Harnessing Molecular Phylogeny and Chemometrics for Taxonomic Validation of Korean Aromatic Plants: Integrating Genomics with Practical Applications
Abstract
1. Introduction
2. Literature Search and Methodology
3. Diversity and Distribution of Korean Aromatic Plants
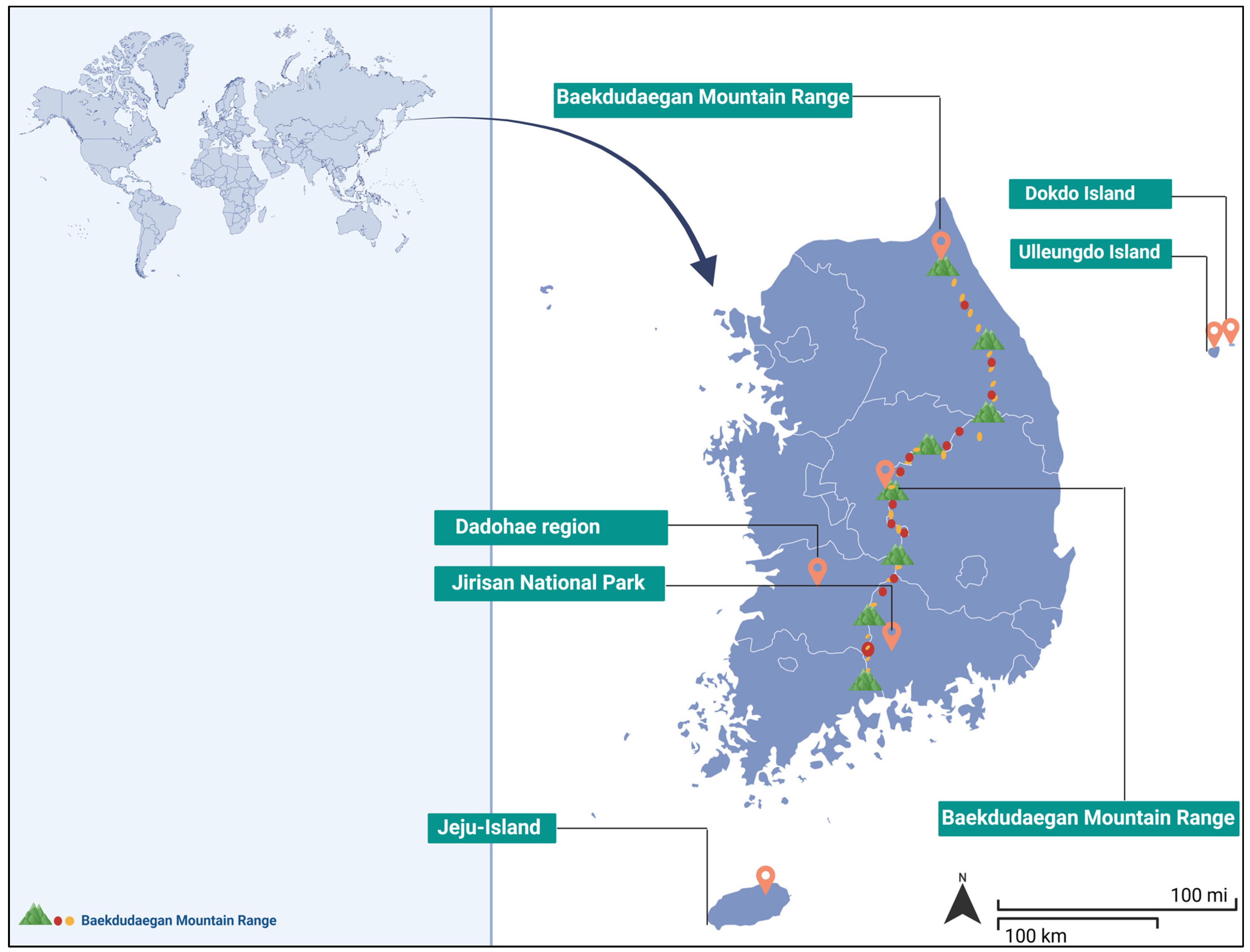
3.1. Geographic Distribution and Habitat Specificity
3.2. Aromatic Plants Hotspots
3.3. Taxonomic Diversity of Aromatic Endemic Species
| Region/Location | Species Name | Family Name | Part Used | Nature of Plants | Citation |
|---|---|---|---|---|---|
| Jeju Island/Hallasan Biosphere Reserve | Pinus thunbergii Parl. | Pinaceae | Wood and resin | Non-endemic | [87] |
| Abies koreana E.H. Wilson | Pinaceae | Wood and needles | Endemic | [88] | |
| Machilus japonica Siebold and Zucc. | Lauraceae | Bark and leaves | Non-endemic | [89] | |
| Cinnamomum camphora (L.) J. Presl | Lauraceae | Leaves and bark | Non-endemic | [90] | |
| Cinnamomum loureirii Nees | Lauraceae | Leaves and bark | Non-endemic | [90] | |
| Neolitsea sericea (Blume) Koidz. | Lauraceae | Leaves and bark | Non-endemic | [83] | |
| Zanthoxylum ailanthoides Siebold and Zucc. | Rutaceae | Fruit and bark | Non-endemic | [91] | |
| Citrus reticulata Blanco. | Rutaceae | Fruit and peel | Non-endemic | [92] | |
| Citrus unshiu (Yu. Tanaka ex Swingle) Marcow. | Rutaceae | Fruit and peel | Non-endemic | [93] | |
| Zanthoxylum coreanum Nakai | Rutaceae | Fruit and bark | Non-endemic | [94] | |
| Cryptomeria japonica (Thunb. ex L.f.) D. Don | Cupressaceae | Wood and bark | Non-endemic | [94] | |
| Agastache rugosa (Fisch. and CA Mey.) Kuntze | Lamiaceae | Leaves and flowers | Non-endemic | [91] | |
| Magnolia kobus DC. | Magnoliaceae | Bark and flowers | Non-endemic | [94] | |
| Vitex rotundifolia L.f. | Verbenaceae | Leaves and flowers | Non-endemic | [94] | |
| Chamaecyparis pisifera (Siebold and Zucc.) Endl. | Cupressaceae | Wood and bark | Non-endemic | [94] | |
| Artemisia hallaisanensis | Asteraceae | Leaves and flowers | Endemic | [95] | |
| Artemisia japonica ssp. littoricola | Asteraceae | Leaves and flowers | Non-endemic | [95] | |
| Baekdudaegan Mountain Range | Pinus rigida Mill | Pinaceae | Wood and resin | Non-endemic | [96] |
| Pinus densiflora for. multicaulis | Pinaceae | Wood and resin | Non-endemic | [97] | |
| Pinus parviflora Siebold and Zucc. | Pinaceae | Wood and resin | Non-endemic | [80] | |
| Agastache rugosa (Fisch. and CA Mey.) Kuntze | Lamiaceae | Leaves and flowers | Non-endemic | [98] | |
| Magnolia kobus DC. | Magnoliaceae | Bark and flowers | Non-endemic | [99] | |
| Jirisan National Park | Pinus densiflora for. multicaulis | Pinaceae | Wood and resin | Non-endemic | [80] |
| Abies nephrolepis (Trautv. ex Maxim.) Maxim. | Pinaceae | Wood and needles | Non-endemic | [100] | |
| Pinus parviflora Siebold and Zucc. | Pinaceae | Wood and resin | Non-endemic | [80] | |
| Machilus japonica Siebold and Zucc. | Lauraceae | Bark and leaves | Non-endemic | [83] | |
| Pinus koraiensis Siebold and Zucc. | Pinaceae | Wood and resin | Non-endemic | [101] | |
| Thuja koraiensis Nakai | Cupressaceae | Wood and bark | Non-endemic | [102] | |
| Chamaecyparis pisifera (Siebold and Zucc.) Endl. | Cupressaceae | Wood and bark | Non-endemic | [102] | |
| Juniperus chinensis L. | Cupressaceae | Wood and leaves | Non-endemic | [91] | |
| Tsuga sieboldii Carriere | Pinaceae | Wood and needles | Non-endemic | [103] | |
| Abies holophylla Maxim. | Pinaceae | Wood and needles | Non-endemic | [91] | |
| Artemisia montana (Nakai) Pamp. | Asteraceae | Leaves and flowers | Non-endemic | [56] | |
| Zanthoxylum schinifolium Siebold and Zucc. | Rutaceae | Fruit and bark | Non-endemic | [104] | |
| Artemisia absinthium L. | Asteraceae | Leaves and flowers | Non-endemic | [56] | |
| Rubus coreanus | Rosaceae | Berries and leaves | Endemic | [104] | |
| Juniperus chinensis L. | Cupressaceae | Wood and leaves | Non-endemic | [54] | |
| Artemisia annua L. | Asteraceae | Leaves and flowers | Non-endemic | [56] | |
| Dokdo Island | Artemisia japonica ssp. littoricola | Asteraceae | Leaves and flowers | Non-endemic | [105] |
| Throughout Korea | Artemisia annua L. | Asteraceae | Leaves and flowers | Non-endemic | [54,106] |
| Artemisia capillaris (Thunb.) Besser | Asteraceae | Leaves and flowers | Non-endemic | [107] | |
| Artemisia iwayomogi Kitamura | Asteraceae | Leaves and flowers | Non-endemic | [107] | |
| Artemisia absinthium L. | Asteraceae | Leaves and flowers | Non-endemic | [107] | |
| Thymus quinquecostatus Celak | Endemic | [108] | |||
| Dadohae region/Ulleungdo island | Artemisia absinthium L. | Asteraceae | Leaves and flowers | Non-endemic | [47] |
| Pinus densiflora for. multicaulis | Pinaceae | Wood and resin | Non-endemic | [97] | |
| Agastache rugosa (Fisch. and CA Mey.) Kuntze | Lamiaceae | Leaves and flowers | Non-endemic | [91] | |
| Pinus parviflora Siebold and Zucc. | Pinaceae | Wood and resin | Non-endemic | [80] |
4. Chemotaxonomy of Aromatic Plants
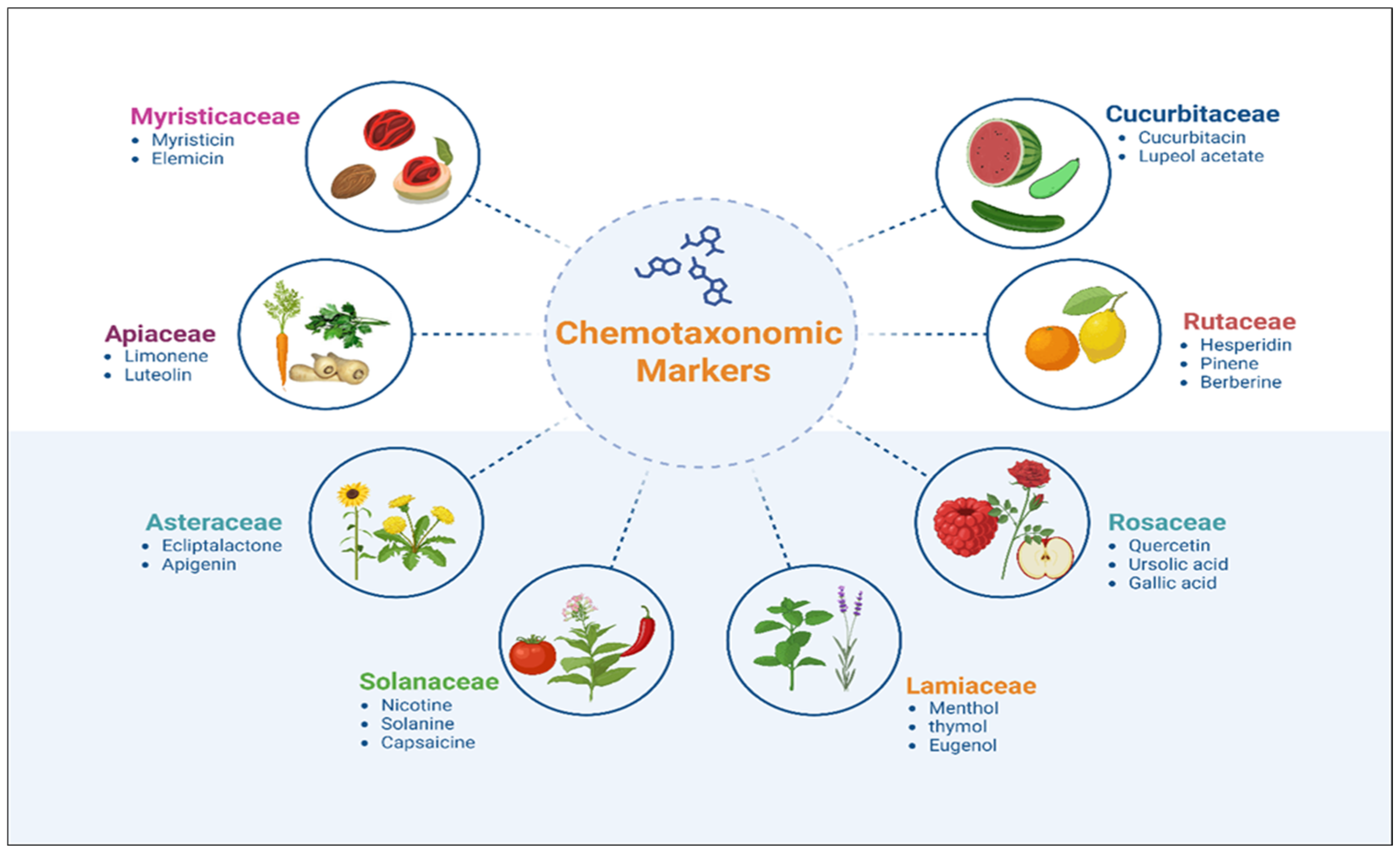
4.1. Phytochemical Markers and Chemotaxonomic Classification
4.2. Essential Oils as Chemotaxonomic Markers
4.3. Essential Oil Composition
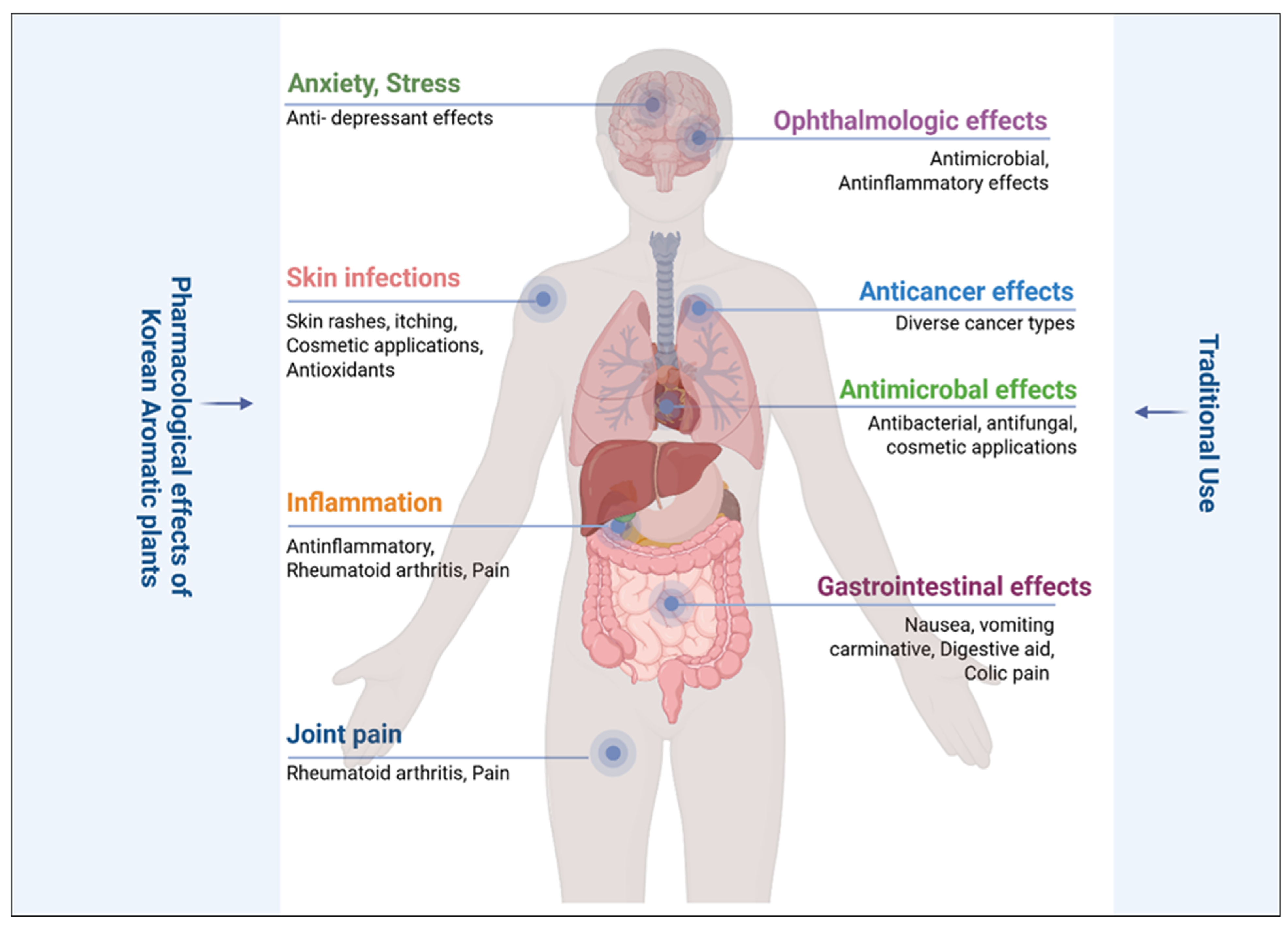
5. Pharmacological Effects of Korean Aromatic Plants
5.1. Thymus quinquecostatus Celak (Lamiaceae; Bak-Ri-Hyang)
5.2. Agastache rugosa (Lamiaceae; Korean Mint)
5.3. Abies koreana (Pinaceae)
5.4. Artemisia spp. (Asteraceae)
5.5. Cinnamon spp. (Lauraceae)
5.6. Citrus Species (Rutaceae)
5.7. Zanthoxylum ailanthoides (Rutaceae, Sichuan Pepper Tree)
5.8. Cryptomeria japonica (Cupressaceae, Japanese Cedar)
5.9. Pinus spp. (Pinaceae, Korean Nut Pine)
5.10. Abies nephrolepis (Pinaceae, Korean Fir)
5.11. Thuja koraiensis (Cupressaceae)
5.12. Magnolia kobus (Kobus Magnolia; Magnoliaceae; Moknyeon)
5.13. Zingiber officinale (Korean Bongdong (Bg) Cultivar; Zingiberaceae)
| S. No | Species Name | Major Chemical Components | Pharmacological Activities | Investigation Type | Citation |
|---|---|---|---|---|---|
| 1 | Pinus thunbergii Parl. | α-pinene, β-pinene, and bornyl acetate | Antioxidant, anti-inflammatory, and antimicrobial | In vitro | [80] |
| 2 | Abies koreana E.H. Wilson | α-pinene, limonene, and camphene | Anti-inflammatory, antioxidant, and antimicrobial | In vitro | [212] |
| 3 | Machilus japonica Siebold and Zucc. | Lignans (syringaresinol and secoisolariciresinol) and terpenoids | Antioxidant, anticancer, and anti-inflammatory | In vitro | [213] |
| 4 | Cinnamomum camphora (L.) J. Presl | Camphor, eugenol, and cinnamaldehyde | Antioxidant, antimicrobial, and anti-inflammatory | In vitro | [90] |
| 5 | Cinnamomum loureirii Nees | Linalool, eugenol, and cinnamaldehyde | Anti-inflammatory, antioxidant, and anticancer | In vitro | [90] |
| 6 | Neolitsea sericea (Blume) Koidz. | β-caryophyllene, α-pinene, and eucalyptol | Antioxidant and anti-inflammatory | In vitro | [91] |
| 7 | Zanthoxylum ailanthoides Siebold and Zucc. | Bergamottin, piperine, and limonene | Antioxidant, antimicrobial, and anti-inflammatory | In vitro | [15] |
| 8 | Citrus reticulata Blanco | Limonene, β-pinene, and α-terpinene | Antioxidant, anticancer, and anti-inflammatory | In vitro | [92] |
| 9 | Citrus unshiu (Yu. Tanaka ex Swingle) Marcow. | Limonene, γ-terpinene, and α-pinene | Anti-inflammatory, antioxidant, and antimicrobial | In vitro | [15] |
| 10 | Zanthoxylum coreanum Nakai | Bergamottin, limonene, and piperine | Antioxidant and anti-inflammatory | In vitro | [15] |
| 11 | Cryptomeria japonica (Thunb. ex L.f.) D. Don | Lignans (cryptomeridiol and α-terpineol) and flavonoids | Anti-inflammatory, antioxidant, and anticancer | In vitro | [214] |
| 13 | Magnolia kobus DC. | Magnolol, honokiol, and eugenol | Anti-inflammatory, antioxidant, and anticancer | In vitro | [99] |
