Drought and Shrub Encroachment Accelerate Peatland Carbon Loss Under Climate Warming
Abstract
1. Introduction
2. Results
2.1. Soil Physicochemical Properties of Different Depths of Peat
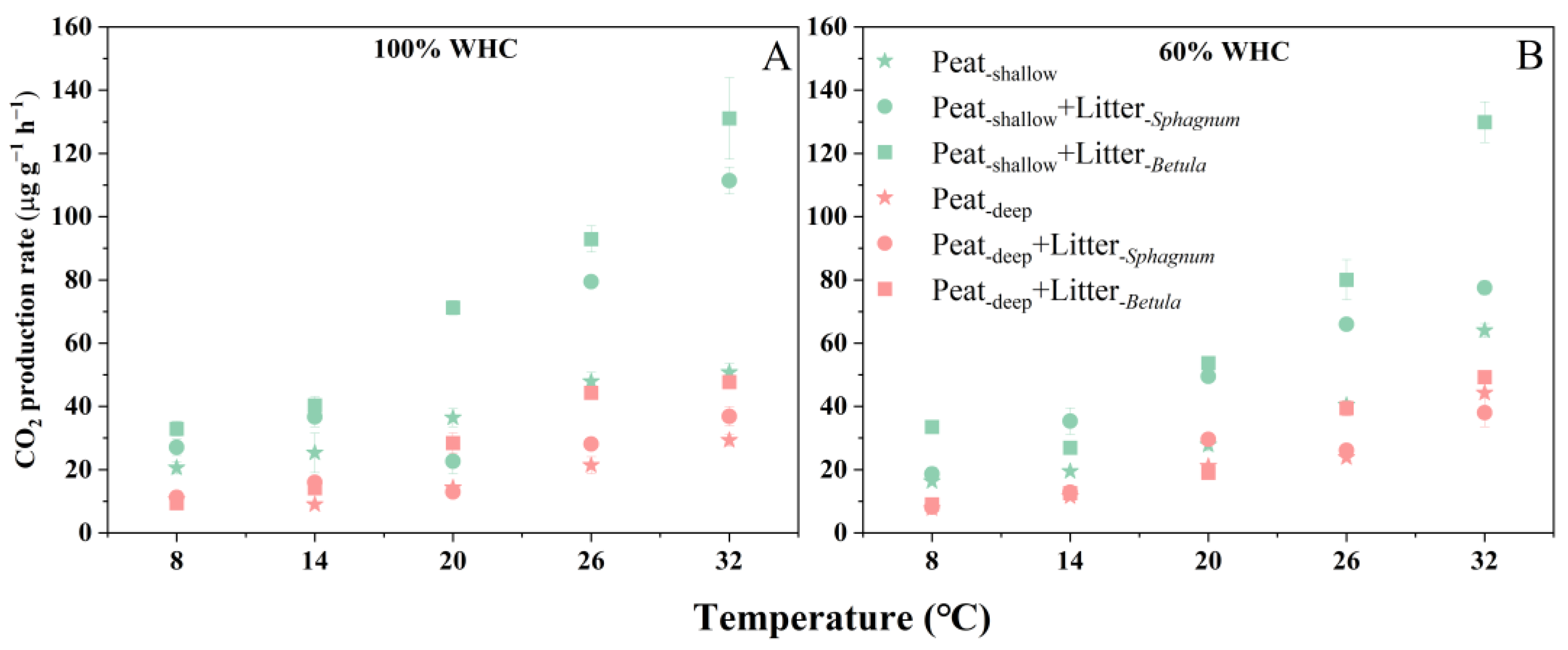
2.2. Peat CO2 Production Rate and Its Temperature Sensitivity
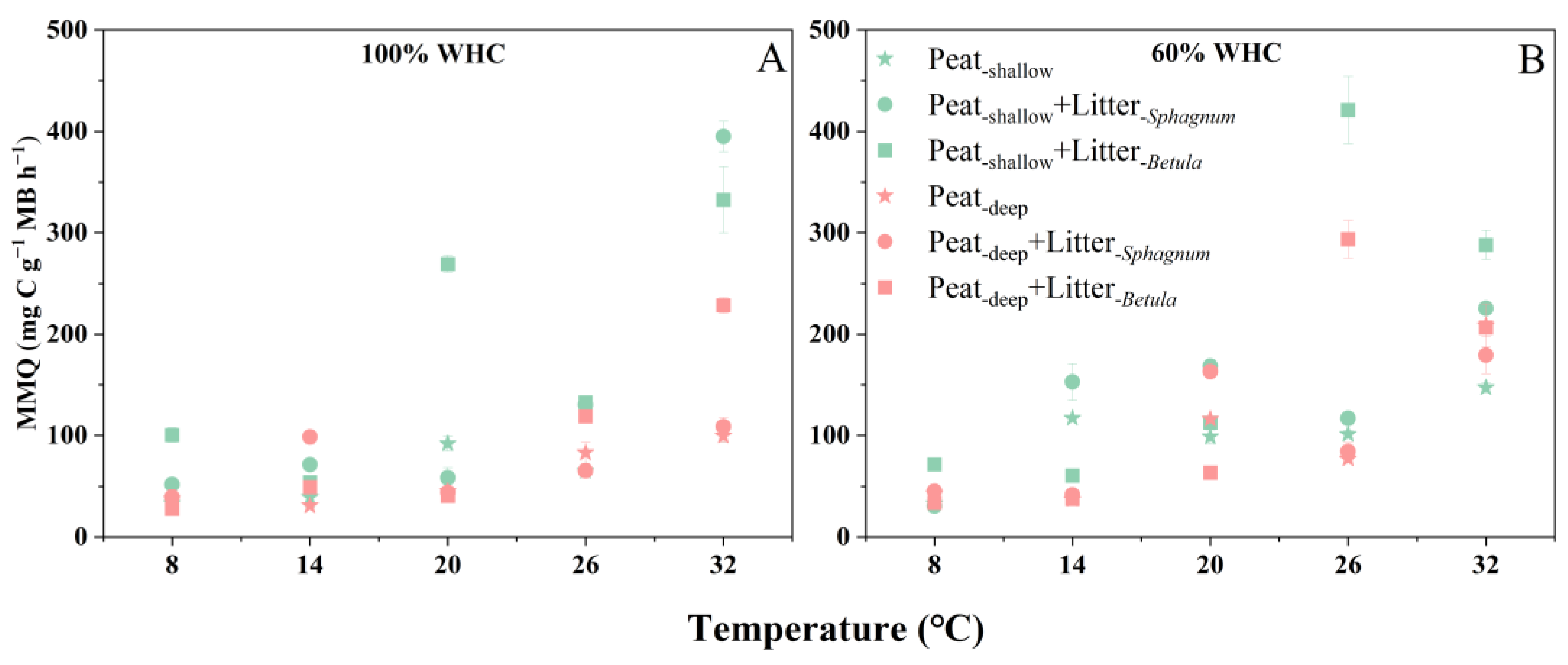
2.3. Microbial Metabolic Quotient
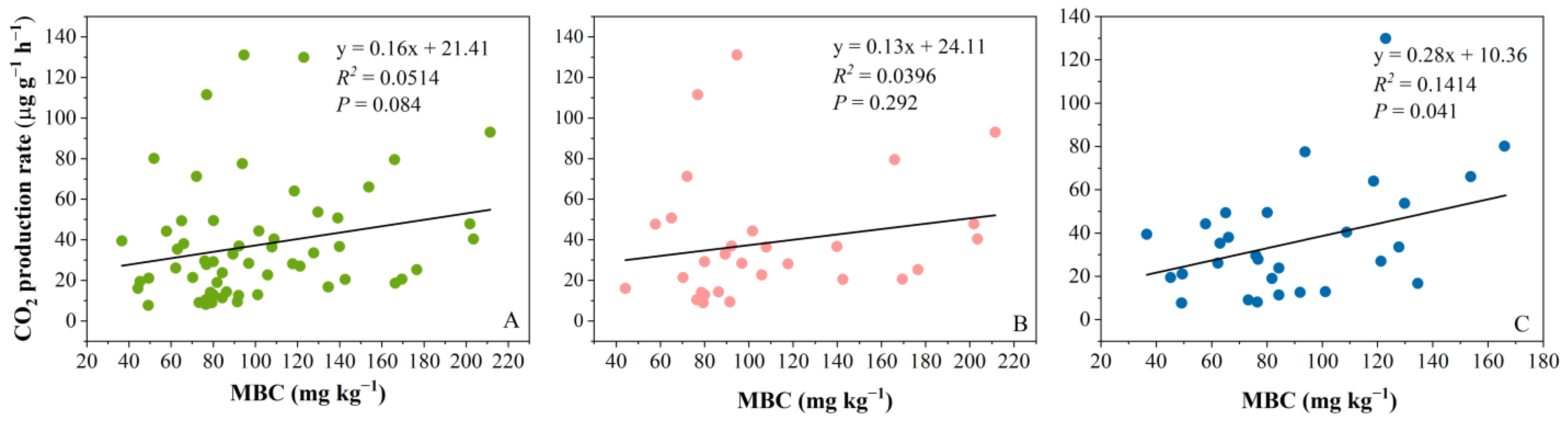
2.4. Relationships Between CO2 Production Rate and Microbial Biomass Carbon (MBC)
3. Discussions
3.1. Deep Peat Decomposes Slowly but Is More Sensitive to Warming
