alterations in stomatal response to fluctuating light increase biomass and yield of rice under drought conditions. Plant J. 2020, 104, 1334–1347. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Cramer, M.D.; Verboom, G.A. The importance of nutritional regulation of plant water flux. Oecologia 2009, 161, 15–24. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Z.; Hou, S. SPEECHLESS speaks loudly in stomatal development. Front. Plant Sci. 2020, 11, 114. [Google Scholar]
- Niinemets, Ü. A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol. Res. 2010, 25, 693–714. [Google Scholar] [CrossRef]
- Vialet-Chabrand, S.; Matthews, J.S.; Simkin, A.J.; Raines, C.A.; Lawson, T. Importance of fluctuations in light on plant photosynthetic acclimation. Plant Physiol. 2017, 173, 2163–2179. [Google Scholar] [CrossRef]
- Roháček, K. Chlorophyll fluorescence parameters: The definitions, photosynthetic meaning, and mutual relationships. Photosynthetica 2002, 40, 13–29. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef]
- Balaguer, L.; Barnes, J.D.; Panicucci, A.; Borland, A.M. Production and utilization of assimilates in wheat (Triticum aestivum L.) leaves exposed to elevated O3 and/or CO2. New Phytol. 1995, 129, 557–568. [Google Scholar] [CrossRef]
- Cazzaniga, S.; Dall’Osto, L.; Szaub, J.; Scibilia, L.; Ballottari, M.; Purton, S.; Bassi, R. Domestication of the green alga Chlorella sorokiniana: Reduction of antenna size improves light-use efficiency in a photobioreactor. Biotechnol. Biofuels. 2014, 7, 157. [Google Scholar] [CrossRef]
- Adireddy, R.G.; Anapalli, S.S.; Ojha, M.; Puppala, N.; Reddy, K.N. Photosynthetic responses to light levels in drought-tolerant novel peanut (Arachis hypogaea L.) genotypes. Sci. Rep. 2025, 15, 25032. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ibrahim, M.; Farid, M.; Adrees, M.; Bharwana, S.A.; Zia-Ur-Rehman, M.; Qayyum, M.F.; Abbas, F. Mechanisms of silicon-mediated alleviation of drought and salt stress in plants: A review. Environ. Sci. Pollut. Res. 2015, 22, 15416–15431. [Google Scholar] [CrossRef]
- Qiao, J.; Li, G.; Liu, M.; Zhang, T.; Wen, Y.; Wang, J.; Ren, J.; Du, H.; Hu, C.; Dong, S. Effects of different planting patterns on growth and yield components of foxtail millet. Agronomy 2025, 15, 840. [Google Scholar] [CrossRef]
- Huang, L.; Niinemets, Ü.; Jianzhong, M.; Schrader, J.; Wang, R.; Shi, P. Plant Age Has a Minor Effect on Non-Destructive Leaf Area Calculations in Moso Bamboo (Phyllostachys edulis). Symmetry 2021, 13, 369. [Google Scholar] [CrossRef]
- Li, F.; Xiao, J.; Chen, J.; Ballantyne, A.; Jin, K.; Li, B.; Abraha, M.; John, R. Global water use efficiency saturation due to increased vapor pressure deficit. Science 2023, 381, 672–677. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A. Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]

| Treatment | Average Pn | Average Tr | WUE |
|---|---|---|---|
| (µmol·m−2·s−1) | (mmol·m−2·s−1) | (µmol·CO2/mmol·H2O) | |
| Zhonggu 19 | 18.49 ± 0.24 c | 2.38 ± 0.08 c | 7.77 ± 0.18 a |
| Zhangza 13 | 16.29 ± 0.52 d | 2.64 ± 0.03 bc | 6.17 ± 0.24 b |
