Comparative Analysis of Habitat Expansion Mechanisms for Four Invasive Amaranthaceae Plants Under Current and Future Climates Using MaxEnt
Abstract
1. Introduction
2. Results and Interpretation
2.1. Model Accuracy Evaluation and Contribution of Environmental Variables
2.2. Potential Distribution Under Current Climate Conditions
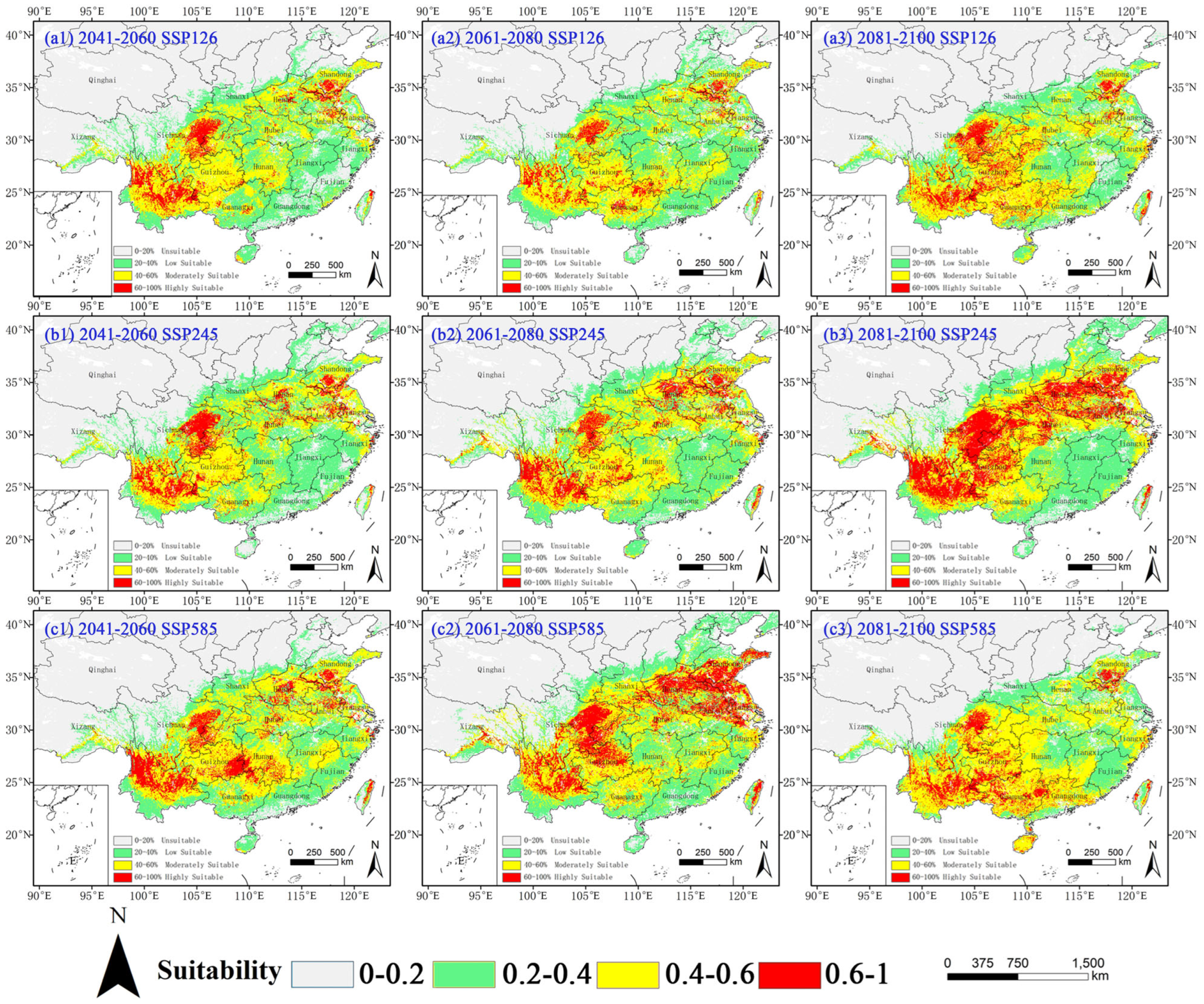
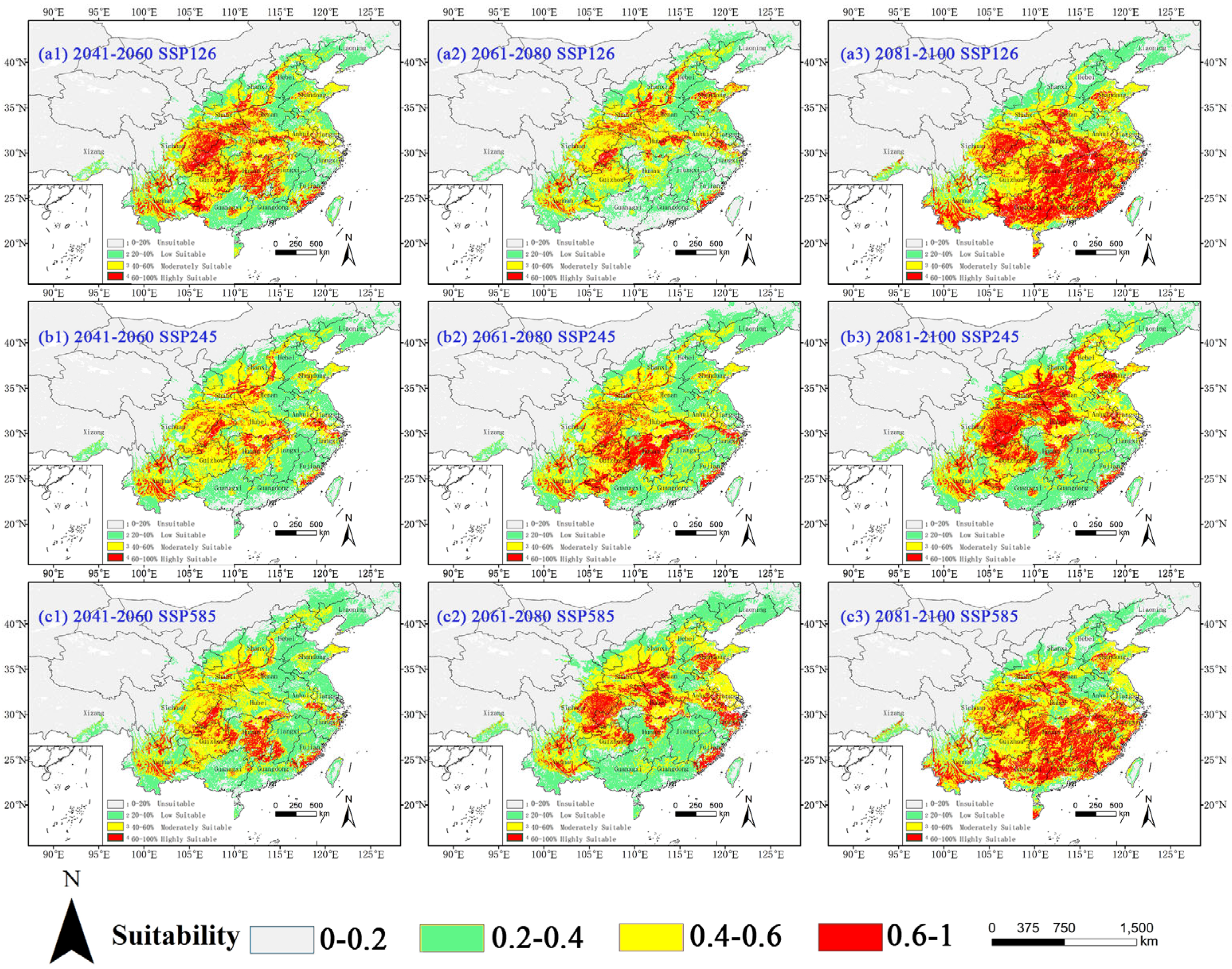
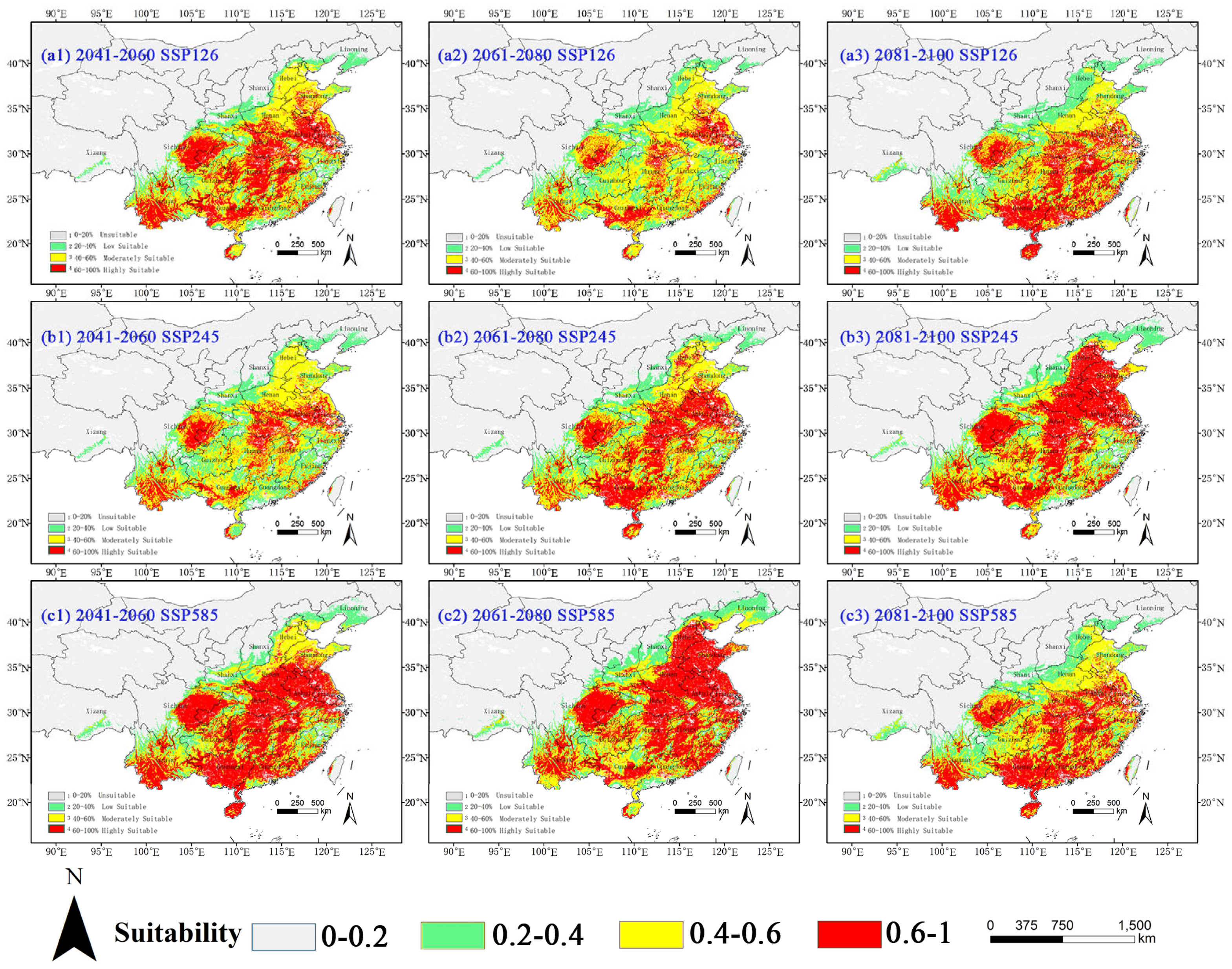
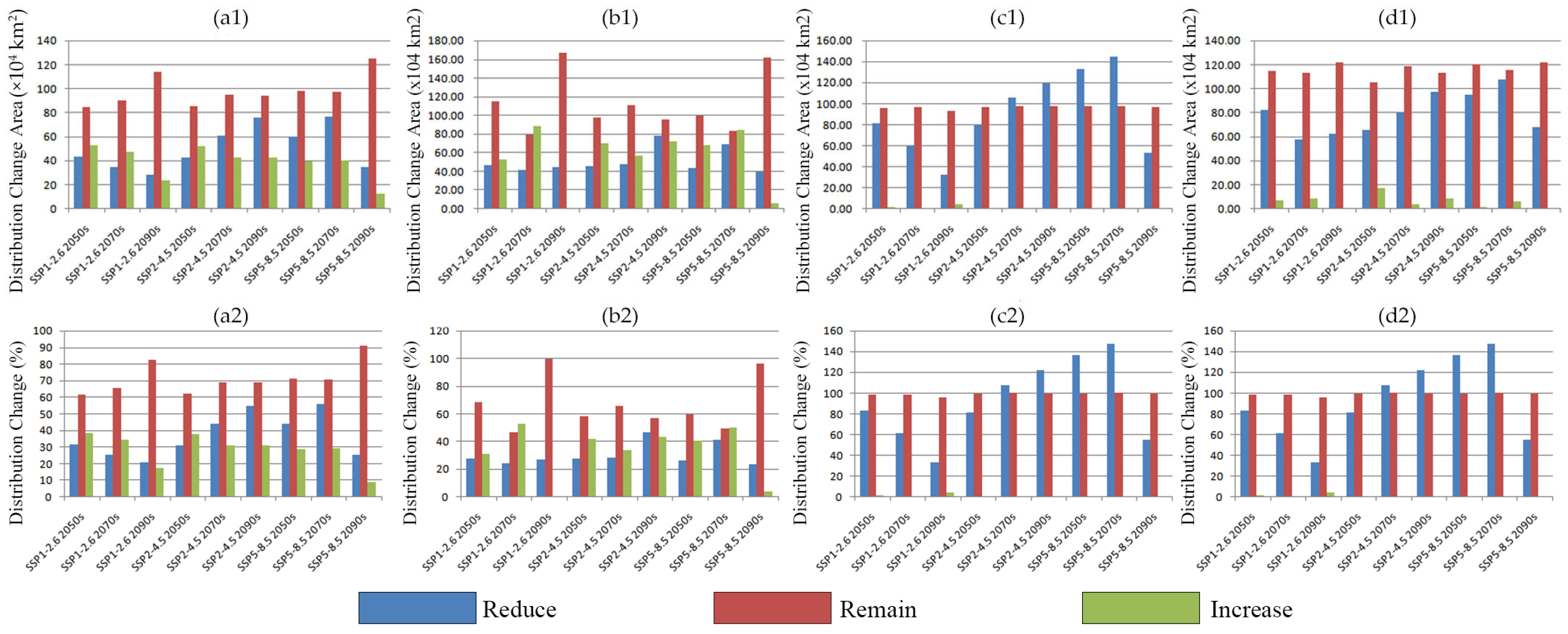
2.3. Potential Distribution of Four Amaranthaceae Under Future Climate Conditions
2.3.1. Potential Habitat for D. ambrosioides Under Climate Change Scenarios
2.3.2. Potential Habitat for C. argentea Under Climate Change Scenarios
2.3.3. Potential Habitat for A. palmeri Under Climate Change Scenarios
2.3.4. Potential Habitat for A. spinosus Under Climate Change Scenarios
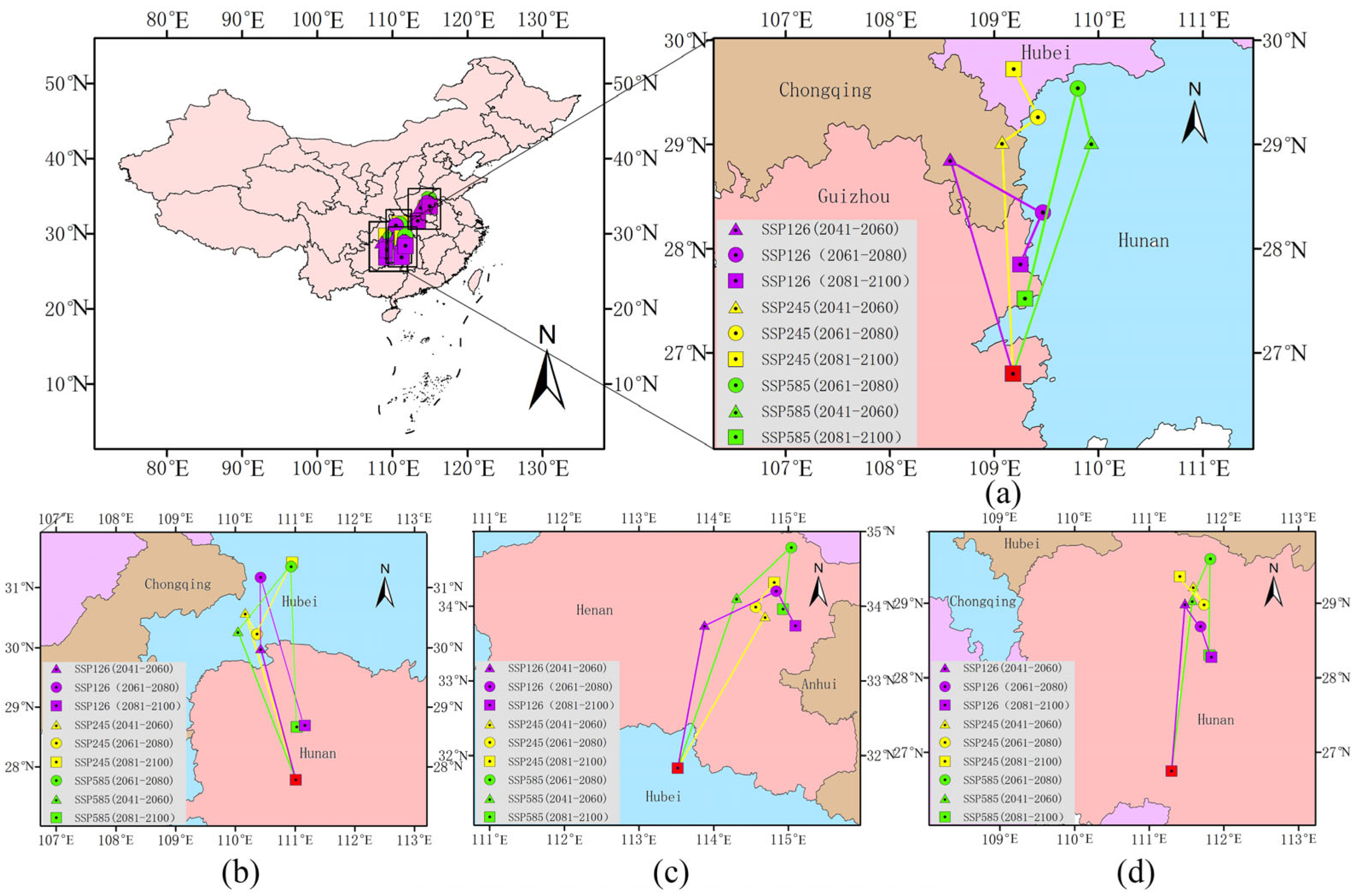
2.4. Centroid Shifts in Direction and Distance of Different Species
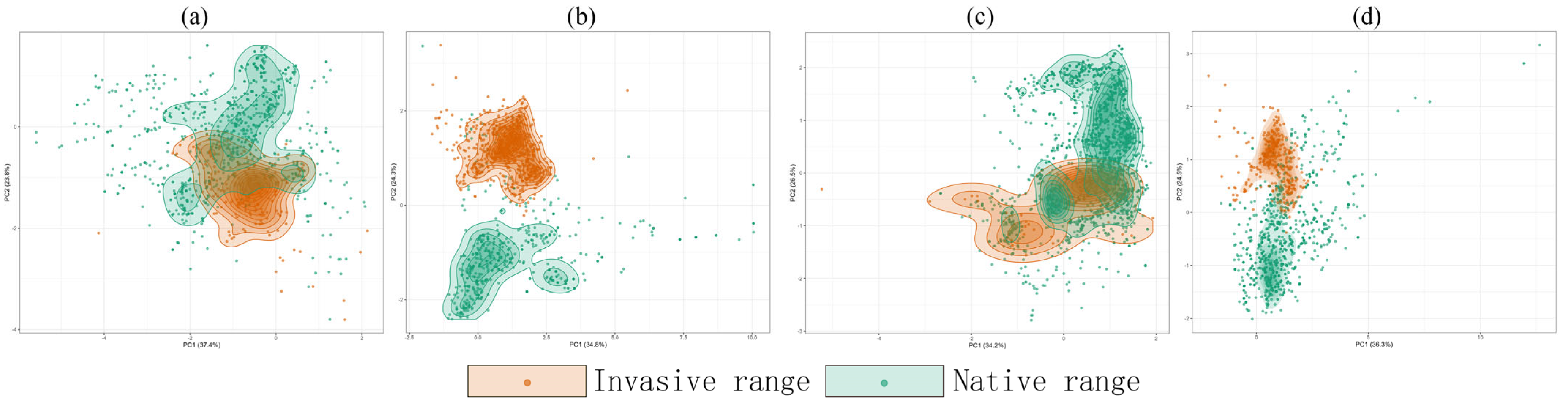
2.5. Niche Divergence Supports Species-Specific Plasticity
3. Discussion
3.1. Model Accuracy Analysis
3.2. The Key Environmental Drivers Influencing Amaranthaceae Invasion
3.3. Suitable Habitat and Its Dynamics Change
3.4. Endangered Status and Prevention Recommendations
4. Conclusions
5. Materials and Methods
5.1. Species Data Source
5.2. Environmental Variables and Processing
5.3. Formatting of Mathematical Components
5.4. Centroid Change Analysis
5.5. Quantifying Niche Shifts Between Native and Invaded Ranges
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Díaz, S.; Settele, J.; Brondízio, E.S.; Ngo, H.T.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.A.; Butchart, S.H.M.; Chan, K.M.A.; et al. Pervasive human-driven decline of life on earth points to the need for transformative change. Science 2019, 366, eaax3100. [Google Scholar] [CrossRef]
- Naidoo, S. Commentary on the contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. S. Afr. J. Sci. 2022, 118, 16–19. [Google Scholar] [CrossRef]
- Zhao, H.X.; Xian, X.Q.; Zhao, Z.H.; Zhang, G.F.; Liu, W.X.; Wan, F.H. Climate change increases the expansion risk of Helicoverpa zea in China according to potential geographical distribution estimation. Insects 2022, 13, 79. [Google Scholar] [CrossRef]
- Pysek, P.; Richardson, D.M. The biogeography of naturalization in alien plants. J. Biogeogr. 2006, 33, 2040–2050. [Google Scholar] [CrossRef]
- Hulme, P.E. Unwelcome exchange: International trade as a direct and indirect driver of biological invasions worldwide. One Earth 2021, 4, 666–679. [Google Scholar] [CrossRef]
- Shrestha, U.B.; Shrestha, B.B. Climate change amplifies plant invasion hotspots in Nepal. Divers. Distrib. 2019, 25, 1599–1612. [Google Scholar] [CrossRef]
- Diagne, C.; Leroy, B.; Vaissière, A.C.; Gozlan, R.E.; Roiz, D.; Jaric, I.; Salles, J.M.; Bradshaw, C.J.A.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef]
- Hao, Q.; Ma, J.S. Invasive alien plants in China: An update. Plant Divers. 2023, 45, 117–121. [Google Scholar] [CrossRef]