3.2. Vegetation Succession Will Alter Peat Decomposition and Its Temperature Sensitivity (Q10)
3.3. Moisture Content Is Vital for Peat Decomposition and Its Temperature Sensitivity
4. Materials and Methods
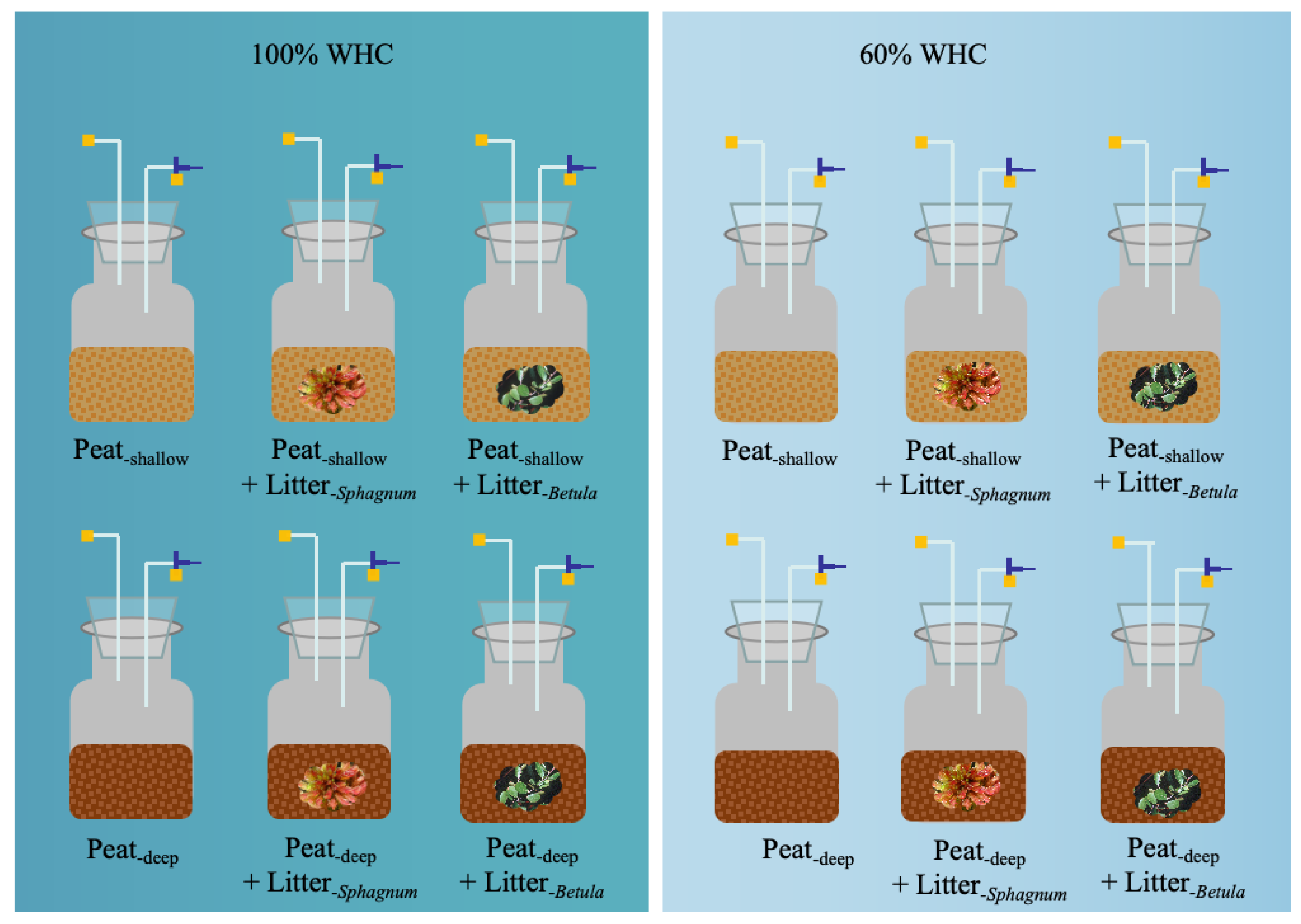
4.1. Site Description
4.2. Soil and Litter Collection
4.3. Incubation and CO2 Flux Measurement
4.4. Soil Physicochemical Properties Analyses
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, Z.; Loisel, J.; Brosseau, D.P.; Beilman, D.W.; Hunt, S.J. Global peatland dynamics since the Last Glacial Maximum. Geophys. Res. Lett. 2010, 37, L13402. [Google Scholar] [CrossRef]
- Rydin, H.; Jeglum, J.K.; Bennett, K.D. The Biology of Peatlands, 2nd ed.; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- United Nations Environment Programme (UNEP). Global Peatlands Assessment—The State of the World’s Peatlands: Evidence for Action Toward the Conservation, Restoration, and Sustainable Management of Peatlands; Main Report; Global Peatlands Initiative, UNEP: Nairobi, Kenya, 2022; Available online: https://www.unep.org/resources/global-peatlands-assessment-2022 (accessed on 3 July 2025).
