Methodology-Dependent Reversals in Root Decomposition: Divergent Regulation by Forest Gap and Root Order in Pinus massoniana
Abstract
1. Introduction
2. Results
2.1. Root Mass Remaining and Decomposition Coefficient
2.2. Different Litterbag Methods
2.3. Link Between Soil Properties and Root Decomposition
3. Discussion
3.1. Effects of Litterbag Methods on Root Decomposition
3.2. Effects of Forest Gap and Root Order on Root Decomposition
3.3. Implications for Plantation Management and Research
4. Materials and Methods
4.1. Study Site
4.2. Experimental Design and Sampling
4.3. Root Decomposition and Nutrient Analysis
4.4. Soil Properties Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silver, W.L.; Miya, R.K. Global patterns in root decomposition: Comparisons of climate and litter quality effects. Oecologia 2001, 129, 407–419. [Google Scholar] [CrossRef]
- Li, A.; Fahey, T.J.; Pawlowska, T.E.; Fisk, M.C.; Burtis, J. Fine root decomposition, nutrient mobilization and fungal communities in a pine forest ecosystem. Soil Biol. Biochem. 2015, 83, 76–83. [Google Scholar] [CrossRef]
- Guo, L.B.; Sims, R.E.H. Litter decomposition and nutrient release via litter decomposition in New Zealand eucalypt short rotation forests. Agric. Ecosyst. Environ. 1999, 75, 133–140. [Google Scholar] [CrossRef]
- Gordon, W.S.; Jackson, R.B. Nutrient concentrations in fine roots. Ecology 2000, 81, 275–280. [Google Scholar] [CrossRef]
- Böhm, W. Methods of Studying Root Systems, Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Maeght, J.L.; Rewald, B.; Pierret, A. How to study deep roots-and why it matters. Front. Plant Sci. 2013, 4, 299. [Google Scholar] [CrossRef]
- Harmon, M.E.; Silver, W.L.; Fasth, B.; Chen, H.; Burke, I.C.; Parton, W.J.; Hart, S.C.; Currie, W.S.; Laundre, J.; Wright, J.; et al. Long-term patterns of mass loss during the decomposition of leaf and fine root litter: An intersite comparison. Glob. Change Biol. 2009, 15, 1320–1338. [Google Scholar] [CrossRef]
- Dornbush, M.E.; Isenhart, T.M.; Raich, J.W. Quantifying fine-root decomposition: An alternative to buried litterbags. Ecology 2002, 83, 2985–2990. [Google Scholar] [CrossRef]
