Water Stress Promotes Secondary Sexual Dimorphism in Ecophysiological Traits of Papaya Seedlings
Abstract
1. Introduction
2. Results
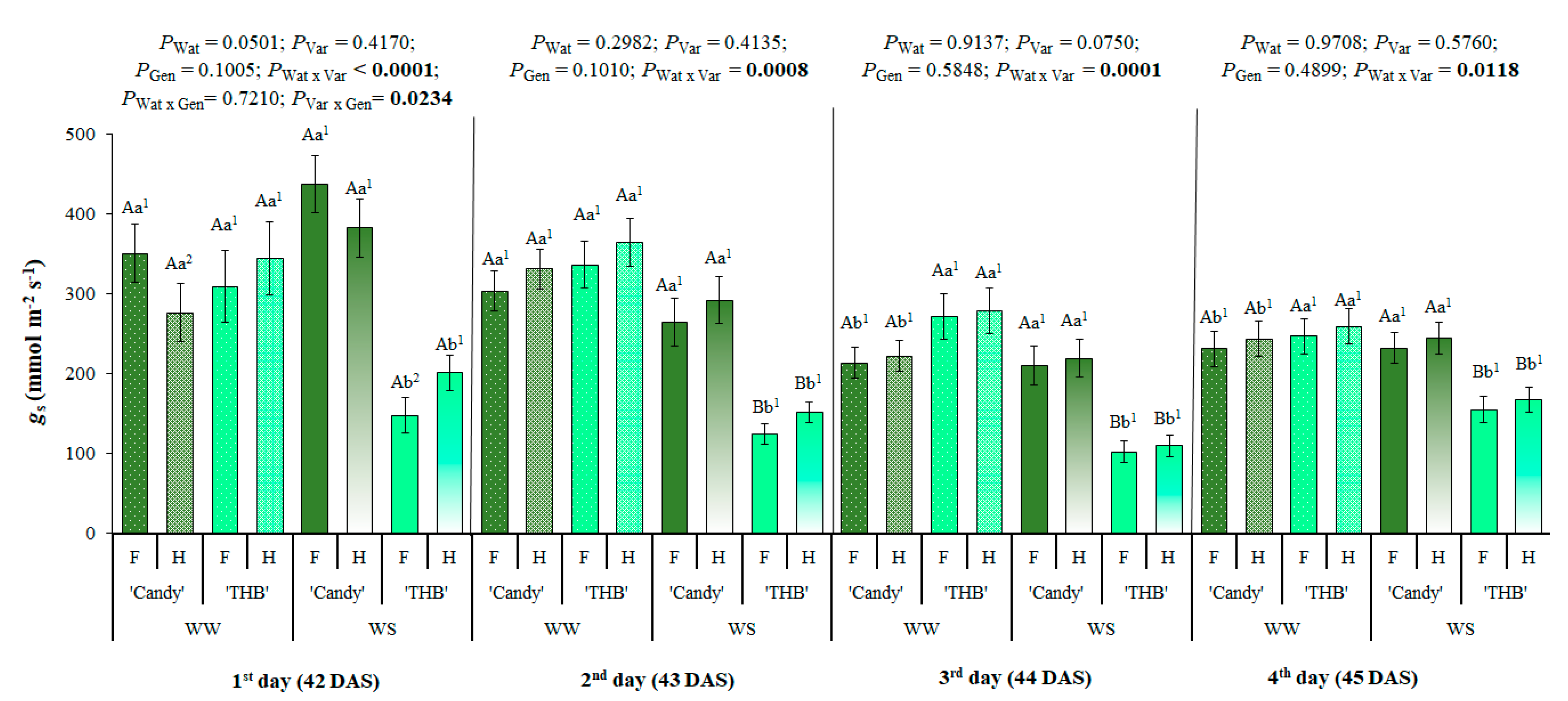
2.1. SSD in Stomatal Conductance After a Water-Shortage Period
2.2. SSD in SPAD and Leaf Spectral Reflectance Indices After a Water-Shortage Period
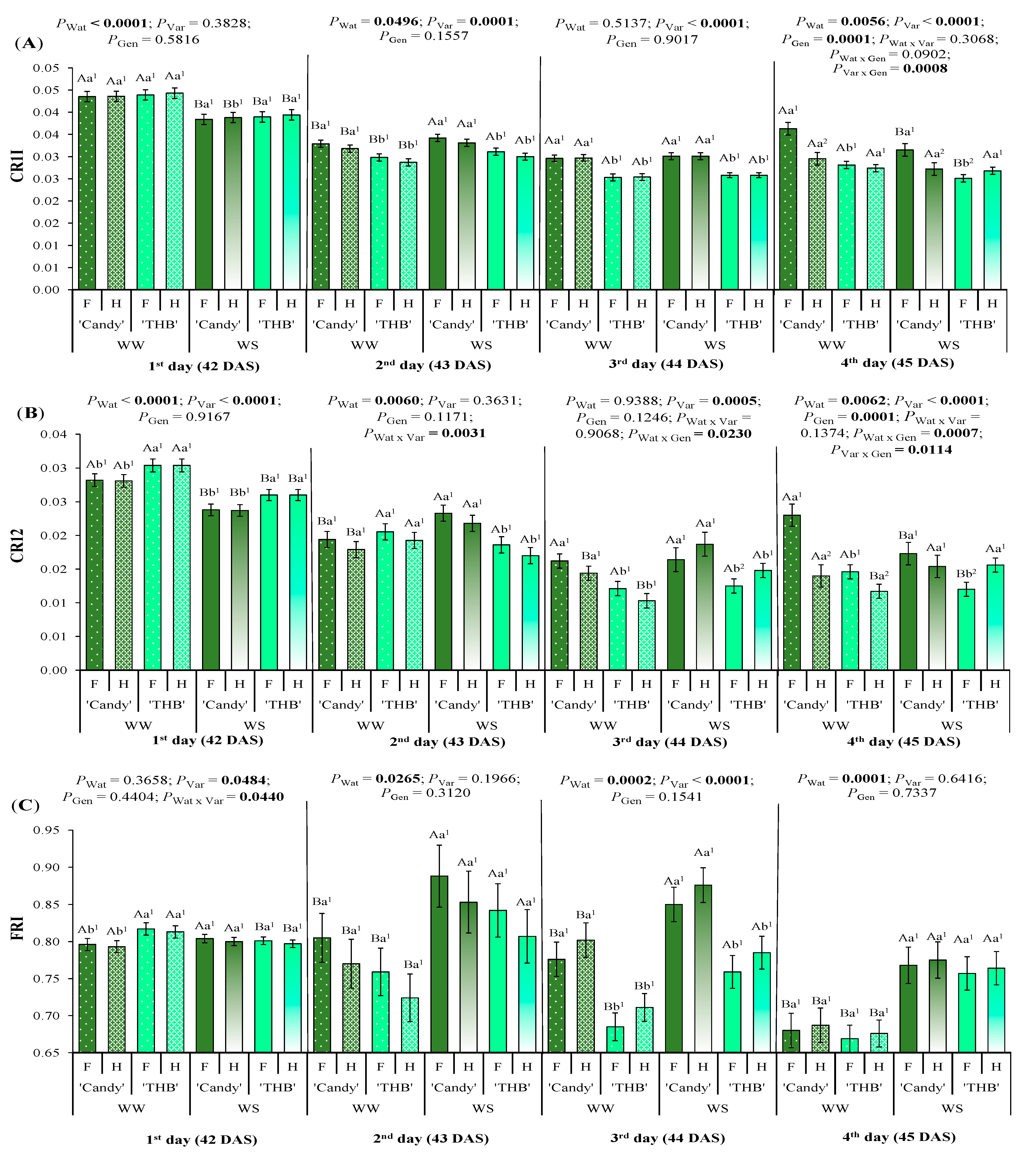
2.2.1. SSD in SPAD and Spectral Indices Associated with Chl Content
2.2.2. SSD in Spectral Indices Associated with Carotenoid and Flavonoid Contents
2.2.3. SSD in Spectral Indices Associated with Water Status
2.2.4. SSD in Spectral Indices Associated with Structure
2.3. SSD in Morphological Traits and Biomass Accumulation
3. Discussion
3.1. Early Expression of SSD in Stomatal Conductance Related to Water Shortage and Variety of Papaya Seedlings
3.2. SSD in Pigment Content Indicators Related to Water Shortage and Variety of Papaya Seedlings
3.3. SSD in Water Indicators Related to Water Shortage and Variety of Papaya Seedlings
3.4. SSD in Structural Indicators Related to Water-Shortage and Variety of Papaya Seedlings
3.5. SSD in Morphological Traits and Biomass Accumulation Related to Water Shortage and Variety of Papaya Seedlings
4. Materials and Methods
4.1. Plant Material and Experimental Conditions
4.2. Determinations of Plant Gender
4.3. Evaluations of Ecophysiological Traits
4.4. Evaluations of Plant Morphology and Dry Mass Accumulation
4.5. Statistical Analysis and Experimental Design
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Anet | Net CO2 assimilation rate |
| Car | Carotenoids |
| Chl | Chlorophyll |
| CNDVI | Combination of normalized difference vegetation index |
| CRI1 | Carotenoid reflectance index 1 |
| CRI2 | Carotenoid reflectance index 2 |
| G index | Greenness index |
| gs | Stomatal conductance |
| FRI | Flavonoid reflectance index |
| LDM | Leaf dry mass |
| LMR | Leaf mass ratio |
| LN | Leaf number |
| PRI | Photochemical reflectance index |
| RDM | Root dry mass |
| RShR | Root-to-shoot ratio |
| SD | Stem diameter |
| SDM | Stem dry mass |
| SIPI | Structure intensive pigment index |