- Clymo, R.; Hayward, P. The Ecology of Sphagnum. In Bryophyte Ecology; Smith, A.J.E., Ed.; Springer: Dordrecht, The Netherlands, 1982; pp. 229–289. [Google Scholar]
- Van Breemen, N. How Sphagnum bogs down other plants. Trends Ecol. Evol. 1995, 10, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, J.T.A.; Liefveld, W.M. The ecological significance of organochemical compounds in Sphagnum. Acta Bot. Neerl. 1997, 46, 117–130. Available online: https://natuurtijdschriften.nl/pub/541086 (accessed on 3 July 2025). [CrossRef]
- Fenner, N.; Freeman, C. Drought-induced carbon loss in peatlands. Nat. Geosci. 2011, 4, 895–900. [Google Scholar] [CrossRef]
- Ward, S.E.; Ostle, N.J.; Oakley, S.; Quirk, H.; Henrys, P.A.; Bardgett, R.D. Warming effects on greenhouse gas fluxes in peatlands are modulated by vegetation composition. Ecol. Lett. 2013, 16, 1285–1293. [Google Scholar] [CrossRef]
- Walker, T.N.; Garnett, M.H.; Ward, S.E.; Oakey, S.; Bargett, R.D.; Ostle, N.J. Vascular plants promote ancient peatland carbon loss with climate warming. Glob. Change Biol. 2016, 22, 1880–1889. [Google Scholar] [CrossRef]
- Chen, X.; Mallik, A.U.; Yu, Z.; Wang, Z.; Wang, S.; Dong, Y.; Zhang, M.; Bu, Z. Drainage-driven loss of carbon sequestration of a temperate peatland in Northeast China. Ecosystems 2024, 27, 207–221. [Google Scholar] [CrossRef]
- Freeman, C.; Ostle, N.; Kang, H. An enzymic ‘latch’ on a global carbon store. Nature 2001, 409, 149. Available online: https://www.nature.com/articles/35051650 (accessed on 25 June 2025). [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F. The temperature dependence of soil organic matter decomposition, and the effect of global warming on soil organic C storage. Plant Soil 1995, 187, 753–760. [Google Scholar] [CrossRef]
- Hartley, I.P.; Ineson, P. Substrate quality and the temperature sensitivity of soil organic matter decomposition. Soil Biol. Biochem. 2008, 40, 1567–1574. [Google Scholar] [CrossRef]
- Bubier, J.L.; Moore, T.R.; Bledzki, L.A. Effects of nutrient addition on vegetation and carbon cycling in an ombrotrophic bog. Glob. Change Biol. 2007, 13, 1168–1186. [Google Scholar] [CrossRef]
- Hirano, T.; Ohkubo, S.; Itoh, M.; Tsuzuki, H.; Sakabe, A.; Takahashi, H.; Kusin, K.; Osaki, M. Large variation in carbon dioxide emissions from tropical peat swamp forests due to disturbances. Commun. Earth Environ. 2024, 5, 221. Available online: https://www.nature.com/articles/s43247-024-01387-7 (accessed on 25 June 2025). [CrossRef]
- Weltzin, J.F.; Pastor, J.; Harth, C.; Bridgham, S.D.; Updegraff, K.; Chapin, C.T. Response of bog and fen plant communities to warming and water-table manipulations. Ecology 2000, 81, 3464–3478. [Google Scholar] [CrossRef]
- Dieleman, C.M.; Branfireun, B.A.; McLaughlin, J.W.; Lindo, Z. Climate change drives a shift in peatland ecosystem plant community: Implications for ecosystem function and stability. Glob. Change Biol. 2015, 21, 388–395. [Google Scholar] [CrossRef]
- Buttler, A.; Bragazza, L.; Laggoun-Défarge, F.; Gogo, S.; Toussaint, M.L.; Lamentowicz, M.; Chojnicki, B.H.; Słowiński, M.; Słowińska, S.; Zielińska, M.; et al. Ericoid shrub encroachment shifts aboveground–belowground linkages in three peatlands across Europe and Western Siberia. Glob. Change Biol. 2023, 29, 6772–6793. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Tian, J.; Zhang, B.; Kang, W.; Zhou, Y.; He, C. Effects of vegetation types on soil wetting pattern and preferential flow in arid mountainous areas of northwest China. J. Hydrol. 2024, 642, 131849. [Google Scholar] [CrossRef]