- Sun, T.; Mao, Z.; Dong, L.; Hou, L.; Song, Y.; Wang, X. Further evidence for slow decomposition of very fine roots using two methods: Litterbags and intact cores. Plant Soil 2013, 366, 633–646. [Google Scholar] [CrossRef]
- Chen, G.-T.; Chen, Y.-Q.; Peng, Y.; Hu, H.-L.; Xie, J.-L.; Chen, G.; Xiao, Y.-L.; Liu, L.; Tang, Y.; Tu, L.-H. Standard litterbags inestimate early-stage lower-order root decomposition rate in a subtropical forest, China. Plant Soil 2021, 469, 335–346. [Google Scholar] [CrossRef]
- Riutta, T.; Slade, E.M.; Bebber, D.P.; Taylor, M.E.; Malhi, Y.; Riordan, P.; Macdonald, D.W.; Morecroft, M.D. Experimental evidence for the interacting effects of forest edge, moisture and soil macrofauna on leaf litter decomposition. Soil Biol. Biochem. 2012, 49, 124–131. [Google Scholar] [CrossRef]
- Mcclaugherty, C.; Formation, H.; Sequestration, C. Plant Litter: Decomposition, Humus Formation, Carbon Sequestration; Springer: Berlin, Germany, 2014; p. 6172. [Google Scholar] [CrossRef]
- Jing, Y.; Tian, P.; Wang, Q.; Li, W.; Sun, Z.; Yang, H. Effects of root dominate over aboveground litter on soil microbial biomass in global forest ecosystems. For. Ecosyst. 2021, 8, 38. [Google Scholar] [CrossRef]
- Saha, S.; Huang, L.; Khoso, M.A.; Wu, H.; Han, D.; Ma, X.; Poudel, T.R.; Li, B.; Zhu, M.; Lan, Q.; et al. Fine root decomposition in forest ecosystems: An ecological perspective. Front. Plant Sci. 2023, 14, 1277510. [Google Scholar] [CrossRef]
- Carvalho, J.I.; An, J.Y.; Tran, L.T.N.; Carayugan, M.B.; Kong, Y.J.; Jo, M.S.; Hintural, W.P.; Rahman, S.K.A.; Lee, H.J.; Park, S.H.; et al. Root decomposition of four temperate species in the Republic of Korea: Associations of root traits and microbial community with root decay. Plant Soil 2025. [Google Scholar] [CrossRef]
- Lin, N.; Bartsch, N.; Heinrichs, S.; Vor, T. Long-term effects of canopy opening and liming on leaf litter production, and on leaf litter and fine-root decomposition in a European beech (Fagus sylvatica L.) forest. For. Ecol. Manag. 2015, 338, 183–190. [Google Scholar] [CrossRef]
