Soil Drenching with Wood Distillate Modifies the Nutritional Properties of Chickpea (Cicer arietinum L.) Seeds by Increasing the Protein Content and Inducing Targeted Changes in the Proteomic Profile
Abstract
1. Introduction
2. Results
2.1. Yield Parameters
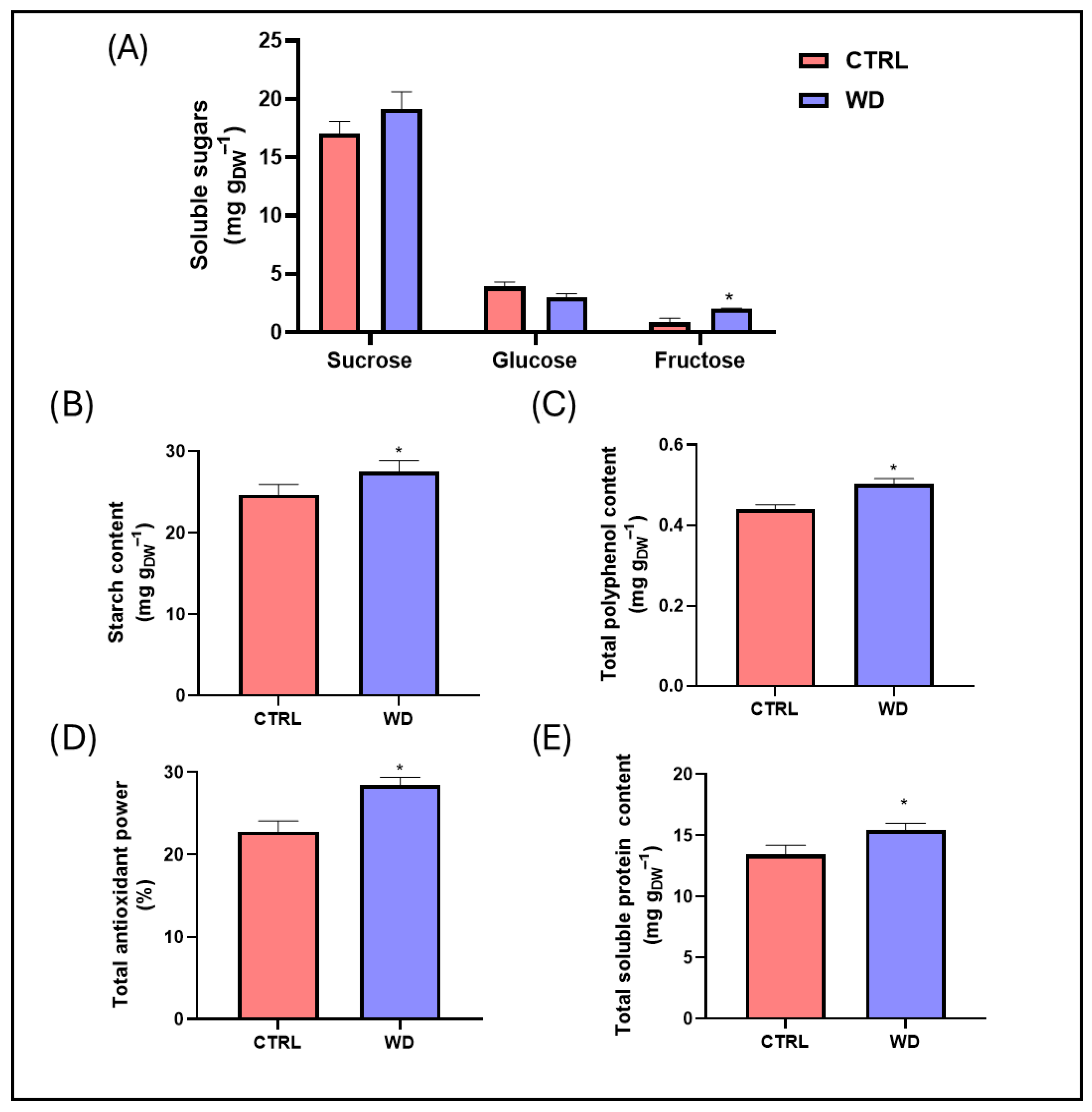
2.2. Flour Nutritional Parameters
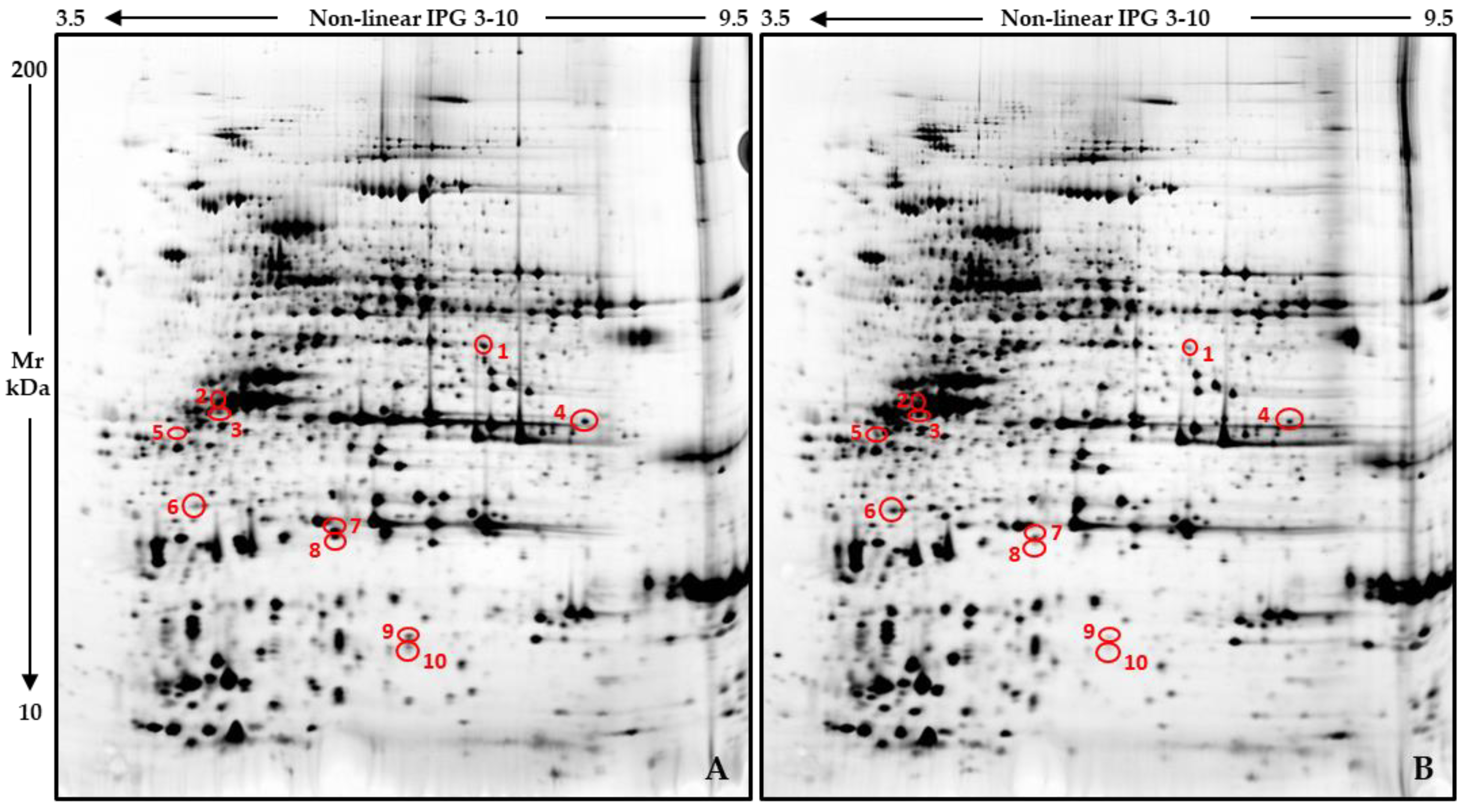
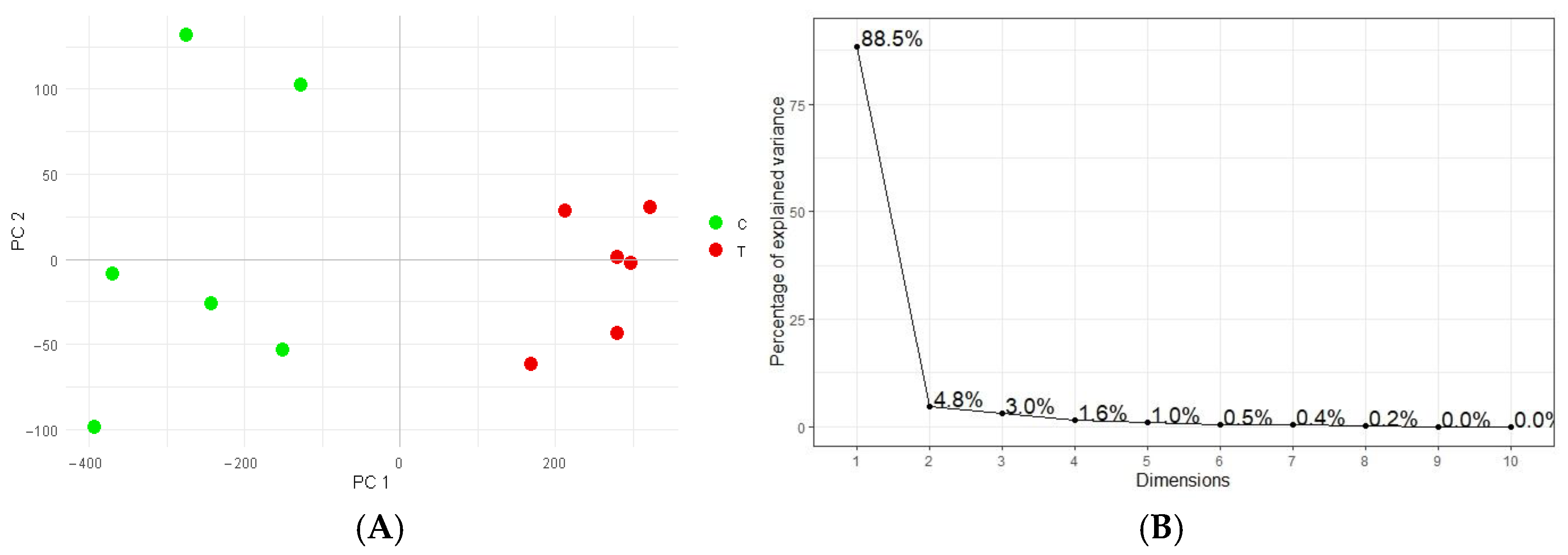
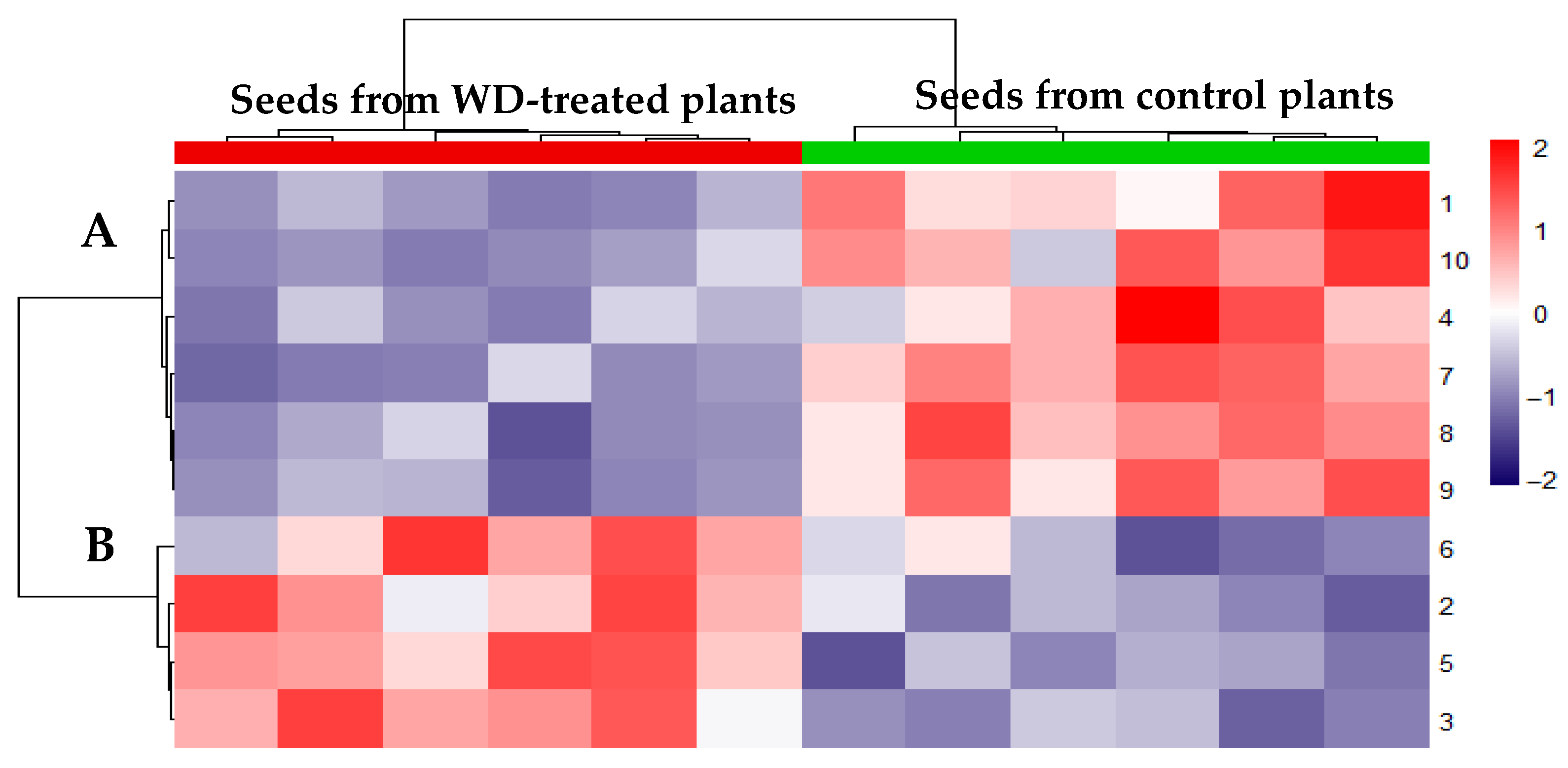
2.3. WD-Induced Proteomic Profile Modulation
3. Discussion
4. Materials and Methods
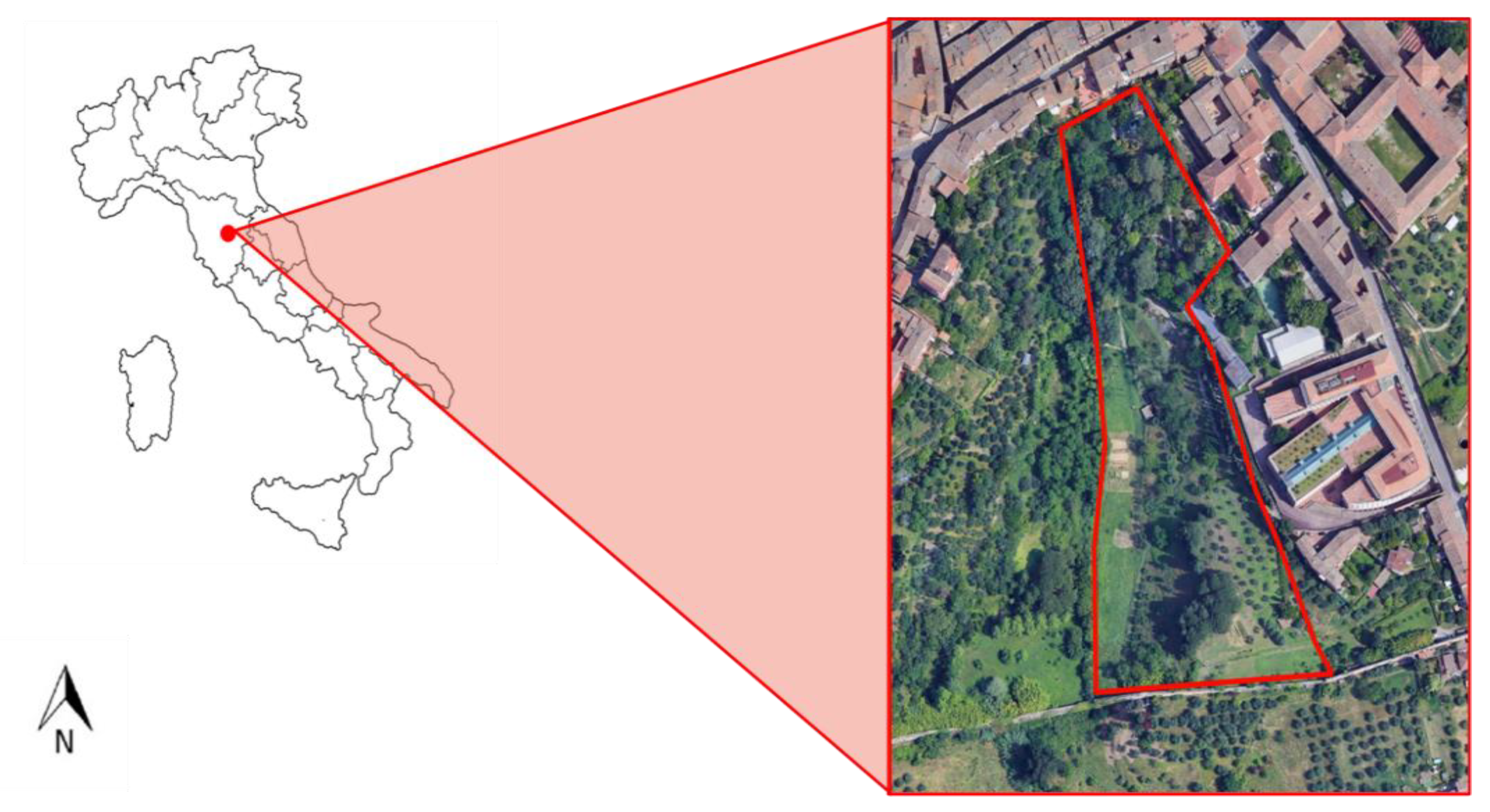
4.1. Experimental Design
4.2. Yield Parameters
4.3. Flour Nutritional Parameters
4.3.1. Mineral Elements
4.3.2. Soluble Sugars
4.3.3. Starch
4.3.4. Total Antioxidant Power
4.3.5. Total Polyphenols
4.3.6. Total Soluble Proteins
4.3.7. Statistical Analysis for Yield, Biochemical, Micro-, and Macronutrient Parameters
4.4. Protein Sample Extraction for Proteomics Analyses
4.5. Proteomic Analyses
4.6. Mass Spectrometry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.L.; Chibbar, R.N. Nutritional quality and health benefits of chickpea (Cicer arietinum L.): A review. Br. J. Nutr. 2012, 108 (Suppl. S1), S11–S26. [Google Scholar] [CrossRef] [PubMed]
- Floret, C.; Monnet, A.F.; Micard, V.; Walrand, S.; Michon, C. Replacement of animal proteins in food: How to take advantage of nutritional and gelling properties of alternative protein sources. Crit. Rev. Food Sci. Nutr. 2023, 63, 920–946. [Google Scholar] [CrossRef] [PubMed]
- Quintieri, L.; Nitride, C.; De Angelis, E.; Lamonaca, A.; Pilolli, R.; Russo, F.; Monaci, L. Alternative protein sources and novel foods: Benefits, food applications and safety issues. Nutrients 2023, 15, 1509. [Google Scholar] [CrossRef]
- Koneswaran, G.; Nierenberg, D. Global farm animal production and global warming: Impacting and mitigating climate change. Environ. Health Perspect. 2008, 116, 578–582. [Google Scholar] [CrossRef]
- McMichael, A.J.; Powles, J.W.; Butler, C.D.; Uauy, R. Food, livestock production, energy, climate change, and health. Lancet 2007, 370, 1253–1263. [Google Scholar] [CrossRef]
- Lal, R. Improving soil health and human protein nutrition by pulses-based cropping systems. Adv. Agron. 2017, 145, 167–204. [Google Scholar]
- Mrabet, R. Sustainable agriculture for food and nutritional security. In Sustainable Agriculture and the Environment; Academic Press: Cambridge, MA, USA, 2023; pp. 25–90. [Google Scholar]