- Bu, Z.J.; Zheng, X.X.; Rydin, H.; Moore, T.; Ma, J.Z. Facilitation vs. competition: Does inter-specific interaction affect drought responses in Sphagnum? Basic Appl. Ecol. 2013, 14, 574–584. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, Y.F.; Yuan, F.H.; Song, C.C.; Xu, M.J.; Wang, Q.W.; Hao, G.Y.; Bao, T.; Zuo, Y.J.; Liu, J.Z.; et al. Warming-induced vapor pressure deficit suppression of vegetation growth diminished in northern peatlands. Nat. Commun. 2023, 14, 7885. [Google Scholar] [CrossRef]
- Leroy, F.; Gogo, S.; Guimabaud, C.; Bernard-Jannin, L.; Hu, Z.; Laggoun-Défarge, F. Vegetation composition controls temperature sensitivity of CO2 and CH4 emissions and DOC concentration in peatlands. Soil Biol. Biochem. 2017, 107, 164–167. [Google Scholar] [CrossRef]
- Bell, M.C.; Ritson, J.P.; Verhoef, A.; Brazier, R.E.; Templeton, M.R.; Graham, N.J.D.; Freeman, C.; Clark, J.M. Sensitivity of peatland litter decomposition to changes in temperature and rainfall. Geoderma 2018, 331, 29–37. [Google Scholar] [CrossRef]
- Moore, T.R.; Bubier, J.L.; Bledzki, L. Litter decomposition in temperate peatland ecosystems: The effect of substrate and site. Ecosystems 2007, 10, 949–963. Available online: https://link.springer.com/article/10.1007/s10021-007-9064-5 (accessed on 25 June 2025). [CrossRef]
- Su, J.; Zhang, H.; Han, X.; Lv, R.; Liu, L.; Jiang, Y.; Li, H.; Kuzyakov, Y.; Wei, C. 5300-year-old soil carbon is less primed than young soil organic matter. Glob. Change Biol. 2023, 29, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.J.; Treffkorn, J.; Silver, W.L. Breaking the enzymatic latch: Impacts of reducing conditions on hydrolytic enzyme activity in tropical forest soils. Ecology 2014, 95, 2964–2973. [Google Scholar] [CrossRef]
- Wang, M.; Wu, J.; Lafleur, P.M.; Luan, J.; Chen, H.; Zhu, X. Can abandoned peatland pasture sequestrate more carbon dioxide from the atmosphere than an adjacent pristine bog in Newfoundland, Canada? Agric. For. Meteorol. 2018, 248, 91–108. [Google Scholar] [CrossRef]
- Durán, N.; Rosa, M.A.; D’Annibale, A.; Gianfreda, L. Applications of laccases and tyrosinases (phenoloxidases) immobilized on different supports: A review. Enzyme Microb. Technol. 2002, 31, 907–931. [Google Scholar] [CrossRef]
- Freeman, C.; Fenner, N.; Ostle, N.J.; Kang, H.; Dowrick, D.J.; Reynolds, B.; Lock, M.A.; Sleep, D.; Hughes, S.; Hudson, J. Export of dissolved organic carbon from peatlands under elevated carbon dioxide levels. Nature 2004, 430, 195–198. [Google Scholar] [CrossRef]
- Boot, C.M.; Hall, E.K.; Denef, K.; Baron, J.S. Long-term reactive nitrogen loading alters soil carbon and microbial community properties in a subalpine forest ecosystem. Soil Biol. Biochem. 2016, 92, 211–220. [Google Scholar] [CrossRef]
- Morugán-Coronado, A.; García-Orenes, F.; McMillan, M.; Pereg, L. The effect of moisture on soil microbial properties and nitrogen cyclers in Mediterranean sweet orange orchards under organic and inorganic fertilization. Sci. Total Environ. 2019, 655, 158–167. [Google Scholar] [CrossRef]
- Juszczak, R.; Humphreys, E.; Acosta, M.; Michalak-Galczewska, M.; Kayzer, D.; Olejnik, J. Ecosystem respiration in a heterogeneous temperate peatland and its sensitivity to peat temperature and water table depth. Plant Soil 2013, 366, 505–520. [Google Scholar] [CrossRef]
- Jassal, R.S.; Black, T.A.; Novak, M.D.; Gaumont-Guay, D.; Nesic, Z. Effect of soil water stress on soil respiration and its temperature sensitivity in an 18-year-old temperate Douglas-fir stand. Glob. Change Biol. 2008, 14, 1305–1318. [Google Scholar] [CrossRef]
- Singh, B.; Bardgett, R.; Smith, P.; Reay, D.S. Microorganisms and climate change: Terrestrial feedbacks and mitigation options. Nat. Rev. Microbiol. 2010, 8, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.P.; Shen, W.J.; Li, Y.E.; Hui, D.F. Interactive effects of temperature and moisture on composition of the soil microbial community. Eur. J. Soil Sci. 2017, 68, 909–918. [Google Scholar] [CrossRef]