- Lyu, Q.; Luo, Y.; Dong, Y.; Xiang, Y.; Zhao, K.; Chen, G.; Chen, Y.; Fan, C.; Li, X. Effects of forest gaps on the structure and diversity of soil bacterial communities in weeping cypress forest plantations. Front. Microbiol. 2022, 13, 882949. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; He, R.; Yin, H.; Guo, M.; Li, X.; Fan, C.; Liu, S. Effects of Cinnamomum septentrionale in hilly area of central sichuan basin on ecological stoichiometry characteristics of Cupressus funebris forest. Acta Ecol. Sin. 2019, 39, 590–598. [Google Scholar] [CrossRef]
- Du, T.; Chen, Y.; Bi, J.; Yang, Y.; Zhang, L.; You, C.; Tan, B.; Xu, Z.; Wang, L.; Liu, S.; et al. Effects of forest gap on losses of total phenols and condensed tannins of foliar litter in a subalpine forest of western Sichuan, China. Chin. J. Plant Ecology 2023, 47, 660–671. [Google Scholar] [CrossRef]
- Kneeshaw, D.D.; Bergeron, Y. Canopy gap characteristics and tree replacement in the southeastern boreal forest. Ecology 1998, 79, 783–794. [Google Scholar] [CrossRef]
- Tong, R.; Ji, B.; Wang, G.G.; Lou, C.; Ma, C.; Zhu, N.; Yuan, W.; Wu, T. Canopy gap impacts on soil organic carbon and nutrient dynamic: A meta-analysis. Ann. For. Sci. 2024, 81, 12. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W. The decomposition of fine and coarse roots: Their global patterns and controlling factors. Sci. Rep. 2015, 5, 9940. [Google Scholar] [CrossRef]
- Guo, D.; Xia, M.; Wei, X.; Chang, W.; Liu, Y.; Wang, Z. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol. 2008, 180, 673–683. [Google Scholar] [CrossRef]
- Fan, P.; Guo, D. Slow decomposition of lower order roots: A key mechanism of root carbon and nutrient retention in the soil. Oecologia 2010, 163, 509–515. [Google Scholar] [CrossRef]
- Guo, L.; Deng, M.; Yang, S.; Liu, W.; Wang, X.; Wang, J.; Liu, L. The coordination between leaf and fine root litter decomposition and the difference in their controlling factors. Glob. Ecol. Biogeogr. 2021, 30, 2286–2296. [Google Scholar] [CrossRef]