| 14 | Vitex rotundifolia L.f. | Casticin, vitexin, and apigenin | Antioxidant, anti-inflammatory, and antimicrobial | In vitro | [15] |
| 15 | Chamaecyparis pisifera (Siebold and Zucc.) Endl. | Isofraxidin, lignans, and α-pinene | Antimicrobial, anti-inflammatory, and antioxidant | In vitro | [15] |
| 16 | Artemisia hallaisanensis | Flavonoids (rutin and quercetin) and terpenoids (camphor and β-caryophyllene) | Anti-inflammatory, antioxidant, and antimicrobial | In vivo | [215] |
| 17 | Artemisia japonica ssp. littoricola | α-pinene, β-caryophyllene, and artemisinin | Anti-inflammatory, antioxidant, and antimicrobial | In vivo | [215] |
| 18 | Pinus rigida Mill | α-pinene, β-pinene, and bornyl acetate | Antioxidant, anti-inflammatory, and antimicrobial | In vitro | [80] |
| 19 | Pinus densiflora for. multicaulis | α-pinene, β-pinene, and bornyl acetate | Antioxidant, anti-inflammatory, and antimicrobial | In vitro | [80] |
| 20 | Pinus parviflora Siebold & Zucc. | α-pinene, β-pinene, and bornyl acetate | Antioxidant, anti-inflammatory, and antimicrobial | In vitro | [80] |
| 21 | Agastache rugosa (Fisch. and CA Mey.) Kuntze | Rosmarinic acid, luteolin, and apigenin | Antimicrobial, anti-inflammatory, and antioxidant | In vitro | [91] |
| 22 | Magnolia kobus DC. | Magnolol, honokiol, and eugenol | Anti-inflammatory, antioxidant, and anticancer | In vitro | [15] |
| 23 | Pinus densiflora for. multicaulis | α-pinene, β-pinene, and bornyl acetate | Antioxidant, anti-inflammatory, and antimicrobial | In vitro | [80] |
| 24 | Abies nephrolepis (Trautv. ex Maxim.) Maxim. | α-pinene, camphene, and terpinolene | Anti-inflammatory, antioxidant, and antimicrobial | In vitro | [100] |
| 25 | Pinus parviflora Siebold and Zucc. | α-pinene, β-pinene, and bornyl acetate | Antioxidant, anti-inflammatory, and antimicrobial | In vitro | [80] |
| 27 | Pinus koraiensis Siebold and Zucc. | α-pinene, β-pinene, and bornyl acetate | Antioxidant, anti-inflammatory, and antimicrobial | In vitro | [80] |
| 28 | Thuja koraiensis Nakai | Lignans (tanoic acid) and phenolic acids | Antimicrobial, anti-inflammatory, and anticancer | In vitro; In vivo | [198] |
| 30 | Juniperus chinensis L. | α-pinene, β-pinene, and sabinene | Antioxidant, anti-inflammatory, and antimicrobial | In vitro | [91] |
6. Molecular Phylogenetics
6.1. Molecular Markers in Phylogenetic Studies
6.2. Phylogenetic Relationships and Evolutionary History
6.3. Integrative Approaches Combining Molecular Phylogenetics and Chemotaxonomy
7. Conservation and Sustainable Utilization
Conservation Status of Korean Aromatic Plants
8. Challenges and Limitations
9. Strategies for Conservation and Sustainable Use
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kant, R.; Kumar, A. Review on essential oil extraction from aromatic and medicinal plants: Techniques, performance and economic analysis. Sustain. Chem. Pharm. 2022, 30, 100829. [Google Scholar] [CrossRef]
- Thakur, M.; Kumar, R. Microclimatic buffering on medicinal and aromatic plants: A review. Ind. Crops Prod. 2021, 160, 113144. [Google Scholar] [CrossRef]
- El Gendy, A.N.G.; Fouad, R.; Omer, E.A.; Cock, I.E. Effects of climate change on medicinal plants and their active constituents. In Climate-Resilient Agriculture, Vol 1: Crop Responses and Agroecological Perspectives; Springer: Berlin/Heidelberg, Germany, 2023; pp. 125–156. [Google Scholar]
- Mansinhos, I.; Gonçalves, S.; Romano, A. How climate change-related abiotic factors affect the production of industrial valuable compounds in Lamiaceae plant species: A review. Front. Plant Sci. 2024, 15, 1370810. [Google Scholar] [CrossRef]
- Dikme, T.G. Use of medicinal and aromatic plants in food. Eu. Clin. Anal. Med. 2023, 11, 6–10. [Google Scholar]
- Baser, K.H.; Bonello, J.M. Global trade of essential oils. J. Essent. Oil Res. 2025, 37, 208–214. [Google Scholar] [CrossRef]
- Wang, S.-Q.; Dong, X.-Y.; Ye, L.; Wang, H.-F.; Ma, K.-P. Flora of Northeast Asia. Plants 2023, 12, 2240. [Google Scholar] [CrossRef]
- Kim, N.S.; Lim, C.H.; Cha, J.Y.; Cho, Y.C.; Jung, S.H.; Jin, S.Z.; Nan, Y. Distribution characteristics of Manchurian and China-Japan-Korea flora in Korean Peninsula. J. Ecol. Environ. 2022, 46, 259–272. [Google Scholar] [CrossRef]
- Jung, S.; Cho, Y.C. Redefining floristic zones in the Korean Peninsula using high-resolution georeferenced specimen data and self-organizing maps. Ecol. Evol. 2020, 10, 11549–11564. [Google Scholar] [CrossRef]
- Chung, G.Y.; Hyun-Do, J.; Chang, K.S.; Hyeok Jae, C.; Young-Soo, K.I.M.; Hyuk-Jin, K.I.M.; Son, D.C. A checklist of endemic plants on the Korean Peninsula II. Korean J. Plant Taxon. 2023, 53, 79–101. [Google Scholar] [CrossRef]
- Yun, H.-G.; Jung, J.-Y.; Shin, H.-T.; An, J.-B. Floristic inventory of forest genetic resources reserve in Korea. J. Asia-Pac. Biodivers. 2021, 14, 410–414. [Google Scholar] [CrossRef]
- Chae, H.-H.; Kim, Y.-C.; Son, S.-W. Korean and worldwide research trends on rare plant and endemic plant in Korea. Korean J. Environ. Ecol. 2022, 36, 257–276. [Google Scholar] [CrossRef]
- Cho, Y.C.; Seol, J.; Lim, C.H. Climate-induced distribution dynamics and niche adaptation of South Korean endemic plants across the Korean Peninsula. Sci. Rep. 2024, 14, 22253. [Google Scholar] [CrossRef]
- East Asian Biodiversity Conservation Network. Important Plants of East Asia II: Endemic Plant Stories; Korea National Arboretum: Pocheon, Republic of Korea, 2015. [Google Scholar]
- Shin, K.H.; Chi, H.J.; Lim, S.S.; Cho, S.H.; Moon, H.I.; Yu, J.H. Antimicrobial activities of volatile essential oils from Korean aromatic plants. Nat. Prod. Sci. 1997, 3, 141–147. [Google Scholar]
- Kim, M.; Sowndhararajan, K.; Kim, S. The chemical composition and biological activities of essential oil from Korean native thyme Bak-Ri-Hyang (Thymus quinquecostatus Celak.). Molecules 2022, 27, 4251. [Google Scholar] [CrossRef]
- Hong, M.J.; Kim, J.H.; Kim, H.Y.; Kim, M.J.; Kim, S.M. Chemical composition and biological activity of essential oil of Agastache rugosa (Fisch. CA Mey.) O. Kuntze. Korean J. Med. Crop Sci. 2020, 28, 95–110. [Google Scholar] [CrossRef]
- Chung, J.-M.; Kim, H.-J.; Park, G.-W.; Jeong, H.-R.; Choi, K.; Shin, C.-H. Ethnobotanical study on the traditional knowledge of vascular plant resources in South Korea. Korean J. Plant Resour. 2016, 29, 62–89. [Google Scholar] [CrossRef]
- Ong, H.G.; Chung, J.-M.; Jeong, H.-R.; Kim, Y.-D.; Choi, K.; Shin, C.-H.; Lee, Y.-M. Ethnobotany of the wild edible plants gathered in Ulleung Island, South Korea. Genet. Resour. Crop Evol. 2016, 63, 409–427. [Google Scholar] [CrossRef]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. genus: A review of bioactive essential oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef]
- Park, C.; Woo, H.; Park, M.-J. Development of Pinaceae and Cupressaceae essential oils from forest waste in South Korea. Plants 2023, 12, 3409. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Faraz, M.; Farid, A.; Ghazanfar, S. Threatened and Endangered Medicinal and Aromatic Plants. In Ethnobotany and Ethnopharmacology of Medicinal and Aromatic Plants: Steps Towards Drug Discovery, 1st ed.; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Woo-Seok, K.; Watts, P. The Plant Geography of Korea: With an Emphasis on the Alpine Zones; Springer Science Business Media: Berlin/Heidelberg, Germany, 2012; Volume 19. [Google Scholar]
- Cadotte, M.W.; Tucker, C.M. Difficult decisions: Strategies for conservation prioritization when taxonomic, phylogenetic and functional diversity are not spatially congruent. Biol. Conserv. 2018, 225, 128–133. [Google Scholar] [CrossRef]
- Umoh, O.T. Chemotaxonomy: The role of phytochemicals in chemotaxonomic delineation of taxa. Taxon 2020, 13, 14. [Google Scholar] [CrossRef]