| SPAD | Green intensity index |
| SRPI | Simple ratio pigment index |
| SSD | Secondary sexual dimorphism |
| TDM | Total plant dry mass |
| VPDair | Air vapor pressure deficit |
| WBI | Water band index |
| WS | Water-shortage conditions |
| WW | Well-watered conditions |
References
- Renner, S.S. The relative and absolute frequencies of angiosperm sexual systems: Dioecy, monoecy, gynodioecy, and an updated online database. Am. J. Bot. 2014, 101, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Campostrini, E.; Glenn, D.M. Ecophysiology of papaya: A Review. Braz. J. Plant Physiol. 2007, 19, 413–424. [Google Scholar] [CrossRef]
- de Oliveira, E.J.; Amorim, V.B.O.; Matos, E.L.S.; Costa, J.L.; da Silva Castellen, M.; Pádua, J.G.; Dantas, J.L.L. Polymorphism of microsatellite markers in papaya (Carica papaya L.). Plant Mol. Biol. Rep. 2010, 28, 519–530. [Google Scholar] [CrossRef]
- De Luca, Y.; Cozzolino, S.; Cristaudo, A.; Widmer, A.; Cafasso, D. Early sex identification by leaflet distance in plantlets of Cycas revoluta. Euphytica 2024, 220, 150. [Google Scholar] [CrossRef]
- Vyskot, B.; Hobza, R. Gender in plants: Sex chromosomes are emerging from the fog. Trends Genet. 2004, 20, 432–438. [Google Scholar] [CrossRef]
- Obeso, J.R. The costs of reproduction in plants. New Phytol. 2002, 155, 321–348. [Google Scholar] [CrossRef]
- Rakočević, M.; Costes, E.; Assad, E.D. Structural and physiological sexual dimorphism estimated from 3D virtual trees of yerba–mate (Ilex paraguariensis St. Hil.) is modified by cultivation environment. Ann. App. Biol. 2011, 156, 178–191. [Google Scholar] [CrossRef]
- Rakočević, M.; Medrado, M.J.S.; Martim, S.F.; Assad, E.D. Sexual dimorphism and seasonal changes of leaf gas exchange in the dioecious tree Ilex paraguariensis grown in two contrasted cultivation types. Ann. App. Biol. 2009, 154, 291–301. [Google Scholar] [CrossRef]
- Rabska, M.; Robakowski, P.; Ratajczak, E.; Żytkowiak, R.; Iszkuło, G.; Pers-Kamczyc, E. Photochemistry differs between male and female Juniperus communis L. independently of nutritional availability. Trees 2021, 35, 27–42. [Google Scholar] [CrossRef]