- King, W.L.; Yates, C.F.; Guo, J.; Fleishman, S.M.; Trexler, R.V.; Centinari, M.; Bell, T.H.; Eissenstat, D.M. The hierarchy of root branching order determines bacterial composition, microbial carrying capacity and microbial filtering. Commun. Biol. 2021, 4, 483. [Google Scholar] [CrossRef]
- King, W.L.; Yates, C.F.; Cao, L.; O’Rourke-Ibach, S.; Fleishman, S.M.; Richards, S.C.; Centinari, M.; Hafner, B.D.; Goebel, M.; Bauerle, T.; et al. Functionally discrete fine roots differ in microbial assembly, microbial functional potential, and produced metabolites. Plant Cell Environ. 2023, 46, 3919–3932. [Google Scholar] [CrossRef] [PubMed]
- Siegwart, L.; Bertrand, I.; Roupsard, O.; Duthoit, M.; Jourdan, C. Root litter decomposition in a sub-Sahelian agroforestry parkland dominated by Faidherbia albida. J. Arid. Environ. 2022, 198, 104696. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, X.; Lian, J.; Cheng, X.; Wang, G.G.; Zhang, J. Soil depth can modify the contribution of root system architecture to the root decomposition rate. Forests 2023, 14, 1092. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, S.; Lu, Y.; Froese, R.E.; Xu, X.; Zeng, J.; Ming, A.; Liu, X.; Xie, Y.; Li, Q. Thinning effects on forest evolution in Masson pine (Pinus massoniana Lamb.) conversion from pure plantations into mixed forests. For. Ecol. Manag. 2020, 477, 118503. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, P.; Qian, Z.; Sun, J.; Wu, T.; Yang, Z.; Sun, Y.; Huang, X. Evolutionary history of mixed tree species improved soil nutrient content of Pinus massoniana plantation. Plant Soil 2025. [Google Scholar] [CrossRef]
- Ma, C.; Wang, X.; Wang, J.; Zhu, X.; Qin, C.; Zeng, Y.; Zhen, W.; Fang, Y.; Shangguan, Z. Interactions of soil nutrients and microbial communities during root decomposition of gramineous and leguminous forages. Land Degrad. Dev. 2023, 34, 3250–3261. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, A.; Li, X.; Kuang, W.; Islam, W. Litter decomposition rate response to multiple global change factors: A meta-analysis. Soil Biol. Biochem. 2024, 195, 109474. [Google Scholar] [CrossRef]