- Ahn, D.K. Illustrated Book of Korean Medicinal Herbs. Kyohaksa Publishing Co., Ltd.: Seoul, Republic of Korea, 1998; Volume 2, pp. 208–214. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Marcelino, S.; Gaspar, P.D.; Paco, A. Sustainable waste management in the production of medicinal and aromatic plants—A systematic review. Sustainability 2023, 15, 13333. [Google Scholar] [CrossRef]
- Lee, T.B. Endemic Plants and Their Distribution in Korea. Seoul National University: Seoul, Republic of Korea, 1983; Volume 14, pp. 21–32. [Google Scholar]
- Paik, W.K. The status of endemic plants in Korea and our tasks in the 21st century. Korean J. Plant Taxon. 1999, 29, 263–274. [Google Scholar] [CrossRef]
- Chung, G.Y.; Chang, K.S.; Chung, J.-M.; Choi, H.J.; Paik, W.-K.; Hyun, J.-O. A checklist of endemic plants on the Korean Peninsula. Korean J. Plant Taxon. 2017, 47, 264–288. [Google Scholar] [CrossRef]
- Kim, K.-O.; Hong, S.-H.; Lee, Y.-H.; Na, C.-S.; Kang, B.-H.; Son, Y.-W. Taxonomic status of endemic plants in Korea. J. Ecol. Environ. 2009, 32, 277–293. [Google Scholar] [CrossRef][Green Version]
- Rotenberry, J.T.; Preston, K.L.; Knick, S.T. GIS-Based Niche Modeling for Mapping Species’ Habitat. Ecology 2006, 87, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-K. Baekdudaegan, the central axis of the Korean peninsular: The path toward management strategies regarding to its concepts. In Ecological Issues in a Changing World: Status, Response and Strategy; Springer: Berlin/Heidelberg, Germany, 2004; pp. 355–383. [Google Scholar]
- Shin, S.; Kim, J.-H.; Dang, J.-H.; Seo, I.-S.; Lee, B.Y. Elevational distribution ranges of vascular plant species in the Baekdudaegan mountain range, South Korea. J. Ecol. Environ. 2021, 45, 7. [Google Scholar] [CrossRef]
- Chung, M.Y.; López-Pujol, J.; Chung, M.G. The role of the Baekdudaegan (Korean Peninsula) as a major glacial refugium for plant species: A priority for conservation. Biol. Conserv. 2017, 206, 236–248. [Google Scholar] [CrossRef]
- Darvon O’g’li, S.E. Learning Geography of Korea by Mountains and Island as Main Resource of Korean Peninsula. Int. J. Adv. Sci. Res. 2024, 4, 30–34. [Google Scholar] [CrossRef]
- Park, J.-S.; An, J.-B.; Yun, H.-G.; Yi, M.-H.; Park, W.-G.; Shin, H.-T.; Hong, Y.-s.; Lee, K.-C.; Shim, Y.-J.; Sung, J.-W. Ecological Characteristics of Natural Habits of Deutzia paniculata, a Rare and Endemic Woody Species in Korea. J. For. Environ. Sci. 2021, 37, 206–216. [Google Scholar]
- Sollins, P. Factors influencing species composition in tropical lowland rain forest: Does soil matter? Ecology 1998, 79, 23–30. [Google Scholar] [CrossRef]
- Chung, M.Y.; Son, S.; Suh, G.U.; Herrando-Moraira, S.; Lee, C.H.; López-Pujol, J.; Chung, M.G. The Korean Baekdudaegan Mountains: A glacial refugium and a biodiversity hotspot that needs to be conserved. Front. Genet. 2018, 9, 489. [Google Scholar] [CrossRef] [PubMed]
- Hh, K.; Mizuno, K.; Ws, K. Present status and distribution of naturalized plants in the island regions of the South Korea. BioInvasions Rec. 2023, 12, 31–42. [Google Scholar] [CrossRef]
- Song, M.-J.; Kim, H.; Heldenbrand, B.; Jeon, J.; Lee, S. Ethnopharmacological survey of medicinal plants in Jeju Island, Korea. J. Ethnobiol. Ethnomedicine 2013, 9, 48. [Google Scholar] [CrossRef]
- Kim, H.-J.; Jeong, H.-S.; Kang, S.-H. Ethnobotany of Jeju Island, Korea. Korean J. Plant Resour. 2015, 28, 217–234. [Google Scholar] [CrossRef]
- Hong, H.-J.; Kim, C.-K.; Lee, H.-W.; Lee, W.-K. Conservation, restoration, and sustainable use of biodiversity based on habitat quality monitoring: A case study on Jeju Island, South Korea (1989–2019). Land 2021, 10, 774. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Jeong, I.; Kim, S.; Choi, J.W.; Won, M.H.; Kim, D.; Kim, D.; Kim, Y.-K.; Jeon, J.; Ryu, J. Checklist for the insect fauna of two East Sea Islands (Ulleungdo Is. and Dokdo Is.) in the Republic of Korea. Biodivers. Data J. 2024, 12, e129360. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.E.; Park, J.-S.; Jung, J.-Y.; Kim, D.-K.; Yang, S.; Choi, H.J. Notes on Allium section Rhizirideum (Amaryllidaceae) in South Korea and northeastern China: With a new species from Ulleungdo Island. PhytoKeys 2021, 176, 1. [Google Scholar] [CrossRef]
- Jung, S.-Y.; Park, S.-H.; Nam, C.-H.; Lee, H.-J.; Lee, Y.-M.; Chang, K.-S. The distribution of vascular plants in Ulleungdo and nearby island regions (Gwaneumdo, Jukdo), Korea. J. Asia-Pac. Biodivers. 2013, 6, 123–156. [Google Scholar] [CrossRef][Green Version]
- Shin, H.T.; Hwang, J.H.; Yoon, K.J. Distribution of vascular plants in the Ulleung forest trail area (Seokpo to Naesujeon). J. Korean Nat. 2011, 4, 79–85. [Google Scholar] [CrossRef]
- Chung, J.-M.; Shin, J.-K.; Kim, H.-M. Diversity of vascular plants native to the Ulleungdo and Dokdo Islands in Korea. J. Asia-Pac. Biodivers. 2020, 13, 701–708. [Google Scholar] [CrossRef]
- Lee, M.-W.; Han, G.-I.; Kim, Y.-S.; Kim, S.-B.; Heo, T.-I. Ecological Strategy for Restoring the Forest Ecosystem in the Taebaek Region of Baekdudaegan Mountains in Korea. Forests 2025, 16, 383. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, J.-S.; Shin, S.; Park, S.-A.; Park, S.; Han, S.K.; Kim, J.-S. Vertical distribution and vascular plants on Joryeongsan Mountain in Baekdudaegan, Korea. Korean J. Environ. Biol. 2024, 42, 95–126. [Google Scholar] [CrossRef]
- Sadiq, A.; Zeb, A.; Ullah, F.; Ahmad, S.; Ayaz, M.; Rashid, U.; Muhammad, N. Chemical characterization, analgesic, antioxidant, and anticholinesterase potentials of essential oils from Isodon rugosus Wall. ex. Benth. Front. Pharmacol. 2018, 9, 623. [Google Scholar] [CrossRef]
- Cho, W.; Chun, B.K. Restoration of the Baekdudaegan mountains in the Republic of Korea. Unasylva 2015, 66, 64. [Google Scholar]
- Shin, H.T.; Yi, M.H.; Yoon, J.W.; Yoo, J.H.; Lee, B.-C.; Park, E.-H. Distribution of rare plants and endemic plants in jirisan national park. J. Korean Nat. 2010, 3, 219–222. [Google Scholar] [CrossRef]
- Noh, I.; Chung, J.-M.; Cho, M.-G.; Kim, T.-W.; Moon, H.-S. The flora of subalpine vascular plants in Seseok area of Jirisan national park. J. Clim. Change Res. 2017, 8, 201–211. [Google Scholar] [CrossRef]
- Kim, H.; Song, M.-J. Analysis of traditional knowledge about medicinal plants utilized in communities of Jirisan National Park (Korea). J. Ethnopharmacol. 2014, 153, 85–89. [Google Scholar] [CrossRef]
- Kim, H.; Song, M.-J. Analysis of traditional knowledge for wild edible mushrooms consumed by residents living in Jirisan National Park (Korea). J. Ethnopharmacol. 2014, 153, 90–97. [Google Scholar] [CrossRef]
- Sung, H.-J.; Kwon, H.-S.; Seo, C.-W.; Park, C.-H. A Study on the Spatial Decision Making Support Model for Protected Areas Boundary (re) Design-A Case of Jirisan National Park. J. Korean Soc. Environ. Restor. Technol. 2011, 14, 101–113. [Google Scholar]
- Cho, T.-D. Analyzing the Problems of Nature Trails of National Park-Case Studies on Odaesan and Seoraksan National Park. J. Environ. Sci. Int. 2009, 18, 715–719. [Google Scholar] [CrossRef]
- Lee, S.-C.; Kang, H.-M.; Kim, D.-H.; Kim, Y.-S.; Kim, J.-H.; Kim, J.-S.; Park, B.-J.; Park, S.-G.; Eum, J.-H.; Oh, H.-K. Subalpine vegetation structure characteristics and flora of Mt. Seoraksan National Park. Korean J. Environ. Ecol. 2022, 36, 118–138. [Google Scholar] [CrossRef]
- Lee, I.W.; Kim, K.D. Analysis of Traditional Knowledge Data Types Related to Plants in Seoraksan and Hallasan Biosphere Reserves. Korean J. Plant Resour. 2024, 37, 321–359. [Google Scholar]
- Lim, E.M. Preferences for Management Strategies Aimed at Conserving Biodiversity in South Koreas National Parks: A Case of Seoraksan National Park. 2017. Available online: https://hdl.handle.net/10371/137597 (accessed on 30 May 2025).