- Nadathur, S.; Wanasundara, J.P.; Scanlin, L. Feeding the globe nutritious food in 2050: Obligations and ethical choices. In Sustainable Protein Sources; Academic Press: San Diego, CA, USA, 2024; pp. 649–668. [Google Scholar]
- Hossain, M.E.; Shahrukh, S.; Hossain, S.A. Chemical fertilizers and pesticides: Impacts on soil degradation, groundwater, and human health in Bangladesh. In Environmental Degradation: Challenges and Strategies for Mitigation; Springer: Cham, Switzerland, 2022; pp. 63–92. [Google Scholar]
- Tripathi, S.; Srivastava, P.; Devi, R.S.; Bhadouria, R. Influence of synthetic fertilizers and pesticides on soil health and soil microbiology. In Agrochemicals Detection, Treatment and Remediation; Butterworth-Heinemann: Oxford, UK, 2020; pp. 25–54. [Google Scholar]
- Prashar, P.; Shah, S. Impact of fertilizers and pesticides on soil microflora in agriculture. In Sustainable Agriculture Reviews; Springer: Cham, Switzerland, 2016; Volume 19, pp. 331–361. [Google Scholar]
- Grewal, A.; Abbey, L.; Gunupuru, L.R. Production, prospects and potential application of pyroligneous acid in agriculture. J. Anal. Appl. Pyrolysis 2018, 135, 152–159. [Google Scholar] [CrossRef]
- Lu, S.N.; Zhou, L.; Wang, L.J.; Liu, L.; Zhang, H.M. Research progress on preparation of wood vinegar and its application in agriculture. J. Shanxi Agric. Sci. 2023, 51, 225–232. [Google Scholar]
- Wei, Q.; Ma, X.; Zhao, Z.; Zhang, S.; Liu, S. Antioxidant activities and chemical profiles of pyroligneous acids from walnut shell. J. Anal. Appl. Pyrolysis 2010, 88, 149–154. [Google Scholar] [CrossRef]
- Gomez, J.P.; Velez, J.P.A.; Pinzon, M.A.; Arango, J.A.M.; Muriel, A.P. Chemical characterization and antiradical properties of pyroligneous acid from a preserved bamboo, Guadua angustifolia Kunth. Braz. Arch. Biol. Technol. 2021, 64, e21190730. [Google Scholar] [CrossRef]
- Becagli, M.; Arduini, I.; Cantini, V.; Cardelli, R. Soil and foliar applications of wood distillate differently affect soil properties and field bean traits in preliminary field tests. Plants 2022, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G.; Fedeli, R.; Mariotti, L.; Pisuttu, C.; Nali, C.; Pellegrini, E.; Loppi, S. Foliar application of wood distillate protects basil plants against ozone damage by preserving membrane integrity and triggering antioxidant mechanisms. Agronomy 2024, 14, 1233. [Google Scholar] [CrossRef]