- Capinha, C.; Essl, F.; Seebens, H.; Moser, D.; Pereira, H.M. The dispersal of alien species redefines biogeography in the Anthropocene. Science 2015, 348, 1248–1251. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, C.; Li, X.Y.; Wang, K.F.; Qiu, Z.L.; Shi, F.C. Changes in the structure and function of rhizosphere soil microbial communities induced by Amaranthus palmeri invasion. Front. Microbiol. 2023, 14, 1114388. [Google Scholar] [CrossRef]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarosík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pysek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef]
- Jin, Z.N.; Yu, W.T.; Zhao, H.X.; Xian, X.Q.; Jing, K.T.; Yang, N.W.; Lu, X.M.; Liu, W.X. Potential global distribution of invasive Alien species, Anthonomus grandis Boheman, under current and future climate using optimal MaxEnt model. Agriculture 2022, 12, 1759. [Google Scholar] [CrossRef]
- Ng, W.T.; Silva, A.C.D.; Rima, P.; Atzberger, C.; Immitzer, M. Ensemble approach for potential habitat mapping of invasive Prosopis spp. in Turkana, Kenya. Ecol. Evol. 2018, 8, 11921–11931. [Google Scholar] [CrossRef]
- Wei, Y.J.; Xian, X.Q.; Zhao, H.X.; Guo, J.Y.; Yang, N.W.; Gong, Z.; Liu, W.X.; Peng, Z.Q. Projection of the potential global geographic distribution of the Solanum fruit fly Bactrocera latifrons (Hendel, 1912) (Diptera: Tephritidae) based on CLIMEX models. Horticulturae 2024, 10, 977. [Google Scholar] [CrossRef]
- Ghahremanian, A.; Ahmadi, A.; Toranjzar, H.; Varvani, J.; Abdi, N. Ecological and statistical evaluation of genetic algorithm (GARP), maximum entropy method, and logistic regression in predicting spatial distribution of Astragalus sp. Scientifica 2025, 2025, 4003408. [Google Scholar] [CrossRef]
- Ma, D.L.; Lu, Z.K.; Xue, Z.Q.; Yu, Z.H.; Duan, X.H.; Gu, X.; Yao, Y.K.; Cai, L.; Zheng, K.Y. Assessment of suitable habitat of Semen Armeniacae Amarum. in China under different climatic conditions by Internal Transcribed Spacer 2 and Maxent model. BMC Plant Biol. 2025, 25, 598. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Yan, H.Y.; Feng, L.; Zhao, Y.F.; Feng, L.; Zhu, C.P.; Qu, Y.F.; Wang, H.Q. Predicting the potential distribution of an invasive species, Erigeron canadensis L., in China with a maximum entropy model. Glob. Ecol. Conserv. 2020, 21, e00822. [Google Scholar] [CrossRef]
- Khwarahm, N.R. MaxEnt-Based Distribution Modeling of the Invasive Species Phragmites australis Under Climate Change Conditions in Iraq. Plants 2025, 14, 768. [Google Scholar] [CrossRef]
- Christina, M.; Limbada, F.; Atlan, A. Climatic niche shift of an invasive shrub (Ulex europaeus): A global scale comparison in native and introduced regions. J. Plant Ecol. 2020, 13, 42–50. [Google Scholar] [CrossRef]
- Cho, K.H.; Park, J.S.; Kim, J.H.; Kwon, Y.S.; Lee, D.H. Modeling the distribution of invasive species (Ambrosia spp.) using regression kriging and Maxent. Front. Ecol. Evol. 2022, 10, 1036816. [Google Scholar] [CrossRef]
- Chu, S.H.; Cong, S.B.; Li, R.H.; Hou, Y.M. Host Range of Herpetogramma basalis (Lepidoptera: Crambidae), a Biological Control Agent for the Invasive Weed Alternanthera philoxeroides (Centrospermae: Amaranthaceae) in China. J. Insect Sci. 2019, 19, 1. [Google Scholar] [CrossRef]