- Zhao, M.; Green, A.; Liu, Y.; Konings, A.G. Evapotranspiration frequently increases during droughts. Nat. Clim. Change 2022, 12, 1024–1030. Available online: https://www.nature.com/articles/s41558-022-01505-3 (accessed on 30 July 2025). [CrossRef]
- Bring, A.; Thorslund, J.; Rosén, L.; Tonderski, K.; Åberg, C.; Envall, I.; Laudon, H. Effects on Groundwater Storage of Restoring, Constructing or Draining Wetlands in Temperate and Boreal Climates: A Systematic Review. Environ. Evid. 2022, 11, 38. [Google Scholar] [CrossRef]
- Peacock, M.; Jones, T.G.; Airey, B.; Johncock, A.; Evans, C.D.; Lebron, I.; Fenner, N.; Freeman, C. The effect of peatland drainage and rewetting (ditch blocking) on extracellular enzyme activities and water chemistry. Soil Use Manag. 2015, 31, 67–76. [Google Scholar] [CrossRef]
- Baysinger, M.R.; Wilson, R.M.; Hanson, P.J.; Kostka, J.E.; Chanton, J.P. Compositional stability of peat in ecosystem-scale warming mesocosms. PLoS ONE 2022, 17, e0263994. [Google Scholar] [CrossRef]
- Lerch, T.Z.; Nunan, N.; Dignac, M.-F.; Chenu, C.; Mariotti, A. Variations in microbial isotopic fractionation during soil organic matter decomposition. Biogeochemistry 2011, 106, 5–21. [Google Scholar] [CrossRef]
- Soong, J.L.; Fuchslueger, L.; Marañón-Jiménez, S.; Torn, M.S.; Richter, A. Microbial carbon limitation: The need for integrating microorganisms into our understanding of ecosystem carbon cycling. Glob. Change Biol. 2020, 26, 1953–1961. [Google Scholar] [CrossRef]
- Fontaine, S.; Barot, S.; Barré, P.; Bdioui, N.; Mary, B.; Rumpel, C. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 2007, 450, 277–280. [Google Scholar] [CrossRef]
- Fontaine, S.; Barot, S. Size and functional diversity of microbe populations control plant persistence and long-term soil carbon accumulation. Ecol. Lett. 2005, 8, 1075–1087. [Google Scholar] [CrossRef]
- Conant, R.T.; Steinweg, J.M.; Haddix, M.L.; Paul, E.A.; Plante, A.F.; Six, J. Experimental warming shows that decomposition temperature sensitivity increases with soil organic matter recalcitrance. Ecology 2008, 89, 2384–2391. [Google Scholar] [CrossRef] [PubMed]
- Hilasvuori, E.; Akujärvi, A.; Fritze, H.; Karhu, K.; Laiho, R.; Mäkiranta, P.; Oinonen, M.; Palonen, V.; Vanhala, P.; Liski, J. Temperature sensitivity of decomposition in a peat profile. Soil Biol. Biochem. 2013, 67, 47–54. [Google Scholar] [CrossRef]
- Karhu, K.; Auffret, M.D.; Dungait, J.A.; Hopkins, D.W.; Prosser, J.I.; Singh, B.K.; Subke, J.A.; Wookey, P.A.; Ågren, G.I.; Sebastià, M.T.; et al. Temperature sensitivity of soil respiration rates enhanced by microbial community response. Nature 2014, 513, 81–84. [Google Scholar] [CrossRef]
- Li, Q.; Leroy, F.; Zocatelli, R.; Gogo, S.; Jacotot, A.; Guimbaud, C.; Laggoun-Défarge, F. Abiotic and biotic drivers of microbial respiration in peat and its sensitivity to temperature change. Soil Biol. Biochem. 2021, 153, 108077. [Google Scholar] [CrossRef]
- Conant, R.T.; Dalla-Betta, P.; Klopatek, C.C.; Klopatek, J.M. Controls on soil respiration in semiarid soils. Soil Biol. Biochem. 2004, 36, 945–951. [Google Scholar] [CrossRef]
- Song, Y.; Song, C.; Hou, A.; Sun, L.; Wang, X.; Ma, X.; Jiang, L.; Liu, C.; Gao, J. Temperature, soil moisture, and microbial controls on CO2 and CH4 emissions from a permafrost peatland. Environ. Prog. Sustain. Energy 2021, 40, e13693. [Google Scholar] [CrossRef]
- Clark, J.M.; Ashley, D.; Wagner, M.; Chapman, P.J.; Lane, S.N.; Evans, C.D.; Heathwaite, A.L. Increased temperature sensitivity of net DOC production from ombrotrophic peat due to water table draw-down. Glob. Change Biol. 2009, 15, 794–807. [Google Scholar] [CrossRef]
- Cleary, J.; Roulet, N.T.; Moore, T.R. Greenhouse gas emissions from Canadian peat extraction, 1990–2000: A life-cycle analysis. Ambio 2005, 34, 456–461. [Google Scholar] [CrossRef]