- Mungkunkamchao, T.; Kesmala, T.; Pimratch, S.; Toomsan, B.; Jothityangkoon, D. Wood vinegar and fermented bioextracts: Natural products to enhance growth and yield of tomato (Solanum lycopersicum L.). Sci. Hortic. 2013, 154, 66–72. [Google Scholar] [CrossRef]
- Akley, E.K.; Ampim, P.A.; Obeng, E.; Sanyare, S.; Yevu, M.; Owusu Danquah, E.; Seidu, A.F. Wood vinegar promotes soil health and the productivity of cowpea. Agronomy 2023, 13, 2497. [Google Scholar] [CrossRef]
- Lau, S.E.; Lim, L.W.T.; Hamdan, M.F.; Chan, C.; Saidi, N.B.; Ong-Abdullah, J.; Tan, B.C. Enhancing plant resilience to abiotic stress: The power of biostimulants. Phyton 2025, 94, 1–31. [Google Scholar]
- Zhu, K.; Gu, S.; Liu, J.; Luo, T.; Khan, Z.; Zhang, K.; Hu, L. Wood vinegar as a complex growth regulator promotes the growth, yield, and quality of rapeseed. Agronomy 2021, 11, 510. [Google Scholar] [CrossRef]
- Ahadiyat, Y.R.; Hadi, S.N.; Herliana, O. Application of wood vinegar coconut shell and NPK fertilizer to maintain sustainable agriculture of upland rice production. J. Degrad. Min. Lands Manag. 2018, 5, 1245. [Google Scholar] [CrossRef]
- Fedeli, R.; Marotta, L.; Frattaruolo, L.; Panti, A.; Carullo, G.; Fusi, F.; Loppi, S. Nutritionally enriched tomatoes (Solanum lycopersicum L.) grown with wood distillate: Chemical and biological characterization for quality assessment. J. Food Sci. 2023, 88, 5324–5338. [Google Scholar] [CrossRef]
- Carril, P.; Bianchi, E.; Cicchi, C.; Coppi, A.; Dainelli, M.; Gonnelli, C.; Colzi, I. Effects of wood distillate (pyroligneous acid) on the yield parameters and mineral composition of three leguminous crops. Environments 2023, 10, 126. [Google Scholar] [CrossRef]
- Noel, R.; Schueller, M.J.; Ferrieri, R.A. Radiocarbon flux measurements provide insight into why a pyroligneous acid product stimulates plant growth. Int. J. Mol. Sci. 2024, 25, 4207. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Melis, A. Solar energy conversion efficiencies in photosynthesis: Minimizing the chlorophyll antennae to maximize efficiency. Plant Sci. 2009, 177, 272–280. [Google Scholar] [CrossRef]
- Shah, L.; Yahya, M.; Shah, S.M.A.; Nadeem, M.; Ali, A.; Ali, A.; Ma, C. Improving lodging resistance: Using wheat and rice as classical examples. Int. J. Mol. Sci. 2019, 20, 4211. [Google Scholar] [CrossRef]