- Rakočević, M.; Batista, E.R.; Matsunaga, F.T.; Wendling, I.; Marcheafave, G.G.; Bruns, R.E.; Scarminio, I.S.; Ribeiro, R.V. Canopy architecture and diurnal CO2 uptake in male and female clones of yerba-mate cultivated in monoculture and agroforestry. Ann. Appl. Biol. 2024, 184, 210–225. [Google Scholar] [CrossRef]
- Marcheafave, G.G.; Pauli, E.D.; Wendling, I.; Rakočević, M.; Scarminio, I.C.; Bruns, R.E. A comparative study using UV–Vis, NIR, and FTIR spectral fingerprinting in yerba mate leaves through mixture design extractions and ASCA models. J. Braz. Chem. Soc. 2025, 36, e-20240073. [Google Scholar] [CrossRef]
- Barrett, S.C.H.; Hough, J. Sexual dimorphism in flowering plants. J. Exp. Bot. 2013, 64, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, D.; Villar-Salvador, P.; García-Fayos, P.; Verdú, M. Genders in Juniperus thurifera have different functional responses to variations in nutrient availability. New Phytol. 2012, 193, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Rakočević, M.; Maia, A.H.N.; Duarte, M.; Wendling, I. Secondary sexual dimorphism in biomass production of Ilex paraguariensis progenies associated with their provenances and morphotypes. Exp. Agric. 2023, 59, E3. [Google Scholar] [CrossRef]
- Muyle, A.; Martin, H.; Zemp, N.; Mollion, M.; Gallina, S.; Tavares, R.; Silva, A.; Bataillon, T.; Widmer, A.; Glémin, S.; et al. Dioecy is associated with high genetic diversity and adaptation rates in the plant genus Silene. Mol. Biol. Evol. 2021, 38, 805–818. [Google Scholar] [CrossRef]
- Matsunaga, F.T.; Rakocevic, M.; Brancher, J.D. Modeling the 3D structure and rhythmic growth responses to environment in dioecious yerba–mate. Ecol. Model. 2014, 290, 34–44. [Google Scholar] [CrossRef]
- Retuerto, R.; Sánchez Vilas, J.; Varga, S. Sexual dimorphism in response to stress. Environ. Exp. Bot. 2018, 146, 1–4. [Google Scholar] [CrossRef]
- Dudley, L.S.; Galen, C. Stage–dependent patterns of drought tolerance and gas exchange vary between sexes in the alpine willow, Salix glauca. Oecologia 2007, 153, 1–9. [Google Scholar] [CrossRef]