- Sage, T.L.; Sage, R.F. The Functional Anatomy of Rice Leaves: Implications for Refixation of Photorespiratory CO2 and Efforts to Engineer C4 Photosynthesis into Rice. Plant Cell Physiol. 2009, 50, 756–772. [Google Scholar] [CrossRef]
- Annaz, H.; Abdelaal, S.; Mandour, D.A.; Mahdi, I.; Mahmoud, M.F.; Sobeh, M. Mexican tea (Dysphania ambrosioides (L.) Mosyakin & Clemants) seeds attenuate tourniquet-induced hind limb ischemia-reperfusion injury by modulating ROS and NLRP3 inflammasome pathways. J. Funct. Foods 2023, 108, 105712. [Google Scholar]
- Cao, J.J.; Wu, Q.M.; Wan, F.H.; Guo, J.Y.; Wang, R. Reliable and rapid identification of glyphosate-resistance in the invasive weed Amaranthus palmeri in China. Pest Manag. Sci. 2022, 78, 2173–2182. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhao, J.; Wang, M.M.; Li, Z.P.; Lin, S.; Chen, H. Potential distribution prediction of Amaranthus palmeri S. Watson in China under current and future climate scenarios. Ecol. Evol. 2022, 12, e9505. [Google Scholar]
- Coelho, L.M.; Silva, P.M.; Martins, J.T.; Pinheiro, A.C.; Vicente, A.A. Emerging opportunities in exploring the nutritional/functional value of amaranth. Food Funct. 2018, 9, 5499–5512. [Google Scholar] [CrossRef]
- Yuan, L.; Zhou, L.; Li, J.M. Effect of microplastics on the allelopathic effects of native and invasive plants on co-occurring invaders. Front. Plant Sci. 2024, 15, 1425815. [Google Scholar] [CrossRef]
- Yang, W.J.; Sun, S.X.; Wang, N.X.; Fan, P.X.; You, C.; Wang, R.Q.; Zheng, P.M.; Wang, H. Dynamics of the distribution of invasive alien plants (Asteraceae) in China under climate change. Sci. Total Environ. 2023, 903, 166260. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, X.W.; Xiang, W.H.; Chen, L.; Ouyang, S. Predicting potential suitable habitats of Chinese fir under current and future climatic scenarios based on Maxent model. Ecol. Inform. 2021, 64, 101393. [Google Scholar] [CrossRef]
- Wang, Y.J.; Xie, L.Y.; Zhou, X.Y.; Chen, R.F.; Zhao, G.H.; Zhang, F.G. Prediction of the potentially suitable areas of Leonurus japonicus in China based on future climate change using the optimized MaxEnt model. Ecol. Evol. 2023, 13, e10597. [Google Scholar] [CrossRef]
- Tu, W.Q.; Xiong, Q.L.; Qiu, X.P.; Zhang, Y.M. Dynamics of invasive alien plant species in China under climate change scenarios. Ecol. Indic. 2021, 129, 101393. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Reich, P.B.; Luo, Y.J.; Bradford, J.B.; Poorter, H.; Perry, C.H.; Oleksyn, J. Temperature drives global patterns in forest biomass distribution in leaves, stems, and roots. Proc. Natl. Acad. Sci. USA 2014, 111, 13721–13726. [Google Scholar] [CrossRef]
- Sharafati, A.; Pezeshki, E. A strategy to assess the uncertainty of a climate change impact on extreme hydrological events in the semi-arid Dehbar catchment in Iran. Theor. Appl. Climatol. 2020, 139, 389–402. [Google Scholar] [CrossRef]
- Guisan, A.; Petitpierre, B.; Broennimann, O.; Kueffer, C.; Randin, C.; Daehler, C. Response to Comment on “Climatic Niche Shifts Are Rare Among Terrestrial Plant Invaders”. Science 2012, 338, 193. [Google Scholar] [CrossRef]