- Nandal, V.; Solanki, M. The Zn as a vital micronutrient in plants. J. Microbiol. Biotechnol. Food Sci. 2021, 11, e4026. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Reid, R.J.; Ayling, S.M. Phosphorus uptake by plants: From soil to cell. Plant Physiol. 1998, 116, 447–453. [Google Scholar] [CrossRef]
- Vandemark, G.J.; Grusak, M.A.; McGee, R.J. Mineral concentrations of chickpea and lentil cultivars and breeding lines grown in the US Pacific Northwest. Crop J. 2018, 6, 253–262. [Google Scholar] [CrossRef]
- Iqbal, A.; Ateeq, N.; Khalil, I.A.; Perveen, S.; Saleemullah, S. Physicochemical characteristics and amino acid profile of chickpea cultivars grown in Pakistan. J. Foodserv. 2006, 17, 94–101. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Iqbal, S.; Ahmad, S.; Imran, M.; Niaz, A.; Bhanger, M.I. Nutritional and compositional study of desi chickpea (Cicer arietinum L.) cultivars grown in Punjab, Pakistan. Food Chem. 2007, 105, 1357–1363. [Google Scholar] [CrossRef]
- Englyst, K.N.; Vinoy, S.; Englyst, H.N.; Lang, V. Glycaemic index of cereal products explained by their content of rapidly and slowly available glucose. Br. J. Nutr. 2003, 89, 329–339. [Google Scholar] [CrossRef]
- Foster-Powell, K.; Miller, J.B. International tables of glycemic index. Am. J. Clin. Nutr. 1995, 62, 871S–890S. [Google Scholar] [CrossRef]
- Singh, M.; Manickavasagan, A.; Shobana, S.; Mohan, V. Glycemic index of pulses and pulse-based products: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 1567–1588. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycaemic level: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Dhital, S.; Warren, F.J.; Butterworth, P.J.; Ellis, P.R.; Gidley, M.J. Mechanisms of starch digestion by α-amylase—Structural basis for kinetic properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 875–892. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, R.; Vannini, A.; Guarnieri, M.; Monaci, F.; Loppi, S. Bio-based solutions for agriculture: Foliar application of wood distillate alone and in combination with other plant-derived corroborants results in different effects on lettuce (Lactuca sativa L.). Biology 2022, 11, 404. [Google Scholar] [CrossRef]
- Lu, X.; Jiang, J.; He, J.; Sun, K.; Sun, Y. Pyrolysis of Cunninghamia lanceolata waste to produce wood vinegar and its effect on the seeds germination and root growth of wheat. BioResources 2019, 14, 8002–8017. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Scarpa, E.S.; Antonelli, A.; Balercia, G.; Sabatelli, S.; Maggi, F.; Caprioli, G.; Micucci, M. Antioxidant, anti-inflammatory, anti-diabetic, and pro-osteogenic activities of polyphenols for the treatment of two different chronic diseases: Type 2 diabetes mellitus and osteoporosis. Biomolecules 2024, 14, 836. [Google Scholar] [CrossRef]