- Zhang, C.; de Pasquale, S.; Hartman, K.; Stanley, C.E.; Berendsen, R.L.; van der Heijden, M.G.A. The microbial contribution to litter decomposition and plant growth. Environ. Microbiol. Rep. 2024, 16, e13205. [Google Scholar] [CrossRef] [PubMed]
- Lindedam, J.; Magid, J.; Poulsen, P.; Luxhøi, J. Tissue architecture and soil fertility controls on decomposer communities and decomposition of roots. Soil Biol. Biochem. 2009, 41, 1040–1049. [Google Scholar] [CrossRef]
- Ulery, A.L.; Graham, R.C.; Goforth, B.R.; Hubbert, K.R. Fire effects on cation exchange capacity of California forest and woodland soils. Geoderma 2017, 286, 125–130. [Google Scholar] [CrossRef]
- Brown, R.W.; Chadwick, D.R.; Bending, G.D.; Collins, C.D.; Whelton, H.L.; Daulton, E.; Covington, J.A.; Bull, I.D.; Jones, D.L. Nutrient (C, N and P) enrichment induces significant changes in the soil metabolite profile and microbial carbon partitioning. Soil Biol. Biochem. 2022, 172, 108779. [Google Scholar] [CrossRef]
- Jones, M.E.; LaCroix, R.E.; Zeigler, J.; Ying, S.C.; Nico, P.S.; Keiluweit, M. Enzymes, manganese, or iron? drivers of oxidative organic matter decomposition in soils. Environ. Sci. Technol. 2020, 54, 14114–14123. [Google Scholar] [CrossRef]
- Zhou, J.; Zang, H.; Loeppmann, S.; Gube, M.; Kuzyakov, Y.; Pausch, J. Arbuscular mycorrhiza enhances rhizodeposition and reduces the rhizosphere priming effect on the decomposition of soil organic matter. Soil Biol. Biochem. 2020, 140, 107641. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.F.; Jin, M.K.; Zhang, W.; Lambers, H.; Hui, D.; Liang, C.; Zhang, J.; Wu, D.; Sardans, J.; et al. Microbial controls over soil priming effects in chronic nitrogen and phosphorus additions in subtropical forests. ISME J. 2023, 17, 2160–2168. [Google Scholar] [CrossRef]
- Li, J.; Alaei, S.; Zhou, M.; Bengtson, P. Root influence on soil nitrogen availability and microbial community dynamics results in contrasting rhizosphere priming effects in pine and spruce soil. Funct. Ecol. 2021, 35, 1312–1324. [Google Scholar] [CrossRef]