- Chang, C.-S.; Kwon, S.Y.; Shin, H.T.; Jung, S.-Y.; Kim, H. Vascular plants occurrences in Dokdo Islands, Korea, based on herbarium collections and legacy botanical literature. Biodivers. Data J. 2021, 9, e77695. [Google Scholar] [CrossRef]
- Seo, H.; Kim, S.-H.; Kim, S.C. Chloroplast DNA insights into the phylogenetic position and anagenetic speciation of Phedimus takesimensis (Crassulaceae) on Ulleung and Dokdo Islands, Korea. PLoS ONE 2020, 15, e0239734. [Google Scholar] [CrossRef]
- Kondratyuk, S.; Lőkös, L.; Halda, J.; Lee, B.G.; Jang, S.-H.; Woo, J.-J.; Park, J.S.; Oh, S.-O.; Han, S.-K.; Hur, J.-S. Arthonia dokdoensis and Rufoplaca toktoana–two new taxa from Dokdo Islands (South Korea). Mycobiology 2019, 47, 355–367. [Google Scholar] [CrossRef]
- Khim, J.S.; Lee, C.; Song, S.J.; Bae, H.; Noh, J.; Lee, J.; Kim, H.-G.; Choi, J.-W. Marine biodiversity in Korea: A review of macrozoobenthic assemblages, their distributions, and long-term community changes from human impacts. Oceanogr. Mar. Biol. 2021, 59, 483–532. [Google Scholar]
- Pandey, A.K.; Kumar, P.; Saxena, M.J.; Maurya, P. Distribution of aromatic plants in the world and their properties. In Feed Additives; Elsevier: Amsterdam, The Netherlands, 2020; pp. 89–114. [Google Scholar]
- Lee, T.B. Korea-Japan Joint Symposium on Plant Taxonomy: Outline of Korean endemic plants and their distribution. Korean J. Plant Taxon. 1984, 14, 21–32. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Kim, S.; Deepa, P.; Kim, M. Variations in the Chemical Composition of Essential Oils in Native Populations of Korean Thyme, Thymus quinquecostatus Celak. Molecules 2022, 27, 7203. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.R.R.; Ferreira, O.O.; Cruz, J.; De Jesus Pereira Franco, C.; Anjos, T.O.D.; Cascaes, M.; Da Costa, W.A.; De Aguiar Andrade, E.H.; De Oliveira, M.S. Lamiaceae Essential Oils, Phytochemical Profile, Antioxidant, and Biological Activities. Evid.-Based Complement. Altern. Med. eCAM 2021, 2021, 6748052. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Choi, Y.-H.; Lee, J.-C. Essential Oils of Thymus quinquecostatus Celakov. and Thymus magnus Nakai. Korean J. Med. Crop Sci. 1994, 2, 234–240. [Google Scholar]
- Leontiev, N.; Shutava, N.; Kavalenka, N.; Shutava, H.; Supichenka, N. Essential Oils of Lamiaceae with High Content of α-, β-Pinene and Limonene Enantiomers. J. Essent. Oil Bear. Plants 2014, 17, 18–25. [Google Scholar] [CrossRef]
- Tran, T.H.; Nguyen, N.L.; Vu, N.; Vu, Q.M.; Le, H.; Ha, M. A Mini Review on the Phytochemical Composition and Biological Activities of Agastache Rugosa. Tạp Chí Nghiên Cứu Khoa Học Phát Triển 2024, 3, 91–92. [Google Scholar] [CrossRef]
- Sytar, O.; Bruckova, K.; Hunkova, E.; Zivcak, M.; Konate, K.; Brestic, M. The application of multiplex fluorimetric sensor for the analysis of flavonoids content in the medicinal herbs family Asteraceae, Lamiaceae, Rosaceae. Biol. Res. 2015, 48, 5. [Google Scholar] [CrossRef] [PubMed]
- Bora, K.S.; Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef]
- Dudkin, R.; Ross, S.; Gorovoi, P.; Khan, I.; Wang, M.; Suleimen, E. Constituent Compositions of Essential Oils from Artemisia littoricola and A. mandshurica. Chem. Nat. Compd. 2015, 51, 790–792. [Google Scholar] [CrossRef]
- Yu, A.N.; Yang, Y.N.; Yang, Y.; Zheng, F.P.; Sun, B.G. Free and bound volatile compounds in the Rubus coreanus fruits of different ripening stages. J. Food Biochem. 2019, 43, e12964. [Google Scholar] [CrossRef] [PubMed]
- Palme, E.; Bilia, A.R.; Morelli, I. Flavonols and isoflavones from Cotoneaster simonsii. Phytochemistry 1996, 42, 903–905. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, P.S.; Lee, D.K. Forest restoration in Korea. Keep Asia Green 2007, 2, 55–79. [Google Scholar]
- Kurose, K.; Okamura, D.; Yatagai, M. Composition of the essential oils from the leaves of nine Pinus species and the cones of three of Pinus species. Flavour Fragr. J. 2007, 22, 10–20. [Google Scholar] [CrossRef]
- Farias, K.S.; Alves, F.M.; Santos-Zanuncio, V.S.; de Sousa Jr, P.T.; Silva, D.B.; Carollo, C.A. Global distribution of the chemical constituents and antibacterial activity of essential oils in Lauraceae family: A review. South Afr. J. Bot. 2023, 155, 214–222. [Google Scholar] [CrossRef]
- Lee, J.-H.; Choi, B.-H. Distribution and northernmost limit on the Korean Peninsula of three evergreen trees. Korean J. Plant Taxon. 2010, 40, 267–273. [Google Scholar] [CrossRef]
- Jeong, M.-J.; Yang, J.; Choi, W.-S.; Kim, J.-W.; Kim, S.J.; Park, M.-J. Chemical compositions and antioxidant activities of essential oil extracted from Neolitsea aciculata (Blume) Koidz leaves. J. Korean Wood Sci. Technol. 2017, 45, 96–106. [Google Scholar] [CrossRef]
- Kim, J.-H. The Origin and Evolution of Botanical Gardens in Korea; DBpia: Seoul, Republic of Korea, 2017. [Google Scholar]
- Lee, J.S.; Kim, S.H.; Lee, S.; Maki, M.; Otsuka, K.; Kozhevnikov, A.E.; Kozhevnikova, Z.V.; Wen, J.; Kim, S.C. New insights into the phylogeny and biogeography of subfamily Orontioideae (Araceae). J. Syst. Evol. 2019, 57, 616–632. [Google Scholar] [CrossRef]
- Zaman, W.; Ayaz, A.; Park, S. Integrating morphological and molecular data in plant taxonomy. Pak. J. Bot. 2025, 57, 1453–1466. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Kim, E.; Lee, E.; Lee, S.; Cho, K.; Lee, Y.; Chung, S.; Jeong, H.; You, Y. Characteristics of vegetation succession on the Pinus thunbergii forests in warm temperate regions, Jeju Island, South Korea. J. Ecol. Environ. 2019, 43, 44. [Google Scholar] [CrossRef]
- Dolezal, J.; Altman, J.; Kopecky, M.; Cerny, T.; Janecek, S.; Bartos, M.; Petrik, P.; Srutek, M.; Leps, J.; Song, J.-S. Plant diversity changes during the postglacial in East Asia: Insights from forest refugia on Halla Volcano, Jeju Island. PLoS ONE 2012, 7, e33065. [Google Scholar] [CrossRef]
- Lee, H.J.; Yeo, J.C.; Youn, W.B.; Kim, H.W.; Park, S.H.; Kong, Y.J.; Park, B.B. The effects of N and P fertilizer application on the growth and nutrient concentrations of Chamaecyparis obtusa, Machilus thunbergii, Quercus salicina seedlings in Jeju Island, Korea. Korean J. Agric. Sci. 2024, 51, 545–563. [Google Scholar] [CrossRef]
- Lee, C.S.; Yeau, S.H.; Chung, S.Y. Study of traditional plants of Jeju island (Five literatures in Joseon Dynasty period). Korean J. Plant Resour. 2016, 29, 225–234. [Google Scholar] [CrossRef]
- Hang-Hwa, H.; Eun-Mi, S.U.N.; Kang-Hyup, L.E.E.; Jin-Suk, K.I.M.; Hyoung-Tak, I.M. Warm-temperate evergreen pterophytes of Korea. Korean J. Plant Taxon. 2024, 54, 151–159. [Google Scholar] [CrossRef]
- Kim, C.M.; Baek, W.S.; Kwon, Y.S.; Ko, K.S.; Huh, I.O. The Pharmacognosical Study on the Citrus Fruit Peels cultivated in Jeju Island by Chemotaxonomy. Korean J. Pharmacogn. 2018, 49, 113–123. [Google Scholar] [CrossRef]
- Kim, G.-J.; Jang, Y.; Kwon, K.-T.; Kim, J.-W.; Kang, S.-I.L.; Ko, H.-C.; Lee, J.-Y.; Apostolidis, E.; Kwon, Y.-I. Jeju Citrus (Citrus unshiu) Leaf Extract and Hesperidin Inhibit Small Intestinal α-Glucosidase Activities In Vitro and Postprandial Hyperglycemia in Animal Model. Int. J. Mol. Sci. 2024, 25, 13721. [Google Scholar] [CrossRef]
- Park, C.; Woo, H. Development of Native Essential Oils from Forestry Resources in South Korea. Life 2022, 12, 1995. [Google Scholar] [CrossRef]
- Lim, C.E.; Kim, G.-B.; Ryu, S.-A.; Yu, H.-J.; Mun, J.-H. The complete chloroplast genome of Artemisia hallaisanensis Nakai (Asteraceae), an endemic medicinal herb in Korea. Mitochondrial DNA Part B 2018, 3, 359–360. [Google Scholar] [CrossRef]
- Park, P.S. Effects of Climate and Rehabilitation on Forests and Forest Disturbances in Korea. In Forests as Complex Social and Ecological Systems: A Festschrift for Chadwick D. Oliver; Springer: Berlin/Heidelberg, Germany, 2022; pp. 137–154. [Google Scholar]
- Lee, J.-H.; Lee, B.-K.; Kim, J.-H.; Lee, S.-H.; Hong, S.-K. Comparison of chemical compositions and antimicrobial activities of essential oils from three conifer trees; Pinus densiflora, Cryptomeria japonica, and Chamaecyparis obtusa. J. Microbiol. Biotechnol. 2009, 19, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Heo, H.; Shin, H.-R.; Chung, J.-W.; Lee, J. Comparison of antioxidant activities in Agastache species. J. Korean Soc. Food Sci. Nutr. 2022, 51, 389–394. [Google Scholar] [CrossRef]
- Lee, J.-W.; Kim, T.-W.; Park, J.-H.; Han, Y.-S.; Lee, S.-H.; Hur, T.; Lim, C.-Y. The Floristic Inventory and Distribution Characteristics of Vascular Plants in the Forest Restoration Post-monitoring Site. Korean J. Plant Resour. 2024, 37, 475–526. [Google Scholar]
- Park, G.E.; Kim, E.-S.; Jung, S.-C.; Yun, C.-w.; Kim, J.-s.; Kim, J.-d.; Kim, J.; Lim, J.-H. Distribution and stand dynamics of subalpine conifer species (Abies nephrolepis, A. koreana, and Picea jezoensis) in Baekdudaegan protected area. J. Korean Soc. For. Sci. 2022, 111, 61–71. [Google Scholar]
- Seo, J.-W.; Sano, M.; Jeong, H.-M.; Lee, K.-H.; Park, H.-C.; Nakatsuka, T.; Shin, C.-S. Oxygen isotope ratios of subalpine conifers in Jirisan National Park, Korea and their dendroclimatic potential. Dendrochronologia 2019, 57, 125626. [Google Scholar] [CrossRef]
- Park, C.; Lee, S.; Lee, D.; Oh, S.H.; Byeon, J. Evaluating the Impact of Climate Change on the Endangered Endemic Species Thuja koraiensis Nakai in Baekdudaegan, South Korea: An Ensemble Modelling Approach. Sens. Mater. 2024, 36, 1501. [Google Scholar] [CrossRef]
- Manabe, T.; Song, J.-S. Gap characteristics and gap regeneration in secondary deciduous broad-leaved forests on Mt. Jiri, South Korea. Bull. Kitakyushu Mus. Nat. Hist. Hum. Hist. Ser. A Nat. Hist. 2011, 9, 157–165. [Google Scholar]
- Park, H.C.; Kim, E.O.; Kim, W.C. A Study on Plant Community Structure Based on the Fourth National Park Resource Survey Plots in Mt. Jirisan National Park. Korean J. Plant Resour. 2020, 33, 482–500. [Google Scholar]
- Kadam, S.K.; Youn, J.-S.; Tamboli, A.S.; Yang, J.; Pak, J.H.; Choo, Y.-S. Complete chloroplast genome sequence of Artemisia littoricola (Asteraceae) from Dokdo Island Korea: Genome structure, phylogenetic analysis, and biogeography study. Funct. Integr. Genom. 2024, 24, 181. [Google Scholar] [CrossRef] [PubMed]
- Choo, G.C.; An, H.C.; Cho, H.S.; Kim, I.K.; Park, E.H.; Park, S.B. Vegetation structure of the Chilseon valley in the Jirisan National Park. Korean J. Environ. Ecol. 2009, 23, 22–29. [Google Scholar]
- Lee, J.H.; Lee, J.W.; Sung, J.S.; Bang, K.H.; Moon, S.G. Molecular authentication of 21 Korean Artemisia species (compositae) by polymerase chain reaction-restriction fragment length polymorphism based on trnL–F region of chloroplast DNA. Biol. Pharm. Bull. 2009, 32, 1912–1916. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Kim, J.H. Antifungal activities of essential oils from Thymus quinquecostatus and T. magnus. Planta Medica 2004, 70, 1090–1092. [Google Scholar] [CrossRef] [PubMed]