- Said, S.; Torre, F.; Derridj, A.; Gauquelin, T.; Mevy, J.P. Gender, Mediterranean drought, and seasonality: Photosystem II photochemistry in Pistacia lentiscus L. Photosynthetica 2013, 51, 552–564. [Google Scholar] [CrossRef]
- Rakočević, M.; Batista, E.R.; de Almeida, R.L.; Wendling, I.; Ribeiro, R.V. Expression of secondary sexual dimorphism in photosynthetic performance of Ilex paraguariensis under contrasted light availability of monoculture and agroforestry during annual rhythmic growth. Front. Photobiol. 2025, 2, 1501826. [Google Scholar] [CrossRef]
- Rakočević, M.; Batista, E.R.; de Almeida, R.L.; Wendling, I.; Ribeiro, R.V. Expression of secondary sexual dimorphism in the diurnal course of leaf gas exchanges is modified by the rhythmic growth of Ilex paraguariensis under monoculture and agroforestry. Forests 2025, 16, 161. [Google Scholar] [CrossRef]
- Correia, O.; Barradas, D.M. Ecophysiological differences between male and female plants of Pistacia lentiscus L. Plant Ecol. 2000, 149, 131–142. [Google Scholar] [CrossRef]
- He, M.; Shi, D.; Wei, X.; Hu, Y.; Wang, T.; Xie, Y. Gender–related differences in adaptability to drought stress in the dioecious tree Ginkgo biloba. Acta Physiol. Plant. 2016, 38, 124. [Google Scholar] [CrossRef]
- Karabourniotis, G.; Liakopoulos, G.; Bresta, P.; Nikolopoulos, D. The optical properties of leaf structural elements and their contribution to photosynthetic performance and photoprotection. Plants 2021, 10, 1455. [Google Scholar] [CrossRef]
- Korres, N.E.; Norsworthy, J.K.; FitzSimons, T.; Roberts, T.L.; Oosterhuis, D.M. Differential response of palmer amaranth (Amaranthus palmeri) gender to abiotic stress. Weed Sci. 2017, 65, 213–227. [Google Scholar] [CrossRef]
- Chen, M.; Lin, C.; Sun, Y.; Yang, R.; Lu, X.; Lou, W.; Deng, X.; Zhao, Y.; Liu, F. Ginkgo biloba sex identification methods using hyperspectral imaging and machine learning. Plants 2024, 13, 1501. [Google Scholar] [CrossRef]
- Crane, J.H. Papaya Growing in the Florida Home Landscape; IntechOpen: London, UK, 2020; Available online: https://edis.ifas.ufl.edu/publication/MG054 (accessed on 4 April 2025).