- Rosa, I.M.D.; Purvis, A.; Alkemade, R.; Chaplin-Kramer, R.; Ferrier, S.; Guerra, C.A.; Hurtt, G.; Kim, H.; Leadley, P.; Martins, I.S.; et al. Challenges in producing policy-relevant global scenarios of biodiversity and ecosystem services. Glob. Ecol. Conserv. 2020, 22, e00886. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Jiang, X.; Chen, Y.; Li, C.C.; Ding, S.; Zhang, X.J.; Luo, L.L.; Jia, Y.; Zhao, G. Prediction of Potential Distribution and Response of Changium smyrnioides to Climate Change Based on Optimized MaxEnt Model. Plants 2025, 14, 743. [Google Scholar] [CrossRef]
- Mayer, K.; Haeuser, E.; Dawson, W.; Essl, F.; Kreft, H.; Pergl, J.; Pysek, P.; Weigelt, P.; Winter, M.; Lenzner, B.; et al. Naturalization of ornamental plant species in public green spaces and private gardens. Biol. Invasions 2017, 19, 3613–3627. [Google Scholar] [CrossRef]
- Petitpierre, B.; Kueffer, C.; Broennimann, O.; Randin, C.; Daehler, C.; Guisan, A. Climatic Niche Shifts Are Rare Among Terrestrial Plant Invaders. Science 2012, 335, 1344–1348. [Google Scholar] [CrossRef]
- Anibaba, Q.A.; Dyderski, M.K.; Jagodzinski, A.M. Predicted range shifts of invasive giant hogweed (Heracleum mantegazzianum) in Europe. Sci. Total Environ. 2022, 825, 154053. [Google Scholar] [CrossRef]
- Huang, J.; Hao, J.M.; Nie, J.T.; Qian, R.H.; Li, H.Y.; Zhao, J.Y.; Wang, Y.A. Possible Mechanism of Dysphania ambrosioides (L.) Mosyakin & Clemants Seed Extract Suppresses the Migration and Invasion of Human Hepatocellular Carcinoma Cells SMMC-7721. Chem. Biodivers. 2023, 20, e202200768. [Google Scholar]
- Wang, S.Y.; Lu, Y.Y.; Han, M.Y.; Li, L.L.; He, P.; Shi, A.M.; Bai, M. Using MaxEnt Model to Predict the Potential Distribution of Three Potentially Invasive Scarab Beetles in China. Insects 2023, 14, 239. [Google Scholar] [CrossRef]
- Roberts, J.; Florentine, S. A review of the biology, distribution patterns and management of the invasive species Amaranthus palmeri S. Watson (Palmer amaranth): Current and future management challenges. Weed Res. 2022, 62, 113–122. [Google Scholar] [CrossRef]
- GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.zmpzmv (accessed on 5 July 2025).
- Fan, Y.; Yao, W.H.; Wang, Z.H.; Fan, X.Y.; Hu, S.Y.; Wang, H.F.; Ou, J. Predicting Potential Suitable Habitats of Three Rare Wild Magnoliaceae Species (Michelia crassipes, Lirianthe coco, Manglietia insignis) Under Current and Future Climatic Scenarios Based on the Maxent Model. Plants 2025, 14, 506. [Google Scholar] [CrossRef]
- Li, Y.L.; Ding, C.Q. Effects of sample size, sample accuracy and environmental variables on predictive performance of MaxEnt model. Pol. J. Ecol. 2016, 64, 303–312. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) experimental design and organization. Geosci. Model Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef]
- He, Y.L.; Ma, J.M.; Chen, G.S. Potential geographical distribution and its multi-factor analysis of Pinus massoniana in China based on the maxent model. Ecol. Indic. 2023, 154, 110790. [Google Scholar] [CrossRef]
- Beckmann, M.; Václavík, T.; Manceur, A.M.; Sprtová, L.; von Wehrden, H.; Welk, E.; Cord, A.F. glUV: A global UV-B radiation data set for macroecological studies. Methods Ecol. Evol. 2014, 5, 372–383. [Google Scholar] [CrossRef]