- Mali, S.; Yadav, R.; Gauttam, V.; Sawale, J. An Updated Review on Taxonomy and Chemotaxonomy. Toxicol. Int. 2023, 30, 121–129. [Google Scholar] [CrossRef]
- Ramos, Y.J.; Gouvêa-Silva, J.G.; de Brito Machado, D.; Felisberto, J.S.; Pereira, R.C.; Sadgrove, N.J.; de Lima Moreira, D. Chemophenetic and chemodiversity approaches: New insights on modern study of plant secondary metabolite diversity at different spatiotemporal and organizational scales. Rev. Bras. De Farmacogn. 2023, 33, 49–72. [Google Scholar] [CrossRef]
- Lawson, P.A.; Patel, N.B. The strength of chemotaxonomy. In Trends in the Systematics of Bacteria and Fungi; CABI Publishing: Wallingford, UK, 2021; pp. 141–167. [Google Scholar]
- Marne, P.A.; Pawar, A.T.; Tagalpallewar, A.A.; Baheti, A.M. Comparative Phytochemistry and Chemotaxonomy. Pharmacogn. Phytochem. Princ. Technol. Clin. Appl. 2025, 17, 333–346. [Google Scholar]
- Moss, C.W. New methodology for identification of nonfermenters: Gas-liquid chromatographic chemotaxonomy. In Glucose Nonfermenting Gram-Negative Bacteria in Clinical Microbiology; CRC Press: Boca Raton, FL, USA, 2020; pp. 171–201. [Google Scholar]
- Upton, R.; David, B.; Gafner, S.; Glasl, S. Botanical ingredient identification and quality assessment: Strengths and limitations of analytical techniques. Phytochem. Rev. 2020, 19, 1157–1177. [Google Scholar] [CrossRef]
- Pradhan, A.K.; Chowra, U.; Nath, M.; Roy, S.J.; Kalita, B.; Kundu, B.; Rajkumari, J.D.; Tanti, B. Biomarkers from Medicinal Plants. In Traditional Resources and Tools for Modern Drug Discovery: Ethnomedicine and Pharmacology; Springer: Berlin/Heidelberg, Germany, 2024; pp. 205–239. [Google Scholar]
- Yin, T.; Yan, Y.; Jiang, H.; Yang, X. Alkaloids from Aconitum brachypodum and their network-based analysis of chemotaxonomic value. Biochem. Syst. Ecol. 2022, 105, 104534. [Google Scholar] [CrossRef]
- Gismondi, A.; Baldoni, M.; Gnes, M.; Scorrano, G.; D’Agostino, A.; Di Marco, G.; Calabria, G.; Petrucci, M.; Müldner, G.; Von Tersch, M. A multidisciplinary approach for investigating dietary and medicinal habits of the Medieval population of Santa Severa (7th-15th centuries, Rome, Italy). PLoS ONE 2020, 15, e0227433. [Google Scholar] [CrossRef]
- Kaigongi, M.M.; Lukhoba, C.W. The chemosystematics of the genus Zanthoxylum L. (Rutaceae) in Kenya. Biochem. Syst. Ecol. 2021, 98, 104319. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Jones, A.D. Alkaloids of the genus datura: Review of a rich resource for natural product discovery. Molecules 2021, 26, 2629. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. Plant-based isoquinoline alkaloids: A chemical and pharmacological profile of some important leads. Asian J. Res. Chem. 2023, 16, 43–48. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; López-Martínez, L.X.; Contreras-Angulo, L.A.; Elizalde-Romero, C.A.; Heredia, J.B. Plant alkaloids: Structures and bioactive properties. In Plant-Derived Bioactives: Chemistry and Mode of Action; Springer: Berlin/Heidelberg, Germany, 2020; pp. 85–117. [Google Scholar]
- Chen, M.; Li, J.-M.; Kan, S.-L.; Liu, Y.-J.; Cao, Y.; Yu, C.-L.; Wang, H.W.; Liu, Y.Y.; Peng, D. An autotetraploid genome of Corydalis sheareri provides insight into the evolution and benzylisoquinoline alkaloids diversity of Corydalis. Genom. Commun. 2025, 2, e002. [Google Scholar] [CrossRef]
- Zheng, X.; Yin, J.; Gao, X.; Liu, Z.; Zheng, W.; Zhou, J.; Han, N. Study on the discrimination between Corydalis Rhizoma and its adulterants based on HPLC-DAD-Q-TOF-MS associated with chemometric analysis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1090, 110–121. [Google Scholar] [CrossRef]
- Emerenciano, V.d.P.; Militão, J.; Campos, C.C.; Romoff, P.; Kaplan, M.A.C.; Zambon, M.; Brant, A.J.C. Flavonoids as chemotaxonomic markers for Asteraceae. Biochem. Syst. Ecol. 2001, 29, 947–957. [Google Scholar] [CrossRef]
- Benalaya, I.; Alves, G.; Lopes, J.; Silva, L.R. A review of natural polysaccharides: Sources, characteristics, properties, food, and pharmaceutical applications. Int. J. Mol. Sci. 2024, 25, 1322. [Google Scholar] [CrossRef]
- Ullah, S.; Khalil, A.A.; Shaukat, F.; Song, Y. Sources, extraction and biomedical properties of polysaccharides. Foods 2019, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Kadam, P.; Shahi, S. Exploring the Medicinal Properties of Underutilized Zingiberaceae Family Plants: A Comparative Study of Thikur, Ginger, and Turmeric. Int. J. Environ. Sci. 2025, 11, 765–777. [Google Scholar]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef]
- Sharma, A.; Gumber, K.; Gohain, A.; Bhatia, T.; Sohal, H.S.; Mutreja, V.; Bhardwaj, G. Importance of essential oils and current trends in use of essential oils (aroma therapy, agrofood, and medicinal usage). In Essential Oils; Elsevier: Amsterdam, The Netherlands, 2023; pp. 53–83. [Google Scholar]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Elkiran, O. Chemotaxonomy and Essential Oils in Plants. Curr. Res. Sci. Math. 2022, 2, 31–40. [Google Scholar]
- da Silva, B.D.; Bernardes, P.C.; Pinheiro, P.F.; Fantuzzi, E.; Roberto, C.D. Chemical composition, extraction sources and action mechanisms of essential oils: Natural preservative and limitations of use in meat products. Meat Sci. 2021, 176, 108463. [Google Scholar] [CrossRef]
- Silveira, R.M.; Menezes, F.H.; Lima, I.G.; Carvalho, A.F.F.U.; de Oliveira Bünger, M.; da Costa, I.R. Essential oil constituents as the chemosystematic markers in Eugenia L. (Myrtaceae): An evolutionary perspective. South Afr. J. Bot. 2023, 160, 309–318. [Google Scholar] [CrossRef]
- Zeng, T.; Chen, Y.; Jian, Y.; Zhang, F.; Wu, R. Chemotaxonomic investigation of plant terpenoids with an established database (TeroMOL). New Phytol. 2022, 235, 662–673. [Google Scholar] [CrossRef]
- Mabou, F.D.; Yossa, I.B.N. TERPENES: Structural classification and biological activities. IOSR J. Pharm. Biol. Sci. 2021, 16, 25–40. [Google Scholar]
- Mohammed, H.A.; Sulaiman, G.M.; Khan, R.A.; Al-Saffar, A.Z.; Mohsin, M.H.; Albukhaty, S.; Ismail, A. Essential oils pharmacological activity: Chemical markers, biogenesis, plant sources, and commercial products. Process Biochem. 2024, 144, 112–132. [Google Scholar] [CrossRef]
- György, Z.; Incze, N.; Pluhár, Z. Differentiating Thymus vulgaris chemotypes with ISSR molecular markers. Biochem. Syst. Ecol. 2020, 92, 104118. [Google Scholar] [CrossRef]
- Kakouri, E.; Daferera, D.; Andriopoulou, A.; Trigas, P.; Tarantilis, P.A. Evaluation of the Essential Oil Composition of Five Thymus Species Native to Greece. Chemosensors 2024, 12, 7. [Google Scholar] [CrossRef]
- Bisht, D.; Kumar, D.; Kumar, D.; Dua, K.; Chellappan, D.K. Phytochemistry and pharmacological activity of the genus artemisia. Arch. Pharmacal Res. 2021, 44, 439–474. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Herrera-Bravo, J.; Semwal, P.; Painuli, S.; Badoni, H.; Ezzat, S.M.; Farid, M.M.; Merghany, R.M.; Aborehab, N.M.; Salem, M.A. Artemisia spp.: An update on its chemical composition, pharmacological and toxicological profiles. Oxidative Med. Cell Longev. 2022, 2022, 5628601. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Singh, P. The Genus Artemisia: A 2012–2017 Literature Review on Chemical Composition, Antimicrobial, Insecticidal and Antioxidant Activities of Essential Oils. Medicines 2017, 4, 68. [Google Scholar] [CrossRef]
- Staneva, J.D.; Todorova, M.N.; Evstatieva, L.N. Sesquiterpene lactones as chemotaxonomic markers in genus Anthemis. Phytochemistry 2008, 69, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cai, Q.; Wu, X.; Tan, Z.; Huang, S.; Wei, C.; Zhang, W.; Chen, Z.; Zhang, L.; Xiang, H. Variation in compositions and biological activities of essential oils from four citrus species: Citrus limon, Citrus sinensis, Citrus paradisi, and Citrus reticulata. Chem. Biodivers. 2022, 19, e202100910. [Google Scholar] [CrossRef]
- Susandarini, R.; Rugayah; Nugroho, L.H.; Subandiyah, S. Chemotaxonomy of Indonesian Citrus maxima based on Leaf Essential Oils. OnLine J. Biol. Sci. 2015, 16, 26–33. [Google Scholar] [CrossRef]
- Tomou, E.-M.; Fraskou, P.; Dimakopoulou, K.; Dariotis, E.; Krigas, N.; Skaltsa, H. Chemometric analysis evidencing the variability in the composition of essential oils in 10 Salvia species from different taxonomic sections or phylogenetic clades. Molecules 2024, 29, 1547. [Google Scholar] [CrossRef]
- Drapal, M.; Enfissi, E.M.A.; Fraser, P.D. The chemotype core collection of genus Nicotiana. Plant J. 2022, 110, 1516–1528. [Google Scholar] [CrossRef]
- Yingngam, B. Chemistry of Essential Oils. In Flavors and Fragrances in Food Processing: Preparation and Characterization Methods; ACS Publications: Washington, DC, USA, 2022; pp. 189–223. [Google Scholar]
- Siddiqui, T.; Khan, M.U.; Sharma, V.; Gupta, K. Terpenoids in essential oils: Chemistry, classification, and potential impact on human health and industry. Phytomedicine Plus 2024, 4, 100549. [Google Scholar] [CrossRef]
- Rosenkranz, M.; Chen, Y.; Zhu, P.; Vlot, A.C. Volatile terpenes–mediators of plant-to-plant communication. Plant J. 2021, 108, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Lemma, B.; Bromm, T.; Zech, W.; Zech, M.; Nemomissa, S.; Glaser, B. Terpenoid profiling of keystone plant species of the Bale Mountains, Ethiopia: Implications for chemotaxonomy and paleovegetation studies. Biochem. Syst. Ecol. 2024, 116, 104865. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, R.; Zhang, L. Simple phenylpropanoids: Recent advances in biological activities, biosynthetic pathways, and microbial production. Nat. Prod. Rep. 2024, 41, 6–24. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, Q.; Wang, Z.; Cao, H.; Zhang, D. Structure-activity relationships of cinnamaldehyde and eugenol derivatives against plant pathogenic fungi. Ind. Crops Prod. 2017, 97, 388–394. [Google Scholar] [CrossRef]
- Kamal, I.K.; Mahmood, A.T.; Mustafa, Y.F. Synthesis of Eugenol-Derived Coumarins as Broad-Spectrum Biosafe Antimicrobial Agents. Russ. J. Bioorganic Chem. 2024, 50, 2240–2251. [Google Scholar] [CrossRef]
- Pratyusha, S. Phenolic Compounds in the Plant Development and Defense. Plant Stress Physiol. Perspect. Agric. 2022, 125, 102873. [Google Scholar]
- Pinto, T.; Aires, A.; Cosme, F.; Bacelar, E.; Morais, M.C.; Oliveira, I.; Ferreira-Cardoso, J.; Anjos, R.; Vilela, A.; Gonçalves, B. Bioactive (poly) phenols, volatile compounds from vegetables, medicinal and aromatic plants. Foods 2021, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A comprehensive review on the biological, agricultural and pharmaceutical properties of secondary metabolites based-plant origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef] [PubMed]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential oils: Chemistry and pharmacological activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef]