- Arunachalam, A.; Arunachalam, K. Influence of gap size and soil properties on microbial biomass in a subtropical humid forest of north-east India. Plant Soil 2000, 223, 187–195. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, B.; Yang, W.; Wang, Q.; Chang, C.; Wang, L.; Li, H.; You, C.; Cao, R.; Jiang, Y.; et al. Forest gaps accelerate the degradation of cellulose and lignin in decaying logs in a subalpine forest. Eur. J. For. Res. 2023, 142, 27–36. [Google Scholar] [CrossRef]
- Iversen, C.M.; McCormack, M.L.; Powell, A.S.; Blackwood, C.B.; Freschet, G.T.; Kattge, J.; Roumet, C.; Stover, D.B.; Soudzilovskaia, N.A.; Valverde-Barrantes, O.J.; et al. A global Fine-Root Ecology Database to address below-ground challenges in plant ecology. New Phytol. 2017, 215, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, N.; Cheng, R.; Xiao, W.; Yang, S.; Guo, Y.; Lei, L.; Zeng, L.; Wang, X. Characteristics of fine roots of Pinus massoniana in the Three Gorges Reservoir area, China. Forests 2017, 8, 183. [Google Scholar] [CrossRef]
- Yang, S.; Cheng, R.; Xiao, W.; Shen, Y.; Wang, L.; Guo, Y.; Sun, P. Heterogeneity in decomposition rates and nutrient release in fine-root architecture of Pinus massoniana in the Three Gorges Reservoir Area. Forests 2019, 11, 14. [Google Scholar] [CrossRef]
- Pregitzer, K.S. Fine roots of trees: A new perspective. New Phytol. 2002, 154, 267–270. [Google Scholar] [CrossRef]
- LY/T 1271-1999; Determination of Total Nitrogen, Phosphorus, Potassium, Sodium, Calcium, Magnesium in Forest Plant and Forest Floor. State Forestry Administration of China: Beijing, China, 1999.
- Gregorich, E.G.; Carter, M.R. Soil Sampling and Methods of Analysis; CRC Press: Boca Raton, USA, 2007; p. 1264. [Google Scholar] [CrossRef]
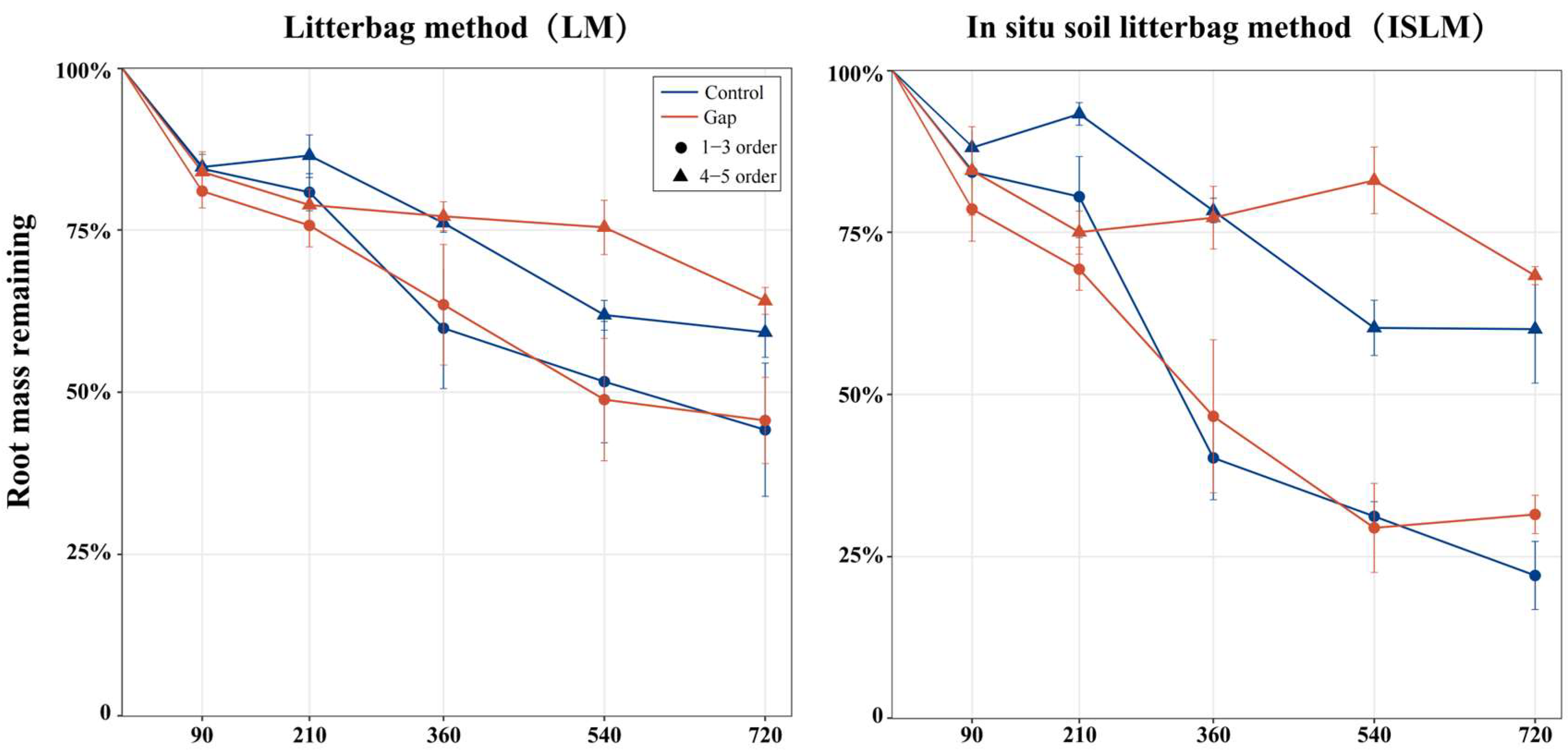

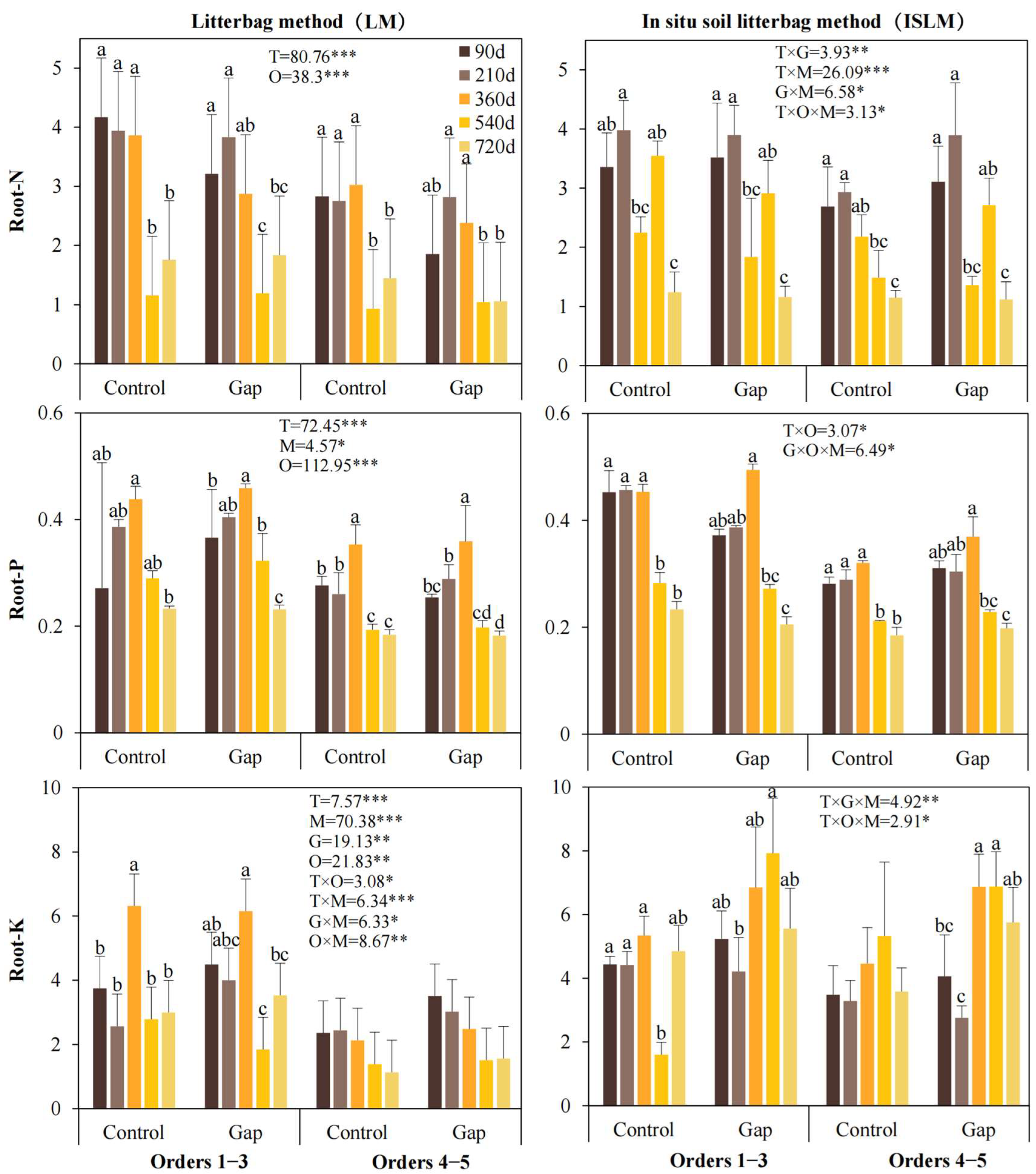
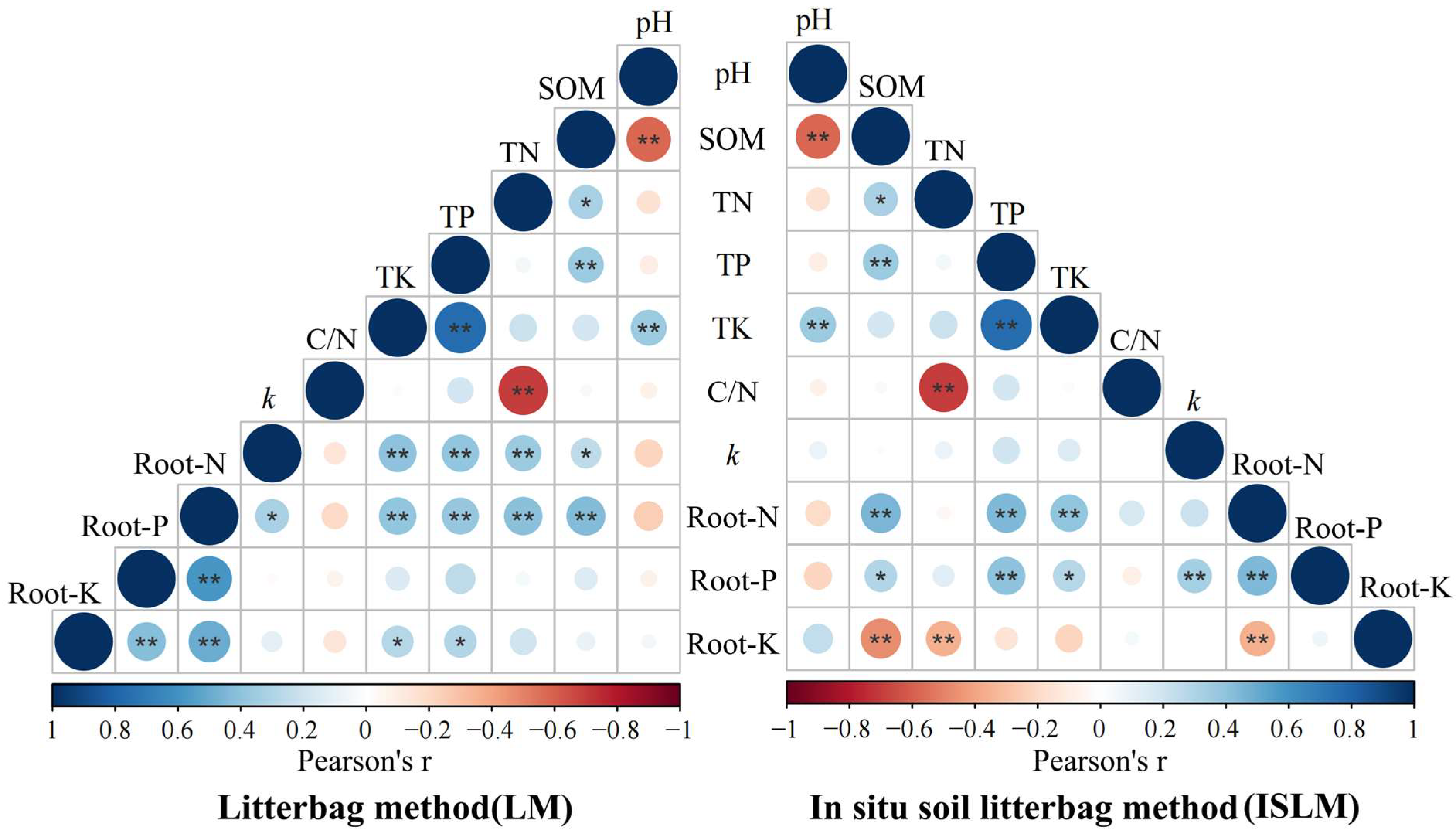
| Overall | Regression Equation | R2 | k/year−1 | T0.5/year | T0.95/year | |
|---|---|---|---|---|---|---|
| ISLM | y = 0.9488 × e−0.459t | 0.4172 | 0.459 | 1.51 | 6.53 | |