- Chi, Y.; Wang, G.G.; Zhu, M.X.; Jin, P.; Hu, Y.; Shu, P.Z.; Wang, Z.X.; Fan, A.F.; Qian, P.H.; Han, Y.N.; et al. Potentially suitable habitat prediction of Pinus massoniana Lamb. in China under climate change using Maxent model. Front. For. Glob. Chang. 2023, 6, 1144401. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Bowen, A.K.M.; Stevens, M.H.H. Temperature, topography, soil characteristics, and NDVI drive habitat preferences of a shade-tolerant invasive grass. Ecol. Evol. 2020, 10, 10785–10797. [Google Scholar] [CrossRef]
- Xie, C.P.; Li, M.; Jim, C.Y.; Chen, R.N. Distribution Pattern of Endangered Cycas taiwaniana Carruth. in China Under Climate-Change Scenarios Using the MaxEnt Model. Plants 2025, 14, 1600. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Warren, D.L.; Wright, A.N.; Seifert, S.N.; Shaffer, H.B. Incorporating model complexity and spatial sampling bias into ecological niche models of climate change risks faced by 90 California vertebrate species of concern. Divers. Distrib. 2014, 20, 334–343. [Google Scholar] [CrossRef]
- Li, M.; Xian, X.Q.; Zhao, H.X.; Xue, L.; Chen, B.X.; Huang, H.K.; Wan, F.H.; Liu, W.X. Predicting the Potential Suitable Area of the Invasive Ant Linepithema humile in China under Future Climatic Scenarios Based on Optimized MaxEnt. Diversity 2022, 14, 921. [Google Scholar] [CrossRef]
- Li, J.J.; Fan, G.; He, Y. Predicting the current and future distribution of three Coptis herbs in China under climate change conditions, using the MaxEnt model and chemical analysis. Sci. Total Environ. 2020, 698, 134141. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Zhao, G.H.; Cui, X.Y.; Sun, J.J.; Li, T.T.; Wang, Q.; Ye, X.Z.; Fan, B.G. Analysis of the distribution pattern of Chinese Ziziphus jujuba under climate change based on optimized biomod2 and MaxEnt models. Ecol. Indic. 2021, 132, 108256. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Liu, Y.L.; Qin, H.; Meng, Q.X. Prediction on spatial migration of suitable distribution of Elaeagnus mollis under climate change conditions in Shanxi Province, China. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2019, 30, 496–502. [Google Scholar] [CrossRef]

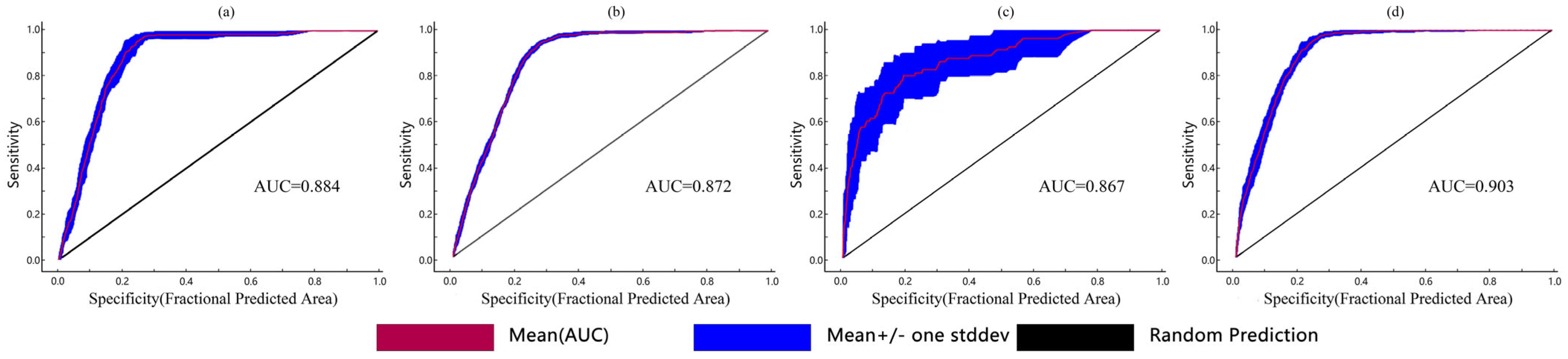
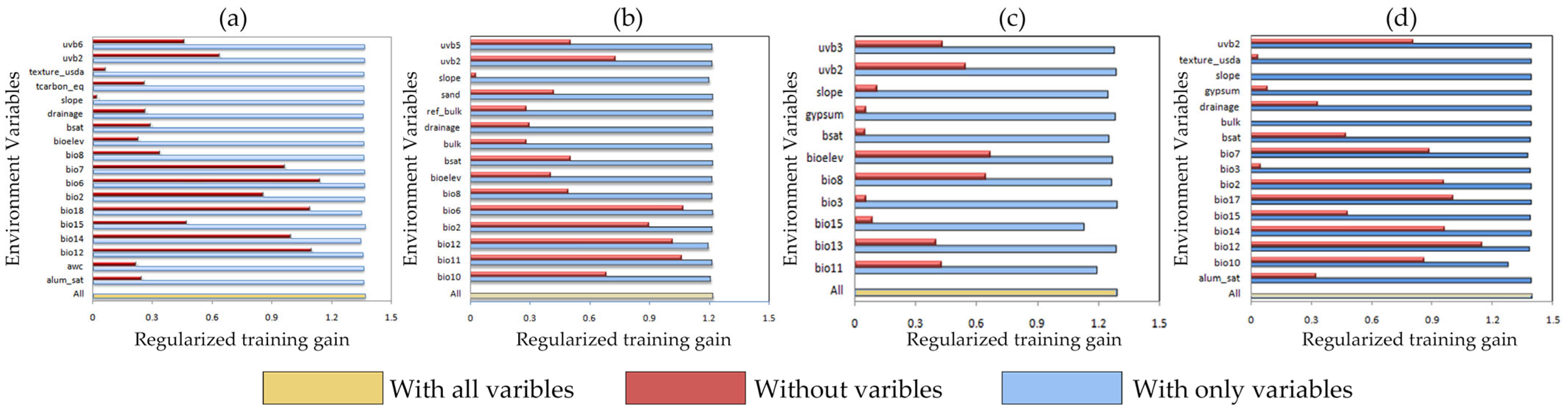
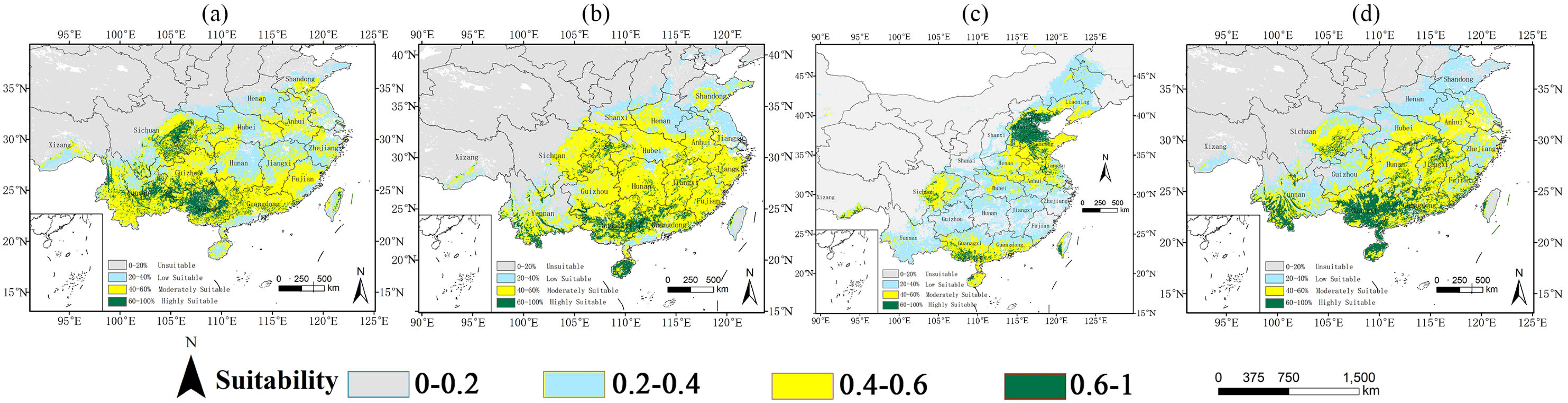


| Species | D. ambrosioides | C. argentea | A. palmeri | A. spinosus |
|---|---|---|---|---|
| Optimized FC | PT | QPT | LQT | LQT |
| Optimized RM | 1.5 | 0.9 | 1.2 | 0.3 |
| ΔAICc | 0 | 0 | 0 | 0 |
| Omission rate (5%) | 0.22 | 0.054217 | 0.166666 | 0.039473 |
| W-AICc | 1.34 × 10−3 | 1.10 × 10−3 | 1.72 × 10−3 | 9.11 × 10−4 |
| TSS | 0.6804 | 0.7057 | 0.712 | 0.7274 |
| Calibration figure |  | |||
| Species | AUC Type | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Avg. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D. ambrosioides | Training AUC | 0.9287 | 0.9348 | 0.9303 | 0.9286 | 0.934 | 0.9277 | 0.9279 | 0.9286 | 0.9324 | 0.929 | 0.9302 |
| Test AUC | 0.897 | 0.8588 | 0.8854 | 0.8708 | 0.8714 | 0.8899 | 0.8978 | 0.8923 | 0.8836 | 0.8929 | 0.884 | |
| C. argentea | Training AUC | 0.8986 | 0.902 | 0.9016 | 0.9036 | 0.9009 | 0.9017 | 0.9 | 0.9032 | 0.9015 | 0.9005 | 0.9014 |