- Pellerin, S.; Huot, J.; Côté, S.D. Long-term effects of deer browsing and trampling on the vegetation of peatlands. Biol. Conserv. 2006, 128, 316–326. [Google Scholar] [CrossRef]
- Herre, M.; Heitkötter, J.; Heinze, S.; Rethemeyer, J.; Preusser, S.; Kandeler, E.; Marschner, B. Differences in organic matter properties and microbial activity between bulk and rhizosphere soil from the top and subsoils of three forest stands. Geoderma 2022, 409, 115589. [Google Scholar] [CrossRef]
- Jobbagy, E.; Jackson, B.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Biester, H.; Knorr, K.H.; Schellekens, J.; Basler, A.; Hermanns, Y.M. Comparison of different methods to determine the degree of peat decomposition in peat bogs. Biogeosciences 2014, 11, 2691–2707. [Google Scholar] [CrossRef]
- Broder, T.; Blodau, C.; Biester, H.; Knorr, K.H. Peat decomposition records in three pristine ombrotrophic bogs in southern Patagonia. Biogeosciences 2012, 9, 1479–1491. [Google Scholar] [CrossRef]
- Kuhry, P.; Vitt, D.H. Fossil carbon/nitrogen ratios as a measure of peat decomposition. Ecology 1996, 77, 271–275. [Google Scholar] [CrossRef]
- Asif, T.; Naeem, I.; Bu, Z.J.; Mallik, A.; Ma, J.Z.; Rochefort, L. Litter mixing effects on decomposition in a peatland partially drained 30 years ago. Wetl. Ecol. Manag. 2021, 29, 883–895. [Google Scholar] [CrossRef]
- Yang, T.; Jiang, J.; He, Q.; Shi, F.; Jiang, H.; Wu, H.; He, C. Impact of drainage on peatland soil environments and greenhouse gas emissions in Northeast China. Sci. Rep. 2025, 15, 8320. Available online: https://www.nature.com/articles/s41598-025-92655-9 (accessed on 30 July 2025). [CrossRef]
- Hamer, U.; Marschner, B. Priming effects in different soil types induced by fructose, alanine, oxalic acid and catechol additions. Soil Biol. Biochem. 2005, 37, 445–454. [Google Scholar] [CrossRef]
- Mason-Jones, K.; Schmücker, N.; Kuzyakov, Y. Contrasting effects of organic and mineral nitrogen challenge the N-mining hypothesis for soil organic matter priming. Soil Biol. Biochem. 2018, 124, 38–46. [Google Scholar] [CrossRef]
- Qian, H.; Joseph, R.; Zeng, N. Enhanced terrestrial carbon uptake in the Northern High Latitudes in the 21st century from the Coupled Carbon Cycle Climate Model Intercomparison Project model projections. Glob. Change Biol. 2010, 16, 641–656. [Google Scholar] [CrossRef]
- Ge, X.; Zeng, L.; Xiao, W.; Huang, Z.; Geng, X.; Tan, B. Effect of litter substrate quality and soil nutrients on forest litter decomposition: A review. Acta Ecol. Sin. 2013, 33, 102–108. [Google Scholar] [CrossRef]
- Laskowski, R.; Berg, B. Litter Decomposition: Guide to Carbon and Nutrient Turnover; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Pietri, J.A.; Brookes, P.C. Substrate inputs and pH as factors controlling microbial biomass, activity and community structure in an arable soil. Soil Biol. Biochem. 2009, 41, 1396–1405. [Google Scholar] [CrossRef]
- Makkonen, M.; Berg, M.P.; van Logtestijn, R.S.P.; van Hal, J.; Aerts, R. Do physical plant litter traits explain non-additivity in litter mixtures? A test of the improved microenvironmental conditions theory. Oikos 2013, 122, 987–997. [Google Scholar] [CrossRef]
- Meyer, N.; Welp, G.; Rodionov, A.; Borchard, N.; Martius, C.; Amelung, W. Nitrogen and phosphorus supply controls soil organic carbon mineralization in tropical topsoil and subsoil. Soil Biol. Biochem. 2018, 115, 152–161. [Google Scholar] [CrossRef]
- Gunina, A.; Kuzyakov, Y. From energy to (soil organic) matter. Glob. Change Biol. 2022, 28, 2169–2182. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.; Zahorka, A.; Holawe, F.; Inselsbacher, E.; Glatzel, S. Incubation of ombrotrophic bog litter and mixtures of Sphagnum, Betula and Calluna results in the formation of single litter-specific decomposition patterns. Geoderma 2023, 440, 116702. [Google Scholar] [CrossRef]