- Souza, G.A.R.d.; Vale, E.; Bernado, W.P.d.; Baroni, D.F.; Sousa, E.F.d.; Rakočević, M.; Rodrigues, W.P.; Campostrini, E. Water relations in fruit trees: Knowing for better irrigation management. In Fruit Crops Science; IntechOpen: London, UK, 2025. [Google Scholar]
- Ming, R.; Yu, Q.; Moore, P.H. Sex determination in papaya. Semin. Cell Dev. Biol. 2007, 18, 401–408. [Google Scholar] [CrossRef]
- Salinas, I.; Salehi, M.; Hueso, J.J.; Cuevas, J. Assessment of two sex–determining procedures in ‘BH–65’ papaya from an economical and developmental point of view. Fruits 2018, 73, 184–190. [Google Scholar] [CrossRef]
- Araya-Valverde, E.; Bogantes, A.; Holst, A.; Vargas-Mora, C.; Gómez-Alpízar, L.; Brenes, A.; Sánchez-Barrantes, E.; Chavarría, M.; Barboza-Barquero, L. Field performance of hermaphrodite papaya plants obtained through molecular selection and micropropagation. Crop Breed. Appl. Biotechnol. 2019, 19, 420–427. [Google Scholar] [CrossRef]
- Carr, M.K.V. The water relations and irrigation requirements of papaya (Carica papaya L.): A review. Exp. Agric. 2014, 50, 270–283. [Google Scholar] [CrossRef]
- Ruas, K.F.; Baroni, D.F.; de Souza, G.A.R.; de Paula Bernado, M.; Paixão, J.S.; dos Santos, G.M.; Machado Filho, J.A.; de Abreu, D.P.; de Sousa, E.F.; Rakočević, M.; et al. A Carica papaya L. genotype with low leaf chlorophyll concentration copes successfully with soil water stress in the field. Sci. Horticul. 2022, 293, 110722. [Google Scholar] [CrossRef]
- Honoré, M.N.; Belmonte-Ureña, L.J.; Navarro-Velasco, A.; Camacho-Ferre, F. Effects of the size of papaya (Carica papaya L.) seedling with early determination of sex on the yield and the quality in a greenhouse cultivation in Continental Europe. Sci. Hortic. 2020, 265, 109218. [Google Scholar] [CrossRef]
- Vasconcellos, M.A.d.S.; Carvalho, J.E.B.d.; de Pádua, T.R.P.; Martelleto, L.A.P.; Oliveira, A.M.G. Implantação da cultura e práticas culturais. In A Cultura do Mamoeiro; Oliveira, A.M.G., Meissner Filho, P.E., Eds.; Embrapa: Brasília, Brazil, 2021; pp. 153–190. [Google Scholar]
- Fernandes, T.F.S.; Silva, R.V.d.O.; de Freitas, D.L.D.; Sanches, A.G.; da Silva, M.B.; Júnior, L.C.C.; de Lima, K.G.; Teixeira, G.H.d.A. Sex type determination in papaya seeds and leaves using near infrared spectroscopy combined with multivariate techniques and machine learning. Comput. Electron. Agric. 2022, 193, 106674. [Google Scholar] [CrossRef]
- Chutteang, C.; Yingjajaval, S.; Wasee, S. Leaf photosynthetic potential of female and hermaphrodite papaya (Carica papaya cv. Khaeg Nuan). Acta Hortic. 2007, 740, 197–202. [Google Scholar] [CrossRef]
- Carminati, A.; Javaux, M. Soil rather than xylem vulnerability controls stomatal response to drought. Trends Plant Sci. 2020, 25, 868–880. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, M.A.; Cock, M.J.H.; Hernandez, A.D.P. Stomatal response to air humidity and its relation to stomatal density in a wide range of warm climate species. Photosynth Res 1985, 7, 137–149. [Google Scholar] [CrossRef]
- Campostrini, E.; Schaffer, B.; Ramalho, J.D.; González, J.C.; Rodrigues, W.P.; da Silva, J.R.; Lima, R.S. Environmental factors controlling carbon assimilation, growth, and yield of papaya (Carica papaya L.) under water-scarcity scenarios. In Water Scarcity and Sustainable Agriculture in Semiarid Environment; Tejero, I.F.G., Zuazo, V.H.D., Eds.; Academic Press: Cambridge, UK, 2018; Chapter 19; pp. 481–505. [Google Scholar]