- Trinidad, T.P.; Mallillin, A.C.; Loyola, A.S.; Sagum, R.S.; Encabo, R.R. The potential health benefits of legumes as a good source of dietary fibre. Br. J. Nutr. 2010, 103, 569–574. [Google Scholar] [CrossRef]
- Maphosa, Y.; Jideani, V.A. The role of legumes in human nutrition. In Functional Food—Improve Health Through Adequate Food; InTech: Rijeka, Croatia, 2017; Volume 1, p. 13. [Google Scholar]
- Martín-Cabrejas, M.Á. (Ed.) Legumes: Nutritional Quality, Processing and Potential Health Benefits; Royal Society of Chemistry: Cambridge, UK, 2019; Volume 8. [Google Scholar]
- Boukid, F. Chickpea (Cicer arietinum L.) protein as a prospective plant-based ingredient: A review. Int. J. Food Sci. Technol. 2021, 56, 5435–5444. [Google Scholar] [CrossRef]
- Di Francesco, A.; De Santis, M.A.; Lanzoni, A.; Pittalà, M.G.G.; Saletti, R.; Flagella, Z.; Cunsolo, V. Mass spectrometry characterization of the SDS-PAGE protein profile of legumins and vicilins from chickpea seed. Foods 2024, 13, 887. [Google Scholar] [CrossRef]
- Boye, J.I.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Day, L. Proteins from land plants—Potential resources for human nutrition and food security. Trends Food Sci. Technol. 2013, 32, 25–42. [Google Scholar] [CrossRef]
- Grasso, N.; Lynch, N.L.; Arendt, E.K.; O’Mahony, J.A. Chickpea protein ingredients: A review of composition, functionality, and applications. Compr. Rev. Food Sci. Food Saf. 2021, 21, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Shevkani, K.; Singh, N.; Chen, Y.; Kaur, A.; Yu, L. Pulse proteins: Secondary structure, functionality and applications. J. Food Sci. Technol. 2019, 56, 2787–2798. [Google Scholar] [CrossRef]
- Fang, C.; Kanemaru, K.; Carvalho, W.S.P.; Fruehauf, K.R.; Zhang, S.; Das, P.P.; Serpe, M.J. Self-assembled poloxamer-legumin/vicilin nanoparticles for the nanoencapsulation and controlled release of folic acid. Int. J. Biol. Macromol. 2024, 268, 131646. [Google Scholar] [CrossRef]
- Clare, G.; Simões, P.; Costa, B.F.; Durães, L. Dual-function starch aerogels: Nutraceutical carriers for iron and folic acid delivery. J. Drug Deliv. Sci. Technol. 2024, 100, 106069. [Google Scholar] [CrossRef]