- Song, Y.; Sun, L.; Song, C.; Li, M.; Liu, Z.; Zhu, M.; Chen, S.; Yuan, J.; Gao, J.; Wang, X.; et al. Responses of soil microbes and enzymes to long-term warming incubation in different depths of permafrost peatland soil. Sci. Total Environ. 2023, 900, 165733. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Richardson, C.J.; Ho, M. Dual controls on carbon loss during drought in peatlands. Nat. Clim. Change 2015, 5, 584–587. [Google Scholar] [CrossRef]
- Fanin, N.; Bertrand, I. Aboveground litter quality is a better predictor than belowground microbial communities when estimating carbon mineralization along a land-use gradient. Soil Biol. Biochem. 2016, 94, 48–60. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, S.; Wang, Z.; Dong, Y.; Zhang, Y.; Liu, S.; Li, J. Effect of drainage on microbial enzyme activities and communities dependent on depth in peatland soil. Biogeochemistry 2021, 155, 323–341. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Powlson, D.S. The effects of biocidal treatments on metabolism in soil—V: A method for measuring soil biomass. Soil Biol. Biochem. 1976, 8, 209–213. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
| Soil Parameters | Shallow Peat | Deep Peat | p |
|---|---|---|---|
| TC (%) | 42.02 ± 0.54 | 30.81 ± 1.89 | <0.001 |
| TN (%) | 0.91 ± 0.03 | 1.44 ± 0.08 | <0.001 |
| C:N ratio | 46.22 ± 1.27 | 21.78 ± 3.46 | <0.001 |
| DOC (mg kg−1) | 108.53 ± 3.32 | 176.94 ± 12.29 | 0.007 |
| MBC (mg kg−1) | 63.29 ± 13.85 | 63.53 ± 9.28 | 0.989 |
| Treatments | (μg g−1 h−1) | ||
|---|---|---|---|
| df | F | p | |
| Depth | 1 | 1395.50 | <0.001 |
| Temperature | 4 | 561.90 | <0.001 |
| Litter | 2 | 243.88 | <0.001 |
| Drought | 1 | 8.51 | <0.001 |
| Depth × Temperature | 4 | 80.99 | <0.001 |
| Depth × Litter | 2 | 93.97 | <0.001 |
| Depth × Drought | 1 | 20.42 | <0.001 |
| Temperature × Litter | 8 | 27.59 | <0.001 |
| Temperature × Drought | 4 | 3.63 | <0.007 |
| Litter × Drought | 2 | 6.61 | <0.002 |
| OM × Temperature × Litter | 8 | 14.73 | <0.001 |
| OM × Temperature × Drought | 4 | 1.59 | 0.179 |
| Depth × Litter × Drought | 2 | 0.15 | 0.865 |
| Temperature × Litter × Drought | 8 | 14.08 | <0.001 |
| Depth × Temperature × Litter × Drought | 8 | 4.00 | <0.001 |
| Treatments | Q10 (100% WHC) | Q10 (60% WHC) | F | p |
|---|---|---|---|---|
| Peat-shallow | 1.47 ± 0.09 b | 1.78 ± 0.03 b | 10.05 | 0.025 |
| Peat-shallow + Litter-Sphagnum | 1.84 ± 0.04 ab | 1.81 ± 0.07 b | 0.13 | 0.730 |
| Peat-shallow + Litter-Betula | 1.83 ± 0.11 ab | 1.86 ± 0.03 ab | 0.11 | 0.756 |
| Peat-deep | 1.76 ± 0.03 ab | 2.13 ± 0.11 a | 7.82 | 0.049 |
| Peat-deep + Litter-Sphagnum | 1.67 ± 0.13 ab | 1.87 ± 0.07 ab | 1.84 | 0.224 |
| Peat-deep + Litter-Betula | 2.10 ± 0.09 a | 2.17 ± 0.01 a | 0.08 | 0.785 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, F.; Yi, B.; Ma, J.-X.; Wang, S.-N.; Feng, Y.-J.; Qin, K.; Tu, Q.; Bu, Z.-J. Drought and Shrub Encroachment Accelerate Peatland Carbon Loss Under Climate Warming. Plants 2025, 14, 2387. https://doi.org/10.3390/plants14152387
Lu F, Yi B, Ma J-X, Wang S-N, Feng Y-J, Qin K, Tu Q, Bu Z-J. Drought and Shrub Encroachment Accelerate Peatland Carbon Loss Under Climate Warming. Plants. 2025; 14(15):2387. https://doi.org/10.3390/plants14152387
Chicago/Turabian StyleLu, Fan, Boli Yi, Jun-Xiao Ma, Si-Nan Wang, Yu-Jie Feng, Kai Qin, Qiansi Tu, and Zhao-Jun Bu. 2025. "Drought and Shrub Encroachment Accelerate Peatland Carbon Loss Under Climate Warming" Plants 14, no. 15: 2387. https://doi.org/10.3390/plants14152387
APA StyleLu, F., Yi, B., Ma, J.-X., Wang, S.-N., Feng, Y.-J., Qin, K., Tu, Q., & Bu, Z.-J. (2025). Drought and Shrub Encroachment Accelerate Peatland Carbon Loss Under Climate Warming. Plants, 14(15), 2387. https://doi.org/10.3390/plants14152387