- Gaião Calixto, M.; Alves Ramos, H.; Veríssimo, L.S.; Dantas Alves, V.; Medeiros, A.C.D.; Alencar Fernandes, F.H.; Veras, G. Trends and application of chemometric pattern recognition techniques in medicinal plants analysis. Crit. Rev. Anal. Chem. 2023, 53, 326–338. [Google Scholar] [CrossRef]
- Lim, Y.S.; Kim, Y.D.; Shin, H.C. Lectotypification and identity of Thymus quinquecostatus var. magnus (Nakai) Kitam. (Labiatae). Korean J. Pl. Taxon. 2006, 36, 129–136. [Google Scholar] [CrossRef]
- Kim, M.; Moon, J.-C.; Kim, S.; Sowndhararajan, K. Morphological, Chemical, and Genetic Characteristics of Korean Native Thyme Bak-Ri-Hyang (Thymus quinquecostatus Celak.). Antibiotics 2020, 9, 289. [Google Scholar] [CrossRef]
- Hernández-Abreu, O.; Castillo-España, P.; León-Rivera, I.; Ibarra-Barajas, M.; Villalobos-Molina, R.; González-Christen, J.; Vergara-Galicia, J.; Estrada-Soto, S. Antihypertensive and vasorelaxant effects of tilianin isolated from Agastache mexicana are mediated by NO/cGMP pathway and potassium channel opening. Biochem. Pharmacol. 2009, 78, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Edris, A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007, 21, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.S.; Salloom, Y.F.; Al-Asadi, R.A.U.H.; Al-Mousswi, Z.J.; Alamrani, H.A. The medicinal importance of Thyme plant (Thymus vulgaris). Biomedicine 2021, 41, 531–534. [Google Scholar] [CrossRef]
- Kim, J.; Park, E.-J. Chemical and Biological Properties of the Genus Abies. In Advances in Plant Phenolics: From Chemistry to Human Health; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2018; Volume 1286, pp. 225–236. [Google Scholar]
- Wajs-Bonikowska, A.; Sienkiewicz, M.; Stobiecka, A.; Maciąg, A.; Szoka, Ł.; Karna, E. Chemical Composition and Biological Activity of Abies alba and A. koreana Seed and Cone Essential Oils and Characterization of Their Seed Hydrolates. Chem. Biodivers. 2015, 12, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Hong, S.-M.; Yoon, D.H.; Ham, S.L.; Kim, J.; Kim, S.Y.; Choi, S.U.; Kim, C.S.; Lee, K.R. Triterpenoids from the leaves of Abies koreana and their biological activities. Phytochemistry 2023, 208, 113594. [Google Scholar] [CrossRef]
- Nurlybekova, A.; Kudaibergen, A.; Kazymbetova, A.; Amangeldi, M.; Baiseitova, A.; Ospanov, M.; Aisa, H.A.; Ye, Y.; Ibrahim, M.A.; Jenis, J. Traditional use, phytochemical profiles and pharmacological properties of Artemisia genus from Central Asia. Molecules 2022, 27, 5128. [Google Scholar] [CrossRef]
- Ekiert, H.; Klimek-Szczykutowicz, M.; Rzepiela, A.; Klin, P.; Szopa, A. Artemisia species with high biological values as a potential source of medicinal and cosmetic raw materials. Molecules 2022, 27, 6427. [Google Scholar] [CrossRef] [PubMed]
- Trifan, A.; Zengin, G.; Sinan, K.I.; Sieniawska, E.; Sawicki, R.; Maciejewska-Turska, M.; Skalikca-Woźniak, K.; Luca, S.V. Unveiling the phytochemical profile and biological potential of five Artemisia species. Antioxidants 2022, 11, 1017. [Google Scholar] [CrossRef]
- Morua, E.; Cuyas, L.; Matías-Hernández, L. The Beneficial Use of Artemisia annua, Artemisinin, and Other Compounds in Animal Health. Animals 2025, 15, 1359. [Google Scholar] [CrossRef]
- Polito, F.; Di Mercurio, M.; Rizzo, S.; Di Vito, M.; Sanguinetti, M.; Urbani, A.; Bugli, F.; De Feo, V. Artemisia spp. essential oils: From their ethnobotanical use to unraveling the microbiota modulation potential. Plants 2024, 13, 967. [Google Scholar] [CrossRef]
- Ahn, C.; Yoo, Y.-M.; Park, M.-J.; Ham, Y.; Yang, J.; Jeung, E.-B. Cytotoxic evaluation of the essential oils from Korean native plant on human skin and lung cells. J. Korean Wood Sci. Technol. 2021, 49, 371–383. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, D.H.; Lee, S.; Seo, H.J.; Park, S.J.; Jung, K.; Kim, S.-Y.; Kang, K.S. Potential and beneficial effects of Cinnamomum cassia on gastritis and safety: Literature review and analysis of standard extract. Appl. Biol. Chem. 2021, 64, 95. [Google Scholar] [CrossRef]
- Lee, S.-y.; Lee, D.-S.; Cho, S.-M.; Kim, J.-C.; Park, M.-J.; Choi, I.-G. Antioxidant properties of 7 domestic essential oils and identification of physiologically active components of essential oils against Candida albicans. J. Korean Wood Sci. Technol. 2021, 49, 23–43. [Google Scholar] [CrossRef]
- Shin, S.C.; Song, J.H.; Yoo, Y.-H.; Lee, J.-S.; Kang, S.-I.; Kim, H.J.; Lee, H.; Kim, H.B. The complete chloroplast genome sequence of a medicinal citrus landrace, Citrus erythrosa Hort. ex Tanaka in Jeju Island, Korea. Mitochondrial DNA Part B 2022, 7, 580–582. [Google Scholar] [CrossRef]
- Kim, J.-W.; Ko, H.C.; Jang, M.-G.; Han, S.H.; Kim, H.J.; Kim, S.-J. Phytochemical content and antioxidant activity in eight citrus cultivars grown in Jeju Island according to harvest time. Int. J. Food Prop. 2023, 26, 14–23. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lee, S.; Park, S.M.; Yun, S.H.; Gab, H.-S.; Kim, S.S.; Kim, H.-J. Comparative metabolomics analysis of citrus varieties. Foods 2021, 10, 2826. [Google Scholar] [CrossRef]
- Alam, F.; Mohammadin, K.; Shafique, Z.; Amjad, S.T.; Asad, M.H.H.b. Citrus flavonoids as potential therapeutic agents: A review. Phytother. Res. 2022, 36, 1417–1441. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lim, C.Y.; Jung, J.I.; Kim, T.Y.; Kim, E.J. Protective effects of red orange (Citrus sinensis [L.] Osbeck [Rutaceae]) extract against UVA-B radiation-induced photoaging in Skh: HR-2 mice. Nutr. Res. Pract. 2023, 17, 641–659. [Google Scholar] [CrossRef]
- Chuang, C.-W.; Wen, C.-H.; Wu, T.-J.; Li, C.-C.; Chiang, N.-T.; Ma, L.-T.; Ho, C.-L.; Tung, G.-S.; Tien, C.-C.; Lee, Y.-R. Sesquiterpene synthases of Zanthoxylum ailanthoides: Sources of unique aromas of a folklore plant in Taiwan. J. Agric. Food Chem. 2021, 69, 12494–12504. [Google Scholar] [CrossRef] [PubMed]
- Okagu, I.U.; Ndefo, J.C.; Aham, E.C.; Udenigwe, C.C. Zanthoxylum species: A comprehensive review of traditional uses, phytochemistry, pharmacological and nutraceutical applications. Molecules 2021, 26, 4023. [Google Scholar] [CrossRef]
- Fan, W.-L.; Wen, C.-H.; Ma, L.-T.; Ho, C.-L.; Tung, G.-S.; Tien, C.-C.; Chu, F.-H. Monoterpene synthases contribute to the volatile production in tana (Zanthoxylum ailanthoides) through indigenous cultivation practices. Plant Physiol. Biochem. 2023, 202, 107969. [Google Scholar] [CrossRef]
- Ma, J.; Ning, L.; Wang, J.; Gong, W.; Gao, Y.; Li, M. GC-MS Analysis and Bioactivity Screening of Leaves and Fruits of Zanthoxylum armatum DC. Separations 2023, 10, 420. [Google Scholar] [CrossRef]
- Baral, J.; Satyal, P.; Adhikari, A. Spatial variation in constituents of essential oils from fruit pericarp of Zanthoxylum armatum DC of Nepali origin and their antibacterial activity. J. Essent. Oil Bear. Plants 2024, 27, 47–56. [Google Scholar] [CrossRef]
- Lima, A.; Arruda, F.; Medeiros, J.; Baptista, J.; Madruga, J.; Lima, E. Variations in essential oil chemical composition and biological activities of Cryptomeria japonica (Thunb. ex Lf) D. Don from different geographical origins—A critical review. Appl. Sci. 2021, 11, 11097. [Google Scholar] [CrossRef]
- Lee, E.-J.; Shin, Y.; Lee, K.; Lee, S.-C.; Cha, J.-Y.; Oh, N.-H. Comparison of organic carbon properties in extracted soil solutions obtained underneath Cryptomeria japonica and Quercus acutissima and its implication on stream dissolved organic carbon. For. Sci. Technol. 2023, 19, 296–308. [Google Scholar] [CrossRef]
- Sharma, R.; Dev, K.; Sharma, S.K.; Kumar, R.; Agnihotri, V.K. Integrative network pharmacology and in vitro antimicrobial assessment of Cryptomeria japonica essential oil from North-western Himalaya. J. Biol. Act. Prod. Nat. 2025, 15, 138–155. [Google Scholar] [CrossRef]
- Rim, S.O.; Roy, M.; Jeon, J.; Montecillo, J.A.V.; Park, S.-C.; Bae, H. Diversity and communities of fungal endophytes from four Pinus species in Korea. Forests 2021, 12, 302. [Google Scholar] [CrossRef]
- Lee, J.-E.; Song, J.-H.; Kim, H.-J.; Cho, H.-J.; Park, W.-G.; Yun, C.-W. Classification of the Vegetation of Pinus densiflora Forests Distributed in Baekdudaegan (From Hyangrobong to Cheonwangbong), South Korea. Forests 2025, 16, 746. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Jakovljevic, V.L.; Bradic, J.V.; Tomovic, M.T.; Petrovic, B.P.; Petrovic, A.M. Korean and Siberian Pine: Review of chemical composition and pharmacological profile. Acta Pol. Pharm. 2022, 79, 785–797. [Google Scholar] [CrossRef]
- Kwak, M.; Nakamura, K.; Xiang, Q.; Wen, J.; Lee, E.S.; Hong, J.; Kovtonyuk, N.; Kryukova, M.; Korchagina, O.; Koo, K.A. Circular genetic structure of the Abies nephrolepis species complex shaped by the circular landform of Northeast Asia. J. Biogeogr. 2024, 51, 1533–1548. [Google Scholar] [CrossRef]
- Lee, S.-J.; Shin, D.-B.; Byeon, J.-G.; Oh, S.-H. Climate Change Vulnerability Assessment and ecological characteristics study of Abies nephrolepis in South Korea. Forests 2023, 14, 855. [Google Scholar] [CrossRef]
- Ancuceanu, R.; Anghel, A.I.; Hovaneț, M.V.; Ciobanu, A.-M.; Lascu, B.E.; Dinu, M. Antioxidant activity of essential oils from Pinaceae species. Antioxidants 2024, 13, 286. [Google Scholar] [CrossRef]
- Kim, E.; Yang, S.; Jeon, B.B.; Song, E.; Lee, H. Terpene compound composition and antioxidant activity of essential oils from needles of Pinus densiflora, Pinus koraiensis, Abies holophylla, and Juniperus chinensis by harvest period. Forests 2024, 15, 566. [Google Scholar] [CrossRef]
- Oh, M.-J.; Yeom, H.-J.; Lee, J.-Y. A Study of the Anti-inflammatory Effects of Abies nephrolepis MAX. Extract in RAW 264.7 Cells. J. Life Sci. 2024, 34, 160–169. [Google Scholar]
- Lee, S.-J.; Byeon, J.-G.; Kim, J.-S.; Cho, J.-h.; Oh, S.-H. Modeling Habitat Suitability of the Climate-vulnerable Plant Thuja koraiensis in Response to Climate Change. Sens. Mater. 2024, 36, 1511–1523. [Google Scholar] [CrossRef]
- Darwish, R.S.; Hammoda, H.; Harraz, F.M.; Shawky, E. Genus Thuja: A comprehensive review on botany, traditional uses, pharmacological activities and phytochemistry. J. Adv. Pharm. Sci. 2024, 1, 100–120. [Google Scholar] [CrossRef]
- Fu, C.; Lan, X.; Yuan, J.; Li, C.; Li, L.; Yu, Z.; Tan, T.; Yuan, M.; Du, F. Research on the optimization, key chemical constituents and antibacterial activity of the essential oil extraction process of Thuja koraiensis Nakai. J. Microbiol. Methods 2022, 194, 106435. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Sharma, K. A review of phyto-chemical constituent and pharmacological activity of Thuja species. Int. J. Pharm. Res. Appl. 2021, 6, 85–95. [Google Scholar]
- Lee, M.-M.; Cho, W.-K.; Cha, M.H.; Yim, N.-H.; Yang, H.J.; Ma, J.Y. The antiviral activity of Thuja orientalis folium against Influenza A virus. Virus Res. 2023, 335, 199199. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Kim, J.K.; Yoon, J.; Lim, J.; Kim, K.; Kim, B.; Park, C.H.; Sathasivam, R.; Kwon, S.-J.; Park, S.U. Identification of metabolite changes and evaluation of biological activities in edible flowers of Magnolia kobus at different developmental stages. Chem. Biol. Technol. Agric. 2024, 11, 98. [Google Scholar] [CrossRef]