| LM | y = 0.8977 × e−0.188t | 0.8202 | 0.188 | 3.68 | 15.95 | |
| Control | y = 0.9623 × e−0.373t | 0.4661 | 0.373 | 1.86 | 8.04 | |
| Gap | y = 0.8851 × e−0.274t | 0.3317 | 0.274 | 2.53 | 10.95 | |
| Orders 1–3 | y = 0.9413 × e−0.455t | 0.4477 | 0.455 | 1.52 | 6.59 | |
| Orders 4–5 | y = 0.9049 × e−0.193t | 0.5752 | 0.193 | 3.59 | 15.54 | |
| Method | Treatment | Regression Equation | R2 | k/year−1 | T0.5/year | T0.95/year |
| ISLM | Control | y = 1.0484 × e−0.5723t | 0.5652 | 0.572 | 1.21 | 5.24 |
| Gap | y = 0.8587 × e−0.3467t | 0.2754 | 0.346 | 2.00 | 8.65 | |
| LM | Control | y = 0.8833 × e−0.1746t | 0.8053 | 0.174 | 3.97 | 17.18 |
| Gap | y = 0.9123 × e−0.2016t | 0.8388 | 0.201 | 3.44 | 14.88 | |
| ISLM | Orders 1–3 | y = 0.9835 × e−0.7382t | 0.8150 | 0.738 | 0.94 | 4.06 |
| Orders 4–5 | y = 0.9154 × e−0.1807t | 0.4219 | 0.180 | 3.84 | 16.60 | |
| LM | Orders 1–3 | y = 0.9010 × e−0.1717t | 0.7825 | 0.171 | 4.04 | 17.47 |
| Orders 4–5 | y = 0.8944 × e−0.2045t | 0.9040 | 0.204 | 3.39 | 14.67 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, H.; Zeng, J.; Liu, S.; Su, Y.; Yu, A.; Li, X. Methodology-Dependent Reversals in Root Decomposition: Divergent Regulation by Forest Gap and Root Order in Pinus massoniana. Plants 2025, 14, 2365. https://doi.org/10.3390/plants14152365
Yin H, Zeng J, Liu S, Su Y, Yu A, Li X. Methodology-Dependent Reversals in Root Decomposition: Divergent Regulation by Forest Gap and Root Order in Pinus massoniana. Plants. 2025; 14(15):2365. https://doi.org/10.3390/plants14152365
Chicago/Turabian StyleYin, Haifeng, Jie Zeng, Size Liu, Yu Su, Anwei Yu, and Xianwei Li. 2025. "Methodology-Dependent Reversals in Root Decomposition: Divergent Regulation by Forest Gap and Root Order in Pinus massoniana" Plants 14, no. 15: 2365. https://doi.org/10.3390/plants14152365
APA StyleYin, H., Zeng, J., Liu, S., Su, Y., Yu, A., & Li, X. (2025). Methodology-Dependent Reversals in Root Decomposition: Divergent Regulation by Forest Gap and Root Order in Pinus massoniana. Plants, 14(15), 2365. https://doi.org/10.3390/plants14152365