| Test AUC | 0.8793 | 0.8705 | 0.8693 | 0.8648 | 0.8774 | 0.8734 | 0.8741 | 0.8683 | 0.8721 | 0.8715 | 0.8721 | |
| A. palmeri | Training AUC | 0.9153 | 0.9354 | 0.9197 | 0.9032 | 0.921 | 0.9239 | 0.9081 | 0.9227 | 0.9139 | 0.8975 | 0.9161 |
| Test AUC | 0.8532 | 0.8131 | 0.8874 | 0.923 | 0.9008 | 0.7818 | 0.7663 | 0.874 | 0.9309 | 0.9443 | 0.8675 | |
| A. spinosus | Training AUC | 0.9118 | 0.9151 | 0.9167 | 0.911 | 0.9076 | 0.9093 | 0.9118 | 0.9111 | 0.9138 | 0.9145 | 0.9123 |
| Test AUC | 0.9073 | 0.8932 | 0.8898 | 0.9052 | 0.9206 | 0.9155 | 0.8994 | 0.9079 | 0.9033 | 0.8942 | 0.9036 |
| Variable | Description | Contribution (%) | ||||
|---|---|---|---|---|---|---|
| D. ambrosioides | C. argentea | A. palmeri | A. spinosus | |||
| Climate | Bio2 | Mean Diurnal Range | — | 0.9 | — | 9.5 |
| Bio3 | Isothermality (Bio2/Bio7)(× 100) | — | — | 0.2 | 2.4 | |
| Bio6 | Min. Temperature of Coldest Month | 44.6 | 18.4 | — | — | |
| Bio7 | Temperature Annual Range | 2.9 | — | — | 20.8 | |
| Bio8 | Mean Temperature of Wettest Quarter | 0.6 | 0.8 | 12.3 | — | |
| Bio10 | Mean Temperature of Warmest Quarter | — | 6.5 | — | 25.2 | |
| Bio11 | Mean Temperature of Coldest Quarter | — | 56.4 | 15.7 | — | |
| Bio12 | Annual Precipitation | 33.3 | 7.1 | — | 1.7 | |
| Bio13 | Precipitation in Wettest Month | — | — | 1.9 | — | |
| Bio14 | Precipitation in Driest Month | 0.6 | — | — | 0.7 | |
| Bio15 | Precipitation Seasonality | 0.5 | — | 12.7 | 0.5 | |
| Bio18 | Precipitation of Warmest Quarter | 9.5 | — | — | — | |
| Altitude | elev | Elevation | 0.8 | 1.9 | 27.3 | — |
| slope | Slope Degree | 1.3 | 2.5 | 5.2 | — | |
| Soil | ALUM_SAT | 0.6 | — | — | 1.1 | |
| AWC | 0.5 | — | — | — | ||
| BSAT | 0.7 | 0.6 | 3.3 | 12.9 | ||
| DRAINAGE | 0.8 | 0.9 | — | 13.7 | ||
| GYPSUM | — | — | 1.7 | — | ||
| TCARBON_EQ | 0.5 | — | — | — | ||
| TETURE_USDA | 0.6 | — | — | 1 | ||
| BULK | — | 0.6 | — | — | ||
| REF_BULK | Reference Bulk Density | — | 0.5 | — | — | |
| UV-B | UVB2 | UV-B Seasonality | 0.7 | 1.6 | 16.2 | 10 |
| UVB3 | Mean UV-B of Highest Month | — | — | 3.6 | — | |
| UVB5 | Sum of Monthly Mean UV-B during Highest Quarter | — | 1 | — | — | |
| UVB6 | Sum of Monthly Mean UV-B during Lowest Quarter | 0.8 | — | — | — | |
| Period | Low Suit | Change% | Middle Suit | Change% | High Suit | Change (%) | Total Suit | Change% | |
|---|---|---|---|---|---|---|---|---|---|
| Current | 83.67 | — | 111.23 | — | 26.30 | — | 137.53 | — | |
| SSP126 | 2050s | 114.15 | 36.42 | 104.03 | −6.48 | 24.16 | −8.12 | 128.19 | −6.79 |
| 2070s | 121.23 | 44.89 | 105.49 | −5.16 | 19.34 | −26.44 | 124.84 | −9.23 | |
| 2090s | 89.38 | 6.82 | 113.89 | 2.38 | 28.25 | 7.43 | 142.14 | 3.35 | |
| SSP245 | 2050s | 120.92 | 44.52 | 99.61 | −10.45 | 28.17 | 7.14 | 127.78 | −7.09 |
| 2070s | 115.56 | 38.11 | 121.92 | 9.60 | 34.04 | 29.44 | 155.95 | 13.40 | |
| 2090s | 111.18 | 32.87 | 103.89 | −6.61 | 66.21 | 151.80 ↑ | 170.10 | 23.68 | |
| SSP585 | 2050s | 98.13 | 17.28 | 122.59 | 10.21 | 35.49 | 34.95 | 158.08 | 14.94 |
| 2070s | 112.84 | 34.86 | 117.18 | 5.35 | 57.04 | 116.91 | 174.22 | 26.68 | |
| 2090s | 76.31 | −8.80 ↓ | 131.34 | 18.07 | 28.32 | 7.70 | 159.66 | 16.09 | |
| Period | Low Suit | Change% | Middle Suit | Change% | High Suit | Change (%) | Total Suit | Change% | |