- Lopes, T.d.S. Gas-Exchange, Thermal Imaging, and Photochemical Efficiency: Relationship with Gender in Young Papaya (Carica papaya L.) Plants. Ph.D. Thesis, Northern Rio de Janeiro State University, Rio de Janeiro, Brazil, 2014; 130p. [Google Scholar]
- Anjum, S.A.; Xie, X.; Wang, L.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Merzlyak, M.N.; Solovchenko, A.E.; Smagin, A.I.; Gitelson, A.A. Apple flavonols during fruit adaptation to solar radiation: Spectral features and technique for non–destructive assessment. J. Plant Physiol. 2005, 162, 151–160. [Google Scholar] [CrossRef]
- Barradas, A.; Correia, P.M.P.; Silva, S.; Mariano, P.; Pires, M.C.; Matos, A.R.; da Silva, A.B.; Marques da Silva, J. Comparing machine learning methods for classifying plant drought stress from leaf reflectance spectra in Arabidopsis thaliana. Appl. Sci. 2021, 11, 6392. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Baret, F. Semi–empirical indices to assess carotenoids/chlorophyll a ratio from leaf spectra reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing carotenoid content in plant leaves with reflectance spectroscopy. Photochem. Photobiol. 2007, 75, 272–281. [Google Scholar] [CrossRef]
- Manivannan, P.; Jaleel, A.; Chang-Xing, Z.; Somasundaram, R.; Azooz, M.M.; Panneerselvam, R. Variations in growth and pigment composition of sunflower varieties under early season drought stress. Glob. J. Mol. Sci. 2008, 3, 50–56. [Google Scholar]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Zarco-Tejada, P.J.; Ustin, S.L.; Whiting, M.L. Temporal and spatial relationships between within–field yield variability in cotton and high–spatial hyperspectral remote sensing imagery. Agron. J. 2005, 97, 641–653. [Google Scholar] [CrossRef]
- Sun, H.; Li, M.; Zheng, L.; Zhang, Y.; Yang, W. Evaluation of maize growth by ground based multi-spectral image. In Proceedings of the 2011 IEEE/SICE International Symposium on System Integration (SII), Kyoto, Japan, 20–22 December 2011; pp. 207–211. [Google Scholar]
- Zarzecka, K.; Gugała, M.; Mystkowska, I.; Sikorska, A. The leaf greenness index SPAD and selected features of potato following an application of herbicides and biostimulants. J. Ecol. Eng. 2021, 22, 54–63. [Google Scholar] [CrossRef]
- Liao, J.; Song, H.; Tang, D.; Zhang, S. Sexually differential tolerance to water deficiency of Salix paraplesia—A female-biased alpine willow. Ecol. Evol. 2019, 9, 8450–8464. [Google Scholar] [CrossRef] [PubMed]
- Gouker, F.E.; Carlson, C.H.; Zou, J.; Evans, L.; Crowell, C.R.; Smart, C.D.; DiFazio, S.P.; Smart, L.B. Sexual dimorphism in the dioecious willow Salix purpurea. Am. J. Bot. 2021, 108, 1374–1387. [Google Scholar] [CrossRef]
- Baskar, V.; Venkatesh, R.; Ramalingam, S. Flavonoids (antioxidants systems) in higher plants and their response to stresses. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Zhang, R.R.; Wang, Y.H.; Li, T.; Tan, G.F.; Tao, J.P.; Su, X.J.; Xu, Z.S.; Tian, Y.S.; Xiong, A.S. Effects of simulated drought stress on carotenoid contents and expression of related genes in carrot taproots. Protoplasma 2021, 258, 379–390. [Google Scholar] [CrossRef]
- Rosa, A.P.; Barão, L.; Chambel, L.; Cruz, C.; Santana, M.M. Early identification of plant drought stress responses: Changes in leaf reflectance and plant growth promoting rhizobacteria selection-the case study of tomato plants. Agronomy 2023, 13, 183. [Google Scholar] [CrossRef]