- Hakim Yahaya, A.A.; Salleh, W.; Ghani, N.A. Magnolia genus-A systematic review on the composition and biological properties of its essential oils. Riv. Ital. Delle Sostanze Grasse 2022, 99, 249–261. [Google Scholar]
- Sowndhararajan, K.; Cho, H.; Yu, B.; Kim, S. Comparison of essential oil compositions of fresh and dried fruits of Magnolia kobus DC. J. Appl. Pharm. Sci. 2016, 6, 146–149. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, S. Antioxidative and anti-inflammatory effect of ethanol extracts from Magnolia kobus DC on LPS-induced in vitro and in vivo model. J. Immunol. 2023, 210, 66.17. [Google Scholar] [CrossRef]
- Cho, H.; Sowndhararajan, K.; Jung, J.-W.; Jhoo, J.-W.; Kim, S. Fragrant chemicals in the supercritical carbon dioxide extract of Magnolia kobus DC. Flower buds increase the concentration state of brain function. J. Essent. Oil Bear. Plants 2015, 18, 1059–1069. [Google Scholar] [CrossRef]
- Kang, J.-N.; Choi, H.-J.; Choi, M.-H.; Yang, S.-H.; Lee, S.-M. Molecular Marker-Based TaqMan PCR Approach for Determination of Origin and Content in Commercial Ginger Powder. In Food Analytical Methods; Springer: Berlin/Heidelberg, Germany, 2025. [Google Scholar] [CrossRef]
- Jo, M.-H.; Ham, I.-K.; Lee, G.-H.; Lee, J.-K.; Lee, G.-S.; Park, S.-K.; Kim, T.-I.; Lee, E.-M. Comparison of active ingredients between field grown and in vitro cultured rhizome of Korean native ginger (Zingiber officinale Roscoe). Korean J. Plant Resour. 2011, 24, 404–412. [Google Scholar] [CrossRef]
- Kim, M.-K.; Lee, B.-E.; Yun, S.; Kim, Y.-H.; Kim, Y.-K.; Hong, J.-S. Changes in Volatile Constituents of Zingiber officinale Roscoe Rhizomes During Storage. Appl. Biol. Chem. 1994, 37, 1–8. [Google Scholar]
- Baek, S.; Han, J.-H.; Park, S.-H. Effects in Blood Pressure and Cerebral Blood Flow with Green Ginger (Zingiber officinale Roscoe) and Development of Health Drink by Using It. J. Korean Soc. Food Cult. 2004, 19, 150–157. [Google Scholar]
- Song, H.; Choi, H.-Y.; Seo, Y.S. Gastroprotective Effect of Ginger Extract against an Ethanol/HCl-Induced Acute Gastric Ulcer Rat Model and a Helicobacter pylori Infection Mouse Model. J. Korean Soc. Food Sci. Nutr. 2025, 54, 391–401. [Google Scholar] [CrossRef]
- Jeong, S.I.; Lim, J.P.; Jeon, H. Chemical composition and antibacterial activities of the essential oil from Abies koreana. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007, 21, 1246–1250. [Google Scholar] [CrossRef]
- Li, S.-L.; Wu, H.-C.; Hwang, T.-L.; Lin, C.-H.; Yang, S.-S.; Chang, H.-S. Phytochemical investigation and anti-inflammatory activity of the leaves of Machilus japonica var. kusanoi. Molecules 2020, 25, 4149. [Google Scholar] [CrossRef]
- Cha, J.D.; Kim, J.Y. Essential oil from Cryptomeria japonica induces apoptosis in human oral epidermoid carcinoma cells via mitochondrial stress and activation of caspases. Molecules 2012, 17, 3890–3901. [Google Scholar] [CrossRef]
- Kim, H.H.; Vetrivel, P.; Ha, S.E.; Bhosale, P.B.; Lee, H.J.; Kim, J.-A.; Park, K.I.; Kim, S.M.; Kim, G.S. Functions of flavonoids in three Korean native varieties of Artemisia species. J. Biomed. Transl. Res. 2020, 21, 39–49. [Google Scholar] [CrossRef]
- Savolainen, V.; Chase, M.W. A decade of progress in plant molecular phylogenetics. TRENDS Genet. 2003, 19, 717–724. [Google Scholar] [CrossRef]
- Seethapathy, G.S. Ethnobotany, Phytochemistry and DNA Metabarcoding Studies on Indian Traditional Medicine. Ph.D. Thesis, University of Oslo, Oslo, Norway, 2019. [Google Scholar]
- Cruzan, M.B. Genetic markers in plant evolutionary ecology. Ecology 1998, 79, 400–412. [Google Scholar] [CrossRef]
- Joshi, S.P.; Ranjekar, P.K.; Gupta, V.S. Molecular markers in plant genome analysis. Curr. Sci. 1999, 77, 230–240. [Google Scholar]
- Bachmann, K. Molecular markers in plant ecology. New Phytol. 1994, 126, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, A.; Ray, S.; Roy, A. Molecular markers in phylogenetic studies-a review. J. Phylogenetics Evol. Biol. 2014, 2, 131. [Google Scholar]
- Zimmer, E.A.; Wen, J. Reprint of: Using nuclear gene data for plant phylogenetics: Progress and prospects. Mol. Phylogenetics Evol. 2013, 66, 539–550. [Google Scholar] [CrossRef]
- Amom, T.; Nongdam, P. The use of molecular marker methods in plants: A review. Int. J. Curr. Res. Rev. 2017, 9, 171. [Google Scholar]
- Small, R.L.; Cronn, R.C.; Wendel, J.F. Use of nuclear genes for phylogeny reconstruction in plants. Aust. Syst. Bot. 2004, 17, 145–170. [Google Scholar] [CrossRef]
- Neigel, J.E. A comparison of alternative strategies for estimating gene flow from genetic markers. Annu. Rev. Ecol. Syst. 1997, 28, 105–128. [Google Scholar] [CrossRef]
- de Groot, G.A.; During, H.J.; Maas, J.W.; Schneider, H.; Vogel, J.C.; Erkens, R.H.J. Use of rbcL and trnL-F as a two-locus DNA barcode for identification of NW-European ferns: An ecological perspective. PLoS ONE 2011, 6, e16371. [Google Scholar] [CrossRef]
- Dong, W.; Liu, J.; Yu, J.; Wang, L.; Zhou, S. Highly variable chloroplast markers for evaluating plant phylogeny at low taxonomic levels and for DNA barcoding. PLoS ONE 2012, 7, e35071. [Google Scholar] [CrossRef]
- Birky, C.W., Jr. Uniparental inheritance of mitochondrial and chloroplast genes: Mechanisms and evolution. Proc. Natl. Acad. Sci. USA 1995, 92, 11331–11338. [Google Scholar] [CrossRef]
- Soltis, D.E.; Moore, M.J.; Burleigh, G.; Soltis, P.S. Molecular markers and concepts of plant evolutionary relationships: Progress, promise, and future prospects. Crit. Rev. Plant Sci. 2009, 28, 1–15. [Google Scholar] [CrossRef]
- De Mandal, S.; Chhakchhuak, L.; Gurusubramanian, G.; Kumar, N.S. Mitochondrial markers for identification and phylogenetic studies in insects–A Review. DNA Barcodes 2014, 2, 1–9. [Google Scholar] [CrossRef]
- Schmidt, T.R.; Wu, W.; Goodman, M.; Grossman, L.I. Evolution of nuclear-and mitochondrial-encoded subunit interaction in cytochrome c oxidase. Mol. Biol. Evol. 2001, 18, 563–569. [Google Scholar] [CrossRef]
- Møller, I.M.; Rasmusson, A.G.; Van Aken, O. Plant mitochondria–past, present and future. Plant J. 2021, 108, 912–959. [Google Scholar] [CrossRef]
- Godden, G.T.; Jordon-Thaden, I.E.; Chamala, S.; Crowl, A.A.; García, N.; Germain-Aubrey, C.C.; Heaney, J.M.; Latvis, M.; Qi, X.; Gitzendanner, M.A. Making next-generation sequencing work for you: Approaches and practical considerations for marker development and phylogenetics. Plant Ecol. Divers. 2012, 5, 427–450. [Google Scholar] [CrossRef]
- Ray, S.; Satya, P. Next generation sequencing technologies for next generation plant breeding. Front. Plant Sci. 2014, 5, 367. [Google Scholar] [CrossRef]
- Satam, H.; Joshi, K.; Mangrolia, U.; Waghoo, S.; Zaidi, G.; Rawool, S.; Thakare, R.P.; Banday, S.; Mishra, A.K.; Das, G. Next-generation sequencing technology: Current trends and advancements. Biology 2023, 12, 997. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.T.; Jae-Hong, P.; Kim, J.S. The complete chloroplast genome sequence of Thymus quinquecostatus var. japonicus (Lamiaceae), an endemic to Ullenung Island of Korea. Mitochondrial DNA Part B 2020, 5, 2401–2402. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-S.; Jo, I.H.; Raveendar, S.; Kim, N.-H.; Gil, J.; Shim, D.; Kim, C.; Yu, J.-K.; So, Y.-S.; Chung, J.-W. A chromosome-level genome assembly of Korean mint (Agastache rugosa). Sci. Data 2023, 10, 792. [Google Scholar] [CrossRef]
- Kim, G.-B.; Lim, C.E.; Kim, J.-S.; Kim, K.; Lee, J.H.; Yu, H.-J.; Mun, J.-H. Comparative chloroplast genome analysis of Artemisia (Asteraceae) in East Asia: Insights into evolutionary divergence and phylogenomic implications. BMC Genom. 2020, 21, 415. [Google Scholar] [CrossRef]
- Yen, L.T.; Park, J. The complete chloroplast genome sequence of Origanum majorana L. Mitochondrial DNA Part B 2021, 6, 1224–1225. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kwak, M.; Park, S. Complete organelle genomes of Korean fir, Abies koreana and phylogenomics of the gymnosperm genus Abies using nuclear and cytoplasmic DNA sequence data. Sci. Rep. 2024, 14, 7636. [Google Scholar] [CrossRef]
- Jeong, H.; Yun, Y.B.; Jeong, S.Y.; Cho, Y.; Kim, S. Identification of putative parental species of yuzu (Citrus junos Sieb. Ex Tanaka) by comparative analyses of variations in chloroplast genomes and nuclear single-copy genes of citrus. Hortic. Sci. Technol. 2022, 40, 420–431. [Google Scholar] [CrossRef]
- Shen, L.; Ding, C.; Zhang, W.; Zhang, T.; Li, Z.; Zhang, J.; Chu, Y.; Su, X. The Populus koreana genome provides insights into the biosynthesis of plant aroma. Ind. Crops Prod. 2023, 197, 116453. [Google Scholar] [CrossRef]
- Han, E.-K.; Tamaki, I.; Heo, T.-I.; Byeon, J.-G.; Gantsetseg, A.; Jang, Y.-J.; Park, J.-S.; Lee, J.-H. Genetic variation and structure shaped by recent population fragmentation in the boreal conifer Thuja koraiensis: Conservation perspectives. Glob. Ecol. Conserv. 2025, 59, e03573. [Google Scholar] [CrossRef]
- Chen, L.; Liu, X.; Wang, Z.; Wu, X.; Hong, K.; Xie, C. Comparative Chloroplast Genome Analyses of Six Hemlock Trees in East Asia: Insights into Their Genomic Characterization and Phylogenetic Relationship. Forests 2023, 14, 2136. [Google Scholar] [CrossRef]
- Zhao, K.; Li, L.; Quan, H.; Yang, J.; Zhang, Z.; Liao, Z.; Lan, X. Comparative Analyses of Chloroplast Genomes From 14 Zanthoxylum Species: Identification of Variable DNA Markers and Phylogenetic Relationships Within the Genus. Front. Plant Sci. 2021, 11, 605793. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, Y.; Erst, A. Recent Advances in the Integrative Taxonomy of Plants. Plants 2023, 12, 4097. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Yang, M.; He, C.-n.; Bi, Y.-q.; Zhang, C.-h.; Li, M.-h.; Xiao, P.-G. Plant pharmacophylogeny: Review and future directions. Chin. J. Integr. Med. 2022, 28, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, L.-E.; Hu, Y.; Wu, X.; Wang, Z.; Miao, Y.; Sun, H.; Nie, Z.; Tan, N. Integrating morphology, molecular phylogeny and chemotaxonomy for the most effective authentication in Chinese Rubia with insights into origin and distribution of characteristic Rubiaceae-type cyclopeptides. Ind. Crops Prod. 2023, 191, 115775. [Google Scholar] [CrossRef]
- Kim, J.-H.; Doh, E.-J.; Lee, G. Chemotaxonomic Classification of Peucedanum japonicum and Its Chemical Correlation with Peucedanum praeruptorum, Angelica decursiva, and Saposhnikovia divaricata by Liquid Chromatography Combined with Chemometrics. Molecules 2022, 27, 1675. [Google Scholar] [CrossRef]
- Soo-jung, K. Taxonomic Study of Korean Chrysanthemum based on Morphological, Molecular, and Chemotaxonomical Characteristics. Seoul Natl. Univ. Grad. Sch. 2015, 175, 278–289. [Google Scholar]