- Brokesh, A.M.; Gaharwar, A.K. Inorganic biomaterials for regenerative medicine. ACS Appl. Mater. Interfaces 2020, 12, 5319–5344. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef]
- Abu Risha, M.; Rick, E.M.; Plum, M.; Jappe, U. Legume allergens pea, chickpea, lentil, lupine and beyond. Curr. Allergy Asthma Rep. 2024, 24, 527–548. [Google Scholar] [CrossRef]
- Wangorsch, A.; Kulkarni, A.; Jamin, A.; Spiric, J.; Bräcker, J.; Brockmeyer, J.; Scheurer, S. Identification and characterization of IgE-reactive proteins and a new allergen (Cic a 1.01) from chickpea (Cicer arietinum). Mol. Nutr. Food Res. 2020, 64, e2000560. [Google Scholar] [CrossRef]
- Celletti, S.; Fedeli, R.; Ghorbani, M.; Aseka, J.M.; Loppi, S. Exploring sustainable alternatives: Wood distillate alleviates the impact of bioplastic in basil plants. Sci. Total Environ. 2023, 900, 166484. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, R.; Di Lella, L.A.; Loppi, S. Suitability of XRF for routine analysis of multi-elemental composition: A multi-standard verification. Methods Protoc. 2024, 7, 496. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, R.; Zhatkanbayeva, Z.; Loppi, S. Wood Distillate as a Solution for Growing Crops Under Water Deficiency. Crops 2025, 5, 22. [Google Scholar] [CrossRef]
- Lamaro, G.P.; Tsehaye, Y.; Girma, A.; Vannini, A.; Fedeli, R.; Loppi, S. Evaluation of yield and nutraceutical traits of orange-fleshed sweet potato storage roots in two agro-climatic zones of northern Ethiopia. Plants 2023, 12, 1319. [Google Scholar] [CrossRef]
- Fedeli, R.; Cruz, C.; Loppi, S.; Munzi, S. Hormetic effect of wood distillate on hydroponically grown lettuce. Plants 2024, 13, 447. [Google Scholar] [CrossRef]
- Fedeli, R.; Vannini, A.; Djatouf, N.; Celletti, S.; Loppi, S. Can lettuce plants grow in saline soils supplemented with biochar? Heliyon 2024, 10, e09724. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 12 May 2025).
- Bianchi, L.; Casini, S.; Vantaggiato, L.; Di Noi, A.; Carleo, A.; Shaba, E.; Caliani, I. A novel ex vivo approach based on proteomics and biomarkers to evaluate the effects of chrysene, MEHP, and PBDE-47 on loggerhead sea turtles (Caretta caretta). Int. J. Environ. Res. Public Health 2022, 19, 4369. [Google Scholar] [CrossRef]