- Danilov, R.; Kremneva, O.; Pachkin, A. identification of the spectral patterns of cultivated plants and weeds: Hyperspectral vegetation indices. Agronomy 2023, 13, 859. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Biel, C.; Serrano, L.; Savé, R. The reflectance at the 950–970 nm region as an indicator of plant water status. Int. J. Remote Sens. 1993, 14, 1887–1905. [Google Scholar] [CrossRef]
- Rossini, M.; Fava, F.; Cogliati, S.; Meroni, M.; Marchesi, A.; Panigada, C.; Giardino, C.; Busetto, L.; Migliavacca, M.; Amaducci, S.; et al. Assessing canopy PRI from airborne imagery to map water stress in maize. ISPRS J. Photogramm. Remote Sens. 2013, 85, 168–177. [Google Scholar] [CrossRef]
- Chou, S.; Chen, J.M.; Yu, H.; Chen, B.; Zhang, X.; Croft, H.; Khalid, S.; Li, M.; Shi, Q. Hyperspectral remote sensing and leaf-level non-photochemical quenching as early indicators of water stress in maize. Remote Sens. 2017, 9, 794. [Google Scholar] [CrossRef]
- Panigada, C.; Rossini, M.; Meroni, M.; Cilia, C.; Busettoa, L.; Amaducci, S.; Boschetti, M.; Cogliati, S.; Picchi, V.; Pinto, F.; et al. Fluorescence PRI and canopy temperature for water stress detection in cereal crops. Int. J. Appl. Earth Obs. 2014, 30, 167–178. [Google Scholar] [CrossRef]
- Peñuelas, J.; Isla, R.; Filella, I.; Araus, J.L. Visible and near–infrared reflectance assessment of salinity effects on barley. Crop Sci. 1997, 37, 198–202. [Google Scholar] [CrossRef]
- Sánchez-Vilas, J.; Retuerto, R. Sex-specific physiological, allocation and growth responses to water availability in the subdioecious plant Honckenya peploides. Plant Biol. 2009, 11, 243–254. [Google Scholar] [CrossRef]
- Letts, M.G.; Phelan, C.A.; Johnson, D.R.E.; Rood, S.B. Seasonal photosynthetic gas exchange and leaf reflectance characteristics of male and female cottonwoods in a riparian woodland. Tree Physiol. 2008, 28, 1037–1048. [Google Scholar] [CrossRef]
- Kior, A.; Sukhov, V.; Sukhova, E. Application of reflectance indices for remote sensing of plants and revealing actions of stressors. Photonics 2021, 8, 582. [Google Scholar] [CrossRef]
- Claeys, H.; Inzé, D. The agony of choice: How plants balance growth and survival under water–limiting conditions. Plant Physiol. 2013, 162, 1768–1779. [Google Scholar] [CrossRef]
- Oliveira, L.F.R.; Oliveira, M.L.R.; Gomes, F.C.; Santana, R.C. Estimating foliar nitrogen in Eucalyptus using vegetation indices. Sci. Agríc. 2017, 74, 142–147. [Google Scholar] [CrossRef]
- Janeczko, A.; Dziurka, M.; Gullner, G.; Kocurek, M.; Rys, M.; Saja, D.; Skoczowski, A.; Tóbiás, I.; Kornas, A.; Barna, B. Comparative studies of compatible and incompatible pepper—Tobamovirus interactions and the evaluation of effects of 24-epibrassinolide. Photosynthetica 2018, 56, 763–775. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non–destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Thenot, F.; Méthy, M.; Winkel, T. The photochemical reflectance index (PRI) as a water–stress index. Int. J. Remote Sens. 2002, 23, 5135–5139. [Google Scholar] [CrossRef]
- Ranjan, R.; Chopra, U.K.; Sahoo, R.N.; Singh, A.K.; Pradhan, S. Assessment of plant nitrogen stress in wheat (Triticum aestivum L.) through hyperspectral indices. Int. J. Remote Sens. 2012, 33, 6342–6360. [Google Scholar] [CrossRef]
- R Core Team. R Development Core Team. Available online: https://www.r-project.org/ (accessed on 11 May 2025).