- Oh, M.; Park, H.-S.; Um, S.; Yang, T.-J.; Kim, S.H. A comparative phytochemical study of nine Lauraceae species by using chemometric data analysis. PLoS ONE 2022, 17, e0273616. [Google Scholar] [CrossRef]
- Peters, K.; Blatt-Janmaat, K.L.; Tkach, N.; van Dam, N.M.; Neumann, S. Untargeted Metabolomics for Integrative Taxonomy: Metabolomics, DNA Marker-Based Sequencing, and Phenotype Bioimaging. Plants 2023, 12, 881. [Google Scholar] [CrossRef] [PubMed]
- Ji-hwan, K. Integrating Ecosystem Services and Connectivity for Prioritizing Conservation Areas in Jeju Island, Republic of Korea. Landsc. Urban Plan. 2023, 239, 104865. [Google Scholar]
- Lee, S.; Park, H.; Jeong, A.; Lee, Y.; Koo, S.; Kim, M. Management plans for Korean national parks to conserve the habitat of the Korean fir (Abies koreana). Biol. Conserv. 2023, 287, 110285. [Google Scholar] [CrossRef]
- Exposito-Alonso, M.; Booker, T.R.; Czech, L.; Gillespie, L.; Hateley, S.; Kyriazis, C.C.; Lang, P.L.M.; Leventhal, L.; Nogues-Bravo, D.; Pagowski, V. Genetic diversity loss in the Anthropocene. Science 2022, 377, 1431–1435. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, M.A.; Weiskopf, S.R.; Bertrand, R.; Carter, S.L.; Comte, L.; Eaton, M.J.; Johnson, C.G.; Lenoir, J.; Lynch, A.J.; Miller, B.W. Climate change and the global redistribution of biodiversity: Substantial variation in empirical support for expected range shifts. Environ. Evid. 2023, 12, 7. [Google Scholar] [CrossRef]
- Vences, M.; Miralles, A.; Dufresnes, C. Next-generation species delimitation and taxonomy: Implications for biogeography. J. Biogeogr. 2024, 51, 1709–1722. [Google Scholar] [CrossRef]
- Mahanayak, B. Ex-situ and in-situ conservation of wild life. World J. Biol. Pharm. Health Sci. 2024, 18, 277–282. [Google Scholar] [CrossRef]
- Vandamme, P.; Sutcliffe, I. Out with the old and in with the new: Time to rethink twentieth century chemotaxonomic practices in bacterial taxonomy. Int. J. Syst. Evol. Microbiol. 2021, 71, 005127. [Google Scholar] [CrossRef]
- Singh, R. Chapter 6—Chemotaxonomy of Medicinal Plants: Possibilities and Limitations. In Natural Products and Drug Discovery; Mandal, S.C., Mandal, V., Konishi, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 119–136. [Google Scholar]
- Teixeira, T.M.; Nazareno, A.G. One step away from extinction: A population genomic analysis of a narrow endemic, tropical plant species. Front. Plant Sci. 2021, 12, 730258. [Google Scholar] [CrossRef]
- Keith, J.A.; Vassilev-Galindo, V.; Cheng, B.; Chmiela, S.; Gastegger, M.; Muller, K.-R.; Tkatchenko, A. Combining machine learning and computational chemistry for predictive insights into chemical systems. Chem. Rev. 2021, 121, 9816–9872. [Google Scholar] [CrossRef] [PubMed]
- Kather, R.; Martin, S.J. Cuticular hydrocarbon profiles as a taxonomic tool: Advantages, limitations and technical aspects. Physiol. Entomol. 2012, 37, 25–32. [Google Scholar] [CrossRef]
- Shukla, S.; Patra, D.; Sinha, A.; Saha, R.; Tripathi, Y.; Borah, N.K.; Shukla, S.K. Medicinal and Aromatic Plant Cultivation, Improvement and Conservation for Sustainable Development. In Industrial Crops Improvement: Biotechnological Approaches for Sustainable Agricultural Development; Springer: Berlin/Heidelberg, Germany, 2025; pp. 183–204. [Google Scholar]
- Naruka, K.P.S.; Reddy, P.B. Assessing the efficacy of in-situ vs. ex-situ biodiversity conservation measures. Recent Adv. Biodivers. Res. 2023, 67, 1–6. [Google Scholar]
- Lee, S.-H.; Park, H.; Kim, J.-G. Current status of and challenges for phytoremediation as a sustainable environmental management plan for abandoned mine areas in Korea. Sustainability 2023, 15, 2761. [Google Scholar] [CrossRef]
- Unal, B.T.; Turker, H.; Ozturk, M. 2 Ex-situ Conservation of Plants as Medicine and Aromatics: Pharmacognosy, Ecology and Conservation; CRC Press: Boca Raton, FL, USA, 2023; Volume 13. [Google Scholar]
- Radomir, A.-M.; Stan, R.; Florea, A.; Ciobotea, C.-M.; Bănuță, F.M.; Negru, M.; Neblea, M.A.; Sumedrea, D.I. Overview of the success of in vitro culture for ex situ conservation and sustainable utilization of endemic and subendemic native plants of Romania. Sustainability 2023, 15, 2581. [Google Scholar] [CrossRef]
- Khater, M.; Ibrahim, O.; Sayed, M.N.E.; Faik, M. Legal frameworks for sustainable tourism: Balancing environmental conservation and economic development. Curr. Issues Tour. 2024, 2024, 1–22. [Google Scholar] [CrossRef]
- Fajardo del Castillo, T. Principles and approaches in the convention on biological diversity and other biodiversity-related conventions in the post-2020 scenario. In Biological Diversity and International Law: Challenges for the Post 2020 Scenario; Springer: Berlin/Heidelberg, Germany, 2021; pp. 15–34. [Google Scholar]
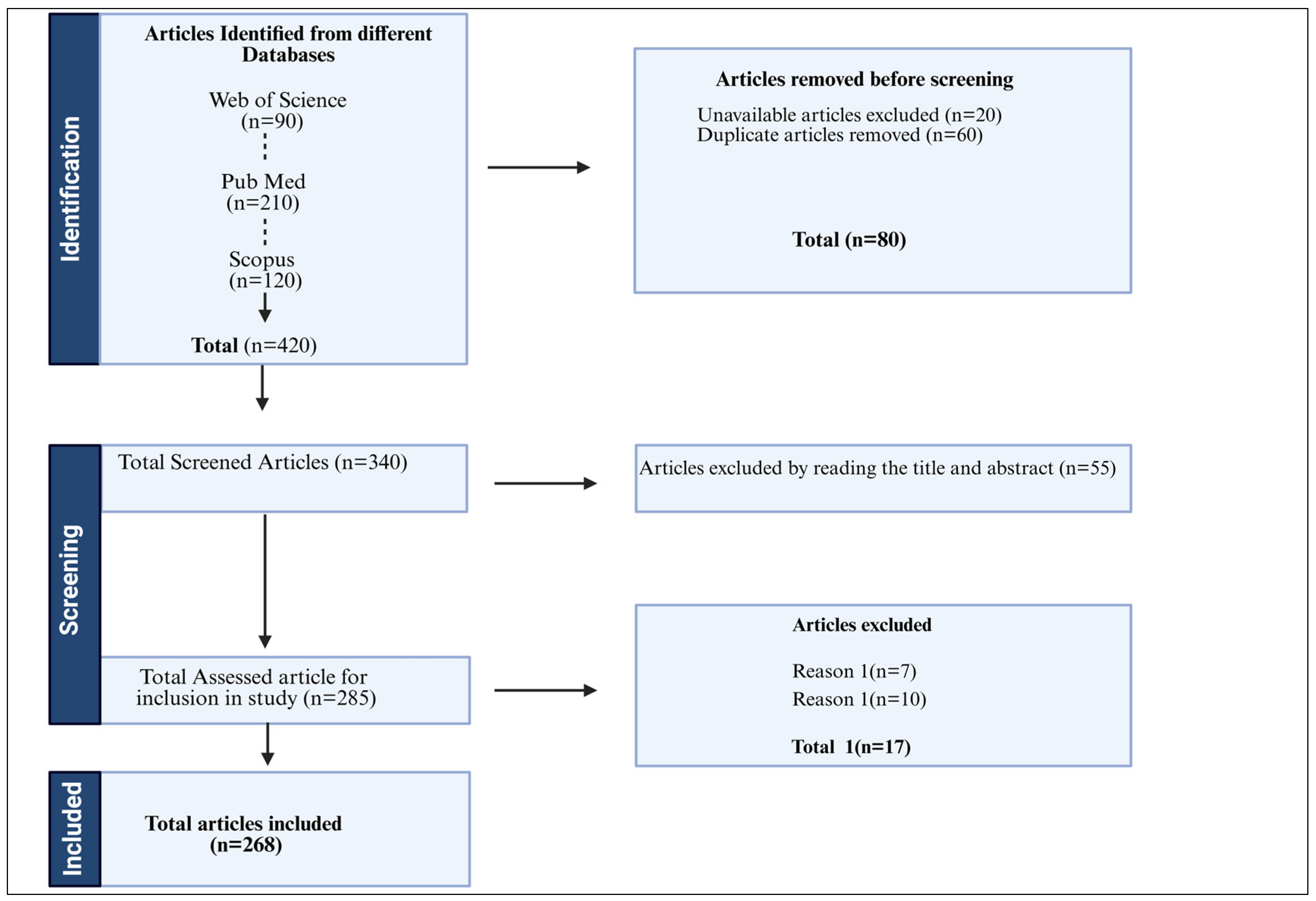

| Search String | Database | Articles Found |
|---|---|---|
| TITLE-ABS-KEY (“Korean aromatic plants” OR “Essential oil containing plants in Korea” OR “aromatic plants from Korean peninsula”) AND (“aromatic plant *” OR “medicinal plant *” OR “aromatic herbs *” OR “Aromatic flowers *” OR “aromatic leaves *” OR “Common Korean Aromatic herbs *”) | PubMed | 16 |
| Web of Science | 21 | |
| Scopus | 28 | |
| TITLE-ABS-KEY (“Korean aromatic plants hotspots” OR “Korean aromatic plants in islands” OR “Korean aromatic plants in mountain range”) AND (“Korean Aromatic plants occurrence *” OR “Korean Aromatic plants prevalence *” OR “distribution of Aromatic plants across South Korea *”) | PubMed | 14 |
| Web of Science | 22 | |
| Scopus | 31 | |
| TITLE-ABS-KEY (“Chemistry of Korean aromatic plants” OR “Essential oil composition of in Korean plants” OR “Phytochemistry of Korean aromatic plants”) AND (“Chemotaxonomy of Korean Aromatic plants *” OR “Chemotaxonomically evaluation of Korean Aromatic plants *” OR “GC-Ms analysis of Korean Aromatic plants *” OR “Chemotaxonomical evaluation of Korean fragrant plants *” OR “aromatic leaves chemotaxonomy *”) | PubMed | 16 |
| Web of Science | 19 | |
| Scopus | 35 | |
| TITLE-ABS-KEY (“Molecular phylogenetic analysis” OR “Phylogenetic analysis” OR “Molecular phylogeny” OR “Molecular systematics”) AND (“Chemotaxonomy” OR “Taxonomy” OR “Plant taxonomy” OR “Phylogenetic relationships” OR “Evolutionary relationships”) AND (“Molecular markers” OR “Genetic markers” OR “DNA barcoding” OR “rDNA sequencing” OR “Next-generation sequencing” OR “Genetic analysis *”) | PubMed | 12 |
| Web of Science | 18 | |
| Scopus | 35 | |
| TITLE-ABS-KEY (“Integrated plant taxonomy” OR “Plant taxonomy” OR “Plant systematics” OR “Taxonomic integration” OR “Taxonomic classification”) AND (“Plant classification” OR “Phylogenetic relationships” OR “Evolutionary relationships” OR “Molecular taxonomy” OR “Molecular systematics”) AND (“Genetic markers” OR “Molecular markers” OR “DNA barcoding” OR “Next-generation sequencing” OR “rDNA sequencing” OR “Genetic analysis”) AND (“Ecological relationships” OR “Plant biodiversity” OR “Plant identification” OR “Phylogenetic analysis *”) | PubMed | 16 |
| Web of Science | 20 | |
| Scopus | 39 | |
| TITLE-ABS-KEY (“Pharmacology” OR “Pharmacological properties of Korean aromatic plants” OR “Pharmacological properties of Korean aromatic plants” OR “Pharmacological evaluation of Korean aromatic plants” OR “Phytopharmacology”) AND (“Traditional uses of Korean aromatic plants” OR “Traditional aromatic Korean medicine” OR “Ethnopharmacology of Korean aromatic plants” OR “Herbal medicine” OR “Traditional healing of Korean aromatic plants”) AND (“Antioxidant Korean Aromatic plants” OR “Anti-inflammatory Korean aromatic plants” OR “Antibacterial Korean aromatic plants” OR “Anticancer Korean aromatic plants” OR “Antifungal Korean aromatic plants” OR “Antiviral Korean aromatic plants” OR “Immunomodulatory Korean aromatic plants”) | PubMed | 16 |
| Web of Science | 20 | |
| Scopus | 42 | |
| Sum | 420 |
| S. No. | Plant Name | Genetic Markers Used | Phylogenetic Analysis Findings | Citation |
|---|---|---|---|---|
| 1 | Thymus quinquecostatus | Complete Chloroplast genome | Clustered with other Lamiaceae species, indicating close evolutionary relationships within the genus Thymus. | [236] |
| 2 | Agastache rugosa | entire protein-coding gene set | Closely related to other Agastache species, showing high genetic similarity within the Lamiaceae family. | [229] |
| 3 | Artemisia spp. | Whole plastome sequences, trnH–psbA, accD, and ycf1 | Nested within the Artemisia genus, sharing high sequence similarity with other Asteraceae members. | [238] |
| 4 | Origanum majorana | Complete chloroplast genome | Phylogenetic analysis confirmed its closest relationship to Origanum vulgare. | [231] |
| 5 | Abies koreana | rDNA cluster, 452 single-copy genes, tRNAs, and rRNAs | Positioned within the Pinaceae family, closely related to other Abies species, indicating a shared ancestor in temperate conifers. | [240] |
| 6 | Citrus junos | Chlororplast matK, rps16, rpoC1, rpoB, psbD; and nuclear 80 single-copy genes | Confirmed to be a hybrid, likely derived from Ichang papeda and mandarin as parental species. | [241] |
| 7 | Populus koreana | RAD-seq | Restriction site-associated DNA sequencing (RAD-seq) | [242] |
| 8 | Abies nephrolepis | Nuclear microsatellite markers, as well as mitochondrial and chloroplast DNA markers | Provided insights into evolutionary processes and genetic diversity in Abies nephrolepis populations in Northeast Asia. | [192] |
| 9 | Thuja koraiensis | Genome dataset (242 SNPs) | Revealed geographically random patterns of genetic variation. | [243] |
| 10 | Tsuga sieboldii | cpSSRs, | Genome sizes and gene contents were relatively conserved among the species, reflecting structural stability within the genus. | [244] |
| 11 | Zanthoxylum schinifolium | SSR and IR | Enhanced understanding of genetic diversity and evolutionary relationships within the Zanthoxylum genus. | [245] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, A.; Park, S. Harnessing Molecular Phylogeny and Chemometrics for Taxonomic Validation of Korean Aromatic Plants: Integrating Genomics with Practical Applications. Plants 2025, 14, 2364. https://doi.org/10.3390/plants14152364
Amin A, Park S. Harnessing Molecular Phylogeny and Chemometrics for Taxonomic Validation of Korean Aromatic Plants: Integrating Genomics with Practical Applications. Plants. 2025; 14(15):2364. https://doi.org/10.3390/plants14152364
Chicago/Turabian StyleAmin, Adnan, and Seonjoo Park. 2025. "Harnessing Molecular Phylogeny and Chemometrics for Taxonomic Validation of Korean Aromatic Plants: Integrating Genomics with Practical Applications" Plants 14, no. 15: 2364. https://doi.org/10.3390/plants14152364
APA StyleAmin, A., & Park, S. (2025). Harnessing Molecular Phylogeny and Chemometrics for Taxonomic Validation of Korean Aromatic Plants: Integrating Genomics with Practical Applications. Plants, 14(15), 2364. https://doi.org/10.3390/plants14152364