- Oakley, B.R.; Kirsch, D.R.; Morris, N.R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal. Biochem. 1980, 105, 361–363. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Gharahdaghi, F.; Weinberg, C.R.; Meagher, D.A.; Imai, B.S.; Mische, S.M. Mass spectrometric identification of proteins from silver-stained polyacrylamide gel: A method for the removal of silver ions to enhance sensitivity. Electrophoresis 1999, 20, 601–605. [Google Scholar] [CrossRef]
| Yield Parameters | CTRL | WD |
|---|---|---|
| Plant height (cm) | 62 ± 13 | 75 ± 4 |
| Plant biomass (g) * | 25 ± 8 | 61 ± 8 |
| Number of pods * | 31 ± 5 | 77 ± 18 |
| Number of seeds * | 21 ± 4 | 52 ± 12 |
| Seed diameter (mm) * | 6.75 ± 0.15 | 7.14 ± 0.08 |
| Seed weight (mg) * | 0.28 ± 0.02 | 0.35 ± 0.02 |
| Mineral Element (mg kg−1) | CTRL | WD |
|---|---|---|
| Al | 340 ± 35 | 284 ± 40 |
| Ca * | 416 ± 32 | 287 ± 7 |
| Cu | 5.2 ± 0.4 | 4.8 ± 0.4 |
| Fe | 24 ± 1 | 22 ± 1 |
| K * | 5966 ± 98 | 5268 ± 127 |
| Mn | 18 ± 1 | 16 ± 1 |
| P * | 2707 ± 40 | 2568 ± 30 |
| S | 977 ± 25 | 938 ± 43 |
| Zn * | 29 ± 1 | 25 ± 1 |
| Spot n. a | UniprotKB b | UniProtKB ID c | Statistics d | Mascot Search e | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p-Value | Ctrl | WD | pI-MW | S | C | MP | E | |||
| 1 | Vicilin-like | A0A1S2XQ88_CICAR | 3.93 × 10−2 | 222.11 ± 70.13 | 63.10 ± 34.32 | 6.10-51,227 | 91 | 18 | 7/13 | 2.7 × 10−5 |
| 2 | Legumin | LEG_CICAR | 3.93 × 10−2 | 188.24 ± 36.03 | 317.08 ± 50.85 | 6.20-56,672 | 60 | 9 | 5/11 | 0.031 |
| 3 | Legumin | LEG_CICAR | 1.55 × 10−2 | 104.76 ± 21.03 | 234.54 ± 41.52 | 6.20-56,672 | 62 | 9 | 4/5 | 0.017 |
| 4 | Vicilin-like | A0A1S2XQ88_CICAR | 3.93 × 10−2 | 450.50 ± 79.44 | 257.03 ± 51.13 | 6.10-51,227 | 70 | 12 | 5/8 | 0.003 |
| 7 | Legumin J-like | A0A1S2XVG1_CICAR | 1.55 × 10−2 | 937.95 ± 208.57 | 300.54 ± 111.15 | 5.50-60,847 | 82 | 20 | 8/27 | 0.0002 |
| Parameter | Value | Measurement Method |
|---|---|---|
| TOC (% DW) | 58.03 | CHNS elemental analysis |
| TN (% DW) | 1.06 | CHNS elemental analysis |
| H (% DW) | 7.27 | CHNS elemental analysis |
| S (% DW) | 0.07 | CHNS elemental analysis |
| pH | 4 | UNI EN ISO 10523:2012 |
| Density (g mL−1) | 1.05 | |
| Flash point (°C) | >60 | ASTM D6450-16a |
| Total organic compounds (g L−1) | 33.8 | |
| Acidity (mg L−1) | 1289 | APAT CNR IRSA 2010 B Man 29 2003 |
| Organic acids (mg L−1) | 32.3 | |
| Acetic acid (mg L−1) | 21.5 | |
| Polyphenols (g L−1) | 24.5 | |
| Phenols (g L−1) | 3 | |
| PCBs (mg L−1) | <0.2 | CNR IRSA 24b Q 64 Vol 3 1988 |
| Hydrocarbons, C < 12 (mg L−1) | <0.1 | EPA 5021A 2014 + EPA 8015D 2003 |