| Index | Full Term | Biological Association | Equation | References |
|---|---|---|---|---|
| Pigment indices | ||||
| G index | Greenness index | Chl content | R554/R677 | [49] |
| CNDVI | Combination of normalized difference vegetation index | Chl content | (R750 − R705)/(R750 + R705) | [50] |
| CRI1 | Carotenoid reflectance index 1 | Chl and Car content | (1/R510) − (1/R550) | [46,69] |
| CRI2 | Carotenoid reflectance index 2 | Total Car content | (1/R510) − (1/R700) | [46,69] |
| FRI | Flavonoid reflectance index | Flavonol content and screening of excessive visible and UV-A radiation | [(1/R410) − (1/R460)] − R800 | [43] |
| Water indices | ||||
| PRI | Photochemical reflectance index | Water status and water stress | (R531 − R570)/(R531 + R570) | [70] |
| WBI | Water band index | Water status and relative water content | R900/R970 | [58] |
| Structural indices | ||||
| SIPI | Structure intensive pigment index | Pest damages through ratio Car/Chl | (R800 − R445)/(R800 + R680) | [45] |
| SRPI | Simple ratio pigment index | Large area monitoring of plants’ N status and ratio Car/Chl | R430/R680 | [45,71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trancoso, I.; de Souza, G.A.R.; de Souza, J.V.P.; Miranda, R.M.d.S.N.d.; Neves, D.d.A.; Rakocevic, M.; Campostrini, E. Water Stress Promotes Secondary Sexual Dimorphism in Ecophysiological Traits of Papaya Seedlings. Plants 2025, 14, 2445. https://doi.org/10.3390/plants14152445
Trancoso I, de Souza GAR, de Souza JVP, Miranda RMdSNd, Neves DdA, Rakocevic M, Campostrini E. Water Stress Promotes Secondary Sexual Dimorphism in Ecophysiological Traits of Papaya Seedlings. Plants. 2025; 14(15):2445. https://doi.org/10.3390/plants14152445
Chicago/Turabian StyleTrancoso, Ingrid, Guilherme A. R. de Souza, João Vitor Paravidini de Souza, Rosana Maria dos Santos Nani de Miranda, Diesily de Andrade Neves, Miroslava Rakocevic, and Eliemar Campostrini. 2025. "Water Stress Promotes Secondary Sexual Dimorphism in Ecophysiological Traits of Papaya Seedlings" Plants 14, no. 15: 2445. https://doi.org/10.3390/plants14152445
APA StyleTrancoso, I., de Souza, G. A. R., de Souza, J. V. P., Miranda, R. M. d. S. N. d., Neves, D. d. A., Rakocevic, M., & Campostrini, E. (2025). Water Stress Promotes Secondary Sexual Dimorphism in Ecophysiological Traits of Papaya Seedlings. Plants, 14(15), 2445. https://doi.org/10.3390/plants14152445