| Jinmiao K1 | 12.87 ± 0.16 e | 2.40 ± 0.10 c | 5.36 ± 0.15 c |
| Jingu 21 | 20.72 ± 0.31 b | 2.72 ± 0.07 b | 7.62 ± 0.26 a |
| Changgu 4 | 18.74 ± 0.14 c | 2.95 ± 0.16 a | 6.35 ± 0.28 b |
| Dabaigu | 22.99 ± 0.23 a | 2.86 ± 0.03 ab | 8.04 ± 0.05 a |
| Treatment | Ci | Gs | RH | Tl |
|---|---|---|---|---|
| (µmol·m−2·s−1) | (mmol·m−2·s−1) | (µmol·CO2/mmol·H2O) | °C | |
| Zhonggu 19 | 196.43 ± 2.05 c | 92.94 ± 3.83 c | 38.33 ± 0.15 a | 35.08 ± 0.91 b |
| Zhangza 13 | 191.76 ± 0.85 c | 132.33 ± 3.01 a | 33.38 ± 0.09 c | 35.86 ± 0.70 b |
| Jinmiao K1 | 182.95 ± 1.70 d | 74.96 ± 1.83 d | 34.21 ± 0.06 bc | 36.14 ± 0.80 ab |
| Jingu 21 | 206.80 ± 2.05 b | 111.30 ± 8.30 b | 35.50 ± 0.24 bc | 37.51 ± 0.92 ab |
| Changgu 4 | 230.42 ± 4.12 a | 104.83 ± 7.76 bc | 37.27 ± 0.05 a | 36.89 ± 0.82 ab |
| Dabaigu | 211.55 ± 2.80 b | 127.90 ± 2.61 a | 36.74 ± 0.36 b | 38.08 ± 1.05 a |
| Principal Component Number | Eigenvalue | Percentage of Variance (%) | Cumulative (%) |
|---|---|---|---|
| 1 | 5.99356 | 37.45978 | 37.45978 |
| 2 | 2.99472 | 18.717 | 56.17678 |
| 3 | 2.42137 | 15.13353 | 71.31031 |
| 4 | 1.93217 | 12.07604 | 83.38635 |
| Total P | Total K | Total N | Available K | Available N | Available P | Organic Matter | PH |
|---|---|---|---|---|---|---|---|
| (g/kg) | (g/kg) | (g/kg) | (mg/kg) | (mg/kg) | (mg/kg) | (g/kg) | Value |
| 0.59 | 15.50 | 0.62 | 89.00 | 69.10 | 21.70 | 11.20 | 9.04 |
| Cultivar | Origin |
|---|---|
| Zhonggu 19 | Institute of Crop Sciences, Chinese Academy of Agricultural Sciences |
| Zhangza 13 | Zhangjiakou Academy of Agricultural Sciences |
| Jinmiao K1 | Chifeng Agriculture and Animal Husbandry Science Research Institute |
| Jingu 21 | Economic Crops Research Institute, Shanxi Agricultural University |
| Changgu 4 | Foxtail Millet Research Institute, Shanxi Agricultural University |
| Dabaigu | Guangling, local variety |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Wang, C.; Yang, X.; Ji, T.; Shang, S.; Li, S.; Wen, Y.; Ren, J.; Li, X.; Zhao, J.; et al. Adaptability of Foxtail Millet Varieties Based on Photosynthetic Performance and Agronomic Traits. Plants 2025, 14, 2502. https://doi.org/10.3390/plants14162502
Gao S, Wang C, Yang X, Ji T, Shang S, Li S, Wen Y, Ren J, Li X, Zhao J, et al. Adaptability of Foxtail Millet Varieties Based on Photosynthetic Performance and Agronomic Traits. Plants. 2025; 14(16):2502. https://doi.org/10.3390/plants14162502
Chicago/Turabian StyleGao, Shulin, Chenxu Wang, Xu Yang, Tianyu Ji, Suqi Shang, Shuo Li, Yinyuan Wen, Jianhong Ren, Xiaorui Li, Juan Zhao, and et al. 2025. "Adaptability of Foxtail Millet Varieties Based on Photosynthetic Performance and Agronomic Traits" Plants 14, no. 16: 2502. https://doi.org/10.3390/plants14162502
APA StyleGao, S., Wang, C., Yang, X., Ji, T., Shang, S., Li, S., Wen, Y., Ren, J., Li, X., Zhao, J., Hu, C., Yuan, X., & Dong, S. (2025). Adaptability of Foxtail Millet Varieties Based on Photosynthetic Performance and Agronomic Traits. Plants, 14(16), 2502. https://doi.org/10.3390/plants14162502