|---|---|---|---|---|---|---|---|---|---|
| Current | 60.36 | 0 | 144.68 | 0 | 23.13 | 0 | 167.81 | 0 | |
| SSP126 | 2050s | 112.85 | 86.96 | 114.72 | −20.71 | 47.55 | 105.60 | 162.27 | −3.30 |
| 2070s | 119.28 | 97.61 | 97.74 | −32.44 ↓ | 22.56 | −2.47 | 120.30 | −28.31 | |
| 2090s | 63.91 | 5.88 | 123.18 | −14.86 | 88.91 | 284.40 ↑ | 212.09 | 26.39 | |
| SSP245 | 2050s | 136.16 | 125.57 | 118.40 | −18.17 | 25.34 | 9.55 | 143.74 | −14.35 |
| 2070s | 134.66 | 123.10 | 114.87 | −20.60 | 44.22 | 91.20 | 159.10 | −5.19 | |
| 2090s | 128.23 | 112.43 | 113.94 | −21.25 | 59.86 | 158.78 | 173.79 | 3.57 | |
| SSP585 | 2050s | 147.404 | 144.20 | 114.584 | −20.80 | 29.32 | 26.76 | 143.90 | −14.25 |
| 2070s | 152.160 | 152.08 | 108.038 | −25.33 | 44.41 | 91.98 | 152.44 | −9.16 | |
| 2090s | 68.690 | 13.80 | 126.611 | −12.49 | 74.49 | 222.07 | 201.11 | 19.84 | |
| Period | Low Suit | Change% | Middle Suit | Change% | High Suit | Change (%) | Total Suit | Change% | |
|---|---|---|---|---|---|---|---|---|---|
| Current | 152.06 | 0 | 74.56 | 0 | 23.10 | 0 | 97.65 | 0 | |
| SSP126 | 2050s | 184.57 | 21.38 | 115.63 | 55.09 | 61.61 | 166.77 | 177.25 | 81.50 |
| 2070s | 164.01 | 7.86 | 92.47 | 24.02 | 63.78 | 176.15 | 156.25 | 60.00 | |
| 2090s | 146.43 | −3.71 ↓ | 87.81 | 17.78 | 37.86 | 63.90 | 125.67 | 28.69 | |
| SSP245 | 2050s | 167.83 | 10.37 | 96.23 | 29.07 | 80.26 | 247.51 | 176.49 | 80.73 |
| 2070s | 206.61 | 35.87 | 98.33 | 31.88 | 104.60 | 352.86 | 202.92 | 107.80 | |
| 2090s | 193.68 | 27.37 | 108.33 | 45.29 | 108.07 | 367.92 | 216.40 | 121.60 | |
| SSP585 | 2050s | 198.69 | 30.66 | 101.03 | 35.51 | 129.51 | 460.75 ↑ | 230.55 | 136.08 |
| 2070s | 223.35 | 46.88 | 120.59 | 61.73 | 121.22 | 424.83 | 241.80 | 147.61 | |
| 2090s | 172.76 | 13.61 | 102.37 | 37.30 | 48.23 | 108.81 | 150.60 | 54.21 | |
| Period | Low Suit | Change% | Middle Suit | Change% | High Suit | Change (%) | Total Suit | Change% | |
|---|---|---|---|---|---|---|---|---|---|
| Current | 89.64 | 0 | 91.19 | 0 | 30.83 | 0 | 122.02 | 0 | |
| SSP126 | 2050s | 51.58 | −42.46 | 104.75 | 14.88 | 92.3673 | 199.56 | 197.12 | 61.55 |
| 2070s | 71.32 | −20.43 | 126.69 | 38.94 | 44.2019 | 43.35 | 170.90 | 40.06 | |
| 2090s | 60.61 | −32.38 | 98.62 | 8.16 | 85.3605 | 176.83 | 183.98 | 50.78 | |
| SSP245 | 2050s | 73.11 | −18.43 | 118.39 | 29.84 | 51.9556 | 68.5 | 170.35 | 39.61 |
| 2070s | 57.10 | −36.30 | 104.71 | 14.83 | 93.7399 | 204.01 | 198.45 | 62.64 | |
| 2090s | 57.09 | −36.31 | 83.04 | −8.93 | 127.493 | 313.47 | 210.54 | 72.54 | |
| SSP585 | 2050s | 45.498 | −49.24 ↓ | 79.617 | −12.69 | 135.5 | 339.44 | 215.12 | 76.30 |
| 2070s | 56.800 | −36.63 | 81.402 | −10.73 | 141.982 | 360.46 ↑ | 223.38 | 83.07 | |
| 2090s | 54.573 | −39.12 | 99.133 | 8.72 | 90.541 | 193.63 | 189.67 | 55.44 | |
| Period | D. ambrosioides | C. argentea | A. palmeri | A. spinosus | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lon(E) | Lat(N) | Dist(km) | Lon(E) | Lat(N) | Dist(km) | Lon(E) | Lat(N) | Dist(km) | Lon(E) | Lat(N) | Dist(km) | ||
| Current | 109.18 | 26.798 | — | 111.01 | 27.78 | — | 113.52 | 31.83 | — | 111.30 | 26.75 | — | |