| Hydrocarbons, C10–C40 (mg L−1) | <0.1 | UNI EN ISO 9377-2:2002 |
| 16 US-EPA PAHs (mg L−1) | EPA 3550C 2007 + EPA 8310 1986 | |
| Acenaphthene | <0.05 | |
| Acenaphthylene | <0.05 | |
| Anthracene | <0.05 | |
| Benzo[a]anthracene | <0.05 | |
| Benzo[a]pyrene | <0.05 | |
| Benzo[b]fluoranthene | <0.05 | |
| Benzo[g,h,i]perylene | <0.05 | |
| Benzo[k]fluoranthene | <0.05 | |
| Chrysene | <0.05 | |
| Dibenz[a,h]anthracene | <0.05 | |
| Fluoranthene | <0.05 | |
| Fluorene | <0.05 | |
| Indeno[1,2,3-cd]pyrene | <0.05 | |
| Naphthalene | <0.05 | |
| Phenanthrene | <0.05 | |
| Pyrene | <0.05 | |
| Macronutrients (mg L−1) | Alkaline melting + ICP-MS analysis | |
| Ca | 325.50 | |
| K | 23.49 | |
| Mg | 6.79 | |
| P | 7.28 | |
| Micronutrients (mg L−1) | Alkaline melting + ICP-MS analysis | |
| Cu | 0.18 | |
| Fe | 21.16 | |
| Mn | 0.58 | |
| Mo | 0.0007 | |
| Zn | 3.22 | |
| Other nutrients | Alkaline melting + ICP-MS analysis | |
| Al | 1.96 | |
| Ba | 0.06 | |
| Cr | 0.03 | |
| Na | 103.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Salvo, R.; Fedeli, R.; Carleo, A.; Bini, L.; Loppi, S.; Bianchi, L. Soil Drenching with Wood Distillate Modifies the Nutritional Properties of Chickpea (Cicer arietinum L.) Seeds by Increasing the Protein Content and Inducing Targeted Changes in the Proteomic Profile. Plants 2025, 14, 2046. https://doi.org/10.3390/plants14132046
De Salvo R, Fedeli R, Carleo A, Bini L, Loppi S, Bianchi L. Soil Drenching with Wood Distillate Modifies the Nutritional Properties of Chickpea (Cicer arietinum L.) Seeds by Increasing the Protein Content and Inducing Targeted Changes in the Proteomic Profile. Plants. 2025; 14(13):2046. https://doi.org/10.3390/plants14132046
Chicago/Turabian StyleDe Salvo, Rossana, Riccardo Fedeli, Alfonso Carleo, Luca Bini, Stefano Loppi, and Laura Bianchi. 2025. "Soil Drenching with Wood Distillate Modifies the Nutritional Properties of Chickpea (Cicer arietinum L.) Seeds by Increasing the Protein Content and Inducing Targeted Changes in the Proteomic Profile" Plants 14, no. 13: 2046. https://doi.org/10.3390/plants14132046
APA StyleDe Salvo, R., Fedeli, R., Carleo, A., Bini, L., Loppi, S., & Bianchi, L. (2025). Soil Drenching with Wood Distillate Modifies the Nutritional Properties of Chickpea (Cicer arietinum L.) Seeds by Increasing the Protein Content and Inducing Targeted Changes in the Proteomic Profile. Plants, 14(13), 2046. https://doi.org/10.3390/plants14132046