| SSP126 | 2050s | 108.58 | 28.858 | 236.44 | 110.42 | 29.99 | 251.64 | 113.88 | 33.74 | 215.36 | 111.48 | 29.00 | 250.52 |
| 2070s | 109.47 | 28.348 | 103.89 | 110.42 | 31.18 | 132.53 | 114.84 | 34.12 | 98.04 | 111.69 | 28.69 | 40.11 | |
| 2090s | 109.25 | 27.85 | 59.3 | 111.16 | 28.69 | 284.98 | 115.1 | 33.73 | 48.98 | 111.84 | 28.27 | 47.91 | |
| SSP245 | 2050s | 109.08 | 29.03 | 247.76 | 110.17 | 30.58 | 322.01 | 114.69 | 33.86 | 250.98 | 111.59 | 29.22 | 276.73 |
| 2070s | 109.42 | 29.26 | 42.6 | 110.36 | 30.22 | 43.91 | 114.57 | 33.99 | 17.91 | 111.74 | 28.98 | 30.59 | |
| 2090s | 109.19 | 29.73 | 56.44 | 110.95 | 31.43 | 144.81 | 114.81 | 34.32 | 42.98 | 111.41 | 29.36 | 52.46 | |
| SSP585 | 2050s | 109.93 | 29.02 | 258.03 | 110.04 | 30.27 | 292.44 | 114.31 | 31.44 | 264.12 | 111.58 | 29.04 | 256.32 |
| 2070s | 109.81 | 29.54 | 58.66 | 110.94 | 31.36 | 147.75 | 115.04 | 34.78 | 100.52 | 111.82 | 29.59 | 65.66 | |
| 2090s | 109.3 | 27.52 | 229.69 | 111.03 | 28.67 | 298.79 | 114.93 | 33.96 | 91.73 | 111.81 | 28.30 | 143.29 | |
| Species | Schoener’s D | Hellinger’s I | Stability (%) | Expansion (%) | Unfilling (%) | Equivalency (p) |
|---|---|---|---|---|---|---|
| D. ambrosioides | 0.2691 | 0.4620 | 0.9718 | 0.0282 | 0.5407 | 0.0693 |
| C. argentea | 0.0064 | 0.0532 | 0.8955 | 0.1045 | 0.9154 | 0.6436 |
| A. palmeri | 0.5094 | 0.7019 | 0.9935 | 0.0065 | 0.5106 | 0.0297 |
| A. spinosus | 0.0266 | 0.051 | 0.027 | 0.973 | 0.973 | 0.07 |
| Species | Top 2 Predictor (Contribution) | Optimal Range |
|---|---|---|
| D. ambrosioides | Bio6 (44.6) | 3.50–10.25 °C |
| Bio12 (33.3) | 1076.12–1956.18 mm | |
| Bio18 (9.5) | 593.96–991.04 mm | |
| C. argentea | Bio11 (56.4) | 4.96–26.79 °C |
| Bio6 (18.4) | 0.14–23.10 °C | |
| Bio12 (7.1) | 1097.36–2026.22 mm | |
| A. spinosus | Elevation (27.3) | 0–256.36 m |
| UVB2 (16.2) | 0.65–1.36 | |
| Bio11 (15.7) | −1.11–24.91 °C | |
| Bio15 (12.7) | 112.79–160.44% | |
| Bio8 (12.3) | 24.42–37.49 °C | |
| A. spinosus | Bio10 (25.2) | 26.06–36.10 °C |
| Bio7 (20.8) | 8.90–27.78 °C | |
| Drainage (13.7) | 0.6–1.02 | |
| BSAT (12.9) | 6.5–63.82% | |
| UVB2 (10) | 0.67–12.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.; Ye, X.; Zhao, Z.; Chen, S.; Liu, B. Comparative Analysis of Habitat Expansion Mechanisms for Four Invasive Amaranthaceae Plants Under Current and Future Climates Using MaxEnt. Plants 2025, 14, 2363. https://doi.org/10.3390/plants14152363
Lin M, Ye X, Zhao Z, Chen S, Liu B. Comparative Analysis of Habitat Expansion Mechanisms for Four Invasive Amaranthaceae Plants Under Current and Future Climates Using MaxEnt. Plants. 2025; 14(15):2363. https://doi.org/10.3390/plants14152363
Chicago/Turabian StyleLin, Mao, Xingzhuang Ye, Zixin Zhao, Shipin Chen, and Bao Liu. 2025. "Comparative Analysis of Habitat Expansion Mechanisms for Four Invasive Amaranthaceae Plants Under Current and Future Climates Using MaxEnt" Plants 14, no. 15: 2363. https://doi.org/10.3390/plants14152363
APA StyleLin, M., Ye, X., Zhao, Z., Chen, S., & Liu, B. (2025). Comparative Analysis of Habitat Expansion Mechanisms for Four Invasive Amaranthaceae Plants Under Current and Future Climates Using MaxEnt. Plants, 14(15), 2363. https://doi.org/10.3390/plants14152363





